Abstract
The production of new biocidal polyester Schiff base metal complexes [PESB–M(II)] via polycondensation reaction between chelated Schiff base diol and adipoyl chloride is reported. The resulting polyesters were characterized by physico-chemical and spectroscopic methods. The analytical data of all the synthesized polyesters were found to be in good agreement with 1:1 molar ratio of chelated Schiff base diol to adipoyl chloride. Thermogravimetric analyses of synthesized polyesters were studied by TG in nitrogen atmosphere up to 1073 K and results indicate that Cu(II) polyester complex exhibited better heat resistant properties than the other polyesters complexes. Magnetic moment and UV–visible spectra were examined to explain the structure of all the polyesters which reveled that Mn(II), Co(II), Ni(II) have octahedral geometry while Cu(II) possess a distorted octahedral geometry. These newly developed polyesters were also tested for their antibacterial activity against several bacteria and fungi. Among all the tested compounds PESB–Cu(II) possess the highest bactericidal and fungicidal activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the recent years, the synthesis of new polymers with unusual thermal, optical, and mechanical properties has gradually gained regards. The branch of the polymer–metal complexes has been developed as an interdisciplinary area involving chemistry, electrochemistry, metallurgy, environmental protection, and material science [1, 2]. A much attraction of many researchers towards the polyesters has been noticed from the early days. The first polyester resin [3] was produced by Berzelius in 1847, and Gay-Lussac and Pelouze in 1888. At the starting, polyesters were produced by the polycondensation of dialcohol and diacid having potential applications, e.g., surface coating, composite, in the manufacture of fibers (terylene, decron) and film (e.g., Melinex, mylar) [4]. Consequently, it was of interest to investigate new polyesters with various linking groups in their backbone like incorporation of metal or azomethine linkage. Polyesters containing metal ions are of special interest, due to the special photophysical, photochemical, and magnetic properties of the utilized transition metal ions. The diverse range of coordination and geometries of the transition elements offers the possibility of accessing polymers with unusual conformational, mechanical, and morphological characters [5, 6]. Azomethine linkage is of special interest due to its interesting properties such as syn–anti isomerism [7], good thermal stabilities [8], non-linear optical activities [9], liquid crystalline property [10], and semiconductivity [11]. A few polyesters having amino acid moieties in the main chain were found to be thermally more stable than the polyester itself [12, 13]. Further, it was found that the nature of metal ions influences the thermal degradation and biological degradation of the polyester–metal complexes. Lately several researcher groups have been actively engaged in the development and study of metal containing polyesters [14, 15].
In the present context we want to give the method of synthesis of Schiff base and their corresponding metal complexes, which further react with adipoyl chloride to produce the desired polyesters [PESB–M(II)] by the polycondensation process. The above synthesized polyesters have been studied by using various spectral techniques such as electronic, FTIR, 1H NMR, and magnetic susceptibility measurement to confirm the type of geometry around the central metal ion. Thermal stability of these polyesters was investigated by using TG technique. The obtained polyesters were also screened for antibacterial activity against Bacillus subtilis, Bacillus megaterium, Streptococcus aureus, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Shigella boydii and for antifungal activity against Candida albicans, Trichophyton longifusus, Aspergillus flavus, Aspergillus niger, Fusarium solani, Microsporum canis, Puccinia graminis by using agar well diffusion method.
Experimental
Materials
2-Hydroxy benzaldehyde (S.D. Fine Chem. India. Ltd.), 1,2-benzenediamine (S.D. Fine Chem. India. Ltd.), adipoyl chloride (Merck India), potassium hydroxide (Merck India), 35% hydrochloric acid (Merck India), conc. sulfuric acid (Merck India), 37% formaldehyde (Merck India), sodium hydroxide (Merck India), manganese(II) acetate tetrahydrate, Mn(CH3COO)2·4H2O; copper(II) acetate monohydrate, Cu(CH3COO)2·H2O; nickel(II) acetate tetrahydrate, Ni(CH3COO)2·4H2O; cobalt(II) acetate tetrahydrate, Co(CH3COO)2·4H2O; zinc(II) acetate dihydrate, Zn(CH3COO)2·2H2O (S.D. Fine Chem India Ltd.) were of analytical grade and used as received without further purification. Solvents DMF, DMSO, methanol, and acetone (Merck, India) and ethanol (Changshu Yangyuan Chemicals, China) were purified by distillation procedure prior to use.
Measurements
The metal content of the polyesters was determined by complexometric titration against EDTA after decomposing with concentrated nitric acid (HNO3) [16]. The FT-IR spectra were recorded over the (4,000–500 cm−1) range on a Perkin Elmer infrared spectrophotometer model 621 by using KBr pellets. Elemental analysis of polyester complexes was performed on an Elemental analyzer system GmbH Vario ELIII (IIT Roorkee). The electronic spectra of synthesized polyesters were carried out on a Perkin Elmer Lambda EZ-201 spectrophotometer by using 10−3 molar solution of the sample in DMSO as a solvent in quartz cuvette. Magnetic moment values of these polyesters were evaluated by Gouy balance by using Hg[Co(SCN)4] as a calibrant at room temperature. Proton and carbon-13 nuclear magnetic resonance spectra (1H NMR and 13C NMR) were recorded on a JEOL-GSX 300-MHz FX-1000 FT-NMR spectrometer using DMSO as a solvent and tetramethylsilane (TMS) as an internal standard. Thermal behavior and glass transition temperature of the polyesters were monitored by using a TGA analyzer Perkin Elmer (Pyris 1 TG–DTA) under nitrogen atmosphere at a heating rate of 283 K min−1 (IIT Roorkee). The solubility of the polymers was tested in various solvents at room temperature. Antimicrobial study of the synthesized polyesters was investigated in the department of microbiology, Aligarh Muslim University, Aligarh, India.
Synthesis
Synthesis of 2,5-dihydroxymethyl benzaldehyde
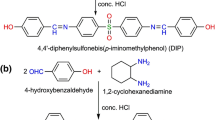
2,5-Dihydroxymethyl benzaldehyde was prepared by the hydrolysis of 2-hydroxy 5-chloromethyl benzaldehyde, which was synthesized and purified according to a procedure described [17]. It was prepared by heating a mixture containing salicylaldehyde, formaldehyde, and 35% hydrochloric acid in aqueous solution at 70 °C with concentrated H2SO4 as a catalyst. The reaction time of 20 h was optimum to furnish a pale yellow solid. It was further hydrolyzed on heating in the presence of aqueous KOH (Scheme 1).
Synthesis of Schiff base diol [bis(2,5-dihydroxymethylbenzylidene)1,2-benzenediamine]
Schiff base diol has been prepared according to the literature [18]. 2,5-Dihydroxymethyl benzaldehyde (3.04 g, 0.02 mol dissolved in 50 mL ethanol) was mixed with 1,2-phenylenediamine (1.08 g, 0.01 mol in 50 mL of methanol) to prepare Schiff base diol [bis{2,5-hydroxymethyl benzylidene}1,2-phenylenediamine]. The reaction mixture was refluxed at 85 °C for 3 h with continuous stirring. Mixture was poured into ice-cooled water to precipitate the Schiff base after removing the excess solvent of the mixture. Obtained colored precipitate was filtered, washed with water, petroleum ether, and then dried in vacuum at 70 °C for 2 h. The product was isolated as yellow powder in 73% yield.
Elemental analyses, calcd (found %) for C22H20N2O4: C, 70.20 (69.09); H, 5.35 (5.39); N, 7.44 (7.45). 1H NMR (300 MHz, DMSO, δ) 14.70 (s, 2H, OH), 7.85–6.62(m, 10H, Ar–H), 4.60 (s, 2H, OH), 8.07 (s, 2H, CH=N), 5.30 (m, 4H, Ar–CH 2–). 13C NMR (DMSO-d 6, δ, ppm): 160.3 (>C=N), 67.54 (Ar–CH2–O), 150 (Ar–OH), 137.4–148.1 (Ar). FT-IR (KBr pellets ν(max) cm−1) 3380–3200, 3083, 2972–2840, 2800–2700, 1635, 1582, 1265 (Scheme 2).
Synthesis of Schiff base metal complexes
A series of polymer metal complexes of Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) were prepared from the by the reaction of Schiff base diol (3.76 g, 0.01 mol in 25 mL methanol) with transition metal acetates (0.01 mol, dissolved in 25 mL methanol).
Procedure for the preparation of Schiff base metal complex is as follows:
The mixture of Schiff base diol and copper acetate was refluxed with continuous stirring for 6 h during which brown colored precipitate obtained. The resulting solid residue was filtered, washed with water, ethanol, and dried at 60 °C in vacuum desiccator for 2 h. The same method was applied for the synthesis of other metal chelated diols of [Mn(II), Co(II), Ni(II) and Zn(II)] by using the same conditions with their respective molar ratio. Crystallization from aqueous ethanol gave the expected metal complex. The data of all the Schiff base metal complexes are as follows (Scheme 3).
Mn(II) complex
Elemental analyses, calcd (found %) for [C22H18N2O4–Mn(II)·2H2O]: C 56.72 (55.87), H 4.76 (4.38), N 6.01 (6.31), Mn 11.80 (10.21). FT-IR (KBr pellets ν(max) cm−1) 3365–3210, 3065, 2940–2880, 1600, 1550, 1260, 465, 375.
Co(II) complex
Elemental analyses, calcd (found %) for [C22H18N2O4–Co(II)·2H2O]: C 56.29 (55.34), H 4.72 (4.56), N 5.96 (5.01), Co 12.50 (13.32). FT-IR (KBr pellets ν(max) cm−1) 3365–3210, 3060, 2940–2880, 1620, 1565, 1258, 470, 372.
Ni(II) complex
Elemental analyses, calcd (found %) for [C22H18N2O4–Ni(II)·2H2O]: C 56.32 (56.79), H 4.72 (4.67), N 5.97 (4.84), Ni 12.51 (11.06). FT-IR (KBr pellets ν(max) cm−1) 3365–3210, 3080, 2940–2880, 1615, 1560, 1265, 468, 374.
Cu(II) complex
Elemental analyses, calcd (found %) for (C22H18N2O4)–Cu(II)·2H2O: C 55.75 (55.79), H 4.67 (4.60), N 5.91 (6.01), Cu 13.40 (13.32). FT-IR (KBr pellets ν(max) cm−1) 3365–3210, 3062, 2970–2840, 1615, 1580, 1258, 848, 465, 370.
Zn(II) complex
Elemental analyses, calcd (found %) for [C22H18N2O4–Zn(II)]: C 60.09 (60.40), H 4.12 (3.75), N 6.37 (6.79), Zn 14.87 (14.11). 1H NMR (300 MHz, DMSO,δ) 4.45–4.60 (s, 2H, OH), 7.52–6.34 (m, 10H, Ar–H), 8.92 (s, 2H, HC=N), 5.32 (4H, Ar–CH 2–), 1.60–2.15(–CH2). 13C NMR (DMSO-d 6, δ, ppm): 162.5 (>C=N), 67.54 (Ar–CH2–O), 154 (Ar–C–O), 137.4–148.1 (Ar). FT-IR (KBr pellets ν(max) cm−1) 3365–3210, 3062, 2940–2880, 1600, 1560, 1264, 470, 374.
Synthesis of polyester complexes
Polyesters were synthesized from the dialcoholic chelates and diacid chloride according to the literature [19] by using the modified method. All the polyesters complexes were obtained by the condensation reaction between a chelated Schiff base diol and adipoyl chloride. In a three necked-round bottom flask, 0.02 mol of the metal containing diol was dissolved in 100 mL of DMF and adipoyl chloride (3.6 g, 0.02 mol, in a minimum amount of freshly distilled carbon tetrachloride) was added drop wise to this solution and then a few drops of aqueous sodium hydroxide. The resulting mixture was heated with continuous stirring at 40 °C for 36 h. The progress of the reaction was monitored by thin layer chromatography (TLC). This reaction mixture was precipitated with excess amount of methanol, filtered, and washed several times with water. Finally the product was dried in vacuum oven at 60 °C for 5 h (Scheme 4).
Antimicrobial assessment
The antimicrobial activities of the synthesized polyesters were tested against different microorganisms in DMSO as a solvent. The sample concentration was 50 μg mL−1 for antibacterial and antifungal study was used. Bacterial strains were nourished in nutrient broth (Difco) and yeasts in malt extract broth (Difco) and incubated for 24 and 48 h, respectively. According to agar diffusion method bacteria were incubated on Mueller–Hinton Agar and yeast on Sabouraud dextrose agar. The wells were dug in the media with the help of a sterile steel borer and 0.1 mL of each sample was introduced in corresponding well. Other wells were supplemented with solvent (DMSO) for positive control and standard drug viz Kanamycin (antibacterial) and Miconazol (antifungal) for negative control [20, 21]. The resulting zones of inhibition on the plates were measured in millimeter.
Results and discussion
Polyesters complexes were produced according to the aforementioned procedure in experimental section. The Schiff base diol was prepared by the condensation of 2,5-dihydroxy methyl benzaldehyde with 1,2-benzenediamine in 2:1 molar ratio. Nitrogen containing compound (1,2-benzenediamine) behave as nucleophilic reagent having a lone pair of electrons, which attacks on carbon with positive charge to form imine linkage (CH=N). The FTIR and NMR spectra of Schiff base show a characteristic absorption at 1,635 cm−1 and 8.07 ppm due to the formation of azomethine (CH=N) group. Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) chelates were prepared by the reaction of metal(II) acetate with Schiff base diol in methanolic solution. The elemental analysis of chelates indicates that they react in 1:1 (metal:ligand) molar ratio. The polyesters were prepared by polycondensation reaction between chelated Schiff base diol and adipoyl chloride. Oxygen atom of alcohol acts as nucleophile, it attacks to the positively charged carbonyl carbon which results in the formation of an intermediate. This intermediate loses HCl with the transfer of a proton (H+) to the second molecule of alcohol thus resulting in the formation of a polyester link.
The geometry of synthesized compounds was discussed with the help of UV–visible, FTIR, NMR, and magnetic moment. The microanalytical data [Calculated (found)] given in Table 1, showed a good agreement for the 1:1 molar ratio (chelated diol to adipoyl chloride).
All the polyester complexes were colored, solid materials, soluble in DMSO and DMF but insoluble in common organic solvents. The molecular mass of the polyester complexes could not be determined by GPC due to their insoluble nature in common organic solvent. Thermal analysis of resulting polyesters was also measured to investigate the presence of water and their thermal behavior.
FT-IR spectra studies
The important infrared spectral bands and their tentative assignments for the synthesized polyesters were recorded and are illustrated in Table 2. Bands in the region 3400–3200 cm−1 were observed due to the stretching vibration of OH. For the Schiff base, the broad bands in the 2800–2700 cm−1 range are assigned to the OH group vibration(ortho position) assigned intramolecular (OH⋯N) with the nitrogen atom of the CH=N group [22]. In Schiff base metal complexes, there was no vibration band at 2800–2700 cm−1 range due to the disappearance of interamolecular hydrogen bonds after coordination of (CH=N) nitrogen atom to the metal ion. The presence of coordinated water molecules in the Mn(II), Co(II), Ni(II), and Cu(II) except Zn(II) polyester complexes were further confirmed by appearance of δHOH deformation bands in the region 1600–1570 and 670–650 cm−1 for the rocking modes of coordinated water, which was also supported by analytical and thermal analysis data. In the spectrum of the Schiff base, ν(HC=N) vibration of azomethine group is shown at 1,635 cm−1 [23]. This vibration shifted to a lower region and can be attributed to the complexation of metal ion and nitrogen atom of the azomethine group [24].
A new band of azomethine ν(HC=N) group at 1620–1600 cm−1 was observed due to the absence of a band at 3452 cm−1 of amino group and a band at 1696 cm−1 of aldehyde group. The bands appeared at 1265–1258 and 1150–1070 cm−1 are due to C–O and CH2–O vibrations [25, 26], respectively. The lowering in frequency of C–O bond (10–36 cm−1) indicates the coordination of phenolic oxygen to the metal ion. The peaks of substituted phenyl ring were observed at 852–833 cm−1. In the low frequency region, two bands of all the polyesters were observed at 375–370 and 470–465 cm−1, which are attributed to ν(M–N) and ν(M–O), respectively. These bands suggested that the coordination with metal ion takes place via the azomethine nitrogen atoms and also via the deprotonated oxygen of the phenol groups [27, 28].
1H NMR and 13C NMR spectra
The 1H NMR of PESB and PESB–Zn(II) were recorded in DMSO-d6 and are shown in Figs. 1 and 2. In the 1H NMR spectrum of PESB–Zn(II) a chemical shift of azomethine proton (CH=N) was observed at 8.94 ppm. The proton of azomethine group (CH=N) was shown at 8.07 ppm in the Schiff base and this group shifted to lower region after the coordination with metal. Peak for aromatic region was seen as a set of multiplets in the range 6.90–7.87 ppm. In the aromatic region some peaks become broad and less intense in comparison with general aromatic region. This effect may be due to the drifting of ring electrons towards metal ion. Polyester showed some other signals assigned label at 1.60, 2.15, and 5.34 ppm due to –CH2–CH 2–CH2–, O–CH 2–CH2– and Ar–CH 2–O– methylene protons in different environments.
In order to get the further information, 13C NMR spectrum was investigated. In the 13C NMR spectrum of Zn(II) polyester complex display signals assigned to CH=N carbons at 162.5 ppm. A sharp peak was found at 67.54 ppm due to the Ar–CH2–O. Other resonance peaks were found at 23.8 and 32.0, which can be assigned to –CH2–CH 2–CH2– and O–CH 2–CH2–. The aromatic carbons were observed in the region 137.4–148.1 ppm [29]. Resonance peaks at 154 and 175 ppm were correlated to the aromatic carbon (C–O) and carbonyl carbon of ester groups, respectively, as shown in Figs. 3 and 4.
Electronic spectra and magnetic moment
The information regarding the geometry of polyesters was obtained by UV–visible spectra by using 10−3 M solution of these compounds. The obtained values of electronic spectral study and magnetic moment are summarized in Table 3. The Mn(II) ion having d5 configuration, generally forms high-spin complex because of the additional stability of the half filled d shell. The magnetic moment value (6.2 B.M.) is in the expected range of octahedral geometry [30]. The electronic spectrum of this complex exhibits three absorption bands at 20,175, 22,640, and 24,660 cm−1, which can be assigned to 4T1g(G) ← 6A1g(F), 4T2g(G) ← 6A1g(F), and 4A1g(G) ← 6A1g(F) transitions, respectively, suggesting octahedral geometry [31]. The above data were used to calculate 10 Dq, Racah parameter (B) and β values. The value of 10 Dq was found to be 9240 cm−1. The Racah parameter (B) was found to be 840 cm−1, which indicates that the magnitude of interelectronic repulsion between various levels in the gaseous ion. The β was evaluated to be 0.87. The magnetic moment of PESB–Co(II) complex was found to be 4.2 B.M. This value is consistent with the high-spin octahedral geometry [32]. The electronic spectrum of the PESB–Co(II) complex showed three bands at 8,010, 11,745, and 21,290 cm−1 which can be assigned due to 4T2g(F) ← 4T1g(F), 4A2g(F) ← 4T1g(F), and 4T1g(P) ← 4T1g (F) transitions, respectively, and suggesting the octahedral geometry around the cobalt ion. The magnetic moment value of Ni(II) octahedral complex generally appear to lie between 2.9 and 3.4 B.M depending upon the magnitude of orbital contribution [33]. The experimental magnetic moment value for the PESB–Ni(II) complex is found to be 3.3 B.M., which is in agreement with the octahedral geometry. PESB–Ni(II) complex exhibits three bands at 10,975, 14,293, and 24,685 cm−1 due to 3T2g(F) ← 3A2g(F), 3T1g(F) ← 3A2g(F), and 3T1g(P) ← 3A2g(F) transitions, respectively and suggested an octahedral environment around the Ni(II) ion.
The magnetic moment of PESB–Cu(II) complex was found to be 2.2 B.M. which is consonance with the distorted octahedral geometry [34]. The electronic spectrum of PESB–Cu(II) complex showed a lower energy weak band at 15,151 and a strong high energy band at 30,235 cm−1. Lower energy band may be assigned to 2A1g ← 2B1g transition and the high energy band assigned to charge transfer spectra. Zn(II) coordination polymer is diamagnetic as expected for d10 system. The values of the electronic parameters, such as the ligand field splitting energy (10 Dq), Racah interelectronic repulsion parameter (B) and nephlauxetic ratio (β) have been calculated and summarized in Table 3.
Thermal analysis
Thermal behavior of the polyesters was investigated by using TG technique. The TG measurements were carried out in the range of 273–1073 K at a rate of 273 K min−1 under nitrogen atmosphere. The resulting data are shown in Fig. 5. The degradation process was found to be taking place in two stages. The first step is faster than the second step this may be due to the fact that non-coordinated part of the polymer decomposed first while the coordinated part of the polymers decomposed later [35]. The initial mass loss of Co(II) and Mn(II) complexes was observed at 413 K which corresponds to the theoretical mass for two water molecules. Therefore, it is suggested that coordinated water molecules are lost up to this temperature range. The loss in mass of Zn(II) complex is 10.23% at 445 K and may be due to the presence of water molecules of crystallization. In the case of Ni(II) and Cu(II) complexes, the mass corresponding to water molecules is gradually lost up to 573 K. It is suggested that the coordinated water molecules present in the polyesters of Ni(II) and Cu(II) did not lost suddenly in a sharp temperature range but gradually over a wider temperature range. The order of stability on the basis of TG results appeared to be PSB-Zn(II) < PSB-Cu(II) > PSB-Co(II) > PSB-Ni(II) > PSB-Mn(II). This order matched with Irving–Williams order of stability of the complexes of divalent metal ions [36]. A continue mass loss was observed up to 863 K indicating the decomposition and volatilization of aromatic part into low molecular mass fractions.
The TG results revealed that a reduced mass 19.07–30.96% for all the polyesters was found at 1073 K which corresponds as metal oxides. The observed reduced mass of the polyesters is more than the calculated value due to the formation of other compounds during thermal reaction. Thermal behavior results indicated that PESB–Cu(II) complex was more stable than the other polyester complexes due to higher stability constant of Cu(II) ion. The glass transition temperature (T g) of PESB–M(II) was obtained from the same instrument in which thermal analysis was done and the obtained T g values are as follows, PESB–Mn(II) = 155 °C, PESB–Co(II) = 160 °C, PESB–Ni(II) = 157 °C, PESB–Cu(II) = 162 °C, and PESB–Zn(II) = 159 °C. All the polyesters showed single T g value while PESB–Cu(II) exhibited highest value. This may be due to the absence or formation of any homopolymers or block copolymers and heterogeneous impurities in the polymers. The higher the T g the better will be the long term thermal stability of a material.
Antimicrobial assessment
The fungicidal and bactericidal effects of these polyesters, Schiff base diol, and chelated diols were determined and described in the experimental section by using agar well diffusion method [37]. This method was employed for the bacteria and yeasts with respect to the Kanamycin and Miconazol as standard drugs. The antimicrobial activity of chelated diols and polyesters was recorded same. So that the antimicrobial studies of resulting polyesters derived from chelated Schiff base diol and Schiff base are summarized in Tables 4 and 5. The results of activity showed that some compounds are very effective on some of microorganisms. The PESB–Mn(II) and PESB–Co(II) complexes showed the maximum zone of inhibition (23 and 21 mm) against P. aeruginosa and moderate zone of inhibition (17, 16, and 16 mm) against B. megaterium, B. subtilis, and S. aureus, respectively. The PESB–Ni(II), Cu(II), and Zn(II) complexes exhibited the maximum zone (23, 25, and 22 mm) against S. typhi while the moderate zone of inhibition (15 and 17 mm) against E. coli and S. aureus was found for PESB–Zn(II). Antibacterial results indicate that the PESB–Cu(II) complex showed the highest zone of inhibition than other metal complexes.
The antifungal activity of all the polyesters was also investigated gainst seven fungi C. albicans, T. longifusus, A. flavus, A. niger, F. solani, M. canis, and P. graminis. The PESB–Cu(II) complex showed the maximum inhibitory zone 24 and 25 mm against A. flavus and M. canis while the minimum (14 mm) was found for PESB–Ni(II) against C. albicans. The inhibitory zone, i.e., 21 and 23 mm observed for the PESB–Mn(II) and PESB–Co(II) complex against F. solani and A. flavus, where as the moderate inhibition zone (19 and 18 mm) against M. canis and P. graminis. The PESB–Ni(II) and PESB–Zn(II) complex showed the zone of inhibition 21 and 22 mm against A. flavus and A. niger while PESB–Ni(II) showed the moderate activity (14, 18, and 19 mm) against C. albicans, F. solani, and M. canis.
The result reveals that antimicrobial activity these polyesters are due to the presence of nitrogen and oxygen groups. It has been suggested that the compound with N and O donor system might have inhibited enzyme production and chelation reduces the polarity of the central metal ion by partial sharing of its positive charge with the donor groups [38], increasing lipophilic nature of the central metal ion, which in turn favors its permeation to the lipid layer of the membrane. Other factors viz., stability constant, molar conductivity, solubility, and magnetic moment, are also responsible for increasing the anti-microbial activity of the complexes [39].
Conclusions
As a conclusion, PESB–Cu(II) was found to be more stable than the other polyester complexes. All the synthesized compounds showed single T g value due to the absence of any homopolymers, block polymers, and heterogeneous impurities in the polymers. Synthesized polyesters were also screened against some microorganism by using agar diffusion methods to investigate their antimicrobial activity. PESB–Cu(II) complex exhibited a wide effective antibacterial and antifungal activity than the other complexes due to higher stability constant of Cu(II) ion.
References
Kaliyappan T, Kanana P. Co-ordination polymers. Prog Polym Sci. 2000;25:343–70.
Kaminiski W, Modrzeiewska Z. Application of chitosan membranes in separation of heavy metal ions. Sep Sci Technol. 1997;32:2659–68.
Brydson JA. Plastic materials. 7th ed. Jordan Hill: Butterworth-Heinemann Ltd; 1999.
John S, Timothy EL. Modern polyester. Chichester: John wiley & sons; 2003.
Manners I. Ring-opening polymerization of strained metallocenophanes: a new route to high molecular weight poly(metallocenes). Pure Appl Chem. 1999;71:1471–6.
Manners I. Polymer science with transition metals and main group elements: towards functional supramolecular inorganic polymeric materials. J Polym Sci Part A Polym Chem. 2002;40:179–91.
Destri S, Khotina IA, Porzio W. 3-Hexyl tetra-substituted sesquithienylene–phenylene polyazomethines with high molecular weight. Mechanistic considerations. Macromolecules. 1998;31:1079–86.
Ahmad T, Nishat N, Parveen S. Synthesis, characterization and anti-microbial studies of a newly developed polymeric Schiff base and its metal-polychelates. J Coord Chem. 2008;61:1963–72.
Zdrozna I, Parzuchowski P, Brzozowski ZK, Milburn GHW. Novel optical material: polyarylates with heterocyclic side chain groups. J Appl Polym Sci. 1999;71:1017–28.
Utkarsh S, Rao KV, Rakshit AK. Thermotropic liquid-crystalline polymers: synthesis, characterization, and properties of poly(azomethine esters). J Appl Polym Sci. 2003;88:153–60.
Atta A. Alternating current conductivity and dielectric properties of newly prepared poly(bis thiourea sulphoxide). Int J Polym Mater. 2003; 52:361–72.
Wang C, Nakamura S. Synthesis of polyester and copolyesters having amino acid moieties in the main chain. J Polym Sci Part-A Polym Chem. 1994;32:413–21.
Bajpai UND, Rai S, Bajpai A. Synthesis and characterization of poly(ethylene aspartate)-metal complexes. Polym Int. 1993;32:215–20.
Liu G, White B, Vancso-Szmercsanyi, Vancso GJ. Thixotropic behavior of metal-containing coordination polymers: melt viscosity of neutral aliphatic polyesters with Zn carboxylates. J Polym Sci Part B Polym Phys. 1996;34:277–82.
Spiratos M, Airinei A, Rusu GJ. Coordination polymers. 9. Chelate polymers derived from bisphenolic complexes and propylenediamine. J Macromol Sci Chem A. 1989;26:1415–23.
Vogel AI. Textbook of qualitative analysis. 4th ed. London: ELBS and Longman gray; 1978.
Wang Q, Wilson C, Blake AJ, Collinson SR, Tasker PA, Schroder M. The one-pot halomethylation of 5-substituted salicylaldehydes as convenient precursors for the preparation of heteroditopic ligands for the binding of metal salts. Tetrahedron Lett. 2006;47:8983–7.
Karakalpan M, Demetgul C, Serin S. Synthesis and thermal properties of a novel Schiff base oligomer with a double azomethine group and its Co(II) and Mn(II) complexes. J Macromol Sci Chem A. 2008;45:406–14.
Gaina C, Gaina V, Ardeleanu R. Preparation and characterization of bismaleimides containing ester groups from bisphenolic chelates and their polymers. Appl Organmet Chem. 2004;18:446–54.
Moon W, Kim JC, Chung K, Park E, Kim M, Yoon J. Antimicrobial activity of a monomer and its polymer based on quinolone. J Appl Polym Sci. 2003;90:1797–801.
Merianos JJ. Disinfection, sterilization and preservation. 4th ed. Philadelphia: Lea & Febiger; 1991.
Dolaz M, Tumer M. Synthesis, spectroscopic characterization and properties of new metal complexes. Trans Met Chem. 2004;29:516.
Avsar G, Altinel H, Yilmaz MK, Guzel B. Synthesis, characterization, and thermal decomposition of fluorinated salicylaldehyde Schiff base derivatives (salen) and their complexes with copper(II). J Therm Anal Calorim. 2010;101:199.
Ferrero JR. Low-frequency vibrations of inorganic and coordination compound. New York: John Wiley and Sons; 1971.
Khalaji A D, Rad S M, Grivani G, Das D. Nickel(II) and copper(II) complexes with an asymmetric bidentate Schiff-base ligand derived from furfurylamine synthesis, spectral, XRD, and thermal studies. J Therm Anal Calorim. 2011;103:747
Haffar D, Douadi T, Chafaa S, Khan M, Bouet G. Synthesis, characterisation and electrochemical study of 4,4′-bis(salicylideneimino) diphenylethane and its complexes with cobalt(II), copper(II) and cadmium(II). Trans Met Chem. 2004;29:245.
Chohan ZH, Praveen M. Synthesis, characterization, coordination and antibacterial properties of novel asymmetric 1,1′-disubstituted ferrocene-derived Schiff-base ligands and their Co(II), Cu(II) Ni(II) and Zn(II) complexes. Appl Organometal Chem. 2001;15:617–25.
Cui XL, Wu YJ, Zou DP, He CH, Chai JJ. Studies on the cyclomercuration of 1,1′-bis[(arylimino)phenylmethyl]ferrocenes. Polyhedron. 1999;18:1023–7.
Samal S, Acharya S, Dey RK, Ray AR. Synthesis, characterization, and metal ion uptake studies of chelating resins derived from formaldehyde/furfuraldehyde condensed phenolic schiff base of 4,4′-diaminodiphenylmethane and o-hydroxyacetophenone. J Appl Polym Sci. 2003;88:570–81.
Bajpai A, Rai S. Synthesis and characterization of coordination polymers of polyesters with pendant amino groups. J Appl Polym Sci. 1998;69:751–9.
Cotton FA. Progress in inorganic chemistry. New York: Wiley Interscience Publishers; 1964.
Chohan Z H, Arif M, Akhtar M A, Supran C T. Metal-based antibacterial and antifungal agents: synthesis, characterization, and in vitro biological evaluation of Co(II), Cu(II), Ni(II), and Zn(II) complexes with amino acid-derived compounds. Bioinorg Chem And Applications. 2006;1.
Lever ABP. Inorganic electronic spectroscopy. Amsterdon: Elsevier; 1984.
Ballhausen CJ. An introduction to ligand field theory. New York: McGraw Hill; 1962.
Roy SM, Juneja HD, Munshi KN. Synthetic and thermal studies of polymeric chelates of some bis-biurets with first transition series metals. J Thermal Anal Cal. 2001;65:197.
Irving H, Willimas RJP. The stability of transition-metal complexes. J Chem Soc. 1953;3:192.
Collins CH, Lyne PM. Microbial methods. Baltimore: University Park Press; 1970. p. 422.
Nishat N, Ahmad S, Rahisuddin, Ahamad T. Synthesis and characterization of antibacterial polychelates of urea–formaldehyde resin with Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), and Zn(II) metal ions. J Appl Polym Sci. 2006;100:928–36.
Nishat N, Ahmad S, Ahamad T. Synthesis, characterization, and antimicrobial studies of newly developed metal-chelated epoxy resins. J Appl Polym Sci. 2006;101:1347–55.
Acknowledgements
One of the authors (Sumaiya Hasnain) wish to thanks the Council of Scientific and Industrial Research (C.S.I.R), New Delhi for financial support in the form of the Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishat, N., Hasnain, S., Ahmad, T. et al. Synthesis, characterization, and biological evaluation of new polyester containing Schiff base metal complexes. J Therm Anal Calorim 105, 969–979 (2011). https://doi.org/10.1007/s10973-011-1442-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1442-8