Abstract
Dendrimers are hyper-branched organic compounds characterized via a three-dimensional structure possessing functional groups on the surface. These terminals groups can be simply modified to enhance the functionality of dendrimers and produce biocompatible and versatile products. They are a promising agent for nanomedicine applications because of their unique properties, including nanoscale size, globular shape, and high reactivity, solubility in water, internal cavities, and comfortable synthesis methods. The use of dendrimers as drug delivery systems have received great attention from researchers. Dendrimers can be applied as carriers for different therapeutic agents. They can reduce the toxicity of drugs and increase their efficacy. This review provides a general outline of the structure and types of dendrimers, the synthesis of dendrimers, and applications in the nanomedicine field with emphasis on drug delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanomedicine is a novel field that utilizes engineered materials of 100 nm or less in size to maintain and improve human health. A broad variety of nanomaterial and nanostructures are involved in nanomedicine including, nanoparticles, nanotubes, nanoporous membranes, nanofibers, and very others [1]. Dendrimers are one of the nanostructures that have a key role in emerging field of nanotechnology. Initially, polymer technology was focused on linear polymers. However, it was later revealed which features of widely branched polymers can be various from common polymers [2]. These hyper branched molecules which are called dendrimers, are identified as the fourth main architectural class of polymers after the 3 good-known types (linear, branched, and cross-linked polymers) [3]. In the two last decades, dendrimers have received very interest due to their applications in the broad range of fields, like electronics, catalysis, and biomedical applications [4, 5]. Dendrimers are highly branched, globular, multivalent, mono-dispersed macromolecules with the well-defined symmetric and three-dimensional structures [6, 7]. Dendritic structures are common motifs in nature that are mostly used when a special function needs to be increased or exposed. The dendrimer structure comprises a core molecule, branches, internal voids, and many terminal groups [8]. Each component of the dendrimer executes a special role and at the same time describes some other features when it grows from generation to generation [9]. The 3-D shape of the dendrimers is affected by the core. The internal voids affect the host–guest properties of the dendrimers. The dendrimers surface can be further modified or polymerized through functional peripheral groups. The overall morphology of the dendrimers is affected by both core and type/number of interior branching units [10].
Most dendrimers are synthesized by either a divergent or convergent route [11]. The first victorious attempt to synthesis dendritic structures by divergent route was performed by Vögtle et al. in 1978 [12], followed by Denkewalter in 1981 [13], Tomalia in 1983 [14] and Newkome in 1985 [15]. The convergent synthetic approach introduced by Frechet in 1990 [16].
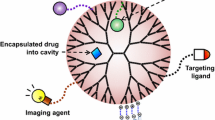
Dendrimers possess unique properties, including globular shape, internal hydrophilic or hydrophobic cavities, functional groups at the periphery, and very low polydispersity due to their 3-D structure [17, 18]. Analytical techniques from both molecular chemistry and polymer chemistry are utilized to characterize these properties. Dendrimers are related to molecular chemistry due to the step by step synthesis method utilized during their synthesis. On the other hand, they are associated with polymer chemistry because of their repetitive structures which are constructed from monomers [19]. The many chain-ends of dendrimers are responsible for their great reactivity and solubility. The physical and chemical properties of dendrimers can be simply change by modification functional groups at the end of the arms [20]. The dendrimers solubility is potently affected by surface groups' nature. So that, dendrimers with hydrophilic surface groups are dissolved in polar solvents, while those with hydrophobic groups on their surface are soluble in nonpolar solvents [21]. Molecular mass and size of dendrimers as monodisperse macromolecules, can be specially regulated during polymerization process. By increasing the molecular mass of dendrimers, their intrinsic viscosity goes via a maximum at the fourth generation and then begins to decrease [22]. The polydispersity of the dendrimers can be minimized by manipulating their size and degree of branching through using special initiator cores [23]. The interesting nanoscale structure of dendrimers gives them some structural benefits over the linear polymers, including fast cellular entry, target-ability, decreased macrophage uptake, and very easy pass across biological barriers through transcytosis [24]. However, dendrimers have disadvantages over the linear polymers such as higher cost of preparation and development of control assays to assure the safety and quality of dendrimers [25]. The unique properties of dendrimers as well as their excellent biocompatibility and nonimmunogenicity, makes them applicable to different biomedical research [26]. Dendrimers possess several unique properties due to their globular morphology and existence of internal voids on their surface. The most significant one is the probability for encapsulating guest molecules in macromolecule interior [27]. The tree-like nanostructures of dendrimers make them attractive systems for drug delivery because of their nanosize range and multiple surface groups that provide simple penetration and excellent targeting, respectively [28]. The main goal of proper drug delivery system is to improve the bioavailability of drugs, reduce the side effects, enhance the retention time, target the drugs cells, and provide prolonged therapeutic effects. Therefore, dendrimers-based drug delivery systems emerge to be the most promising system to meet the needs of an ideal drug delivery system. At present, different dendrimers are made based on their structural properties, thereby presenting novel scaffolds for drug delivery. VivaGel, is the first dendrimer-based commercial biomedical product [29]. Because of the promising structures of dendrimers, drugs can be effectively loaded into them through several interactions [30]. The modification and optimization of the ratio or number of dendrimers surface groups can be affect biodistribution, controlled release of drug from the interior cavities of dendrimers, therapy dosage, and receptor-mediated targeting [31]. The globular structure of dendrimers gives them similar shapes and sizes as special proteins and biomolecules and therefore makes them ideal as biomimics [32]. Unlike conventional polymers, dendrimers have received significant attention in drug delivery applications because of their exact molecular weight, polyvalency, biocompatibility, and high water solubility [33]. The surfaces of dendrimers are amenable to the chemical modifications, and the interior is determined through the availability of a considerable amount of solvent-filled empty space which might be appropriate for host–guest chemistry [34]. The exact nano sizes, different shapes, surface chemistry, and architecture of dendrimers differentiate them as proper model systems for understanding a broad range of important nanomedicine issues such as drug or DNA delivery carriers [35], nanosensors [36, 37], Magnetic Resonance Imaging (MRI) contrast agents [38], and tissue engineering scaffolds [39]. Wide types of compositionally differentiated dendrimers are available from various sources. The Frechet-type poly(ether) dendrimers, Majoral-type phosphorous-containing dendrimers, Meijer-type poly(propyleneimine) (i.e. PPI, Astramol) dendrimers, Tomalia-type poly(etherhydroxylamine) (i.e. PEHAM, Priostar) dendrimers, and Tomalia-type poly(amidoamine) (i.e. PAMAM, Starburst) have all been applied extensively for biomedical evaluation.
The present review provides a general outline of the structure and types of dendrimers, dendrimers synthesis, and their applications in nanomedicine with emphasis on drug delivery.
2 Structure and Types of Dendrimers
2.1 Structure
The term “dendrimer” originated from two Greek words; “dendron” meaning tree and “meros” meaning part. Structure of typical dendrimers comprises of 3 main components: (a) a central core with a single atom or molecule which have at least two similar chemical functions; (b) interior layers formed with repetitive branching units; and (c) the terminal functional groups present in the outer surface, which describe the major properties of dendrimer (Fig. 1) [40, 41]. The first component, central core, encapsulates different chemical groups that show unique properties because of the specific nanoenvironment surrounded through wide dendritic branching. The second component, different inside layers formed of repetitive units supply an adaptable space produced within cavities of dendritic building blocks, that have the ability to entrap different tiny guest molecules. The third component of the dendrimers is the multivalent surface that can incorporate a great number of functionalities that can interact with the external environment, therefore describing macroscopic properties of dendrimers [17]. The generation of the dendrimer is specified as the number of branching points from the central core to the surface groups (Fig. 1) [42]. For instance, a dendrimer having two branching points is denoted as generation (G) 2 dendrimer. Enhancement in each generation of a dendrimer, about doubles the molecular mass of the dendritic structure [43].
2.2 Types of Dendrimers
Dendrimers are distinguished on the base of their shape, terminal functional groups, and internal cavities (Table 1).
3 Synthesis of Dendrimers
3.1 Divergent Synthesis
The name divergent comes from the synthesis method in which dendrimer grows around the core. In this method, the dendrimers growth originates from a core, that renders functional groups available to react with monomers, achieving generation 1 dendrimer. Then, the fresh periphery of dendrimer is activated to react with more monomers (Fig. 2). Divergent growth method results into the 3-D architecture of dendrimers by consecutive addition of generations to the core [47]. The formation of defective dendrimers that arise from incomplete growth and side reactions, is the main disadvantage of this method. To avoid these imperfections and the side reactions, it’s recommended to utilize a large excess of reagents [2].
3.2 Convergent Synthesis
This method begins from the dendrimer periphery and proceeds towards its core. The branching units are grown and connected to other groups. When growing branches are large sufficient, they attached to a core for the formation of a complete dendrimer (Fig. 2) [16]. The convergent synthesis has many advantages over the divergent synthesis. (a). It allowing better structure control of dendrimers because of the low possibility of side reactions, that leads to probability to rise functional groups throughout the structure of the dendrimer, also the formation of symmetric and well-defined dendrimers [60]. (b). The amount of reagents is reduced in this method. (c). Purer products are obtained in this method because of easy purification after each synthesis step [61]. Despite the mentioned advantages, this method offers several problems: (a) the surface functionalization of dendrimer is exactly difficult and (b) the production of high generation dendrimers can be a problem because of steric barrier observed in them [62].
3.3 Hypercores and Branched Monomer Growth
This method is characterized by pre-assembly of oligomeric moieties to produce the large dendrimers in relatively few steps. Since for the synthesis of higher generations of dendrimers the fewer steps are required, hypercores and branched monomers growth is the useful method than to convergent method [22].
3.4 Double Exponential Growth
In this method the monomers are provided from a single starting material for divergent and convergent growth. Then, two products obtained from before step, are reacted together to give an orthogonally protected trimer. This trimer can be applied to repeat the growth process of the dendrimer [63].
3.5 Click Chemistry
This method was rapidly used to prepare novel structures of dendrimers. The approach is to produce carbon-rich dendrimers and safe for the environment. Copper-assisted azide-alkyne cycloaddition (CuAAC), thiol-ene click reaction (TEC), and thiol-yne click reaction (TYC) are the most reactions which used to synthesis of dendrimers. Due to mild reaction conditions, coupling specificity, and quantitative synthetic yields of click reactions, these reactions received very attention [64].
4 Applications of Dendrimers
Dendrimers can be conjugated to various bio-functional moieties including imaging and detection agents, targeting ligands, pharmaceutical agents, and biomolecules [65]. Dendrimer technology has different potential applications in the biomedical field due to their three unique structural properties, core, branching points, and terminal functional groups [66].
4.1 Dendrimers in Photodynamic Therapy (PDT)
PDT is a hopeful method for cancer treatment based on a combination of light, photosensitizers, and oxygen [67]. These photosensitizers have limitations, including low specificity for tumor tissues and weak water solubility. Hence, dendrimers are being used to overcome these limitations, as well as to increase photodynamic therapeutic effects of photosensitizers [68]. The photosensitizer can be conjugated into dendrimer in various ways. It may construct a covalent bond with the dendrimer core by binding to a terminally placed functional group, or conjugate with interior component. Dendrimers have been utilized for delivery of 5-aminolevulinic acid (a natural precursor of photosensitizer protoporphyrin 1X) that enhanced level of porphyrin accumulation in cells, which further leaded to toxicity [69]. Recently, poly(ethylene glycol)-cholesterol molecules were utilized to modify PAMAM dendrimers to form self-assembled dendrimer-PEG-Chol nanoparticles (termed DPC NPs). The cholesterol modification has been found to enhance the cellular uptake of DPC NPs. To obtain DPCC NPs for PDT, the DPC NPs are developed as drug carriers to encapsulate the chlorin e6 (Ce6, a photosensitizer). Also, to enhance the PDT efficacy of the DPCC NPs in solid tumors, MnO2 was synthesized in the Ce6-loaded DPC NPs in situ to obtain TME-responsive Ce6/MnO2@DPC NPs (termed DPCCM NPs) [70].
4.2 Dendrimers as MRI Contrast Agents
The dendrimers with conjugated MRI contrast agents are useful because of their tumor-targeting ability. MRI is an extensively used technique for diagnosis of disease, however, utilization of the contrast agents, typically gadolinium-based is essential because of low sensitivity of MRI. Even though contrast agents increase the MRI signal, they have several disadvantages such as no tissue specificity, low contrast efficiency, and fast excretion. To avoid requiring to exert a high dose of contrast agent to overcome these disadvantages, researchers have focused on adding contrast ligands to a single core scaffold [10]. As mentioned earlier, dendrimers can be used as MRI agents due to their unique properties, including adaptable nature, increased relaxivities when gadolinium is conjugated, the facility of conjugation to a broad diversity of diagnostic agents, and potential for targeting [71]. Among various types of dendrimers that are available in commercial, Starburst PAMAM dendrimers are most commonly utilized [72]. Availability generations 1–9 dendrimers and the presence of various terminal groups (including amino, succinamic acid, amidoethylethanolamine, amidoethanol, and carboxylate) on their surface, are advantages of Starburst PAMAM dendrimers. One of the main properties for imaging is the great rise in the density of surface groups when the increase of the dendrimer generation [73]. The charge of the terminal groups affects the intracellular transport route, solubility, and cytotoxicity of dendrimers [74, 75]. All of these are design considerations to optimize interactions between dendrimers and their microenvironment in the tissue to be imaged with MRI [71]. Recently, radical dendrimers have developed with the aim to be used as MRI contrast agent. Zhang and colleagues have synthesized two generations of biocompatible oligoethylene glyco dendrimers containing 5 and 20 equivalent oligoethylene glycol (OEG) branches. The obtained radical dendrimers exhibited high molecular relaxivity and full water-solubility at physiological pH [76].
4.3 Dendrimers in Tissue Engineering
The goal of applications of tissue engineering is the regeneration of the native extracellular matrix (ECM) and finally replacement of the scaffold by using encapsulated cells. Hence, the scaffold should be biodegraded at a rate matching the rate of novel ECM biosynthesis [31]. Dendrimers are used in tissue engineering because of their capability to incorporate lipophilic and hydrophilic drugs and also their ability to direct site-specific delivery. They can be produced with favorite nontoxic properties in the surface of a 3-D scaffold which serves as a platform for the cells' growth in the ECM comprising the necessary biomolecules, hormones, and growth factors [77]. Moreover, the extremely branched and multivalent nature of dendrimers is a property that permits them to be applied in tissue engineering. This property makes them proper for use in different tissue engineering applications, as modulators of surface charge, crosslinking agents, and surface chemistry [78]. Dendrimers can have more control over their factors such as proliferation rates and biodegradation profiles through systematically changing their terminal group chemistry, generation size, and concentration [79]. The dendrimers integration into common scaffold polymers like proteins, synthetic polymers, and carbohydrates, leads to the development of hybrid scaffolds that have novel mechanical, physical, and biochemical properties [77]. Some scaffolds were designed with dendrimers, including hydroxyapatite composite containing poly(caprolactone) chains conjugated to a poly(L-lysine) dendritic core [80], linear polycaprolactone/hydroxyapatite hybrids [81], dexamethasone-loaded carboxymethyl chitosan/poly(amidoamine) dendrimers [82], and N-hydroxy succinimide/1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (NHS/EDC) cross-linked scaffold [10].
4.4 Dendrimers in Gene Transfection
The ability to deliver genetic material to the required parts of eukaryotic cells can permit treatment of a diversity of genetic disorders [31]. However, there are several challenges for gene delivery into the nucleus and cytoplasm of a cell. The main challenge is the successful delivery of genes utilizing safe and efficient vectors without deactivating or damaging the DNA [83]. Dendrimers can be used as suitable vectors for the transfection of gene. The dendrimers unique properties such as flexible and hyperbranched structure, tunable surface functional groups, the high charge density, and ability to deliver cells without induction of toxicity, make them capable of directing gene delivery [33]. These properties allow dendrimers to form nanostructures with DNA, which are known as “dendriplexes”. Dendriplexes (dendrimer–nucleic acid complexes) protect nucleic acids from being degraded by nuclease as they are able to condense them. Nucleic acids conjugate with dendrimers by electrostatic interactions and then they are entered into the internal structure by various endocytic mechanisms [84]. PAMAM dendrimers have been examined as genetic materials vectors. They possess terminal amino groups which interact with phosphate groups of nucleic acids. These tertiary amino groups due to their great density are responsible for the “proton sponge” effect which eventually results in the endosomal escape of dendriplexes [83]. Several reports have been published about the utilization of PAMAM dendrimers as gene delivery systems. TEA-cored PAMAM dendrimers are greatly flexible which enhances their binding with DNA. The G2 PAMAM is highly effective in delivering as well as it is less cytotoxic [56]. Recently, Gorzkiewicz et al. have synthesized the two new lysine-based dendrimers (D3K2 and D3G2) and have investigated their transfection potential in two cell line models. They showed that cationic D3K2 dendrimer possess high transfection efficiency, so that it is able to enhance intracellular accumulation of large nucleic acid molecules like plasmids [85].
4.5 Dendrimers in Drug Delivery
Dendrimers have been proved as an ideal drug-delivery system because of a particular kind of their structure which can be controlled, as well as their terminal functional groups which showed higher chemical reactivity compared with other polymers [86]. Dendrimer with positively charged surface possesses well features to interact with negatively charged cell membranes that resulting in the dendrimer' applicability for intracellular delivery of drugs [87]. The ability of dendrimers to conjugate or encapsulate high molecular weight drugs and their high uptake by cells are the main properties of dendrimers that make them suitable drug carriers [88]. In addition, dendrimers have the ability to extend the circulation time of the drugs, increase the drugs' stability, protect drugs from the environment, and target the drugs into the tissue [89].
4.5.1 Mechanism of Drug–Dendrimer Interactions
The interactions of the drug molecules with dendrimers are chiefly by interactions with dendrimers' exterior surfaces. The structure of the dendrimers determines the number and type of drug molecule which can be complexed with dendrimers or incorporated into them. The dendrimer loading capacity can be extremely increased through moderating the relation of drug molecules with end groups of dendrimers [90]. The generation number of dendrimers affects the drug loading potential: a high number of generations provides more space for guest molecule and possesses a larger number of functional groups for interaction with the guest molecule [91]. Drugs can be covalently conjugated to the dendrimer terminal groups or enclosed within the core by hydrogen bonding, electrostatic interaction, or hydrophobic linkage [92]. PAMAM and PPI dendrimers have been mostly evaluated for efficient drug delivery. These are useful drug carriers because of the availability of a great density of amine groups on their surface [84]. Biodegradable dendrimers are the most proper ones for the progression of new drug-delivery systems for efficient targeting. These dendrimers show high importance and use in drug delivery because of their biodegradable nature [93]. Biodegradable dendrimers offer the following virtues as drug delivery carriers:
-
a.
Enhancement of the drugs' residence time through inhibiting their excretion.
-
b.
The ability to design drug delivery systems with controlled release.
-
c.
Improvement of the hydrophobic drugs' solubility and their permeation, and inhibition of their degradation in the internal fluids.
-
d.
The ability to target the tumor for its removal.
-
e.
The ability to modulate the pharmacodynamics of drugs by their conjugating or entrapping.
-
f.
The probability to decrease side effects and to relieve the patients’ pain, therefore preparing a painless platform in the form of a drug carrier [93].
The drugs molecules interact with dendrimers through various mechanisms, covalent conjugation, physical encapsulation, and electrostatic interaction (Fig. 3).
4.5.1.1 Physical Encapsulation
Dendrimers can encapsulate guest molecules within their branches because of the open nature of the dendritic structure [94]. The poorly soluble drugs can hydrophobically interact with internal cavities of dendrimers because these cavities are hydrophobic in nature [95]. As an outcome of this physical relationship between the drug molecules and the internal cavities of dendrimers, the encapsulated drugs release in an aqueous environment is controlled via hydrophobic forces or electrostatic interactions or hydrogen bonding between ionic groups of drugs and oppositely charged surfaces of dendrimers [30]. To maximize the drug-loading potential inside the dendrimer, characteristics of internal cavities should be carefully considered in the design of polymer architecture [22]. The primary investigates of dendrimer as a drug delivery system concentrated on its utilization as dendritic box and unimolecular micelle for drug molecule encapsulation [96, 97]. Dendritic boxes are potential hosts to incorporate hydrophilic/hydrophobic molecules that can be produced through constructing a densely-packed shell on dendrimer surface [98, 99]. The number of guest molecules which can be entrapped in dendritic boxes is dependent on the shape of the molecules and the box structure and its internal voids [100]. The unimolecular dendritic micelles are constructed from hydrophobic cores surrounded with hydrophilic shells. These have an important advantage compared to typical polymeric micelles so that the micellar structure is retained at all concentrations since the hydrophobic parts are connected covalently [101].
4.5.1.2 Electrostatic Interactions
The dendrimers surface includes ionizable functional groups (like carboxyl and amine groups) with high density, that attract drug molecules possessing opposite charges and hence provides an electrostatic bonding. Electrostatic interaction can increase the solubility of hydrophobic drugs [102]. For instance, such interaction can happen between carboxyl groups of ibuprofen and amine groups of PAMAM dendrimers. It was evaluated that almost 40 molecules of ibuprofen are conjugated with G4 PAMAM dendrimers at pH 10.2 leading to a significant enhancement of ibuprofen solubility [103]. In addition to ibuprofen, other drugs such as benzoic acid [104], indomethacin [105], and piroxicam [106] have been shown to interact electrostatically with various dendrimers for the formation of steady complexes.
4.5.1.3 Covalent Conjugation
Drugs can be conjugated covalently or noncovalently to dendrimers. When drugs are covalently attached to dendrimers, their release happens by enzymatic or chemical cleavage of hydrolytically unstable bonds [107]. Some chemical inserts such as paminobenzoic acid and polyethyleneglycol (PEG) or biodegradable linkages like ester or amide bonds are being used for the formation of covalent bonding between terminal groups of dendrimer and drug molecules. This type of binding enhances the stability of drugs and provides their better controlled release [108]. Several drugs have successfully conjugated with PAMAM dendrimers. For example, Yang et al. conjugated penicillin V with G2.5 and G3 PAMAM dendrimers by a PEG spacer via amide and ester linkages, respectively [109]. The findings of researchers have shown increased solubility of drugs and their controlled release from dendrimer-drug complexes than to the plain drug. Apart from these, the epirubicin anticancer drug was formulated as a prodrug through conjugating it with PEG dendrimers containing aminoadipic acid. The resulting conjugated system through enhancement of stability of the drug reduced its degradation, also improved therapeutic action of the drug by the increase of blood residence time [94]. Some of the disadvantages of physical encapsulation of drugs can be decreased through their covalent conjugation to the dendrimers by utilizing a chemical approach that will also be causing a selective release of drug in vivo. Such an approach can produce a structure with pre-measured drug concentration and increased stability. The release of the drug can be achieved through a change in the biological microenvironment like change in temperature, pH, or special enzyme concentration [110].
4.5.2 Mechanisms of Drug Delivery Through Dendrimers
Because of globular morphology and multiple functional groups on the surface, dendrimers can be utilized for formation of covalent/ electrostatic bonds between their terminal functional groups and drugs or for drugs encapsulation within the dendritic structure [111]. The covalent bonds are made between the drugs and the dendrimers' exterior surfaces that are introduced as potential sites of interaction. The structural design of a dendrimer is important for the determination of the number of drug molecules that can incorporate into it. The loading potential of dendrimers is enhanced through the formation of a complex with several functional groups on the dendrimers' surface [112]. The number of functional groups existent on the surface of a dendrimer is enhanced by increasing its generation [113]. The dendrimers are delivered drugs through two mechanisms:
-
(1)
In vivo degradation of the covalent bonds between drugs and dendrimers, that is dependent on the existence of enzymes or an environment able of breaking bonding (Fig. 4).
-
(2)
Releasing of the drugs from dendrimers because of alterations in physical conditions like pH and temperature that are independent of the external factors [114].
4.5.3 Dendrimers as Carriers of Different Drugs
4.5.3.1 Non-steroidal Anti-inflammatory Drugs (NSAIDs)
The use of dendrimers as carriers of NSAIDs is increased during the last two decades. Although these drugs are one of the most commonly applied groups, their utilization is restricted due to significant cytotoxicity and side effects. Moreover, NSAIDs are hydrophobic molecules (poor soluble in water), therefore, dendrimers soluble in water because of the existence of terminal amino groups are the proper candidates for their solubilization. Indeed, this is done through the encapsulation of NSAIDs within the structure of dendrimers [115]. It should be noted which the higher solubility of drugs is contributed to their higher bioavailability [116]. The interaction between NSAIDs and dendrimers is a thing that happens between carboxylate groups of NSAIDs and amine groups on the surface of dendrimers [117]. Hydrogen bonding, hydrophobic interactions, and micellar solubilization are other ways for the increase of the dendrimer-mediated solubility. The many factors such as the size of the generation, dendrimer concentration, core, temperature, pH, surface groups, and internal branching units can affect the process of NSAIDs' solubilization [87]. Several NSAIDs have been successfully complexed with or encapsulated into different types of dendrimers and results these studies are presented in Table 2.
Apart from unmodified dendrimers, dendrimers modified with PEG on their surface also could be applied for design drug-delivery and solubilizing systems. Conjugation of PEG moieties into the surface of the dendrimer provides a hydrophilic shell around a hydrophobic dendritic core [129]. The site-specific drug delivery can be increased by modified dendrimers with a targeting moiety like folic acid (FA) [130]. For example, FA was conjugated to amino groups on G4 PAMAM dendrimers surfaces by a carbodiimide reaction for encapsulation of indometacin drug. The findings demonstrated that encapsulation capacity, targeting efficiency, and controlled release profile of the drug, were enhanced with the increase of FA content [131]. In general, the complexation of NSAIDs with dendrimers results in greater biological activity, increased bioavailability, enhanced solubility, and controlled or prolonged release of the tested drugs than to the pure drugs.
4.5.3.2 Anticancer Drugs
Cancer therapy is one of the important biological applications of dendrimers [132]. The dendrimers are utilized to overcome the formulation difficulties of drugs and improve their pharmacokinetic and physical properties [133]. When drugs are conjugated with dendrimers, they are characterized by higher solubility and stability, efficient therapeutic time, extended half-life, and reduced antigenicity and immunogenicity. In addition, dendrimers enable passive targeting of drugs into solid tumours [134]. The results of scientific studies relating to unmodified and modified dendrimers application in the anticancer drugs delivery are presented in Table 3. The use of dendrimers as carriers of anticancer drugs in targeted therapy of cancer has high importance, because, in this method, the drug is guided only to pathological tissues. In this method, a drug therapeutic index is increased due to enhancing its efficacy and reducing its side effects. By engineering the surface groups and branching units of dendrimers, they can be prepared for targeted delivery of drugs by both active and passive targeting [87]. The properties that make dendrimers proper for targeted delivery are their monodispersity, their terminal groups, and their well-defined architecture [2]. Dendrimers can passively target a tumor by the “enhanced permeation and retention” (EPR) effect. Conjugation of specific targeting ligands of cancer cells (such as folic acid, antibodies, polyunsaturated fatty acids, polysaccharides, oligosaccharides, and oligopeptides) to the surface of dendrimers provides targeting of drug to its suitable site of function [135]. A preferentially binding of dendrimers conjugated with FA to tumor cells with overexpressed folate receptor was reported [136]. The high specificity of FA-targeted methotrexate conjugates for human epithelial cancerous cells with overexpressed folate receptors showed by Kukowska-Latallo and Baker. These conjugates delivered the drug by receptor-mediated endocytosis [137]. In several studies, PAMAM dendrimers have been conjugated with monoclonal antibodies and tested for special targeting of tumour cells with overexpressed specific antigens [138,139,140]. The glycosylation is another way for targeted delivery of drugs, that is related to the incorporation of sugar moieties into the structure of dendrimers [141,142,143].
4.5.4 Route of Administration of Dendrimer-Drug Complexes
The drugs conjugated or incorporated with dendrimers can be applied in various administration routes.
4.5.4.1 Dendrimers in Transdermal Drug Delivery
Transdermal drug delivery is a delivery system via the skin which provides a stable blood concentration of the drug. The use of this method facilitates the dosing schedule and eliminates degradation in gastrointestinal tract [150]. Nonetheless, because of the low penetration abilities of drugs and subsequently slow rate of delivery, their transdermal delivery is restricted. Dendrimers have been found to provide advantages like improved drug solubility, increased plasma circulation time, and controlled release of drugs, because of their water-solubility and biocompatibility. The dendrimers permeability through the skin is distinguished by parameters of physicochemical, including composition, size of the generation, concentration, molecular weight, and surface charge [151]. Dendrimers are proved to be efficient as a transdermal delivery system of the drug for anticancer, antiviral, antimicrobial, NSAIDs, or antihypertensive drugs. Several researchers have indicated which the PAMAM dendrimer complex with NSAIDs can increase the permeation of drugs through penetration enhancers (Table 2) [121, 124, 125]. By encapsulation of cisplatin anticancer drug into PAMAM dendrimers, dendrimer-drug conjugates are obtained, which offered greater accumulation in solid tumours, less toxicity, and slower release than the free drug [152].
4.5.4.2 Dendrimers in Oral Drug Delivery
This type of delivery is preferred by patients and clinicians. In general, for anticancer drugs, the oral route is preferred since it decreases administration costs and simplifies utilization of more chronic treatment regimes. Nonetheless, poor solubility of the drug and its low permeability across the cell membranes restrict its intake [153]. To overcome this drawback and to guaranty high oral absorption, the usage of efficient oral delivery systems of drugs is important. An efficient oral delivery carrier should possess the capability to preserve drug from degradation. The carrier might decrease non-specific interactions with proteins of food and permit increased absorption via intestinal epithelium [154]. The potential application of dendrimers as oral drug delivery vehicles have been proved by several studies [155,156,157]. These studies have demonstrated that unmodified and modified dendrimers possess capacity to enhance transepithelial permeability [87]. For example, Jevprasesphant et al. evaluated permeation of unmodified and surface-modified PAMAM dendrimers across Caco-2 cells monolayers and showed that both dendrimers could effectively traverse epithelial monolayers through transcellular and paracellular pathways [158]. The delivery of dendrimer-drug conjugates across intestine membrane has been investigated in several studies. For instance, it was observed which dendrimer-propranolol conjugate had the ability to decrease the effect of the glycoprotein P efflux transporter, hence, could simplify the oral administration of drugs [159].
4.5.4.3 Dendrimers in Ocular Drug Delivery
Two main problems of ocular drug delivery are poor bioavailability of drugs because of rapid drainage through tear turnover or nasolacrimal duct that results in the elimination of formulation, and their short residence time on the cornea, corneal epithelia, and conjunctiva. Moreover, formulation which is to be used through the ocular route must be biocompatible, biodegradable, non-sensitive, isotonic, and non-irritative with proper maintenance within eyes [111, 160]. Therefore, researchers applied from dendrimers for ocular delivery of drugs. Applicability of PAMAM dendrimers with hydroxyl or carboxyl surface groups for ocular delivery of pilocarpine has been reported by Vandamme and Brobeck. They have been shown that dendrimers can enhance the residence time of pilocarpine and its bioavailability within the eyes [161]. Yavuz et al. developed conjugates based on PAMAM dendrimers and dexamethasone for investigation of the ocular absorption of dexamethasone. The resulting conjugate indicated increased transport of the drug across sclera and cornea tissues [28]. Dendrimers are suitable candidates to synthesis the hydrogels, cross-linked networks of polymers in water. Hydrogels which possess applications for sealing ophthalmic injuries and for the production of cartilage tissue are created through conjugating of dendrimers with PEG. These hydrogels effectively deliver ocular drug molecule attached to dendrimer, to eyes [162, 163].
4.5.4.4 Dendrimers in Pulmonary Drug Delivery
PAMAM dendrimers for assessment of pulmonary absorption of enoxaparin were used by Bai et al. Their results showed that G2 and G3 PAMAM dendrimers (dendrimers with a positive charge) enhanced the bioavailability and pulmonary absorption of enoxaparin. In contrast, G2.5 PAMAM dendrimers with carboxyl end groups (dendrimers with a negative charge) did not influence the enoxaparin bioavailability [164]. In another study, the role of G3 and G4 PAMAM dendrimers is demonstrated in the solubility increase of beclometasone. The sustained release of beclometasone in complex with dendrimers proved by in vitro studies. Nebulization studies indicated which aerosol efficiency was not related to the generation of dendrimer but the type of nebulizers, that means which PAMAM dendrimers possess the capacity for beclometasone pulmonary delivery [165]. Applicably of dendrimers for inhalable chemotherapeutic nanomedicines proved by Kaminskas et al.. When doxorubicin conjugated to PEGylated polylysine dendrimers, its exposure to lung metastases is increased [166].
4.5.5 Dendrimers as Nano-drug
A clear example of the use of dendrimers as nano-drug is their utilization as an antiviral drug on herpes simplex virus. So that, poly(lysine) dendrimers modified with sulfonated naphthyl groups can inhibit virus adsorption in early-stage and replication of the virus in the later stage through interfering with integrase and/or reverse transcriptase enzyme actions. Therefore, these dendrimers have the ability for prevention/reduction of transmission of HIV and other sexually transmitted diseases (STDs) [167]. Strong antibacterial effects of PPI dendrimers modified with tertiary alkylammonium groups against Gram-negative and Gram-positive bacteria have been proved. Application of chitosan–dendrimer hybrids as antibacterial agents has also been found [168].
5 Conclusions and Future Perspectives
Dendrimers are characterized by properties, including structure with a great degree of branching, well-defined molecular weight, globular shape, and nanoscale size. Dendrimers have different types based on their structural properties that providing novel platforms for the delivery of various therapeutic agents. Dendrimers offer attractive properties such as high control over branching length, size, and shape, and great flexibility in design, which make them an ideal candidate for nanomedicine, particularly drug delivery. Because of the promising structure of dendrimers, drugs can be effectively incorporated into their structure through various interactions between drug and dendrimer. Bioavailability, biocompatibility, permeability, toxicity, and solubility of drugs can be improved by using of dendrimers. The advantages offered by the dendrimers nano-structures have received significant attention from researchers not only in the delivery of drug but also in diagnosis and therapy of the diseases. Recently, patents and scientific publications on dendrimer-based nanomedicines have enhanced widely. A number of these nanomedicines have been commercialized or under clinical trials. With rapid progress in the field of nanomedicines based on dendrimers, some products are expected to enter clinical trials in the future. However, there are some challenges in the use of dendrimer-based nanomedicines such as the analytical characterization and scale-up generation of dendrimers and their products. The complex structure of dendrimer and its complexes or conjugates with ligands or drugs may need advanced analytical techniques to completely characterize the obtained product. Besides, the scale-up producing process exhibits another important challenge in the development of dendrimer-based nanomedicines because the production of dendrimer usually needs multiple processes. Great reproducibility and stability of dendrimer production are needs for scale-up manufacturing. Hopefully, this review of applications of dendrimers in nanomedicine obviously explains the potential of dendrimers and reaffirms a greater level of optimism for dendrimers' future role in delivery of drug, diagnosis, and therapy.
Data Availability
Hereby we state that data sharing is not applicable in our submission.
References
C.L. Ventola, Progress in nanomedicine: approved and investigational nanodrugs. Pharm. Ther. 42(12), 742 (2017)
A.K. Rai et al., Dendrimers: a potential carrier for targeted drug delivery system. Pharm. Biol. Eval. 3(3), 275–287 (2016)
A.M. Reddy, P.S. Babu, Dendrimers in antimicrobial therapy—an overview. Res. J. Pharm. Technol. 9(3), 322–332 (2016)
M. Stevanović, Biomedical applications of nanostructured polymeric materials, in Nanostructured Polymer Composites for Biomedical Applications. (Elsevier, Amsterdam, 2019), pp. 1–19
M.J. Dunlop et al., Nanocomposites derived from molybdenum disulfide and an organoiron dendrimer. J. Inorg. Organomet. Polym Mater. 27(1), 84–89 (2017)
K. Rajavelu, P. Rajakumar, Synthesis and DSSC application of donor-acceptor stilbenoid dendrimers with triphenylamine as core and benzothiazole as surface unit. Org. Electron. 56, 192–200 (2018)
R. Bissessur et al., Nanocomposites based on dendrimers and layered molybdenum disulfide. J. Inorg. Organomet. Polym Mater. 30, 4771–4782 (2020)
M. Dabrzalska et al., Phosphorus dendrimers and photodynamic therapy. Spectroscopic studies on two dendrimer-photosensitizer complexes: cationic phosphorus dendrimer with rose Bengal and anionic phosphorus dendrimer with methylene blue. Int. J. Pharm. 492(1–2), 266–274 (2015)
D.R.D. Carmo, L.L. Paim, Investigation about the copper adsorption on the chloropropylsilica gel surface modified with a nanostructured dendrimer DAB-Am-16: an analytical application for determination of copper in different samples. Mater. Res. 16(1), 164–172 (2013)
M.A. Mintzer, M.W. Grinstaff, Biomedical applications of dendrimers: a tutorial. Chem. Soc. Rev. 40(1), 173–190 (2011)
A.A. Joraid et al., Thermal degradation behavior of a new family of organometallic dendrimer. J. Inorg. Organomet. Polym. Mater. (2020). https://doi.org/10.1007/s10904-020-01444-6
E. Buhleier, " Cascade"-and" nonskid-chain-like" syntheses of molecular cavity topologies. Synthesis 1978(2): 155–158 (1978)
R. Denkewalter, J. Kolc, W. Lukasavage. US Pat. 4289872, 1981. in Chem. Abstr. (1985)
D.A. Tomalia et al., A new class of polymers: starburst-dendritic macromolecules. Polym. J. 17(1), 117–132 (1985)
G.R. Newkome et al., Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol. J. Org. Chem. 50(11), 2003–2004 (1985)
C.J. Hawker, J.M. Frechet, Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 112(21), 7638–7647 (1990)
B. Noriega-Luna et al., Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J. Nanomater. 2014, 1–19 (2014)
K. Albrecht, Y. Kasai, K. Yamamoto, The fluorescence and electroluminescence properties of the carbazole–phenylazomethine double layer-type dendrimer. J. Inorg. Organomet. Polym Mater. 19(1), 118–123 (2009)
R.W. Scott, O.M. Wilson, R.M. Crooks, Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J. Phys. Chem. B. 109(2), 692–704 (2005)
B. Klajnert, M. Bryszewska, The interaction of tryptophan and ANS with PAMAM dendrimers. Cell. Mol. Biol. Lett. 7(4), 1087–1094 (2002)
M. Nasr, E. Elmowafy, M.E. Soliman, The evolution of dendrimers to composite dendrimers: a review of the state of the art, in Nanoparticles in Pharmacotherapy. (Elsevier, Amsterdam, 2019), pp. 217–249
H. Chaudhari et al., Dendrimers: novel carriers for drug delivery. J. Appl. Pharm. Res. 4(1), 01–19 (2016)
C. Loo et al., Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 5(4), 709–711 (2005)
X.-Y. Zhang, P.-Y. Zhang, Nanotechnology for multimodality treatment of cancer. Oncol. Lett. 12(6), 4883–4886 (2016)
A.P. Dias et al., Dendrimers in the context of nanomedicine. Int. J. Pharm. 573, 118814 (2020)
D. Li, S. Wen, X. Shi, Dendrimer-entrapped metal colloids as imaging agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 7(5), 678–690 (2015)
R. Akki, et al., A novel approach in drug delivery system using dendrimers. Pharma. Innovation J. 8(5), 166–174 (2019)
B. Yavuz et al., In vitro/in vivo evaluation of dexamethasone—PAMAM dendrimer complexes for retinal drug delivery. J. Pharm. Sci. 104(11), 3814–3823 (2015)
D. Patton et al., Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob. Agents Chemother. 50(5), 1696–1700 (2006)
A.P. Sherje et al., Dendrimers: a versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 548(1), 707–720 (2018)
S.K. Prajapati et al., Dendrimers in drug delivery, diagnosis and therapy: basics and potential applications. J. Drug Deliv. Ther. 6(1), 67–92 (2016)
R. Esfand, D.A. Tomalia, Poly(amidoamine)(PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 6(8), 427–436 (2001)
S.K. Parajapati et al., Potential application of dendrimers in drug delivery: a concise review and update. J. Drug Deliv. Ther. 6(2), 71–88 (2016)
R. Yang et al., Synthesis of a novel polyamidoamine dendrimer conjugating with alkali blue as a lymphatic tracer and study on the lymphatic targeting in vivo. Drug Deliv. 23(7), 2298–2308 (2016)
F. Abedi-Gaballu et al., PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today 12, 177–190 (2018)
A.O. Idris, Development of Electrochemical Biosensors Based on Carbon Nanoparticles-Dendrimer Derivatives for Carcinoembryonic Antigen and Alpha-Feto Protein Cancer Biomarkers (University of Johannesburg, Johannesburg, 2019).
L. Liang, J. Ruiz, D. Astruc, “Click” synthesis of a heterobifunctional ferrocenyl dendrimer with molecular recognition properties and influence of the ferrocenyl redox potential on the formation of gold nanoparticles. J. Inorg. Organomet. Polym Mater. 20(3), 503–510 (2010)
E. Esmaeili et al., Dendrimer functionalized magnetic nanoparticles as a promising platform for localized hyperthermia and magnetic resonance imaging diagnosis. J. Cell. Physiol. 234(8), 12615–12624 (2019)
S. Wongin et al., Maintenance of human chondrogenic phenotype on a dendrimer-immobilized surface for an application of cell sheet engineering. BMC Biotechnol. 18(1), 1–11 (2018)
H. Ahmadi, M. Abdollahi, Synthesis and structural characterization of lignin/silica hybrid nanoparticles functionalized with sulfonic acid-terminated polyamidoamine. Wood Sci. Technol. 54(1), 249–268 (2020)
M. Kalomiraki, K. Thermos, N.A. Chaniotakis, Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 11, 1 (2016)
D.K. Hawamdeh, K. Qamhieh, A. Sayyed-Ahmad, Complexation of DNA with nano-cationic dendrimers as nonviral vectors in gene therapy. Technical Report. https://doi.org/10.13140/RG.2.2.30392.44803
H.J. Hsu et al., Dendrimer-based nanocarriers: a versatile platform for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 9(1), e1409 (2017)
V. Mishra et al., Dendrimer based nanoarchitectures in diabetes management: an overview. Curr. Pharm. Des. 25(23), 2569–2583 (2019)
L. Salvi et al., A synthesis, properties and application as a possible drug delivery systems dendrimers—a review. Asian J. Pharm. Res. Dev. 8(2), 107–113 (2020)
V.P. Chavda, Nanobased nano drug delivery: a comprehensive review, in Applications of Targeted Nano Drugs and Delivery Systems. (Elsevier, Amsterdam, 2019), pp. 69–92
T. Baig et al., A review about dendrimers: synthesis, types, characterization and applications. Int. J. Adv. Pharm. Biol. Chem 4, 44–59 (2015)
A.S. Ertürk, G. Elmacı, PAMAM dendrimer functionalized manganese ferrite magnetic nanoparticles: microwave-assisted synthesis and characterization. J. Inorg. Organomet. Polym Mater. 28(5), 2100–2107 (2018)
E. Abbasi et al., Dendrimers: synthesis, applications, and properties. Nanoscale Res. Lett. 9(1), 247 (2014)
A. Gothwal et al., Toxicity and biocompatibility aspects of dendrimers, in Pharmaceutical Applications of Dendrimers. (Elsevier, Amsterdam, 2020), pp. 251–274
C.J. Hawker, K.L. Wooley, J.M. Fréchet, Unimolecular micelles and globular amphiphiles: dendritic macromolecules as novel recyclable solubilization agents. J. Chem. Soc. Perkin Trans. 1 12, 1287–1297 (1993)
U. Gupta, O. Perumal, Dendrimers and its biomedical applications, in Natural and Synthetic Biomedical Polymers. (Elsevier, Amsterdam, 2014), pp. 243–257
X. Xu et al., Cooperative hierarchical self-assembly of peptide dendrimers and linear polypeptides into nanoarchitectures mimicking viral capsids. Angew. Chem. 124(13), 3184–3187 (2012)
K. Sadler, J.P. Tam, Peptide dendrimers: applications and synthesis. Rev. Mol. Biotechnol. 90(3–4), 195–229 (2002)
P. Niederhafner, J. Šebestík, J. Ježek, Peptide dendrimers. J. Pept. Sci. 11(12), 757–788 (2005)
P. Kesharwani et al., Dendrimers in targeting and delivery of drugs, in Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes. (Elsevier, Amsterdam, 2017), pp. 363–388
A. Pushechnikov, S.S. Jalisatgi, M.F. Hawthorne, Dendritic closomers: novel spherical hybrid dendrimers. Chem. Commun. 49(34), 3579–3581 (2013)
K. Jain et al., Dendrimer toxicity: let’s meet the challenge. Int. J. Pharm. 394(1–2), 122–142 (2010)
P. Antoni et al., Bifunctional dendrimers: from robust synthesis and accelerated one-pot postfunctionalization strategy to potential applications. Angew. Chem. 121(12), 2160–2164 (2009)
S.M. Grayson, J.M. Frechet, Convergent dendrons and dendrimers: from synthesis to applications. Chem. Rev. 101(12), 3819–3868 (2001)
D.A. Tomalia, J.M. Fréchet, Discovery of dendrimers and dendritic polymers: a brief historical perspective. J. Polym. Sci. A 40(16), 2719–2728 (2002)
A.-M. Caminade et al., Synthetic pathways towards phosphorus dendrimers and dendritic architectures. Curr. Org. Chem. 10(18), 2333–2355 (2006)
T. Kawaguchi et al., Double exponential dendrimer growth. J. Am. Chem. Soc. 117(8), 2159–2165 (1995)
M. Arseneault, C. Wafer, J.-F. Morin, Recent advances in click chemistry applied to dendrimer synthesis. Molecules 20(5), 9263–9294 (2015)
Z. Iqbal et al., The edge versions of degree-based topological descriptors of dendrimers. J. Cluster Sci. 31(2), 445–452 (2020)
I. Gheorghe, C. Curutiu, L.-M. Ditu, Therapeutic nanostructures: novel approaches, in Materials for Biomedical Engineering. (Elsevier, Amsterdam, 2019), pp. 1–22
S. ho Hng, Y. Choi, Mesoporous silica-based nanoplatforms for the delivery of photodynamic therapy agents. J. Pharm. Invest. 48(1), 3–17 (2018)
P.K. Pandey et al., Nanogold-core multifunctional dendrimer for pulsatile chemo-, photothermal-and photodynamic-therapy of rheumatoid arthritis. J. Colloid Interface Sci. 544, 61–77 (2019)
S. Battah et al., Enhanced porphyrin accumulation using dendritic derivatives of 5-aminolaevulinic acid for photodynamic therapy: an in vitro study. Int. J. Biochem. Cell Biol. 38(8), 1382–1392 (2006)
K.-F. Xu et al., Cholesterol-modified dendrimers for constructing a tumor microenvironment-responsive drug delivery system. ACS Biomater. Sci. Eng. 5(11), 6072–6081 (2019)
M.T. McMahon, J.W. Bulte, Two decades of dendrimers as versatile MRI agents: a tale with and without metals. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 10(3), e1496 (2018)
J.K. Patra et al., Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 16(1), 71 (2018)
L.H. Bryant et al., Pharmacokinetics of a high-generation dendrimer–Gd-DOTA. Acad. Radiol. 9(1), S29–S33 (2002)
O.P. Perumal et al., The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials 29(24–25), 3469–3476 (2008)
H.B. Agashe et al., Investigations on the toxicological profile of functionalized fifth-generation poly (propylene imine) dendrimer. J. Pharm. Pharmacol. 58(11), 1491–1498 (2006)
S. Zhang et al., Radical dendrimers based on biocompatible oligoethylene glycol dendrimers as contrast agents for MRI. Pharmaceutics 12(8), 772 (2020)
B. Gorain et al., The use of nanoscaffolds and dendrimers in tissue engineering. Drug Discov. Today 22(4), 652–664 (2017)
A. Taheri-Kafrani, H. Shirzadfar, E. Tavassoli-Kafrani, Dendrimers and dendrimers-grafted superparamagnetic iron oxide nanoparticles: synthesis, characterization, functionalization, and biological applications in drug delivery systems, in Nano-and Microscale Drug Delivery Systems. (Elsevier, Amsterdam, 2017), pp. 75–94
S. Shukla et al., Biodegradable polymeric nanostructures in therapeutic applications: opportunities and challenges. RSC Adv. 6(97), 94325–94351 (2016)
K.A. Boduch-Lee et al., Design and synthesis of hydroxyapatite composites containing an mPEG−dendritic poly (l-lysine) star polycaprolactone. Macromolecules 37(24), 8959–8966 (2004)
I. Rajzer, Fabrication of bioactive polycaprolactone/hydroxyapatite scaffolds with final bilayer nano-/micro-fibrous structures for tissue engineering application. J. Mater. Sci. 49(16), 5799–5807 (2014)
J.M. Oliveira et al., The osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly (amidoamine) dendrimer nanoparticles. Biomaterials 30(5), 804–813 (2009)
N. Tomar, Dendrimers as nanocarriers in cancer chemotherapy. Anticancer Res. 8, 12 (2019)
J. Hu, K. Hu, Y. Cheng, Tailoring the dendrimer core for efficient gene delivery. Acta Biomater. 35, 1–11 (2016)
M. Gorzkiewicz et al., Application of new lysine-based peptide dendrimers D3K2 and D3G2 for gene delivery: Specific cytotoxicity to cancer cells and transfection in vitro. Bioorg. Chem. 95, 103504 (2020)
J.M. Fréchet, Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy. Science 263(5154), 1710–1715 (1994)
P. Kesharwani, K. Jain, N.K. Jain, Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 39(2), 268–307 (2014)
A.S.P. Kumar, S. Latha, P. Selvamani, Dendrimers: multifunctional drug delivery carriers. Int. J. Technol. Res. Engine 2, 1569–1575 (2015)
W.E. Bawarski et al., Emerging nanopharmaceuticals. Nanomed. Nanotechnol. Biol. Med. 4(4), 273–282 (2008)
D.G. Mullen et al., Design, synthesis, and biological functionality of a dendrimer-based modular drug delivery platform. Bioconjug. Chem. 22(4), 679–689 (2011)
J. Singh et al., Dendrimers in anticancer drug delivery: mechanism of interaction of drug and dendrimers. Artif. Cells Nanomed. Biotechnol. 44(7), 1626–1634 (2016)
G.A. Hughes, Nanostructure-mediated drug delivery. Nanomed. Nanotechnol. Biol. Med. 1(1), 22–30 (2005)
D. Huang, D. Wu, Biodegradable dendrimers for drug delivery. Mater. Sci. Eng. C 90, 713–727 (2018)
G. Pasut et al., PEG-epirubicin conjugates with high drug loading. J. Bioact. Compat. Polym. 20(3), 213–230 (2005)
M. Liu, J.M. Fréchet, Designing dendrimers for drug delivery. Pharm. Sci. Technol. Today 2(10), 393–401 (1999)
W.-D. Jang et al., Bioinspired application of dendrimers: from bio-mimicry to biomedical applications. Prog. Polym. Sci. 34(1), 1–23 (2009)
Y. Cheng et al., New insights into the interactions between dendrimers and surfactants: 2. Design of new drug formulations based on dendrimer−surfactant aggregates. J. Phys. Chem. B 113(24), 8339–8346 (2009)
J.J. Michels et al., Well-defined assemblies of adamantyl-terminated poly (propylene imine) dendrimers and β-cyclodextrin in water. J. Chem. Soc. Perkin Trans. 2 (2000). https://doi.org/10.1039/b002689l
V. Mishra, U. Gupta, N. Jain, Surface-engineered dendrimers: a solution for toxicity issues. J. Biomater. Sci. Polym. Ed. 20(2), 141–166 (2009)
H. Yang, W.J. Kao, Dendrimers for pharmaceutical and biomedical applications. J. Biomater. Sci. Polym. Ed. 17(1–2), 3–19 (2006)
M.J. Cloninger, Biological applications of dendrimers. Curr. Opin. Chem. Biol. 6(6), 742–748 (2002)
O. Milhem et al., Polyamidoamine Starburst® dendrimers as solubility enhancers. Int. J. Pharm. 197(1–2), 239–241 (2000)
F. Wang et al., Reducing cytotoxicity while improving anti-cancer drug loading capacity of polypropylenimine dendrimers by surface acetylation. Acta Biomater. 8(12), 4304–4313 (2012)
P. Kolhe et al., Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int. J. Pharm. 259(1–2), 143–160 (2003)
V. Kabanov et al., Polyelectrolyte behavior of astramol poly (propyleneimine) dendrimers. Macromolecules 31(15), 5142–5144 (1998)
E. Murugan et al., Evaluation of surface acetylated and internally quaternized poly (propylene imine) dendrimer as a biocompatible drug carrier for piroxicam as a model drug. RSC Adv. 5(129), 106461–106475 (2015)
A.K. Patri, J.F. Kukowska-Latallo, J.R. Baker Jr., Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 57(15), 2203–2214 (2005)
V. Patel et al., Dendrimers as novel drug-delivery system and its applications, in Drug Delivery Systems. (Elsevier, Amsterdam, 2020), pp. 333–392
H. Yang, S.T. Lopina, Penicillin V-conjugated PEG-PAMAM star polymers. J. Biomater. Sci. Polym. Ed. 14(10), 1043–1056 (2003)
R. Shukla et al., HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology 19(29), 295102 (2008)
B.K. Nanjwade et al., Dendrimers: emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 38(3), 185–196 (2009)
J.B. Wolinsky, M.W. Grinstaff, Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Deliv. Rev. 60(9), 1037–1055 (2008)
S.H. Medina, M.E. El-Sayed, Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 109(7), 3141–3157 (2009)
A.S. Chauhan, Dendrimers for drug delivery. Molecules 23(4), 938 (2018)
M. Markowicz-Piasecka, E. Mikiciuk-Olasik, Dendrimers in drug delivery, in Nanobiomaterials in Drug Delivery. (Elsevier, Amsterdam, 2016), pp. 39–74
M. Markowicz et al., Evaluation of poly (amidoamine) dendrimers as potential carriers of iminodiacetic derivatives using solubility studies and 2D-NOESY NMR spectroscopy. J. Biol. Phys. 38(4), 637–656 (2012)
M. Najlah et al., Synthesis, characterization and stability of dendrimer prodrugs. Int. J. Pharm. 308(1–2), 175–182 (2006)
M. Ficker et al., Complexes of indomethacin with 4-carbomethoxy-pyrrolidone PAMAM dendrimers show improved anti-inflammatory properties and temperature-dependent binding and release profile. Mol. Pharm. 15(8), 3573–3582 (2018)
M. Gorzkiewicz et al., Pyrrolidone-modified PAMAM dendrimers enhance anti-inflammatory potential of indomethacin in vitro. Colloids Surf. B 181, 959–962 (2019)
L.D. Pedro-Hernández et al., Synthesis, characterization, and nanomedical applications of conjugates between resorcinarene-dendrimers and ibuprofen. Nanomaterials 7(7), 163 (2017)
J. Manikkath et al., Influence of peptide dendrimers and sonophoresis on the transdermal delivery of ketoprofen. Int. J. Pharm. 521(1–2), 110–119 (2017)
Ł Uram et al., The effect of biotinylated PAMAM G3 dendrimers conjugated with COX-2 inhibitor (celecoxib) and PPARγ agonist (Fmoc-L-Leucine) on human normal fibroblasts, immortalized keratinocytes and glioma cells in vitro. Molecules 24(20), 3801 (2019)
Ł Uram et al., Biotinylated PAMAM G3 dendrimer conjugated with celecoxib and/or Fmoc-l-Leucine and its cytotoxicity for normal and cancer human cell lines. Eur. J. Pharm. Sci. 124, 1–9 (2018)
J. Manikkath et al., Low frequency ultrasound and PAMAM dendrimer facilitated transdermal delivery of ketoprofen. J. Drug Deliv. Sci. Technol. 41, 334–343 (2017)
B. Huang et al., Dendrimer-coupled sonophoresis-mediated transdermal drug-delivery system for diclofenac. Drug Des. Dev. Therapy 9, 3867 (2015)
A.J. Perisé-Barrios et al., Improved efficiency of ibuprofen by cationic carbosilane dendritic conjugates. Mol. Pharm. 13(10), 3427–3438 (2016)
A.S. Ertürk, M.U. Gürbüz, EDA Çekirdekli Amin, TRIS ve Karboksil Sonlu PAMAM Dendrimerleri Kullanarak Ketoprofenin Çözünürlüğünü Geliştirme. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi 22(2), 768–773 (2018)
S. Al-Azzawi et al., Dendrimeric poly (Epsilon-Lysine) delivery systems for the enhanced permeability of flurbiprofen across the blood-brain barrier in Alzheimer’s disease. Int. J. Mol. Sci. 19(10), 3224 (2018)
A. D’Emanuele, D. Attwood, Dendrimer–drug interactions. Adv. Drug Deliv. Rev. 57(15), 2147–2162 (2005)
P. Singh et al., Folate and folate− PEG− PAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjug. Chem. 19(11), 2239–2252 (2008)
D. Chandrasekar et al., Folate coupled poly (ethyleneglycol) conjugates of anionic poly (amidoamine) dendrimer for inflammatory tissue specific drug delivery. J. Biomed. Mater. Res. A 82(1), 92–103 (2007)
R.I. Castro, O. Forero-Doria, L. Guzman, Perspectives of dendrimer-based nanoparticles in cancer therapy. Anais da Academia Brasileira de Ciências 90(2), 2331–2346 (2018)
S. Kim et al., Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 71(3), 420–430 (2009)
G. Pasut, F. Veronese, Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 32(8–9), 933–961 (2007)
S.G. Sampathkumar, K.J. Yarema, in Dendrimers in Cancer Treatment and Diagnosis, Nanotechnologies for the Life Sciences (Wiley, 2007)
Y. Yue et al., Synthesis and characterization of G5 PAMAM dendrimer containing daunorubicin for targeting cancer cells. Arch. Pharmacal. Res. 35(2), 343–349 (2012)
J.R. Baker, Dendrimer-based nanoparticles for cancer therapy. Hematology 2009(1), 708–719 (2009)
M. Marcinkowska et al., Multicomponent conjugates of anticancer drugs and monoclonal antibody with PAMAM dendrimers to increase efficacy of HER-2 positive breast cancer therapy. Pharm. Res. 36(11), 154 (2019)
D. Yoyen-Ermis et al., Tumor-induced myeloid cells are reduced by gemcitabine-loaded PAMAM dendrimers decorated with anti-Flt1 antibody. Mol. Pharm. 15(4), 1526–1533 (2018)
M. Marcinkowska et al., Conjugate of PAMAM dendrimer, doxorubicin and monoclonal antibody—trastuzumab: the new approach of a well-known strategy. Polymers 10(2), 187 (2018)
S.A. Torres-Pérez, M. del Pilar Ramos-Godínez, E. Ramón-Gallegos, Glycosylated one-step PAMAM dendrimers loaded with methotrexate for target therapy in breast cancer cells MDA-MB-231. J. Drug Deliv. Sci. Technol. 58, 101769 (2020)
D.-D. Chang et al., Click synthesis of glycosylated porphyrin-cored PAMAM dendrimers with specific recognition and thermosensitivity. J. Polym. Res. 25(12), 257 (2018)
A. Sharma et al., Effect of mannose targeting of hydroxyl PAMAM dendrimers on cellular and organ biodistribution in a neonatal brain injury model. J. Control. Release 283, 175–189 (2018)
H. Kulhari et al., Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci. Rep. 6(1), 1–13 (2016)
H. Kulhari et al., Optimization of carboxylate-terminated poly (amidoamine) dendrimer-mediated cisplatin formulation. Drug Dev. Ind. Pharm. 41(2), 232–238 (2015)
H. Bhatt, B. Ghosh, S. Biswas, Cell-penetrating peptide and α-tocopherol-conjugated poly (amidoamine) dendrimers for improved delivery and anticancer activity of loaded paclitaxel. ACS Appl. Bio Mater. 3(5), 3157–3169 (2020)
S.V.K. Rompicharla et al., Octa-arginine modified poly (amidoamine) dendrimers for improved delivery and cytotoxic effect of paclitaxel in cancer. Artif. Cells Nanomed. Biotechnol. 46(sup2), 847–859 (2018)
K. Tokarczyk, B. Jachimska, Characterization of G4 PAMAM dendrimer complexes with 5-fluorouracil and their interactions with bovine serum albumin. Colloids Surf. A 561, 357–363 (2019)
C. Song et al., Efficient co-delivery of microRNA 21 inhibitor and doxorubicin to cancer cells using core–shell tecto dendrimers formed via supramolecular host–guest assembly. J. Mater. Chem. B 8(14), 2768–2774 (2020)
D.F. Argenta, S.M. Martelli, T. Caon, Dendrimer as a platform for drug delivery in the skin, in Materials for Biomedical Engineering. (Elsevier, Amsterdam, 2019), pp. 331–367
R.V. Movliya, P.M. Patel, Role of dendrimer in drug solubilisation—a review. Drug Deliv. Lett. 9(4), 265–276 (2019)
S. Svenson, D.A. Tomalia, Dendrimers in biomedical applications—reflections on the field. Adv. Drug Deliv. Rev. 64, 102–115 (2012)
V.K. Yellepeddi, H. Ghandehari, Pharmacokinetics of oral therapeutics delivered by dendrimer-based carriers. Expert Opin. Drug Deliv. 16(10), 1051–1061 (2019)
J.D.A.K. Twibanire, T.B. Grindley, Polyester dendrimers: smart carriers for drug delivery. Polymers 6(1), 179–213 (2014)
K.M. Kitchens et al., Transport of poly (amidoamine) dendrimers across Caco-2 cell monolayers: influence of size, charge and fluorescent labeling. Pharm. Res. 23(12), 2818–2826 (2006)
V.K. Yellepeddi et al., Pediatric oral formulation of dendrimer-N-acetyl-l-cysteine conjugates for the treatment of neuroinflammation. Int. J. Pharm. 545(1–2), 113–116 (2018)
H.S. Sardo et al., A review on 5-aminosalicylic acid colon-targeted oral drug delivery systems. Int. J. Pharm. 558, 367–379 (2019)
R. Jevprasesphant et al., Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm. Res. 20(10), 1543–1550 (2003)
A. D’emanuele et al., The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J. Control. Release 95(3), 447–453 (2004)
M.G. Lancina III., H. Yang, Dendrimers for ocular drug delivery. Can. J. Chem. 95(9), 897–902 (2017)
T.F. Vandamme, L. Brobeck, Poly (amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 102(1), 23–38 (2005)
P.N. Desai, H. Yang, Synthesis and characterization of photocurable polyionic hydrogels. MRS Online Proc. Lib. Arch. (2008). https://doi.org/10.1557/PROC-1095-EE05-05
U. Kandekar et al., Dendrimers: novel drug nanocarriers. Int. J. Pharm. Sci. Res. 2(5), 1086 (2011)
S. Bai, C. Thomas, F. Ahsan, Dendrimers as a carrier for pulmonary delivery of enoxaparin, a low-molecular weight heparin. J. Pharm. Sci. 96(8), 2090–2106 (2007)
M. Nasr et al., PAMAM dendrimers as aerosol drug nanocarriers for pulmonary delivery via nebulization. Int. J. Pharm. 461(1–2), 242–250 (2014)
L.M. Kaminskas et al., Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J. Control. Release 183, 18–26 (2014)
U.J. Chigbo, A.E. Ugochukwu, D.F. John, Dendrimers: a novel tool for drug delivery and targeting. Univers. J. Pharm. Res. 2(3), (2017). https://doi.org/10.22270/ujpr.v2i3.RW5
A. Castonguay et al., Dendrimers as bactericides. New J. Chem. 36(2), 199–204 (2012)
Acknowledgements
This manuscript was supported by Pharmacotherapy Department, Faculty of Pharmacy, Bagyattallah University of Medical Sciences, and Tehran Iran.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikzamir, M., Hanifehpour, Y., Akbarzadeh, A. et al. Applications of Dendrimers in Nanomedicine and Drug Delivery: A Review. J Inorg Organomet Polym 31, 2246–2261 (2021). https://doi.org/10.1007/s10904-021-01925-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-01925-2