Abstract
Some new Schiff bases, (Z)-4-amino-3-((E)-(R-methoxybenzylidene)hydrazono)-6-methyl-3,4-dihydro-1,2,4-triazin-5(2H)-one (R = 2 (L2), R = 3 (L3) and R = 4 (L4)), were synthesized by the condensation reactions of 4-amino-3-hydrazinyl-6-methyl-1,2,4-triazin-5(4H)-one (L1) and corresponding methoxybenzaldehyde in a molar ratio 1:1.5 in high yields. The reaction of L2 and L4 with an excess amount of the corresponding aldehydes gave the unsymmetrical bis-Schiff bases (E)-3-((E)-(R-methoxybenzylidene)hydrazono)-4-((E)-R-methoxybenzylideneamino)-6-methyl-3,4-dihydro-1,2,4-triazin-5(2H)-one (R = 2 (L22) and R = 4 (L44)), respectively. Furthermore, the reaction of L2–L4 with silver(I) nitrate in a molar ratio 2:1 led to the silver(I)-complexes with the general formula [Ag(Lx)2]NO3 (Lx = L2 (2), L3 (3) and L4 (4)). All synthesized Schiff base compounds and complexes were characterized by a combination of IR-, 1H-NMR spectroscopy, mass spectrometry and elemental analyses. In addition, the structures of L2, L4·CH3CN, L22·CH3OH and L44·CH3OH and complexes 2 and 4 were determined by X-ray diffraction studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The synthetic methodologies and reactivity of 1,2,4-triazine and its substituted derivatives have been widely studied and reviewed [1–4]. Compounds containing 1,2,4-triazine moiety are known to be endowed with a wide spectrum of biological activities. In particular, 1,2,4-triazin-5(2H)-one derivatives exhibit anticancer [5–8], antimicrobial [9–11], herbicidal [12, 13] and anti-HIV [14] activities. Numerous compounds containing 1,2,4-triazine moieties are well-known in natural materials and show interesting biological and antiviral properties [15, 16]. In addition to the mentioned properties and applications of 1,2,4-triazine and its substituted derivatives, the presence of three nitrogen atoms in the six-membered cycle combined with substituents of various nature allows one to produce metal complexes displaying different coordination modes. On the other hand, a number of Schiff bases have been studied due to their potent biological, physiological and pharmacological activities and they have industrial, antifungal, antibacterial, anticancer and herbicidal applications [17–23]. The Schiff bases containing heterocycles with donor atoms such as nitrogen, oxygen, sulfur, etc., have been playing an important role in the development of coordination chemistry, giving metal complexes which serve as models for biological systems [24–28]. They also exhibit biological activities and have potential applications in many fields of chemistry, such as oxidation catalysis, electrochemistry, etc. [29–38]. It is also known that the existence of metal ions bonded to biologically active compounds may enhance their activities.
We have previously reported that heterocycles such as 1,2,4-triazoles and 1,2,4-triazines can be used as good stabilization agents for late transition metal ions such as copper(I), silver(I), palladium(II) and platinum(II) ion in their low oxidation states [39–44]. For instance, we have found that according to the molecular structures of silver(I) complexes containing 4-amino-6-methyl-1,2,4-triazin-3-thion-5-one (AMTTO), the heterocycle AMTTO act as S-monodentate ligand with a weak Ag···N interaction, which leads to 2 + 2 coordination with the silver(I) atom [39]. We have also reported that the sulfur atom of each 4-amino-6-methyl-1,2,4-triazol-3-thione (AMTT) moiety in its silver(I) complex acts simultaneously as S-monodentate one, as well as bridging agent between two metal centers forming the polymeric coordination complex [{Ag(AMTT)2}NO3] n [44]. We have also reported the synthesis and characterization of the silver(I) complex [{[Ag(AETT)]NO3}2] n (AETT = 4-amino-5-ethyl-2H-1,2,4-triazol-3(4H)-thione) with new coordination modes between silver(I) ions and the sulfur atoms of the 1,2,4-triazole-3-thione moieties. These coordination modes lead to the formation of a ten-membered Ag–S ‘‘mosaic” pattern and cause a two-dimensional endless framework [45]. Furthermore, we have synthesized and characterized several Schiff bases derived from AMTTO and AMTT using different aldehydes and studied their behavior toward late transition metals such as copper(I), silver(I) and palladium(II) ions [46–49].
In our ongoing interest in the study of the behavior of nitrogen-donor ligands toward late transition metals, this work focuses on the synthesis and characterization of new nitrogen-donor Schiff base compounds based on 1,2,4-triazine and their complexation ability toward silver(I) ion.
Experimental section
Chemicals and starting materials
All chemicals were purchased from Merck AG and Fluka and were used without further purification. L1 was prepared according to the literature procedure [50]. Ethanol was purified and dried by the standard method.
Physical measurements
Infrared (IR) spectra were recorded on a Perkin-Elmer 883 spectrometer (KBr pellets 4,000–400 cm−1). Melting points were recorded on a Büchi B545 melting point apparatus and are uncorrected. 1H NMR spectra were recorded on the Bruker-AQS AVANCE 300 using TMS (δ = 0.0 ppm) as internal standard. Mass spectra were recorded on a Fisons Instruments Trio 1000 spectrometer in positive mode with EI (70 eV). Elemental analyses (C, H, and N) were performed on a Thermo Finnigan Flash EA 1112 series elemental analyzer and Costech ECS 4010 CHNS analyzer.
Synthesis of mono-Schiff bases L2, L3 and L4—general procedure
A solution of L1 (0.31 g, 2 mmol) in ethanol (40 mL) was treated with a solution of the corresponding methoxy-substituted benzaldehyde (0.40 g, 3 mmol) in the same solvent (10 mL) and was refluxed for 8 h. In the case of L3, the solution of the corresponding aldehyde was acidified with acetic acid (3 drops). The progress of the reaction was monitored by thin layer chromatography (TLC) using ethyl acetate:petroleum ether (1:2) as eluent. After completion of the reaction, the solvent was evaporated to 20 mL, and the solid crude was filtered and washed with hexane and cold water (2 × 5 mL). The clear filtrate was kept at 4 °C to give the colorless crystals of the corresponding Schiff bases L2–L4.
Selected data for L2: Yield: 0.52 g (95%), mp.: 185 °C. Elemental analysis for C12H14N6O2 (274.12), Calcd.: C, 52.55; H, 5.14; N, 30.64. Found: C, 52.35; H, 5.10; N 30.38; 1H-NMR (DMSO-d6); δ, 2.08 (s, 3H, CH3), 3.69 (s, 3H, O–CH3), 5.36 (s, 2H, NH2), 6.74–7.20 (d, 4H, Ar), 8.60 (s, 1H, H-imine), 11.03 (s, 1H, NH); IR \( \tilde{v} \) (KBr disc, cm−1): 3,319 (N–H, triazine), 3,226–3,153 (NH, NH2), 1,689 (C=O), 1,633 (C=N, imine), 1,562 (C=N, triazine), 1,244, 1,024 (CH3–O–Ar); MS (70 eV): m/z: 276 [M+ +2], 275 [M++1], 274 [M+], 243, 228, 217, 186, 167, 156, 141, 134, 119, 114, 104, 92, 91, 77, 42, 29.
Selected data for L3: Yield: 0.41 g (76%), mp.: 202 °C. Elemental analysis calculated for C12H14N6O2 (274.12): C, 52.55; H, 5.14; N, 30.64. Found: C, 52.14; H, 5.31; N, 30.12. 1H-NMR (DMSO-d6); δ, 2.32 (s, 3H, CH3), 3.91 (s, 3H, O–CH3), 5.40 (s, 2H, NH2), 6.99–7.38 (d, 2H, Ar), 8.41(s, 1H, H-imine), 10.03 (s, 1H, NH). IR \( \tilde{v} \) (KBr disc, cm−1): 3,286 (N–H, triazine), 3,143–3,211 (NH, NH2), 1,695 (C=O), 1,630 (C=N, imine), 1,570 (C=N, triazine), 1,265, 1,033 (CH3–O–Ar). MS (70 eV) m/z: 275 [M+ + 1], 274 [M+], 168, 141, 134, 119, 114, 91, 77, 43, 32, 27.
Selected data for L4: Yield: 0.50 g (90%), mp.: 165 °C. Elemental analysis calculated for C12H14N6O2 (274.12): C, 52.55; H 5.14; N, 30.64. Found: C, 52.42; H, 5.12; N, 30.58. 1H-NMR (DMSO-d6); δ, 2.31 (s, 3H, CH3), 3.90 (s, 3H, O–CH3), 5.38 (s, 2H, NH2), 6.97–7.72 (d, 2H, Ar), 8.38 (s, 1H, H-imine), 10.00 (s, 1H, NH). IR \( \tilde{v} \) (KBr disc, cm−1): 3,286 (N–H, triazine), 3,199–3,213 (NH, NH2), 1,689 (C=O), 1,635 (C=N, imine), 1,610 (C=N, triazine), 1,251, 1,026 (CH3–O–Ar). MS (70 eV); m/z: 276 [M+ +2], 275 [M+ + 1], 274 [M+], 259, 167, 156, 141, 134, 120, 91, 77, 43, 31.
Synthesis of bis-Schiff bases L22 and L44: general procedure
A solution of mono-Schiff bases (L2 or L4, 0.55 g, 2 mmol) in hot dry ethanol (25 mL) was treated with a solution of corresponding aldehyde (0.41 g, 3 mmol) in the same solvent (10 mL). The reaction mixture was acidified with hydrochloric acid (pH 4.5–5) and refluxed for 15 h. The progress of the reaction was monitored by TLC using ethyl acetate: petroleum ether (1:2) as eluent. After completion of the reaction, the solvent was evaporated to 20 mL, and the solid crude was filtered and washed with hexane (2 × 5 mL). The clear filtrate was kept at 4 °C to give the yellowish crystals of the corresponding bis-Schiff base compounds L22 and L44.
Selected data for L22: Yield: 0.47 g (60%), mp.: 189 °C. Elemental analysis calculated for C20H20N6O3 (392.16): C, 61.21; H, 5.14; N, 21.42. Found: C, 61.11; H, 5.12; N, 21.22. 1H-NMR (DMSO-d6); δ, 2.16 (s, 3H, CH3), 3.80 (s, 3H, O–CH3), 3.89 (s, 3H, O–CH3), 6.98–8.35 (8H, Ar), 8.46 (s, 1H, H-imine), 8.88 (s, 1H, H-imine), 12.07 (s, 1H, NH). IR \( \tilde{v} \) (KBr disc, cm−1): 3,367 (N–H, triazine), 1,689 (C=O), 1,610 (C=N, imine), 1,246, 1,020 (CH3–O–Ar). MS (70 eV); m/z: 392 [M+], 359, 259, 258, 229, 228, 215, 187, 133, 119, 104, 103, 92, 91, 85, 78, 27.
Selected data for L44: Yield: 0.43 g (55%), mp.: 180 °C. Elemental analysis calculated for C20H20N6O3 (392.16): C, 61.21; H, 5.14; N, 21.42. Found: C, 60.98; H, 5.22; N, 21.13. 1H NMR (DMSO-d6); δ, 2.16 (s, 3H, CH3), 3.81 (s, 3H, O–CH3), 3.87 (s, 3H, O–CH3), 6.95–7.14 (d, 2H, Ar), 7.86–7.89 (d, 2H, Ar), 8.18 (s, 1H, H-imine), 8.57(s, 1H, H-imine), 12.04 (s, 1H, NH). IR \( \tilde{v} \) (KBr disc, cm−1): 3,325 (N–H, triazine), 1,681 (C=O), 1,604 (C=N, imine), 1,577 (C=N, triazine), 1,242, 1,018 (CH3–O–Ar). MS (70 eV); m/z: 394 [M++2], 392 [M+], 274, 258, 228, 217, 187, 133, 119, 92, 77, 43, 27.
Synthesis of the complexes 2, 3 and 4: general procedure
A solution of mono-Schiff bases (L2, L3 or L4, 0.27 g, 1 mmol) in methanol/acetonitrile (40 mL, 1/1) was treated with silver(I) nitrate (0.08 g, 0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and was refluxed for a further 1 h. After completion of the reaction, which was monitored by TLC using ethyl acetate:petroleum ether (1:2) as eluent, the solid crude was filtered and washed with cold ethanol (2 × 5 mL). The clear filtrate was kept at 4 °C to give the colorless crystals of 2 and 4.
Selected data for 2: Yield: 0.22 g (62%), mp.: >290 °C (dec.). Elemental analysis calculated for C24H28AgN13O7 (718.43): C, 40.12; H, 3.93; N, 25.35. Found: C, 40.03; H, 3.88; N, 25.29. IR \( \tilde{v} \) (KBr disc, cm−1): 3,481 (N–H, triazine), 3,323, 3,238, 1,627 (C=O), 1,604 (C=N, imine), 1,589, 1,489 (C=N, triazine), 1,429, 1,409, 1,385, 1,301, 1,267, 1,186, 1,035 (CH3–O–Ar), 974, 920, 830, 771, 692, 501. MS (70 eV); m/z: 276 [M++2], 275 [M+], 274, 259, 244, 243, 228, 167, 133, 119, 91, 77, 42, 28.
Selected data for 3: Yield: 0.21 g (60%), mp.: >290 °C (dec.). Elemental analysis calculated for C24H28AgN13O7 (718.43): C, 40.12; H, 3.93; N, 25.35. Found: C, 39.98; H, 3.95; N, 25.40. IR \( \tilde{v} \) (KBr disc, cm−1): 3,454 (N–H, triazine), 3,284, 1,695 (C=O), 1,629 (C=N, imine), 1,577 (C=N, triazine), 1,469, 1,386, 1,323, 1,265, 1,195, 1,033 (CH3–O–Ar), 983, 941, 877, 837, 786, 732, 684, 567, 536, 503, 460, 412. MS (70 eV); m/z: 275 [M+], 274, 245, 244, 243, 215, 167, 141, 134, 119, 91, 77, 42, 28.
Selected data for 4: Yield: 0.23 g (65%), mp.: >290 °C (dec.). Elemental analysis calculated for C24H28AgN13O7 (718.43): C, 40.12; H, 3.93; N, 25.35. Found: C, 40.03; H, 3.88; N, 25.29. IR \( \tilde{v} \) (KBr disc, cm−1): 3,491 (N–H, triazine), 3,286, 1,689 (C=O), 1,635, 1,608 (C=N, imine), 1,577 (C=N, triazine), 1,508, 1,463, 1,367, 1,317, 1,249, 1,168, 1,026, 975, 937, 898, 829, 773, 732, 665, 638, 592, 534, 503, 459, 414. MS (70 eV); m/z: 274, 264, 173, 167, 147, 133, 119, 103, 90, 77, 76, 64, 63, 38, 36.
Crystal structure analysis of L2, L4·CH3CN, L22·CH3OH, L44·CH3OH and complexes 2 and 4
Table 1 shows the crystallographic data of L2, L4·CH3CN, L22·CH3OH, L44·CH3OH, 2 and 4. The crystals of all compounds were covered with perfluorinated oil and mounted on the top of a glass capillary under a flow of cold gaseous nitrogen. The orientation matrix and unit cell dimensions were determined from ca. 6,000 (L2; Stoe IPDS I), 7,500 (L4·CH3CN, Stoe IPDS I), 12,000 (L22·CH3OH, Stoe, IPDS II), 5,500 (L44·CH3OH, Stoe, IPDS I), 5,700 (2, Stoe, IPDS I) and from ca. 7,400 (4; Stoe IPDS I) reflections (graphite-monochromated Mo–Kα radiation (λ = 71.073 pm) for all compounds. The intensities were corrected for Lorentz and polarization effects. In addition, absorption corrections were applied for L4·CH3CN, L22·CH3OH, L44·CH3OH, 2 and 4 (numerical). The structures were solved by the direct methods for L4·CH3CN and L44·CH3OH (SHELXS-97), for L2, L22·CH3OH and 2 (SIR-92) and by the Patterson method for 4 (SHELXTL-Plus). No absorption correction was used for L2 and all data were refined against F 2 by full-matrix least-squares using the program SHELXL-97. The position of carbon bonded hydrogen atoms in all compounds except those in L4·CH3CN, and those mentioned below were calculated for ideal positions and refined with a common displacement parameter. H1–H3 atoms for L2, 2 and 4 and H1 and H2 atoms for L22·CH3OH and L44·CH3OH were included with a free refinement, respectively. Programs used were SHELXS-97 [51], SIR-92 [52], SHELXL-97 [53], SHELXTL-Plus [54], ORTEP [55] and PLATON-98 [56].
Results and discussion
Syntheses and characterization
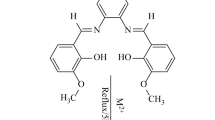
Mono-Schiff base compounds L2, L3 and L4 can be obtained as colorless solid materials by the treatment of L1 with the corresponding o-, m- or p-methoxybenzaldehyde in molar ratio 1:1.5 in ethanol under reflux conditions in high yields. The reaction of L2 and L4 with an excess amount of the same methoxybenzaldehyde in dry methanol gave the bis-Schiff bases as yellowish crystalline solids in good yields according to Eq. (1).

The complexes 2, 3 and 4 can be prepared by the reaction of L2, L3 and L4 with silver(I) nitrate in a molar ratio 1:2 in methanol under reflux conditions in good yields according to Eq. (2).

All synthesized compounds are air stable. In IR spectra of the compounds L2–L4, L22 and L44, the absorptions ν(C=O) and ν(C=N) of the triazine heterocycles were obtained at 1,689 and 1,562 cm−1 (L2), 1,695 and 1,570 cm−1 (L3), 1,689 and 1,610 cm−1 (L4), 1,689 and 1,610 cm−1 (L22) and 1,681 and 1,604 cm−1 (L44), and new absorption bands at 1,633 cm−1 (L2), 1,630 cm−1 (L3), 1,635 cm−1 (L4), 1,633 cm−1 (L22), 1,630 cm−1 (L44) could be assigned to ν(C=N, iminic). The νC=O and νC=N (iminic) function cause vibrations at 1,627 and 1,604 cm−1 for 2, at 1,695 and 1,629 cm−1 for 3, and at 1,689 and 1,608 cm−1 for 4. The NO3 − anion in 2–4 presents its N–O asymmetric stretching mode (E′) at 1,385 cm−1 (2), 1,386 cm−1 (3) and 1,367 cm−1 (4), and its out-of-plane absorptions (A2″) at 830, 837 and 829 cm−1, respectively. In 1H-NMR of the ligands, the signals at 8.60 ppm (L2), 8.41 ppm (L3) and 8.38 ppm (L4) can be assigned to N=CH, and no signal at 4.36 ppm corresponding to NH2 group of L1 was observed. In 1H-NMR spectra of diiminic compounds, the disappearance of the signals at 4.36 and 5.51 ppm corresponding to NH2 groups of the L1 and the presence of new signals at 8.46 and 8.88 ppm (L22) and 8.18 and 8.57 ppm (L44) confirm the formation of diiminic compounds. The highest fragment in the mass spectra of the ligands was observed at m/z = 274 for L2–L4 and at m/z = 392 for L22 and L44 and can be assigned to corresponding M+. The mass spectra of 2, 3 and 4 show only the main peak of their corresponding ligands.
Table 1 shows the crystallographic data of all Schiff base compounds and the synthesized silver(I)-complexes.
Crystal structures of compounds and complexes
Crystal structures of compounds L2, L4·CH3CN, L22·CH3OH and L44·CH3OH
The Schiff bases derived from L1 can exist in two tautomeric forms I and II as shown in Scheme 1.
Due to the determined molecular structures of all synthesized mono- and bis-iminic compounds, the Schiff bases exist in the form I containing one endocyclic NH group and one exocyclic C=N moiety in the solid state. They exhibit no significant differences in their endocyclic-, exocyclic- and azomethine C=N bond distances (129.1(3), 130.4(2) and 128.7(2) pm in L2 (Fig. 1), 128.8(2), 129.1(2) and 127.3(2) pm in L4·CH3CN (Fig. 2), 128.5(2), 130.2(2), 128.0(2) and 127.7(2) pm in L22·CH3OH (Fig. 3) and 129.2(3), 130.4(3), 128.5(3) and 123.5(3) pm in L44·CH3OH (Fig. 4), respectively). The C=N bond lengths and the bond distances in the six-membered heterocycles compare well with those observed in previously reported 4-amino-6-methyl-3-thio-3,4-dihydro-1,2,4-triazin-5(2H)-one (AMTTO) and its Schiff base derivatives [47, 48, 57].
Molecular structure and numbering scheme of L2 (ORTEP plot at 40% probability level). Selected bond lengths [pm] and angles [°]: N1–C2 137.9(2), N1–C1 138.1(2), N1–N2 142.1(2), N3–C3 129.1(3), N3–N4 136.2(2), N4–C1 135.3(2), N5–C1 130.4(2), N5–N6 140.4(2), N6–C5 128.7(2), C2–C3 147.03, C3–C4 148.2(3), N5–C1–N4 125.9(2), N5–C1–N1 118.1(2), C5–N6–N5 111.8(2), C1–N5–N6 111.7(2)
Molecular structure and numbering scheme of L4·CH3CN; the thermal ellipsoids are drawn at the 40% probability level. Selected bond lengths [pm] and angles [°]: N1–C1 136.4(2), N2–C2 128.8(2), N3–C1 137.6(2), N1–N2 135.7(2), N3–N4 141.9(2), N5–C1 129.1(2), N5–N6 140.6(2), N6–C5 127.3(2), C1–N5–N6 109.8(1), C5–N6–N5 113.3(1), N6–C5–C6 122.1(2)
Molecular structure and numbering scheme of L22·CH3OH; the thermal ellipsoids are drawn at the 40% probability level. Selected bond lengths [pm] and angles [°]: N1–C1 138.0(2), N5–C1 130.2(2), C3–N3 128.5(3), N5–N6 141.1(2), N6–C5 128.0(2), N1–N2 142.1(2), N2–C13 127.7(2), C13–C14 1.471(2), N6–C5–C6 121.0(2), C5–N6–N5 113.3(1), C1–N5–N6 110.0(1), C13–N2–N1 114.2(2), N2–C13–C14 117.9(2)
Molecular structure and numbering scheme of L44·CH3OH; the thermal ellipsoids are drawn at the 40% probability level. Selected bond lengths [pm] and angles [°]: N1–C3 138.9(3), N1–N2 142.7(3), N2–C5 123.5(3), N3–C2 129.2(3), C5–C6 1.476(4), N5–C3 130.4(3), N5–N6 141.1(2), N6–C15 128.5(3), C15–C16 1.462(3), N2–C5–C6 121.7(3), C5–N2–N1 112.4(2), N6–C15–C16 122.1(2), C15–N6–N5 113.2(2), C(3)–N(5)–N(6) 109.7(2)
L4·CH3CN molecules are strictly planar, while the dihedral angle between the “best” planes through basic six-membered ring of 1,2,4-triazine moiety and the aldehyde rest in L2 is 16°.
The dihedral angles between the “best” planes in the molecules of L22·CH3OH (A: N1 N3 N4 C1 C2 C3, B: C6 C7 C8 C9 C10 C11 and C: C14 C15 C16 C17 C18 C19) are 6° (A, B) and 84° (A, C), and those observed in L44·CH3OH (A: N1 C1 C2 N3 N4 C3, B: C6 C7 C8 C9 C10 C11 and C: C16 C17 C18 C19 C20 C21) are 101° (A, B) and 20° (A, C).
In L2, the molecules are arranged along the 41-axis and are associated via H bonds (Figs. 1, 5). The NH2 group of each molecule acts as bridging agent between two adjacent molecules. It links the imine nitrogen atom of one ligand to the oxygen atom of the carbonyl group of the adjacent one via hydrogen bondings (N2–H1···N6a: 359.9(2) pm and N2–H2···O1a: 318.9(2) pm). Another H bonding is formed between the endocyclic NH group of one ligand and the exocyclic imine nitrogen atom (N4–H3···N5a: 289.2(2) pm).
In L4·CH3CN, the nitrogen atom of solvate molecule acts as bridging agent and links the NH2 group of each ligand to the endocyclic NH group of the adjacent one via hydrogen bondings (N1–H1···N7: 308.0(2) pm and N4–H3···N7a: 325.9(2) pm). The latter coordinates additionally to the iminic N6 atom via intramolecular H bonding (N1–H1···N6: 259.0(2) pm). The H2 atom of the NH2 moiety of each ligand coordinates simultaneously with its own exocyclic iminic nitrogen atom as well as with the endocyclic nitrogen atom of the adjacent molecule (N4–H2···N5: 265.8(2) pm and N4–H2···N2a: 333.8(2) pm). These coordination modes are responsible for the formation of layers parallel to (10-1), which are also networked among each other (Fig. 6).
The endocyclic NH group in L22·CH3OH acts as bridging agent between two adjacent diimine compounds and links the hydrazine nitrogen group of the own molecule to the oxygen atom of the methoxy moiety of the adjacent one via hydrogen bondings (N4–H1···N6: 257.0(2) pm and N4–H1···O3a: 304.8(2) pm). This coordination mode is responsible for the formation of chains of L22·CH3OH along [010]. In addition, the solvate molecule links the nitrogen atoms of the exocyclic imine group to nitrogen atom of the side chain imine group via hydrogen bondings (O4–H2···N2: 320.0(2) pm and O4–H2···N5: 287.7(2) pm) (Fig. 7).
The OH group of the methanol molecule in L44·CH3OH acts as bridging agent between two unsymmetrically bis-imine compounds and links the endocyclic NH group of one compound to the nitrogen atom of one of the exocyclic C=N group of the adjacent one via hydrogen bonding (N4–H1···O4: 289.8(3) pm and O4–H2···N5a: 292.7(3) pm, Fig. 8). The latter is responsible for the formation of centro-symmetric dimers of L44·CH3OH as depicted in Fig. 9.
Crystal structures of complexes 2 and 4
Complexes 2 and 4 are ionic compounds and consist of [Ag(Lx)2]+-cations (Lx = L2 or L4) and nitrate anions. In these complexes, the metal atom sits on an inversion center and each ligand acts as a monodentate one and coordinates with the metal center through its on ring bonded exocyclic imine nitrogen atom (Ag–N: 227.3(3) pm for 2 (Fig. 10) and 226.1(2) pm for 4 (Fig. 11)). These Ag–N bond lengths are relatively long compared with those found in complexes containing two coordinated silver ions [58–60], but they are shorter than the average values of 231–244 pm found for four-coordinated silver complexes [61–64]. The Ag–NH2 distances of 265.5(2) pm for 2 and the Ag–O bond lengths of 270.0(2) pm for 4 are longer than the maximum atom distance generally accepted for Ag(I)–N bonds of 234 pm and Ag(I)–O bonds of 232 pm [65], but they are clearly shorter than the sum of the van der Waals radii of silver, nitrogen and oxygen atoms (Ag: 170 pm, N: 155 pm and O: 150 pm [66]). The steric hindrance induced by the position of the methoxy substituents in each ligand is responsible for the direct coordination of nitrate anion to the metal center, which was only observed in complex 4. In spite of observed Ag–NH2 contacts of 265.5(2) pm in 2, no distortion in the linear geometry of the metal center is measured (N–Ag–N-axis: 180°). The N–Ag–N bond angle of 169.5(1)° in 4 is characteristic for a distorted linear coordination sphere caused by two additional two Ag–O contacts of 270.0(2) pm in 4. Therefore, the coordination sphere for the metal centers in all complexes can be described as 2 + 2.
Molecular structure and numbering scheme of complex 2; the thermal ellipsoids are drawn at the 40% probability level. Selected bond lengths [pm] and angles [°]: Ag1–N5 227.3(3), Ag1–N2 265.5(2), N1–N2 140.8(4), N1–C1 138.3(5), N5–C1 129.7(5), N5–N6 141.5(4), N6–C5 125.8(5), C5–C6 1.476(5), N7–O5#2 1.04(1), N7–O5 1.04(1), N7–O3#2 1.24(1(, N7–O3 1.24(1), N7–O4#2 1.414(9), N7–O4 1.414(9(, N5–Ag1–N5#1 180.0, N6–N5–Ag1 124.5(2), N6–C5–C6 120.6(3), O5–N7–O3 137(2), O5#2–N7–O4#2 119(2), O5#2–O3–O4#2 112(2)
Molecular structure and numbering scheme of complex 4; the thermal ellipsoids are drawn at the 40% probability level. Selected bond lengths [pm] and angles [°]: Ag(1)–N(5) 2.261(2), Ag(1)–N(5)#1 2.261(2), Ag(1)–O(4) 2.700(2), Ag(1)–O(4)#1 2.700(2), N(1)–C(1) 1.373(3), N(5)–C(1) 1.306(3), N(5)–N(6) 1.401(3), N(6)–C(5) 1.277(3), N(7)–O(4) 1.233(3), N(7)–O(4)#1 1.233(3), N(7)–O(3) 1.246(4), N(5)–Ag(1)–N(5)#1 169.5(1), N(6)–N(5)–Ag(1) 120.7(1), C(5)–N(6)–N(5) 112.1(2), N(6)–C(5)–C(6) 124.0(2), O(4)–N(7)–O(4)#1 120.7(4), O(4)–N(7)–O(3) 120(2), O(4)#1–N(7)–O(3) 120(2)
In 2, the nitrate anions are disordered around an inversion center and their positions could not be fixed exactly (Fig. 12). In this complex, the molecules are stacked along the crystallographic axis [100] and are linked through nitrate anions via hydrogen bondings (N2–H1···O2a: 312.2(4) pm, N2–H2···O2a: 312.2(4) pm, N4–H3···N6: 254.9(4) pm, N2–H1···O3b: 313.5(1) pm, N2–H1···O4b: 316.2(1) pm, N2–H1···O5c: 279.3(1) pm, N4–H3···O3d: 279.6(1) pm, N4–H3···O4e: 287.7(1) pm).
In 4, the molecules are stacked along [010] and they are linked along (010) via H bonds (N2–H1···O3a: 294.5(3) pm, N2–H2···O1: 266.0(3) pm, N4–H3···O4a: 290.6(3) pm, Fig. 13).
The dihedral angle between the “best” planes in 2 (A: N1 N3 N4 N5 C1 C2 C3 and B: N6 C5 C6 C7 C8 C9 C10 C11) is 4°, while 4 is practically planar.
Conclusion
In conclusion, we have synthesized and characterized some mono- and bis-Schiff base compounds based on 1,2,4-triazine by the condensation reaction of 4-amino-3-hydrazinyl-6-methyl-1,2,4-triazin-5(4H)-one with o-, m- and p-methoxybenaldehyde in good to high yields. According to the determined molecular structure, we have found that all synthesized mono- and bis-Schiff bases exist in the tautomeric form I (Scheme 1) in their solid states. We have also studied the behavior of the synthesized Schiff bases toward silver(I) ion and found that the mono-Schiff bases was allowed to react in a 2:1 molar ratio with silver(I) ion giving complexes with the general formula [Ag(Lx)2]NO3 (2, 3, 4). We have also found that under the same conditions, the bis-Schiff bases (L22 and L44) undergo solvolysis reactions to form the corresponding mono-Schiff bases L2 and L4, before the complexation reactions take place. According to the molecular structures of the complexes 2 and 4, the corresponding ligands coordinate with the metal ion as unidentate ones via their on the ring bonded exocyclic nitrogen atoms. This linear geometry around the metal center is distorted by the η2-coordination of oxygen atoms of nitrate anion to the metal center in 4. Therefore, the coordination sphere for the metal centers in all complexes can be described as 2 + 2.
Supplementary data
CCDC 800143, 800144, 800145, 800146, 800147 and 800149 contain the supplementary crystallographic data for compounds L2, L4·CH3CN, L22·CH3OH and L44·CH3OH and complexes 2 and 4. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, U.K.; Fax.: 44 (0)1223-336-033 or e-mail: deposit@ccdc.cam.ac.uk.
References
H. Neunhoeffer, P.F. Wiley, in Chemistry of 1,2,3-Triazines and 1,2,4-Triazines, Tetrazines and Pentazines, ed. by A. Weissberger, E.C. Taylor, (John Wiley and Sons, New York, Chichester, Brisbane, Toronto, 1978), pp. 1001–1004
H. Neunhoeffer, in Comprehensive Heterocyclic Chemistry, vol. 3, eds. by A.R. Katritzky, C.W. Rees (Pergamon Press, Oxford, 1984), pp. 385–456
M.A. Ali, S.E. Livingstone, Coord. Chem. Rev. 13, 101–132 (1974)
R.M. Abdel-Rahman, Pharmazie 56, 275–286 (2001)
R.M. Abdel-Rahman, J.M. Morsy, S. El Edfawy, H.A. Amine, Pharmazie 54, 667–671 (1999)
Z. El-Gengy, J.M. Morsy, H.A. Allimony, W.R. Abdel-Monem, R.M. Abdel-Rahman, Pharmazie 56, 376–383 (2001)
S.K. Padney, A. Singh, A. Singh, A. Nizamuddin, Eur. J. Med. Chem. 44, 1188–1197 (2009)
A.A. El-Barbary, A.M. Sakran, A.M. El-Madani, N. Claus, J. Heterocycl. Chem. 42, 935–941 (2005)
K. Singh, M.S. Barwa, P. Tyagi, Eur. J. Med. Chem. 42, 394–402 (2007)
T.E. Ali, Phosphorus Sulfur Silicon Rel. Elem. 182, 1717–1726 (2007)
D.J. Parsad, M.S. Karthikeyan, P.B. Karegoudar, B. Poojary, B.S. Holla, N.S. Kumari, Phosphorus Sulfur Silicon Rel. Elem. 182, 1083–1091 (2007)
B. Boehner, A.G. Ciba-Geigy, Eur. Pat. Chem. Abstr. 105, 6522 (1986)
M. Timer, S. Sauvard, C.M. Georgescu, Biochem. Pharmacol. 15, 408–410 (1996)
R.M. Abdel-Rahman, J.M. Morsy, F. Hanafy, H.A. Amene, Pharmazie 54, 347–351 (1999)
Z.H. Cohan, H. Pervez, A. Rauf, K.M. Khan, G.M. Maharvi, C.T. Supuran, J. Enzym. Inhib. Med. Chem. 19, 161–168 (2004)
M. Mashali, H.A. Bayoumi, A. Taha, Chem Papers Chem. Zvesti 53, 299–308 (1999)
S. Kumar, D. Nath Dhar, P.N. Saxena, I. Kanpur, J. Sci. Ind. Res. 68, 181–187 (2009)
M.S. Refat, S.A. El-Korashi, D. Nandan Kumar, A.S. Ahmed, Spectrochim. Acta Part A 70, 898–906 (2008)
M. Sithambaram Karthikeyan, D. Jagadeesh Parsad, B. Poojary, K. Subrahmanya Bhat, B. Shivarama Holla, N. Suchetha Kumari, Bioorg. Med. Chem. 14, 7482–7489 (2006)
L. Shi, H.-M. Ge, S.-H. Tan, H.-Q. Li, Y.-C. Song, H.-L. Zhu, R.-X. Tan, Eur. J. Med. Chem. 42, 558–564 (2007)
A.A. Bekhit, O.A. El-Sayed, T.A.K. Al-Allaf, H.Y. Aboul-Enein, M. Kunhi, S.M. Pulicat, K. Al-Hussain, F. Al Khodairy, J. Arif, Eur. J. Med. Chem. 39, 499–505 (2004)
R. Nair, A. Shah, S. Baluja, S. Chanda, J. Serb. Chem. Soc. 71, 733–744 (2006)
J. Lv, T. Liu, S. Cai, X. Wang, L. Liu, Y. Wang, J. Inorg. Biochem. 100, 1888–1896 (2006)
N. Raman, S. Ravichandran, C. Thangaraja, J. Chem. Sci. 116, 215–219 (2004)
A. Garoufis, S.K. Hadjikakou, N. Hadjiliadia, Coord. Chem. Rev. 253, 1384–1397 (2009)
W. Radecka-Paryzek, V. Patroniak, J. Lisowski, Coord. Chem. Rev. 249, 2156–2175 (2005)
K. Sundaravel, E. Suresh, M. Palaniandavar, Inorg. Chim. Acta 362, 199–207 (2009)
J. Costamagna, J. Vargas, R. Latorre, A. Alvarado, G. Mena, Coord. Chem. Rev. 119, 67–88 (1992)
N. Yoshida, K. Ichikawa, Chem. Commun., pp. 1091–1092 (1997)
M.J. Hannon, C.L. Painting, A. Jackson, J. Hamblin, W. Errington, Chem. Commun., pp. 1807–1808 (1997)
M.J. Hannon, S. Bunce, A.J. Clarke, N.W. Alcock, Angew. Chem. Int. Ed. 38, 1277–1278 (1998)
A.F. Williams, C. Floriani, A.E. Merbach, Perspectives in Coordination Chemistry (VCH Verlagsgesellschaft, Weinheim, 1992)
J.L. Atwood, J.E.D. Davies, D.D. MacNicol, F. Vögtle (Eds.), in Comprehensive Supramolecular Chemistry, Vol. 9, Eds. by J.-P. Sauvage, M. Wais Hosseini (Volume Eds.), (Templating Self-Assembly and Self-Organisation, Pergamon, Oxford, 1996)
S.C. Bhatia, J.M. Bindlish, A.R. Saini, P. C. Jain, J. Chem. Soc., Dalton Trans., pp. 1773–1779 (1981)
T. Kaliyappan, P. Kannan, Prog. Polym. Sci. 25, 343–370 (2000)
R.A. Archer, Coord. Chem. Rev. 128, 49–68 (1993)
R. Ziesel, Coord. Chem. Rev. 195, 216–217 (1993)
K.C. Gupta, A. Kumar, Satur Coord. Chem. Rev. 252, 1420–1450 (2008)
M. Ghassemzadeh, F. Adhami, M.M. Heravi, A. Taeb, S. Chitsaz, B. Neumüller, Z. Anorg, Allg. Chem. 628, 2887–2893 (2002)
M. Ghassemzadeh, M. Bolourtchian, S. Chitsaz, B. Neumüller, M.M. Heravi, Eur. J. Inorg. Chem., pp. 1877–1882 (2000)
T. Sharafi, M.M. Heravi, M. Ghassemzadeh, B. Neumüller, Z. Anorg. Allg. Chem. 631, 2297–2299 (2005)
M. Yazdanbakhsh, M. Hakimi, M.M. Heravi, M. Ghassemzadeh, B. Neumüller, Z. Anorg, Allg. Chem. 631, 924–927 (2005)
M. Ghassemzadeh, M.M. Pooramini, M. Tabatabaee, M.M. Heravi, B. Neumüller, Z. Anorg. Allg. Chem. 630, 403–406 (2004)
M. Ghassemzadeh, M.M. Heravi, B. Neumüller, Z. Anorg. Allg. Chem. 631, 2401–2407 (2005)
M. Ghassemzadeh, L. Fallahnedjad, M.M. Heravi, B. Neumüller, Polyhedron 27, 1655–1664 (2008)
M. Ghassemzadeh, M. Tabatabaee, S. Soleimani, B. Neumüller, Z. Anorg. Allg. Chem. 631, 1871–1876 (2005)
F. Heshmatpour, M. Ghassemzadeh, M. Semsarha, B. Neumüller, Z. Anorg, Allg. Chem. 633, 465–469 (2007)
M. Ghassemzadeh, A. Sharifi, J. Malakootikhah, B. Neumüller, E. Iravani, Inorg. Chim. Acta 357, 2245–2252 (2004)
M. Ghassemzadeh, M. Tabatabaee, M.M. Pooramini, M.M. Heravi, A. Eslami, B. Neumüller, Z. Anorg, Allg. Chem. 632, 786–792 (2006)
A. Dornow, H. Menzel, P. Marx, Chem. Ber. 97, 2173–2178 (1964)
G.M. Sheldrick, SHELXS-97 (Universität Göttingen, Germany, 1997)
A. Altmore, G. Cascarano, C. Giacovazzo, A. Guagliardi, M.C. Burla, G. Polidori, M. Camalli, SIR-92 (Bari, Perugia, 1992)
G.M. Sheldrick, SHELXL-97 (Göttingen, Germany, 1997)
G.M. Sheldrick, SHELXTL-Plus, Release 5.05/VMS for Siemens R3 Crystallographic Research Systems (Siemens Analytical X-Ray Instruments Inc., Madison (WI), 1996)
C.K. Johnson, ORTEP, ORNL-3794 (Oak Ridge National Laboratory, Tennessee, 1965)
A.L. Spek, PLATON-98 (Utrecht, The Netherland, 1998)
M. Tabatabaee, M. Ghassemzadeh, B. Zarabi, B. Neumüller, Z. Naturforsch. 61b, 1421–1425 (2006)
H.W. Roesky, J. Schimkowiak, K. Meyer-Baese, Angew. Chem. Int. Ed. Engl. 25, 1006–1007 (1986)
D. Britton, Y.M. Chow, Acta Crystallogr. Sect. B 33, 697–699 (1977)
T.G. Richmond, E.P. Kelson, A.T. Patton, J. Chem. Soc. Chem. Commun., pp. 96–97 (1988)
T.G. Richmond, E.P. Kelson, A.M. Arif, G.B. Carpenter, J. Am. Chem. Soc. 110, 2334–2335 (1988)
A.M. Arif, T.G. Richmond, J. Chem. Soc. Chem. Commun. pp. 871–872 (1990)
C. Stockheim, K. Wieghardt, B. Nuber, J. Weiss, U. Folerke, H.J. Haupt, J. Chem. Soc., Dalton Trans. pp. 1487–1490 (1991)
M.G.B. Drew, D. McDowell, J. Nelson, Polyhedron 7, 2229–2232 (1988)
International Table for X-ray Crystallography, Vol. IV, Kynoch Press, Birmingham, U.K. (1974): covalent radii (pm): Ag 159, N 75 and O 73
A. Bondi, J. Phys. Chem. 68, 441–451 (1964)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghassemzadeh, M., Rezaeirad, B., Bahemmat, S. et al. Syntheses, characterization and crystal structures of new mono- and bis-Schiff base compounds derived from 1,2,4-triazine and the silver(I) complexes containing mono-Schiff base ligands. J IRAN CHEM SOC 9, 285–296 (2012). https://doi.org/10.1007/s13738-011-0023-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-011-0023-x