Abstract
The present work introduced the new strategy for direct preparation of Schiff base as well as oxime compounds through oxidation of primary benzylic or allylic alcohols in the presence of amines by complexation of Mn(III) to a polymeric Schiff base ligand based on polysalicylaldehyde (PSA-Schiff base-Mn(III) complex). As a new environmentally benign protocol, manganese heterogeneous polymeric catalytic system demonstrated promising oxidation of alcohols in ethanol using molecular oxygen. PSA was synthesized through polycondensation reaction of 2-hydroxy-5-chloromethyl-benzaldehyde and then treated with 2-aminophenol to form polymeric ligand. Average molecular weight of PSA was studied by an analytical method as well as GPC analysis. Formation of the catalyst was characterized step by step by FTIR, UV–Vis, 1H NMR, TGA, CHN and EDX analyses. Loading amounts of metal ions as well as leaching amount of the catalysis were studied by ICP-OES instrument. The catalyst shows up to high yields for oxidation of primary and secondary primary benzylic or allylic alcohols to carbonyl compounds, especially direct imine formation in a mild, inexpensive and efficient method which can be successfully recovered from the reaction mixture and reused for several times without any remarkable reactivity loss. Effect of solvent, temperature, catalyst amount and oxygen donors along with some blank experiments to elucidation of catalyst activity was evaluated in this work. Also chemoselectivity behavior of the catalyst was investigated with some combinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schiff base (C=N, also known as azomethine or imine) and oxime (=N–OH) compounds are one of the most applicable compounds in chemistry and medicine and widely used in organic transformation and pharmaceutical synthesis [1]. In coordination chemistry, they are known as powerful ligands for various transition metals and due to the versatility preparation, their roles as efficient catalysis in wide variety of fields are inevitable [2]. Schiff base compounds and their complexes have noteworthy properties in medicine which among them can be point to: antitumor [2], antifungal [3], antibacterial [4,5,6], antimicrobial [7], antiviral [8], anticonvulsant [9], anticancer [10, 11], neoplasm inhibitors [12, 13], and antitubercular [14] properties. Moreover, they showed applications in electronic devices [15, 16], catalysis [17, 18], solvatochromism in solution [19]. Schiff base complexes can be polymerized [20] or linked to various nanoparticles [21,22,23] to nailing desire purposes such as catalysis [24, 25] and coating [26].
Traditionally, imine compounds were prepared from condensation of aldehyde and amines; but in last decades, aerobic oxidation of alcohols and amines (known as cross-coupling of alcohols with amines) was another new approach in order to form imines [27]. However, traditional method has some limitations including using unstable aldehyde as well as using Lewis acid as catalyst in some cases and removing water formation during the reaction [27,28,29,30,31,32,33]. Oxidation of alcohols that lead to the preparation of carbonyl compounds are an important class in organic synthesis due to their applications in production of various pharmaceutical and chemical materials [34, 35]. In the other hand, Schiff base compounds which formed through condensation of amines and carbonyl compounds were under-effect to oxidation of alcohols; because in this point of view, alcohols are more commercially available, cheaper and stable than aldehydes [36]. So, the attentions have been attracted to oxidation of alcohols in the presence of amines to formation of Schiff base compounds as an alternative method which covers the above-mentioned drawbacks of traditional method [36]. Various protocols and attempts to oxidation of alcohols (primary and secondary) in order to form carbonyl compounds show the importance of this basic reaction in organic synthesis [37].
Due to lower toxicity as well as low cost of Mn complexes than other transition metal complex systems, they attracted great deal of attentions to themselves in order to oxidation and epoxidation protocols [38, 39]. Various methods have been reported for Mn(III) and Mn(II) salts [40,41,42,43] as well as their complexes for oxidation of alcohols [44]. Mn-salen-type complexes have been known as efficient reagents for catalytic oxidation reactions such as epoxidation of olefins, oxidation of alcohols and aldehydes [45,46,47]. A few Mn catalysts have been reported for cross-coupling of alcohols with amines some of which are as follows: (1) manganese octahedral molecular sieves (OMS-2) [48], (2) MnOx/HAP [49] and (3) meso-Cs/MnOx [50]. Previously, Nasseri et al. [51] used polymer-bounded Mn(III) complex base on cellulose for selective oxidation of benzyl alcohols. Recently, Räisänen et al. [52] studied catalytic homogeneous oxidation of alcohol by Mn(OAc)2. Chen et al. [53] have reported various methods for imine formation through oxidation of alcohols.
Although various methods have been reported for oxidation of alcohols, most of them involve some drawbacks such as lack of selectivity, high consumption of energy, including various by-product, toxic, expensive and unstable materials, corrosive and dangerous procedure and low efficiency. So, finding a method to be felt for oxidation of alcohols to corresponding aldehydes or ketones that not only conquer the above-mentioned drawbacks, but also can be recovered from the reaction mixture.
Salicylaldehyde is one of the safe, readily available organic compounds in health and reactivity [54] point of view and can be extracted from natural sources such as buckwheat [55]. Also, salicylaldehyde is known as one of the constituents of beaver castoreum [56]. Previously, oxidative polycondensation reaction has been used for preparation of polysalicylaldehyde [57]. In the present work, we synthesized polysalicylaldehyde by polycondensation reaction of 2-hydroxy-5-chloromethyl-benzaldehyde and then designed a polymer-Mn complex through complexation of a PSA-Schiff base ligand to Mn(III) metals as a new heterogeneous, low-cost polymeric catalyst for oxidation of different alcohols as well as direct preparation of various Schiff base (such as salen-type ligands) and oxime compounds in the presence of molecular oxygen and ethanol as green oxidant and green solvent, respectively.
Experimental
Materials and instrumentation
All materials were purchased from Sigma-Aldrich and Merck corporations and used as received without any purification. FTIR spectra were obtained using a BRUKER EQUINOX 55 FTIR spectrophotometer using KBr pellet. Analytical thin layer chromatography (TLC) was carried out on glass plates covered with silica gel. The 1H-NMR spectra were recorded in CDCl3 and DMSO-d 6 using 300 MHz instrument. All chemical shifts are reported in δ units downfield from TMS. Elemental analyses (C, H, N) were performed on Perkin Elmer-2004 instrument. UV–Vis analyses performed on UV Spectrolab BEL photonics. Thermal studies (TGA-DTG) have been performed on a NETZSCH STA 409 PC/PG in nitrogen atmosphere with a heating rate of 20 °C/min in the temperature ranges of 25–750 °C. ICP analysis was performed by VARIAN VISTA-PRO CCD simultaneous ICP-OES instrument. Gas chromatography (GC) analyses were performed on a Shimadzu-14B gas chromatography equipped with HP-1 capillary column (30 m, 0.25 mm, 0.25 μm) and N2 as carrier gas. Gel permeation chromatography (GPC) was acquired by Knauer advanced scientific instrument, Germany, with RI detector (Smartline 2300) PL gel 10 µm, 10E3 A° column. Monodispersed poly(methyl methacrylate) (PMMA) standards were used for calibration. Injected volume was 20 µL. An Oxford INCA 350 energy-dispersive X-ray microanalysis system connected with the Hitachi S-4800 field emission scanning electron microscope was used for the energy-dispersive X-ray spectrometry (EDX) measurement. Melting points were measured using Electrothermal IA 9000 melting point apparatus. The Schiff base and oxime products were characterized by their melting points, elemental analysis, 1H NMR and FTIR spectra and compared with literature values.
Preparation of PSA and PSA-Schiff base ligand
2-Hydroxy-5-chloromethyl-benzaldehyde was synthesized and purified according to the procedure described in [58]; in a 100-mL round-bottom flask, salicylaldehyde (10 mmol), paraformaldehyde (0.49 g, 16.4 mmol) and HCl 37% (80 mmol) along with several drop of concentrated H2SO4 as a catalyst at 70°C were mixed together and stirred for 20 h (Scheme 1). The reaction mixture was cooled to room temperature, then water (20 mL) was added to the mixture, and the product was extracted into CH2Cl2 (20 mL). Some anhydrous Na2SO4 was used for drying the organic phase. Remaining CH2Cl2 removed with rotary evaporator. Then, a pale purple product 1 as powder was isolated (9.5 mmol, 95% yield). Polymerization of 2-hydroxy 4-chloromethyl-benzaldehyde 1 (Scheme 1) (6 mmol) was done in the presence of concentrated KOH (50% (v/v), 20 mmol) at 80°C from which after stirring for 16 h a dark yellow solid (PSA, 2) was obtained. The mentioned solid was filtered and washed with distilled water (3 × 10 mL) in order to complete elimination of acid and inorganic salts impurities (such as KOH and KCl that can be formed in polycondensation reaction and release of HCl molecules) and put in the oven (50 °C) overnight. Final mass for 2 was 0.83 g (m.p. > 400 °C). PSA (1 g, Mw ~ 2278) along with 2-aminophenol (6 mmol) was added to 10 mL of ethanol. The reaction was stirred at room temperature for 12 h until pale orange product was obtained which represented the formation of the intended Schiff base ligand. The product was filtered, washed with methanol (2 × 5 mL) and isolated (1.6 g) after drying in the oven (50 °C).
The average molecular weight of the PSA was measured through acylation of hydroxyl groups (end group analysis), an analytical method that determined hydroxyl number (%OH) by Eq. (1): [59, 60]
where V 1 (mL) and V 2 (mL) are the consumed volumes of potassium hydroxide required for titration of blank and polymer sample, respectively, f is the coefficient of KOH solution 0.5 N that is equal to 0.732 [61], and finally m p (g) is mass of polymer sample weighted for titration. The molecular weight obtained by this method will be numerical molecular weight (\( \overline{{M_{n} }} \)) that was measured by insertion of %OH into Eq. (2) [59, 60]:
To measure the hydroxyl number of PSA, 0.017 g of PSA was weighted and dissolved in pyridine/acetic anhydride mixture (88:12) along with stirring at 50 °C for 8 h. One or two droplets of phenolphthalein indicator were added and the reaction mixture then titrated with KOH (0.5 N). Hydroxyl number of PSA was measured 25.62 (V 2 − V 1 = 0.7 mL, Eq. 1). So the \( \overline{{M_{n} }} \) was calculated by Eq. 2, equal to 2190 for PSA.
Molecular weight of the PSA also was studied with gel permeation chromatography (GPC). The results obtained from GPC are tabulated in Table 1. The numerical molecular weight (\( \overline{{M_{n} }} \)) by GPC was equal to 2226. Small differences (Just 36 unit) between analytical and instrumentational measuring (GPC) of number average molecular weight (\( \overline{{M_{n} }} \)) of PSA exhibited the accuracy of the methods as well as obtained amount for \( \overline{{M_{n} }} \). PSA has well polydispersity index (PSI = 1.43). An explanation for this low PSI was step-growth polymerization of 2-Hydroxy-5-chloromethyl-benzaldehyde in alkali medium. The alkali medium neutralized HCl molecules that formed during the condensation reaction. This act as a driving force for forward of the polymerization reaction and provided such low PSI. Obtained molecular weight for PSA clearly confirms the formation of PSA through polycondensation reaction.
Complexation of Mn to PSA-Schiff base ligand
PSA-Schiff base-Mn(III) complex was prepared by the addition of ethanol (15 mL) to PSA-Schiff base ligand (0.40 g) at 70 °C (Underwent reflux). The reaction was stirred for 20 min, and then Mn(OAc)2 (2 mmol) was added wisely during 10 min. Stirring lasted for another 4 h. The brown sediments were filtered and washed with methanol (2 × 5 mL). The resultant product was put on oven overnight and then isolated as brown powder (Isolated weight: 0.69 g).
General procedure for oxidation of alcohols
In a 10-mL round-bottom flask equipped with O2-filled balloon (~1 atm.), a mixture of alcohol (1 mmol), absolute ethanol (3 mL) and 10 mg of catalyst 4 (3.2 mol%) was stirred at room temperature. After the completion of the reactions that monitored by thin layer chromatography (TLC), the catalyst was filtered off and the reaction mixture was extracted to CH2Cl2 and were purified with silica gel plate chromatography (Scheme 2). The oxidation of primary alcohols proceeds to aldehyde stage and was not observed any carboxylic acid product identified by TLC. The reactions also were performed in the absence of catalyst or in the absence of oxidant in the same conditions.
General procedure for direct preparation of Schiff base and oxime compounds catalyzed by PSA-Schiff base-Mn(III) complex
In an oven-dried 10-mL round-bottom flask, 10 mg catalyst 4 (3.2 mol%) along with 1 mmol amine or hydroxyl amine was added to 7 mL absolute ethanol at ambient temperature. Then, 1 mmol of alcohol was added to the reaction mixture and stirred under O2 atmosphere (~1 atm.). After 14–60 min, yellow to red sediments were appeared which show successful oxidation of alcohols followed by imine formation. The product was filtered and added to 20 mL of hot methanol which just dissolved the imine compound. The filtration of the reaction mixture left a solid on the filter that was the catalyst 4. The remained solution set aside overnight for crystallization, and the recovered catalyst was reused for the next run (Scheme 2).
Activity of the catalyst was expressed as turn-over frequency (TOF) and calculated from the moles of benzyl alcohol converted per mole of Mn contained in the catalyst using following equation:
Conversion and selectivity of each product were calculated by the following equations:
Results and discussion
Characterization of PSA
FTIR spectra of the samples well explained their structures and are shown in Fig. 1a–d. Advent of a peak at 1481 cm−1 in the spectrum of 1 was more related to the bending vibration of methylene groups (–CH2–) in m-substitution than that of aldehyde group (Fig. 1a). Another characteristic peak for 1 was 725 cm−1 for vibration of C–Cl which demonstrated successfully the formation of chloromethylene substitution on salicylaldehyde. The presence of C–Cl vibration in the all spectra at the region of ~725–763 cm−1 demonstrated chloromethylene groups in polymer terminal chains (Scheme 2). Also, two peaks at 800–900 cm−1 were assigned to 1,2,4-trisubstituted pattern on the benzene ring. A series vibration around 2750 and 2850 cm−1 represented the C–H of aldehyde stretching (Fig. 1a, b). Also, the spectra showed C–H bending vibrations between 1400 and 1480 cm−1. Vibration of C–H for benzene rings was found at 3000–3040 cm−1 for the samples. Due to the polymerization of salicylaldehyde, stretching vibration of hydroxyl groups at 3200 cm−1 have been reduced (Fig. 1b, dotted circle) which exhibited the formation of ether bonds. After the formation of Schiff base moiety on PSA, a broad peak related to O–H phenolic with H-bonding was appeared in the spectrum of PSA-Schiff base ligand (Fig. 1c). Vibration of imine bond for c at 1612 cm−1 also was another confirmation for imine bond formation. Chelation of Mn(III) to N and O atoms in PSA causes to shifting of C=N stretching vibrations to lower wavenumbers at 1589 cm−1 (Fig. 1d) [62]. Furthermore, considerable reduction of hydroxyl stretching (great elimination of H-bonding) at catalyst 4 illustrates that the chelation of Mn metals also happens through oxygen atoms. Vibrations correspond to Mn–O and Mn–N were found at 547 and 586 cm−1, respectively [63].
Physical properties of the samples including elemental analysis and color are given in Table 1.
Elemental analysis also proved the formation of 1 (Table 2). Also, it showed the presence of nitrogen atom in amount of 11.84% for PSA-Schiff base ligand which exhibited the presence of N result in imine formation. Also after coordination of Mn to PSA-Schiff base ligand, the nitrogen percentage reduced to 9.03% which shows the presence of Mn in the complex.
2-Hydroxy-5-chloromethyl-benzaldehyde 1 has been previously synthesized, and due to the presence of two directing groups on the phenyl, the chloromethylene group is substituted at 5-position as shown in scheme 1 [20]. 1H NMR of 2-hydroxy-5-chloromethyl-benzaldehyde 1 and PSA 2 is shown in Fig. 2. Resonance for methylene protons was found at 4.58 ppm. Methylene protons for PSA 2 were represented with three peaks at 4.09, 4.74 and 4.98 ppm. Aromatic protons were set in the region of 6.97–7.57 ppm for 1 and 7.11–8.3 ppm for PSA. Chemical shifts for aldehyde and hydroxyl protons were found at 9.09 and 11.06 ppm for 1. 1H NMR spectrum of PSA shows two peaks for aldehyde at 10.22 and 1.40 ppm due to different chemical environments. Hydroxyl groups at terminal PSA chains exhibit a single peak at 11.33 ppm (Fig. 2).
The electronic spectra for the samples were scrolled at region of 200–800 nm. Figure 3a shows UV–Vis spectra for Mn(OAc)2, PSA, PSA-Schiff base ligand and PSA-Mn(II) complex. PSA shows a single peak at ~336 nm with a shoulder at 296 nm which can be assigned to π→π* and n→π* related to phenyl and carbonyl groups, respectively. PSA-Schiff base ligand exhibits three adsorption bands at 298, 356 and 444 nm. These adsorptions were due to n→π* and π→π*, resulting in the formation of imine bond and also π→π* transitions for benzene rings [64]. Mn(OAc)2 shows a single peak at 296 nm which was attributed to the Mn(II) adsorption. Elimination of this peak in PSA-Schiff base-Mn(III) complex and advent of a broad peak with center of 428 nm show the changing valence of Mn(II) to (III) [65, 66] as well as metal to ligand charge transfer (MLCT). Furthermore, shift of π→π* adsorption for imine bond in catalyst 4 to longer wavelengths was as a result of coordination of Mn(III) to PSA-Schiff base ligand [67]. Adsorptions of π electrons related to benzene rings appeared as shoulder at 374 nm for the catalyst 4 (Fig. 3a). The data obtained from electronic studies along with brown color reported for Mn(III) complexes [67] confirm the Mn metal was +3 in valence.
Thermal studies of the PSA, PSA-Schiff base ligand and PSA-Schiff base-Mn(III) complex are shown in Fig. 3b. The spectra show good thermal stability for all three samples; remaining weight for the samples was 50–55% until 750 °C. Thermal decomposition of PSA (Fig. 3b) occurs only in one step in the temperature span of 200–525 °C corresponding to 47% weight loss. After that, the weight loss continues to 750 °C with a gentle slope which shows carbonization of the polymer. Advent of an additional step in the TGA curve of PSA-Schiff base ligand was attributed to decomposition of the Schiff base moiety from PSA chain and confirmed the Schiff base formation on the PSA framework. The mentioned decomposition occurs in temperature span of 99–360 °C (8% weigh loss). Second stage which starts at 420 °C shows decomposition of PSA. Insertion of Mn on PSA-Schiff base ligand leads to about 25% (230–510 °C) weight loss in the thermal spectrum of PSA-Schiff base-Mn(III) complex (Fig. 3b) in first stage. According to thermal degradation of Schiff base moiety in PSA-Schiff base ligand, this amount was consistence with loading amount of Mn obtained from ICP analysis. Second step also was belonging to PSA decomposition.
Loading amount of Mn on the PSA-Schiff base ligand was measured by inductive coupled plasma (ICP) analyzer. The experiments indicate that 17.56 mg of Mn was loaded on 100 mg of PSA-Schiff base ligand framework.
The elements of the catalyst 4 were detected by energy-dispersive spectroscopy (EDX) analysis (Fig. 4). Related information including intensity % and weigh % of the elements is shown at inset table in Fig. 4. EDX spectrum clearly confirmed the Mn complex formation on PSA framework. Also, the spectrum showing the attendance of Cl atoms set at the end chains of PSA framework (Fig. 4).
Optimization of effective parameters for oxidation reactions
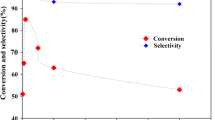
Catalytic oxidation of alcohols is strongly affected by parameters such as oxygen donors, temperature, amount of the catalyst and solvent [67]. To find optimum conditions required for oxidation reactions catalyzed by PSA-Schiff base-Mn(III) complex 4, we investigated these effective parameters for oxidation of alcohols. For this purpose, the oxidation of benzyl alcohol was choosing as model reaction, and so temperature, catalyst amount, solvent and type of oxygen donors were toward our optimization steps and the corresponding results are given in Table 3. Temperature had the least influence for the oxidation of benzyl alcohol which raising the temperature from room temperature to 60 °C or 60–78 °C (Reflux) in ethanol as solvent did not show higher efficiency (Table 3, entries 3, 5 and 6). The effect of temperature for the other solvent was same as ethanol. On the other hand, solvent plays a vital role in the oxidation reactions where the oxidation did not take place at solvent-free conditions (Table 3, entry 15). Ethanol illustrated the best performance at room temperature among the used solvents (Table 3, entry 5). The reaction did not show any progress in water as solvent (Table 3, entry 1). Besides ethanol, other solvents such as DCE, CH3CN, CH2Cl2 and n-hexane showed high progresses for oxidation of benzyl alcohol. Benzaldehyde conversion was low in DMF and toluene (Table 3, entries 8, 9). 3.2 mol% of the catalyst 4 was sufficient in order to nailing to the most possible efficiency (Table 3, entry 5, 96% yield). Amounts more or less than 3.2 mol%, yields lower efficiencies (Table 3, entries 2 and 4) for oxidation reaction at room temperature in ethanol. Progress of the reactions was screened by TLC which in optimum conditions the oxidation of benzyl alcohol was done for 30 min (Table 3, entry 5).
Effect of various oxidant and also catalyst activity on oxidation of benzyl alcohol is given in Fig. 5a, b, respectively, as donut charts. Also, TOF amounts (h−1) for every oxidant as well as used catalysts were calculated and are shown in Fig. 5c, d, respectively. Effect of oxidant was studied by some oxidizing agents: air, tert-BuOOH, NaOCl, oxone, H2O2 and KIO4 (Fig. 5a). In air atmosphere, without O2 balloon equipment, yield of oxidation reduced to 73%. Selectivity also lost in air atmosphere. Moreover, in order to clarify the role of O2 molecules we checked the reaction at argon atmosphere and the conversion was further reduced to 12% (not shown in Fig. 5a). Oxone showed the well performance equal to efficiency with O2 as oxidizing agent (Fig. 5a). But due to green and readily accessible O2 than oxone, we choice the first one for the oxidation reactions. The most activity of the PSA-Schiff base-Mn(III) complex was shown by oxone and O2 as oxygen donors in terms of TOF (TOF = 60 h−1). So, the optimum conditions obtained from the optimization experiments for oxidation of alcohols as well as direct preparation of Schiff base/oxime compounds come as follows: ethanol (Solvent), room temperature, 3.2 mol% of catalyst 4, time and O2 balloon as oxidizing agent.
Experiments in order to show a effect of oxidizing agent and b catalyst activity; c TOF (h−1) calculated for various oxidizing agents; d TOF (h−1) calculated for different catalysts. * Reaction conditions: benzyl alcohol (1 mmol), catalyst 4 (3.2 mol %), room temperature, ethanol (3 mL), oxidant (2 mmol), 30 min. ** KHSO5. *** Reaction conditions: benzyl alcohol (1 mmol), catalyst (Except none), room temperature, ethanol (3 mL), O2 balloon, 30 min
For the elucidation of activity of the catalyst 4, the monomeric complex 5, N-salicylidene-o-aminophenol resulting in the reaction of 2-aminophenol with salicylaldehyde was prepared with previously described procedure [68, 69] and complexed to Mn and used as homogeneous catalyst for oxidation of benzyl alcohol at optimum conditions (Scheme 3). As shown in Fig. 5b, monomeric complex 5 did not show good selectivity and yield for oxidation of benzaldehyde with regard to its homogeneous performance. Conversion to benzaldehyde was 68%, and also benzoic acid was obtained. Furthermore, recovering of the catalyst 5 was a serious problem and suffered from tedious work-up of the carbonyl compounds. Oxidation of manganese may be responsible for low selectivity and activity in 5 [70,71,72] which using solid support for Mn complex not only reduced the formation of oxomanganese species but also improved the recycling ability of the catalyst with low activity loss [72,73,74,75].
On the basis of reported structure for 5 [70] along with achieved results from FTIR, UV–Vis, TGA and EDX analysis, the most probably structure for catalyst 4 is shown at scheme 1.
Furthermore, we checked the blank experiment, in the absence of the catalyst 4 which didn’t observe any noticeable product (Fig. 5b). Conversion with Mn(OAc)2 and PSA also was 36 and 20%, respectively, for benzaldehyde formation. Activity of the PSA-Schiff base-Mn complex as an efficient catalyst can be displayed in terms of TOF which the catalyst has higher TOF amounts than Mn(OAc)2 and Cat. 5. These experiments well addressed the activity of the PSA-Schiff base-Mn(III) complex as an efficient and promising catalyst for oxidation of alcohols.
The oxidation of a variety of primary and secondary alcohols to corresponding carbonyl compounds carried out in obtained optimum conditions and the data is shown in Table 4. The best result was belonging to 4-methoxy benzyl alcohol with 98% yield for 25 min (Table 4, entry 4). Oxidation reactions showed dependency on electron donating/withdrawing substituent on benzene ring in which electron-rich substrates provide higher oxidation yields (Table 4 entries 4, 8, 9). Instead, electron-deficient substrates have lower efficiency (Table 4, entries 3, 6, 7). It believes donating groups stabilized the intermediates produced along the reaction pathway for the incorporation of oxygen into the molecule [51]. Aldehydes did not bear the oxidation which obviously demonstrated selectivity of the method (Table 4, entries 16 and 17). Secondary alcohols represented lower efficiency than primary alcohols (Table 4, entries 13-15). TOF amounts were calculated for every reaction, and the related data are given in Table 4 which represent good activity for the catalyst 4.
Direct synthesis of imines and oximes were taken with various primary and secondary alcohols in the presence of amines and PSA-Schiff base-Mn(III) complex as a catalyst. Up to high yields whether for oximes or imines were achieved. Also, we can point to direct preparation of salen derivatives, the most known strong and applicable ligand so far, with high yields in suitable time (Table 5, entries 2-4) by the present method. Moreover, catalytic oxidation of 1,4-benzenedimethanol yields terephthaladehyde in amount of 95% (Table 5, entry 8) which in the presence of 2-aminophenol the bis-imino compound was prepared. However, due to steric hindrance of ketones, they provided lower efficiency than aldehydes for preparation of ketoximes (Table 5, entries 9, 10) and need to heat in some cases.
The oxidation was inert to amine and aldehyde substrates. This property provides the advantageous of the oxidation conditions for selective preparation of aldehydes as well as imine compounds. Moreover, chemoselectivity behavior of the catalyst was studied by oxidation of benzylic alcohol, n-butanol and ally alcohol through 4 combinations as shown in Table 6.
The results demonstrated high selectivity for oxidation of benzyl alcohol in the presence of aliphatic alcohol or mixture of aliphatic/allyl alcohols (Table 6). Benzaldehyde was the major product in the reaction mixtures of benzyl alcohol with ally alcohol (Table 6, entry 1, > 99%), benzyl alcohol with n-butanol (Table 6, entry 2, 94%) and with mixture of n-butanol/allyl alcohol (Table 6, entry 4, 97%). Also the catalyst exhibited selectivity for oxidation of n-butanol than ally alcohol (Table 6, entry 3, 67%).
Retrieval and recoverability of a catalyst is one of the significant matters for a heterogeneous catalysis which manifest the stability, and metal leaching of the catalyst and was related to some issues such as cost-effective and clean in a reaction [22]. Stability and recoverability of the catalyst was examined on the model reaction (Oxidation of benzyl alcohol) in obtained premium conditions. The catalyst was successfully recovered after completion of the reaction, rinsed with water and reused for eight runs without noticeable reactivity or weight loss. Also amounts of metal leaching were measured by ICP analysis after each run. Metal leaching form the catalyst 4 reached to 3.8% after 8 runs which shows 0.5% in average per cycle (Fig. 6).
The recovered catalyst after eighth run was characterized with FTIR and UV–Vis techniques (Fig. 7). As shown at Fig. 7, UV-Vis spectrum of the recovered catalyst hasn’t changed than the fresh catalyst. Adsorption related to Mn(III) was found at 428 nm for the recovered catalyst as same as the fresh catalyst. With comparison between FTIR spectra of primary catalyst (Orange curve) and used catalyst (Green curve), it could be concluded that the structure of the catalyst remains intact during the reactions and any change was not observed in the main peaks for recovered than primary catalyst; for example, 1612 cm−1 related to imine bond and 547 cm−1 and 586 cm−1 related to vibrations of Mn–O and Mn-N, respectively. No change in spectra of the recycled catalyst as well as negligible leaching metal and loss in activity confirm the stability of the catalyst 4.
Also, in order to measure the exact species responsible for the observed reactions and to measure the extent of Mn leaching after the reactions, we have used the hot filtration test [91,92,93]. For this goal, the model reaction (oxidation of benzyl alcohol) was carried out in the presence of PSA-Schiff base-Mn(III) complex under optimized conditions. The catalyst was removed in situ from the reaction mixture after 44% conversion (GC), and the reactants were allowed to undergo further reaction in the solution. Continuation of the reaction (Without catalyst 4) under the same conditions showed 48% conversion after 1 h. These results indicate that after the removal of heterogeneous catalyst, its leaching amount into the reaction mixture should be low and the catalytic reactions in absence of the catalyst were low, confirming that the catalyst acts heterogeneously in the reaction and only slight leaching take place during the reaction.
To elucidation of advantage, novelty and uniqueness of PSA-Schiff base-Mn(III) complex, we comprised our catalyst with the reported catalysts in the literature for direct preparation of N-benzylidenebenzylamine. So, the reaction of benzyl alcohol with benzyl amine was selected as the model reaction. The results exhibited in Table 7 appeared superiority of PSA-Schiff base-Mn(III) complex over the other systems in environmental and economic aspects. N-benzylidenebenzylamine was formed by Schiff base-Mn(III) complex at short reaction time in ethanol at room temperature.
Conclusion
PSA was synthesized by self-polycondensation reaction of 2-hydroxy-5-chloromethyl-benzaldehyde in the presence of concentrated KOH. PSA-Schiff base ligand was prepared through reaction of PSA with 2-aminophenol. Then, PSA-Schiff base-Mn(III) complex as an efficient catalyst for oxidation of alcohols was prepared by complexation of PSA-Schiff base ligand with a Mn salt. The catalyst was characterized with FTIR, UV–Vis, 1H NMR, CHN, ICP-OES, TGA and EDX analyses. Average molecular weight and PDI of PSA were found 2278 and 1.4, respectively. The catalyst can be prepared through simple procedure, cheap and commercially accessible organic materials which can be selectively catalyze oxidation of primary and secondary alcohols to carbonyl compounds as well as direct formation of Schiff base and oxime compounds specially salen-type ligands through an efficient, mild, heterogeneous, recoverable, cost-effective and novel method by molecular oxygen in ethanol as a green oxidant and solvent, respectively. The catalyst demonstrated well performance in the presence of oxone. Moreover, homogenous monomeric type of the catalyst exhibited lower efficiency than PSA-Schiff base-Mn(III) complex. Chemoselectivity studies on the catalyst toward oxidation of various types of alcohols revealed very high selectivity for oxidation of benzyl alcohol than n-butanol and allyl alcohol. The catalyst can be simply recovered by simple filtration from the reaction mixture and reused for eight runs without any notable reactivity loss. Low leaching, short reaction times, stability, up to high yields and selectivity were some properties of the catalyst discussed in this work.
References
M. Ghassemzadeh, B. Rezaeirad, S. Bahemmat, B. Neumüller, J. Iran. Chem. Soc. 9, 285 (2012)
K. Poonia, S. Siddiqui, M. Arshad, D. Kumar, Spectrochim. Acta A Mol. Biomol. Spectrosc. 155, 146 (2016)
A. D. Khalaji, H. Mighani, M. Kazemnejadi, K. Gotoh, H. Ishida, K. Fejfarova M. Dusek, Arab. J. Chem. (2013), in press
J.R. Anacona, N. Noriega, J. Camus, Acta A Mol. Biomol. Spectrosc. 137, 16 (2015)
H. Keypour, M.H. Zebarjadian, M. Rezaeivala, M. Shamsipur, S.J. Sabounchei, J. Iran. Chem. Soc. 10, 1137 (2013)
H. Keypour, M. Liyaghati-Delshad, M. Rezaeivala, M. Bayat, J. Iran. Chem. Soc. 12, 621 (2015)
C.M. da Silva, D.L. da Silva, L.V. Modolo, R.B. Alves, M.A. de Resende, C.V. Martins, Â. de Fátima, J. Adv. Res. 2, 1 (2011)
K.S. Kumar, S. Ganguly, R. Veerasamy, E. De Clercq, Eur. J. Med. Chem. 45, 5474 (2010)
S.N. Pandeya, A.S. Raja, J.P. Stables, J. Pharm. Pharm. Sci. 5, 266 (2002)
S. Ren, R. Wang, K. Komatsu, P. Bonaz-Krause, Y. Zyrianov, C.E. McKenna, C. Csipke, Z.A. Tokes, E.J. Lien, J. Med. Chem. 45, 410 (2002)
Z. Asadi, M. Asadi, F.D. Firuzabadi, R. Yousefi, M. Jamshidi, J. Iran. Chem. Soc. 11, 423 (2014)
S.P. Chatterjee, B. Sur, S.R. Chaudhary, Oncology 47, 433 (1990)
S. Adsule, V. Barve, D. Chen, F. Ahmed, Q.P. Dou, S. Padhye, F.H. Sarkar, J. Med. Chem. 49, 7242 (2006)
H. Polasa, Indian J. Pharm. Sci. 47, 202 (1985)
S. Ershad, L. Sagathforoush, G. Karim-Nezhad, S. Kangari, Int. J. Electrochem. Sci. 4, 846 (2009)
J. Gradinaru, A. Forni, V. Druta, F. Tessore, S. Zecchin, S. Quici, N. Garbalau, Inorg. Chem. 46, 884 (2007)
E. Lindbäck, H. Norouzi-Arasi, E. Sheibani, D. Ma, S. Dawaigher, K. Wärnmark, ChemistrySelect 1, 1789 (2016)
M. Salavati-Niasari, F. Davar, M. Bazarganipour, Dalton Trans. 39, 7330 (2010)
K. Tiwari, M. Mishra, V.P. Singh, RSC Adv. 4, 27556 (2014)
N. Nishat, S. Hasnain, T. Ahmad, A. Parveen, J. Therm. Anal. Calorim. 105, 969 (2011)
L. Moradi, M. Bina, T. Partovi, Curr. Chem. Lett. 3, 147 (2014)
M. Salavati-Niasari, E. Esmaeili, H. Seyghalkar, M. Bazarganipour, Inorg. Chim. Acta 375, 11 (2011)
M. Salavati-Niasari, M. Bazarganipour, Appl. Surf. Sci. 255, 2963 (2008)
A. Bilici, F. Doğan, I. Kaya, Ind. Eng. Chem. Res. 53, 104 (2013)
A. Bi̇li̇ci̇, İ. Kaya, F. Doğan, J. Polym. Sci., Part A: Polym. Chem. 47, 2977 (2009)
S. Waśkiewicz, K. Zenkner, E. Langer, M. Lenartowicz, I. Gajlewicz, Prog. Org. Coat. 76, 1040 (2013)
E. Zhang, H. Tian, S. Xu, X. Yu, Q. Xu, Org. Lett. 15, 2704 (2013)
J.T. Reeves, M.D. Visco, M.A. Marsini, N. Grinberg, C.A. Busacca, A.E. Mattson, C.H. Senanayake, Org. Lett. 17, 2442 (2015)
Q. Kang, Y. Zhang, Green Chem. 14, 1016 (2012)
L. Jiang, L. Jin, H. Tian, X. Yuan, X. Yu, Q. Xu, Chem. Commun. 47, 10833 (2011)
H. Tian, X. Yu, Q. Li, J. Wang, Q. Xu, Adv. Synth. Catal. 354, 2671 (2012)
M.S. Kwon, S. Kim, S. Park, W. Bosco, R.K. Chidrala, J. Park, J. Org. Chem. 74, 2877 (2009)
S. Sithambaram, R. Kumar, Y.C. Son, S.L. Suib, J. Catal. 253, 269 (2008)
H.K. Kwong, P.K. Lo, K.C. Lau, T.C. Lau, Chem. Commun. 47, 4273 (2011)
H. Tsunoyama, H. Sakurai, Y. Negishi, T. Tsukuda, J. Am. Chem. Soc. 127, 9374 (2005)
D.K.T. Yadav, B.M. Bhanage, RSC Adv. 5, 12387 (2015)
A.R. Hajipour, S.E. Mallakpour, I. Mohammadpoor-Baltork, S. Khoee, Chem. Lett. 2, 120 (2000)
E.P. Talsi, K.P. Bryliakov, Coord. Chem. Rev. 256, 1418 (2012)
K. Schröder, K. Junge, B. Bitterlich, M. Beller, Top. Organomet. Chem. 33, 83 (2011)
A. Onopchenko, J. Schulz, J. Org. Chem. 40, 3338 (1975)
C.C. Cosner, P.J. Cabrera, K.M. Byrd, A.M.A. Thomas, P. Helquist, Org. Lett. 13, 2071 (2011)
H.Y. Sun, Q. Hua, F.F. Guo, Z.Y. Wang, W.X. Huang, Adv. Synth. Catal. 354, 569 (2012)
T.K.M. Shing, Y.Y. Yeung, P.L. Su, Org. Lett. 8, 3149 (2006)
P.G. Cozzi, Chem. Soc. Rev. 33, 410 (2004)
K. Srinivasan, P. Michaud, J.K. Kochi, J. Am. Chem. Soc. 108, 2309 (1986)
T.C. Mac Leod, M.V. Kirillova, A.J. Pombeiro, M.A. Schiavon, M.D. Assis, Appl. Catal. A Gen. 372, 191 (2010)
N.C. Gianneschi, P.A. Bertin, S.T. Nguyen, C.A. Mirkin, L.N. Zakharov, A.L. Rheingold, J. Am. Chem. Soc. 125, 10508 (2003)
S. Sithambaram, R. Kumar, Y.C. Son, S.L.J. Suib, Catal. 253, 269 (2008)
B. Chen, J. Li, W. Dai, L. Wang, S. Gao, Green Chem. 16, 3328 (2014)
S. Biswas, B. Dutta, K. Mullick, C.H. Kuo, A.S. Poyraz, S.L. Suib, ACS Catal. 5, 4394 (2015)
M.A. Nasseri, A. Mohammadinezhad, M. Salimi, J. Iran. Chem. Soc. 12, 81 (2015)
M.T. Räisänen, A. Al-Hunaiti, E. Atosuo, M. Kemell, M. Leskelä, T. Repo, Catal. Sci. Technol. 4, 2564 (2014)
B. Chen, L. Wang, S. Gao, ACS Catal. 5, 5851 (2015)
Food Cosmet, Toxicol. 17, 903 (1979)
D. Janeš, S. Kreft, Food Chem. 109, 293 (2008)
R. Tang, F.X. Webster, D. Müller-Schwarze, J. Chem. Ecol. 19, 1491 (1993)
A. Dehno Khalaji, M. Kazemnejadi, H. Mighani, D. Das, J. App. Chem. 7, 77 (2013)
Q. Wang, C. Wilson, A.J. Blake, S.R. Collinson, P.A. Tasker, M. Schröder, Tetrahedron Lett. 47, 8983 (2006)
M. Pramanik, S.K. Mendon, J.W. Rawlins, Polym. Test. 31, 716 (2012)
V.P. Boiko, V.K. Grischenko, Rev. Acta Polym. 36, 459 (1985)
J.F. Zemaitis Jr., D.M. Clark, M. Rafal, N.C. Scrivner, Handbook of Aqueous Electrolyte Thermodynamics: Theory & Application (Wiley, New York, 1986), pp. 577–579
İ. Şakiyan, N. Gündüz, T. Gündüz, Synth. React. Inorg. Met. Org. Chem. 31, 1175 (2001)
İ. Şakıyan, Transit. Met. Chem. 32, 131 (2007)
S.M.D.M. Romanowski, S.P. Machado, G.R. Friedermann, A.S. Mangrich, M.D.F. Hermann, H.O. Lima, S. Nakagaki, J. Braz. Chem. Soc. 21, 842 (2010)
D.R. Learman, B.M. Voelker, A.S. Madden, C.M. Hansel, Front. Microbial. 4, 262 (2013)
J.E. Jee, O. Pestovsky, A. Bakac, Dalton Trans. 39, 11636 (2010)
B. Bahramian, V. Mirkhani, M. Moghadam, A.H. Amin, Appl. Catal. A Gen. 315, 52 (2006)
M.M. Najafpour, M.Z. Ghobadi, B. Sarvi, B. Haghighi, Dalton Trans. 44, 15271 (2015)
H.R. Godini, A. Gili, O. Görke, S. Arndt, U. Simon, A. Thomas, R. Schomäcker, G. Wozny, Catal. Today 236, 12 (2014)
A.A.A. Aziz, A.N.M. Salem, M.A. Sayed, M.M. Aboaly, J. Mol. Struct. 1010, 130 (2012)
V.D. Chaube, S. Shylesh, A.P. Singh, J. Mol. Catal. A: Chem. 241, 79 (2005)
S. Shylesh, A.P. Singh, J. Catal. 228, 333 (2004)
S. Shylesh, S. Sharma, S.P. Mirajkar, A.P. Singh, J. Mol. Catal. A: Chem. 212, 219 (2004)
T. Joseph, D.P. Sawant, C.S. Gopinath, S.B. Halligudi, J. Mol. Catal. A: Chem. 184, 289 (2002)
P. Karandikar, M. Agashe, K. Vijaymohanam, A.J. Chandwadkar, Appl. Catal. A Gen. 257, 133 (2004)
A. Tashiro, A. Mitsuishi, R. Irie, T. Katsuki, Synlett 2003, 1868 (2003)
H. Tanak, M. Macit, M. Yavuz, Ş. Işık, Acta Crystallogr. Sect. E: Struct. Rep. Online 65, o3056 (2009)
D. Şahin, S. Koçoğlu, Ö. Şener, C. Şenol, H. Dal, T. Hökelek, Z. Hayvalı, J. Mol. Struct. 1102, 302 (2015)
K.W. Mukonyi, I.O. Ndiege, Bull. Chem. Soc. Ethiop. 15, 137 (2001)
A.J. Bosco, S. Lawrence, C. Christopher, S. Radhakrishnan, J. Rosario, A. Arul, S. Raja, D. Vasudevan, J. Phys. Org. Chem. 28, 591 (2015)
A.R. Katritzky, M. Szajda, S. Bayyuk, Synthesis 1986, 804 (1986)
T.M. Fasina, O.O. Ogundele, I. Ayeni, J. Chem. Pharm. Res. 6, 816 (2014)
E.C. Niederhoffer, J.H. Timmons, A.E. Martell, Chem. Rev. 84, 137 (1984)
M.B. Fugu, N.P. Ndahi, B.B. Paul, A.N. Mustapha, J. Chem. Pharm. Res. 5, 22 (2013)
K. Guo, Y. Chen, Anal. Methods 2, 1156 (2010)
İ. Kaya, S. Çulhaoğlu, Chin. J. Polym. Sci. 26, 131 (2008)
L. Saikia, J.M. Baruah, A.J. Thakur, Org. Med. Chem. Lett. 1, 12 (2011)
L. Dinparast, H. Valizadeh, Iran. J. Catal. 5, 73 (2015)
J. Yu, M. Lu, Synlett 25, 1873 (2014)
X. Liang, Z. Mi, Y. Wang, L. Wang, X. Zhang, React. Kinet. Catal. Lett. 82, 333 (2004)
R.H. Crabtree, Chem. Rev. 112, 1536 (2012)
M. Esmaeilpour, J. Javidi, M. Divar, J. Magn. Magn. Mater. 423, 232 (2017)
M. Esmaeilpour, J. Javidi, S. Zahmatkesh, Appl. Organomet. Chem. 30, 897 (2016)
B. Gnanaprakasam, J. Zhang, D. Milstein, Angew. Chem. Int. Ed. Engl. 122, 1510 (2010)
S. Kegnæs, J. Mielby, U.V. Mentzel, C.H. Christensen, A. Riisager, Green Chem. 12, 1437 (2010)
L. Blackburn, R.J. Taylor, Org. Lett. 3, 1637 (2001)
Acknowledgement
The authors are grateful to Golestan University research council for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kazemnejadi, M., Shakeri, A., Mohammadi, M. et al. Direct preparation of oximes and Schiff bases by oxidation of primary benzylic or allylic alcohols in the presence of primary amines using Mn(III) complex of polysalicylaldehyde as an efficient and selective heterogeneous catalyst by molecular oxygen. J IRAN CHEM SOC 14, 1917–1933 (2017). https://doi.org/10.1007/s13738-017-1131-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1131-z