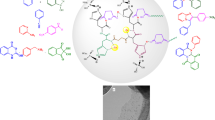
Abstract
Self-assembled peptide nanofibers have attracted extensive attention; they offer unique templating possibilities, which allow the synthesis of nanostructured materials with high surface areas, and also act as organocatalysts for various transformations in organic chemistry. In the present work, peptide nanofibers as hydrogen-bonding organocatalysts have been developed as efficient organocatalysts for three-component Groebke condensation reactions of aldehydes, isocyanides, and 2-aminopyridines in PEG to afford the corresponding 3-aminoimidazo [1, 2-a] pyridines in high yields without any additives. The key advantages of catalytic systems are (1) using peptide nanofibers as powerful hydrogen-bonding organocatalysts for the synthesis of 3-aminoimidazo [1, 2-a] pyridines, (2) having high catalytic activity, and (3) performing the reactions which can be carried out in PEG, as green solvent instead of the usually used organic solvents. This catalyst could be recycled and reused at least for four times without noteworthy loss of its activity.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among nitrogen-fused azoles, imidazo [1, 2-a] pyridines have received considerable attention in recent years, due to their applications in medicinal chemistry [1], organometallics [2], and material science [3]; hence, a variety of synthetic methodologies have been developed for the preparation of these heterocyclic compounds.
There exist three important approaches: (1) condensation of 2-aminopyridine with α-halocarbonyl compounds [4], (2) copper-catalyzed three-component reaction of 2-aminopyridines, aldehydes, and alkynes [5], and (3) reaction of 2-aminopyridine with aldehydes and isonitriles [6]. For the third approach, reaction involves non-concerted [4 + 1] cycloaddition combination between the iminium species and isocyanides, which gives direct access to various imidazoazines.
However, some of these procedures for the synthesis of 3-aminoimidazo [1, 2-a] pyridines were designed based on reaction of 2-aminopyridine with aldehydes and isonitriles. This reaction was carried out by using numerous catalysts such as scandium triflate [7] and Tin(II) chloride dihydrate [8] and by using some acidic catalysts such as silica sulfuric acid [9], p-toluenesulfonic acid [10], as well as ionic liquid [11] and solid-phase synthesis [12]. Although all of these methods are beneficial, some of them have one or more of the following drawbacks: long reaction times, low yields of products, use of toxic organic solvents, difficulties in work-up procedure, harsh reaction conditions, and special instrumentation. On the other hand, green chemistry involves design for reduction or elimination of hazardous materials through chemical production that this can be achieved by designing the appropriate catalyst.
Recently, considerable attention toward the use of nanoparticles in catalysis studies has been widely developed [13,14,15]. In this regard, a variety of nanomaterials or nanosupports were introduced in catalytic reactions such as graphene oxide [16], nanofibers [17], magnetic nanoparticles [18], polymers [19], boehmite [20], zeolites [21], dendrimers [22], and MCM-41 [23]. Nature inspires synthetic peptide nanofiber networks that have a wide range of potential applications due to their several unique properties such as high surface area that makes them templates for inorganic materials. For example, Indrajit Maity reported the fabrication of peptide-capped Pd nanoparticles, for efficient Suzuki coupling reactions under aqueous conditions. Shao reported coupling reactions of aromatic halides in the presence of palladium catalyst immobilized on poly(vinylalcohol) nanofiber. Furthermore, Sahay reported synthesis and characterization of CuO nanofibers and investigation for its suitability as blocking layer in ZnO NPs-based dye-sensitized solar cell and as photocatalyst in organic dye degradation [24,25,26]. Alternatively, the self-assembly approach is more attractive for the preparation of nanofiber. Self-assembly method is the way that molecules organize and arrange themselves into a template through noncovalent interactions including van der Waals, electrostatic, hydrogen-bonding, and stacking interactions.
In the present work, peptide nanofibers as hydrogen-bonding organocatalyst have been developed as efficient organocatalyst for the synthesis of 3-aminoimidazo [1, 2-a] pyridine derivatives.
In our newly published work, we have reported the synthesis of peptide nanofibers using arginine as building block (Scheme 1). We showed that self-assembled peptide formed at pH 7 [17]. Peptide nanofiber features led us to further study the scope of peptide nanofiber in the catalysis of 3-aminoimidazo [1, 2-a] pyridines.
It was proposed that at pH 7 the acidity of the guanidinium cation, (C(NH2)+3) N–H, is increased by the formation of a hydrogen bond between 2-aminopyridine and guanidinium cation, thus providing a stronger hydrogen-bonding interaction with the aldehyde.
Experimental
Chemicals and solvents used in this work were obtained from Sigma-Aldrich, Fluka, or Merck chemical companies and used without further purification.
Preparation of peptide nanofiber (PNF)
Peptide nanofiber was prepared via our previously published method [17]; also the structure of this nanofiber is fully characterized in this report.
General procedure for the synthesis of 3-aminoimidazo [1, 2-a] pyridines
A catalytic amount of peptide nanofibers (PNF) (200 µl) was added to a mixture of benzaldehyde (1 mmol), 2-aminoazine (1 mmol), and cyclohexylisocyanide (1 mmol). The reaction mixture was then stirred at 80 °C under atmospheric conditions. Reaction progress was monitored by TLC. Upon the reaction completion, the product was extracted with ethyl acetate (2 × 10 ml). Then, the resultant organic layer was washed with distilled water, dried over anhydrous sodium sulfate, and concentrated to give the crude solid crystalline product.
Spectral data of synthesized 3-aminoimidazo [1, 2-a] pyridines
2-(4-Hydroxyphenyl)-N-cyclohexyl-imidazo[1,2-a] pyridin-3-amine
White crystals; mp: 230–233 °C; 1H NMR (CDCl3, 400 MHz): δ = 1.11–1.32 (10H, m), 2.51–2.54 (1 H, m), 4.72 (1H, s), 6.84–6.87 (3 H, m), 7.11–7.16 (1 H, m), 7.42–7.44 (d, J = 9.2 Hz, 1H), 8.07–8.04 (d, J = 9.6 Hz, 2H), 8.29–8.27 (d, J = 9.7 Hz, 1H), 9.48 (1H, s). 13C NMR (CDCl3, 100 MHz): δ = 24.9, 25.9, 34.0, 56.7, 111.4, 115.5, 116.8, 123.6, 123.7, 124.7, 126.2, 128.3, 135.9, 140.7, 156.9 (Table 2, entry 8).
2-(3-Hydroxyphenyl)-N-cyclohexyl-imidazo[1,2-a]pyridin-3-amine
White crystals; mp: 261–263 °C; 1HNMR (CDCl3, 400 MHz): δ = 1.11–1.74 (10H, m), 2.53 (1 H, m), 4.72 (1H, s), 6.86 (1H, m), 6.90–6.88 (1H, m), 7.15–7.21 (2H, m), 7.47 (d, J = 9.2 Hz, 1H), 7.67–7.69 (2H, m), 8.32 (t, J = 3.4 Hz, 1H), 9.35(1H,s). 13CNMR (CDCl3, 100 MHz): δ = 25.0, 25.9, 34.0, 56.9, 111.6, 113.9, 114.3, 117.2, 117.8, 123.7, 124.0, 126.2, 129.5, 135.1, 136.5, 147.7, 157.7 (Table 2, entry 9).
2-(Cinnamaldehyde)-N-cyclohexyl-imidazo[1,2-a] pyridin-3-amine
White crystals; mp: 107–110 °C; 1H NMR (CDCl3, 400 MHz): δ = 1.11–1.74 (10H, m), 2.53 (1 H, m), 4.72 (1H, s), 6.84–6.87 (1H, m), 7.11–7.16 (1 H, m), 7.24–7.28 (1H, m), 7.34–7.42 (m, 5H), 7.59 (d, J = 9.7 Hz, 2H), 8.1 (d, J = 6.8 Hz, 1H). 13C NMR (CDCl3, 100 MHz): δ = 25.0, 25.9, 34.0, 56.9, 115.5, 116.8, 120.35, 123.5, 124.3, 126.6, 127,5 128.5, 129.2, 134.3, 138.0, 141.7 (Table 2, entry 9).
Catalytic studies
After preparation and characterization of peptide nanofiber, catalytic activity of this compound was investigated for the direct synthesis of 3-aminoimidazo [1, 2-a] pyridines. The model reaction of benzaldehyde, 2-aminopyridine, and isocyanide in the presence of woven peptide nanofiber was designed, and various parameters were optimized to improve. The reaction is further explained, and the results are summarized in Table 1. We initially screened different organic solvents in the model reaction, including PEG, H2O, DMSO, and DMF. We found that by employing PEG as solvent the best yield of coupled product was achieved at 80 °C. It should be noted that in the absence of catalyst the observed yield was very low. Then, the amount of woven nanofiber was controlled; desired product was obtained in 95% yield with 200 µl of catalyst; when the reaction was conducted at 25 °C, yield was very low (Table 1, entries 6). The ideal temperature for the reaction was found to be 80 °C.

With optimal conditions in hand, a broad range of structurally diverse aldehydes bearing electron-releasing or electron-withdrawing substituents; 2-aminopyridine; and cyclohexyl isocyanide were successfully condensed via a one-pot combination (Table 2).
It seems that the nature of the substituents on the aromatic ring of benzaldehyde exerts different influences. It is of interest to note that electron-withdrawing groups give higher yields of products compared to the electron-donating groups. Furthermore, halogen-bearing aldehydes give suitable reaction yields in reasonable times (Table 2).
Proposed mechanism of described condensation reaction is outlined in Scheme 2 based on previously reported mechanism for this combination reaction [18].
Although the exact reaction mechanism is not understood completely, we suggest woven nanofiber can activate the reaction of 2-aminopyridine and aldehyde to give the corresponding iminium compound. Then subsequent [4 + 1] cycloaddition between the iminium species and the isocyanide gives direct access to various imidazoazines. Finally, in order to assess the present protocol with respect to other reported methods for the synthesis of 3-aminoimidazo [1, 2-a] pyridines the catalytic performance of the peptide nanofiber was compared with some of the previously reported catalysts (Table 3). However, some of the reported procedures have some demerits such as the requirement of expensive and excess amount of catalyst, longer reaction time, difficulties in work-up procedure, harsh reaction conditions, and using toxic solvents. In contrast, eco-friendly nature and convenient recoverability of described catalytic system make this method as an efficient candidate for the potential applications in organic synthesis.
The possibility of recovering and recycling of the catalyst is an important aspect from a different point of views such as environmental concerns and commercial applicability. In order to this issue, reusability of PNF has been examined for the model reaction. After the reaction completion, the reaction mixture was extracted twice with ethyl acetate because catalyst was insoluble in ethyl acetate, it could be easily isolated for reuse in this solvent. The catalyst could be recycled and reused at least four times without noteworthy loss of activity of PNF (Fig. 1).
References
P. Liu, L.S. Fang, X.S. Lei, G.Q. Lin, Tetrahedron Lett. 51, 4505–4608 (2010)
G. Song, Y. Zhang, X. Li, Organometallics 27, 1936–1943 (2008)
A. Hu, G.T. Yee, W. Lin, J. Am. Chem. Soc. 127, 12486–12487 (2005)
A. Gueiffier, S. Mavel, M. Lhassani, A. Elhakmaoui, R. Snoeck, G. Andrei, O. Chavignon, J.C. Teulade, M. Witvrouw, J. Balzarini, E.D. Clercq, J.P. Chapat, J. Med. Chem. 41, 5108–5112 (1998)
N. Chernyak, V. Gevorgyan, Angew. Chem. Int. Ed. 49, 2743–2746 (2010)
E.F. DiMauro, J.M. Kennedy, J. Org. Chem. 72, 1013–1016 (2007)
G.B. Blackburn, P. Fleming, K. Shiosaki, S. Tsai, Tetrahedron Lett. 39, 3635–3638 (1998)
A. Shaabani, E. Soleimani, A. Sarvary, A.H. Rezayan, A. Maleki, Chin. J. Chem. 27, 369–371 (2009)
A. Shaabani, E. Soleimani, A. Maleki, Monatshefte Chem. 138, 73–76 (2007)
A. Shaabani, E. Soleimani, A. Maleki, Synth. Commun. 38, 1090–1095 (2008)
A. Shaabani, E. Soleimani, A. Maleki, Tetrahedron Lett. 47, 3031–3034 (2006)
C. Blackburn, B. Guan, Tetrahedron Lett. 41, 1495–1500 (2000)
M.A. Zolfigol, N. Bahrami-Nejad, F. Afsharnadery, S. Bagheri, J. Mol. Liq. 221, 851–859 (2016)
M.A. Zolfigol, M. Navazeni, M. Yari, R. Ayazi-Nasrabadi, RSC Adv. 6, 92862–92868 (2016)
K. Amiri, A. Rostami, S. Samadi, A. Rostami, Catal. Commun. 86, 108–112 (2016)
A. Sengupta, C.L. Su, C.L. Bao, C.T. Nai, K.P. Loh, ChemCatChem 6, 2507–2511 (2014)
A. Ghorbani-Choghamarani, Z. Taherinia, RSC Adv. 6, 59410–59421 (2016)
A. Ghorbani-Choghamarani, L. Shiri, G. Azadi, RSC Adv. 6, 32653–32660 (2016)
D. Astruc, F. Lu, J.R. Aranzaes, Angew. Chem. Int. Ed. 44, 7852–7872 (2005)
M. Hajjami, A. Ghorbani-Choghamarani, R. Ghafouri-Nejad, B. Tahmasbi, New J. Chem. 40, 3066–3074 (2015)
S. Rayati, S. Shokoohi, E. Bohloulbandi, J. Iran. Chem. Soc. 13, 1983–1991 (2016)
J. Kofoed, J.L. Reymond, Curr. Opin. Chem. Biol. 9, 656–664 (2005)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Khanmoradi, RSC Adv. 6, 56549–56561 (2016)
I. Maity, D.B. Rasale, A.K. Das, RSC Adv. 4, 2984–2988 (2014)
L. Shao, W. Ji, P. Dong, M. Zeng, C. Qi, X.M. Zhang, J. Appl. Catal. 267, 413–417 (2012)
R. Sahay, J. Sundaramurthy, P.S. Kumar, V. Thavasi, S.G. Mhaisalkar, S. Ramakrishna, Solid State Chem. 186, 261–267 (2012)
R.S. Varma, D. Kumar, Tetrahedron Lett. 40, 7665–7669 (1999)
M. Adib, E. Sheikhi, N. Rezaei, Tetrahedron Lett. 52, 3191–3194 (2011)
A. Shaabani, E. Soleimani, A. Maleki, J. Moghimi-Rad, Synth. Commun. 38, 1090–1095 (2008)
A. Habibi, Z. Tarameshloo, S. Rostamizadeh, A. M. Amani, Lett. Org. 9, 155–159 (2012)
S. Sadjadi, M. Eskandari, Monatshefte Chem. 143, 653–656 (2012)
M.L. Bode, D. Gravestock, S. Moleele, S.C.W. van der Westhuyzen, S.C. Pelly, P.A. Steenkamp, L.A. Nkabinde, Bioorg. Med. Chem. 19, 4227–4237 (2011)
A. Habibi, Z. Tarameshloo, S. Rostamizadeh, A.M. Amani, Lett. Org. Chem. 9, 155–159 (2012)
S. Rostamnia, A. Hassankhani, RSC Adv. 3, 1826–18629 (2013)
S. Vidyacharan, A.H. Shinde, B. Satpathi, D.S. Sharada, Green Chem. 16, 1168–1175 (2014)
Acknowledgements
Authors thank the research facilities of Ilam University, Ilam, Iran, for financial support of this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbani-Choghamarani, A., Taherinia, Z. Eco-friendly synthesis of 3-aminoimidazo [1, 2-a] pyridines via a one-pot three-component reaction in PEG catalyzed by peptide nanofibers: as hydrogen-bonding organocatalyst. J IRAN CHEM SOC 17, 59–65 (2020). https://doi.org/10.1007/s13738-019-01744-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01744-w