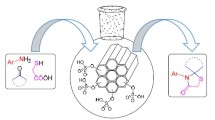
Abstract
Silica-bonded ionic liquids is employed as a recyclable catalyst for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] via one-pot condensation reaction of isatin, activated methylene reagents, and 3-methyl-l-phenyl-5-pyrazolone in refluxing aqueous medium in good to high yields. Also, these silica-bonded ionic liquids were used as catalysts for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]chromene derivatives in refluxing aqueous medium in high yields. Catalyst could be recycled for several times without any additional treatment.
Graphical Abstract
Silica-bonded ionic liquids is employed as a recyclable catalyst for the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] and spiro[indoline-3,4′-pyrano[2,3-c]chromene via one-pot condensation reaction of isatin, activated methylene reagents, and 3-methyl-l-phenyl-5-pyrazolone or 4-hydroxy coumarin respectively in refluxing aqueous medium in good to high yields.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spirooxindoles are commonly occurring heterocyclic ring systems and are important structural motifs found in many natural products and pharmaceuticals [1, 2]. The key structural characteristic of these compounds is the spiro ring fused at the C3 position of the oxindole core with varied heterocyclic motifs [3]. Among them, those with an indole moiety exhibit antibacterial and antifungal activities [4, 5]. The significant biological activity and recent synthetic advances of these natural products encouraged the development of biologically promising analogs that would be more efficacious and selective than the original natural products [6]. The privileged spirooxindole skeletons have high potential for the development of some medicinal agents [7]. Two representative examples are NITD609 (1) and MI-888 (2) in Fig. 1.
The one-pot condensation reaction of isatin, activated methylene reagents, and 1,3-dicarbonyl compounds could readily afford various spirooxindole structures and has attracted wide attention, and this type of reaction has been achieved by a number of different catalysts and conditions, such as SnCl4 [8], triethanolamine [9], basic alumina under microwave irradiation [10], surfactant TEBA (triethylbenzylammonium chloride) [11], electrocatalytic multi-component assembling [12], β-cyclodextrin [13], NaCl under sonication [14], ZnS nanoparticles [15], piperidine under ultrasound irradiation [16], CaCl2 under ultrasonic irradiation [17], 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) [18], ethylenediammonium diformate (EDDF) [19], SBA-Pr-NH2 [20], sulfated choline based heteropolyanion [21], and L-prolin [22].
Immobilization of acidic ionic liquids on solid supports has been designed and it can offer important advantages in handling, separation and reuse procedures. Based on economic criteria, it is desirable to minimize the amount of ionic liquid utilized in a potential process. Immobilized acidic ionic liquids have been used as novel solid catalysts, e.g., for esterification, nitration reactions [23], acetal formation [24], Baeyer–Villiger reaction [25], synthesis of α-aminonitriles [26] bis-pyrazolones [27], chromenes [28], 4,4-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) [29], 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones [30], and 1-(benzothiazolylamino)-phenylmethyl-2-naphthols [31].
In this regard, we introduced a series of silica-bonded ionic liquids such as: silica propyl imidazolium chloride ([Sipim]Cl), silica propyl imidazolium triflate ([Sipim]OTf), N-(3-silicapropyl) imidazolium hydrogen sulfate ([Sipim]HSO4), and N-(3-silicapropyl) imidazolium hydrogen phosphate ([Sipim]H2PO4) as recyclable catalysts and investigated their catalytic activities in organic transformations [26–28] (Scheme 1).
In continuation of our study on the preparation of solid acids and bases and investigation of their applications in heterocyclic synthesis [32–40], Herein, we describe the synthesis of spirooxindole derivatives via three-component condensation reaction of isatin, reactive methylene reagents, and 1,3-dicarbonyl compounds using silica-bonded N-propylimidazolium chloride ([Sipim]Cl) in aqueous media at reflux conditions (Scheme 2).
Experimental
Chemicals and reagents
Chemicals were purchased from Merck and Aldrich Chemical Companies and use as is. 1H-NMR spectra we recorded on a Bruker Avance (500 MHz DRX) or Bruker Ultrashield (400 MHz) using DMSO-d6 as deuterated solvent and with tetramethylsilane (TMS) as internal standard. Melting points were determined in open capillary tubes in a Barnstead Electrothermal 9100 BZ circulating oil melting point apparatus and uncorrected. Reactions were monitored by TLC using silica gel Poly Gram SILG/UV254 plates. All the products were characterized by IR, NMR, Mass spectra and known compounds compared with those reported in literature [11–20]. Solid silica-supported bases such as [Sipim]HSO4, [Sipim]H2PO4, [Sipim]OTf, and [Sipim]Cl were prepared according to our reported procedures [26–28].
Preparation of silica propyl imidazolium chloride [Sipim]Cl
Silicapropyl chloride (10.0 g) was added to a flask containing 100 mL of anhydrous toluene and a large excess of imidazole (10.0 g). The reaction mixture was refluxed with stirring for 24 h. Then, the reaction mixture was cooled to room temperature, transferred to a vacuum glass filter, and washed with toluene, ethanol, and methanol in turn. The silica chemically bonded with imidazolium ([Sipim]Cl) was dried under vacuum at 50 °C for 4 h [26].
General procedure for the synthesis of spirooxindole derivatives
A mixture of isatin (1 mmol), reactive methylene compound (1 mmol) and 1,3-dicarbonyl compound (1 mmol) in the presence of [Sipim]Cl (0.05 g) in water (5 mL) was refluxed with stirring in an oil bath. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was filtered and remaining was washed with warm ethanol (3 × 10 mL) in order to separate [Sipim]Cl catalyst. After cooling the ethanol phase, the precipitates were filtered. The crude products were purified by recrystallization from ethanol (95 %).
6′-Amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4a) (Table 2, entry 1)
mp 235–237 °C, (Ref.: [12] 236–237 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 1.55 (s, 3H, CH3), 6.91–7.09 (m, 2H, Ar), 7.18 (d, 1H, J = 6.8 Hz, Ar), 7.25–7.42 (m, 2H, Ar), 7.48–7.56 (m, 2H, Ar), 7.59 (s, 2H, NH2), 7.79 (d, 2H, J = 7.3 Hz, Ar), 10.75 (s, 1H, NH). 13C-NMR (125 MHz, DMSO-d6): δ 12.2, 48.2, 56.6, 96.8, 110.3, 118.4, 120.6, 123.1, 125.4, 127.0, 129.8, 129.9, 132.6, 137.7, 142.0, 144.4, 145.4, 161.5, 178.0.
6′-Amino-5-chloro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4b) (Table 2, entry 2)
mp 232–233 °C, (Ref.: [14] 232–234 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 1.60 (s, 3H, CH3), 6.97 (d, 1H, J = 8.3 Hz, Ar), 7.32–7.39 (m, 3H, Ar), 7.53 (t, 2H, J = 8.0 Hz, Ar), 7.65 (s, 2H, NH2), 7.79 (d, 2H, J = 7.8 Hz, Ar), 10.90 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 12.2, 48.5, 56.0, 96.1, 111.8, 118.4, 120.7, 125.7, 127.1, 127.2, 129.7, 129.9, 134.8, 137.7, 140.9, 144.2, 145.5, 161.5, 177.8.
6′-Amino-5-nitro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4c) (Table 2, entry 3)
mp 227–229 °C, (Ref.: [14] 226–228 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 1.60 (s, 3H, CH3), 7.18 (d, 1H, J = 8.6 Hz, Ar), 7.37 (t, 1H, J = 7.3 Hz, Ar), 7.53 (t, 2H, J = 7.9 Hz, Ar), 7.74 (s, 2H, NH2), 7.80 (d, 2H, J = 7.8 Hz, Ar), 8.23 (d, 1H, J = 1.8 Hz, Ar), 8.27 (dd, 1H, J 1 = 8.7, J 2 = 2.1 Hz, Ar), 11.49 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 11.8, 47.9, 56.0, 94.9, 110.2, 117.8, 120.4, 121.0, 126.5, 126.7, 129.4, 133.3, 137.1, 143.1, 143.6, 145.2, 147.7, 161.2, 178.1.
Methyl-6′-amino-5-chloro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4d) (Table 2, entry 4)
mp 224–226 °C; 1H-NMR (500 MHz, DMSO-d6): δ (ppm) 1.61 (s, 3H, CH3), 3.34 (s, 3H, OCH3), 6.88 (d, 1H, J = 10.0 Hz, Ar), 7.11 (d, 1H, J = 2.5 Hz, Ar), 7.22 (dd, 1H, J 1 = 10.0, J 2 = 2.5 Hz, Ar), 7.53 (t, 1H, J = 10.0 Hz, Ar), 7.52 (t, 2H, J = 10.0 Hz, Ar), 7.82 (d, 2H, J = 10.0 Hz, Ar), 8.21 (s, 2H, NH2), 10.66 (s, 1H, NH). 13C-NMR (125 MHz, DMSO-d6): δ 12.2, 48.3, 51.0, 74.8, 98.0, 110.7, 120.6, 124.0, 126.2, 126.9, 128.2, 129.9, 137.8, 138.2, 141.3, 144.4, 144.6, 161.8, 168.3, 179.4. Anal calcd for C22H17ClN4O4: C, 60.49; H, 3.92; Cl, 8.12; N, 12.83. Found: C, 60.18; H, 3.98; N, 12.71.
Methyl-6′-amino-5-nitro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro-[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4e) (Table 2, entry 5)
mp 241–243 °C. 1H-NMR (500 MHz, DMSO-d6): δ 1.61 (s, 3H, CH3), 3.33 (s, 3H, OCH3), 7.09 (d, 1H, J = 8.8 Hz, Ar), 7.36 (t, 1H, J = 7.3 Hz, Ar), 7.53 (t, 2H, J = 7.8 Hz, Ar), 7.83 (d, 2H, J = 7.8 Hz, Ar), 7.97 (d, 1H, J = 2.0 Hz, Ar), 8.18 (dd, 1H, J 1 = 8.5, J 2 = 2.2 Hz, Ar), 8.27 (s, 2H, NH2), 11.27 (s, 1H, NH).13C-NMR (125 MHz, DMSO-d6): δ 12.3, 48.2, 51.1, 74.3, 97.3, 109.6, 120.7, 125.9, 127.0, 129.4, 129.8, 137.2, 137.8, 143.0, 144.2, 144.8, 148.9, 161.9, 168.1, 180.2. Anal calcd for C22H17N5O6: C, 59.06; H, 3.83; N, 15.65. Found: C, 58.78; H, 3.90; N, 15.51.
Ethyl-6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4f) (Table 2, entry 6)
mp 239–241 °C, (Ref.: [14] 238–240 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 0.75 (t, 3H, J = 7.2 Hz CH3), 1.59 (s, 3H, CH3), 3.68–3.81 (m, 2H, OCH2), 6.84–6.92 (m, 2H, Ar), 6.97 (d, 1H, J = 7.1 Hz, Ar), 7.17 (dt, 1H, J 1 = 7.1, J 2 = 1.2 Hz, Ar), 7.34 (t, 1H, J = 7.3 Hz, Ar), 7.52 (t, 2H, J = 8.1 Hz, Ar), 7.81 (d, 2H, J = 7.6 Hz, Ar), 8.21 (s, 2H, NH2), 10.52 (s, 1H, NH). 13C-NMR (125 MHz, DMSO-d6): δ 12.2, 13.6, 47.9, 59.5, 75.0, 98.7, 109.3, 120.4, 122.2, 123.7, 126.8, 128.2, 129.9, 136.2, 137.8, 142.7, 144.4, 144.7, 161.8, 168.3, 179.7.
Ethyl-6′-amino-5-chloro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4 g) (Table 2, entry 7)
mp 246–247 °C, (Ref.: [15] 246–248 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 0.79 (t, 3H, J = 7.1 Hz CH3), 1.63 (s, 3H, CH3), 3.72–3.85 (m, 2H, OCH2), 6.87 (d, 1H, J = 8.3 Hz, Ar), 7.12 (d, 1H, J = 2.0 Hz, Ar), 7.23 (dd, 1H, J 1 = 8.3, J 2 = 2.3 Hz, Ar), 7.35 (t, 1H, J = 7.4 Hz, Ar), 7.52 (t, 2H, J = 7.9 Hz, Ar), 7.82 (d, 2H, J = 7.8 Hz, Ar), 8.27 (s, 2H, NH2), 10.66 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 12.2, 13.6, 48.2, 59.6, 74.5, 98.0, 110.7, 120.6, 124.0, 126.2, 126.9, 128.1, 129.9, 137.8, 138.4, 141.6, 144.5, 144.6, 161.9, 168.2, 179.5.
Ethyl-6′-amino-5-nitro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4 h) (Table 2, entry 8)
mp 267–269 °C, (Ref.: [15] 268–270 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 0.78 (t, 3H, J = 7.0 Hz CH3), 1.62 (s, 3H, CH3), 3.71–3.83 (m, 2H, OCH2), 7.09 (d, 1H, J = 8.8 Hz, Ar), 7.36 (t, 1H, J = 7.4 Hz, Ar), 7.53 (t, 2H, J = 8.0 Hz, Ar), 7.83 (d, 2H, J = 7.5 Hz, Ar), 7.98 (d, 1H, J = 2.3 Hz, Ar), 8.2 (dd, 1H, J 1 = 8.9, J 2 = 2.3 Hz, Ar), 8.34 (s, 2H, NH2), 11.28 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 12.3, 13.7, 48.0, 59.7, 74.0, 97.3, 109.6, 120.7, 125.9, 127.0, 129.4, 129.9, 137.4, 137.7, 143.0, 144.3, 144.8, 149.1, 162.1, 167.9, 180.3.
6′-Amino-1,3′-dimethyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4i) (Table 2, entry 9)
mp 224–226 °C, (Ref.: [12] 226–227 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 1.46 (s, 3H, CH3), 3.25 (s, 3H, CH3), 7.10–7.18 (m, 2H, Ar), 7.25 (d, 1H, J = 8.3 Hz, Ar), 7.34–7.42 (m, 2H, Ar), 7.52 (t, 2H, J = 7.9 Hz, Ar), 7.63 (s, 2H, NH2), 7.79 (dd, 2H, J 1 = 8.7, J 2 = 1.0 Hz, Ar). 13C-NMR (125 MHz, DMSO-d6): δ 12.2, 27.0, 47.9, 56.1, 96.6, 109.4, 118.3, 120.6, 123.8, 125.0, 127.1, 129.9, 131.8, 137.7, 143.5, 144.3, 145.4, 161.6, 176.3.
6′-Amino-3′,5-dimethyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4j) (Table 2, entry 10)
mp > 270 °C, (Ref.: [12] 288–289 °C); 1H-NMR (400 MHz, DMSO-d6): δ (ppm) 1.51 (s, 3H, CH3), 2.19 (s, 3H, CH3), 6.78 (d, 1H, J = 6.4 Hz, Ar), 6.95 (s, 1H, Ar), 7.01–7.04 (m, 1H, Ar), 7.29–7.32 (m, 1H, Ar), 7.45–7.49 (m, 4H, Ar and NH2), 7.74 (d, 2H, J = 6.4 Hz, Ar), 10.57 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 19.4, 21.4, 48.7, 97.4, 110.4, 118.9, 121.0, 126.2, 127.4, 130.3, 130.4, 132.5, 133.2, 138.1, 140.0, 144.9, 145.8, 161.8, 178.3.
2′-Amino-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carbonitrile (5a) (Table 3, entry 1)
mp > 270 °C, (Ref. [18] 292–294 °C); 1H-NMR (400 MHz, DMSO-d6): δ 6.86 (d, 1H, J = 8.0 Hz, Ar), 6.94 (t, 1H, J = 7.0 Hz, Ar), 7.20–7.25 (m, 2H, Ar), 7.50 (d, 1H, J = 8.3 Hz, Ar), 7.55 (t, 1H, J = 7.7 Hz, Ar), 7.68 (s, 2H, NH2), 7.77 (t, 1H, J = 7.9 Hz, Ar), 7.95 (dd, 1H, J 1 = 8.0, J 2 = 1.5 Hz, Ar), 10.69 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 48.1, 57.5, 101.9, 110.0, 112.9, 117.2, 117.5, 122.5, 123.1, 124.6, 125.5, 129.4, 133.5, 134.2, 142.7, 152.5, 155.6, 158.8, 158.9, 177.6.
2′-Amino-5-chloro-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carbonitrile (5b) (Table 3, entry 2)
mp > 270 °C, (Ref. [20] >300 °C); 1H-NMR (400 MHz, DMSO-d6): δ 6.87 (d, 1H, J = 8.3 Hz, Ar), 7.27 (dd, 1H, J 1 = 8.3, J 2 = 2.2 Hz, Ar), 7.45 (d, 1H, J = 2.3 Hz, Ar), 7.51 (d, 1H, J = 8.3 Hz, Ar), 7.56 (dt, 1H, J 1 = 7.7, J 2 = 0.8 Hz, Ar), 7.75 (s, 2H, NH2), 7.79 (dt, 1H, J 1 = 7.8, J 2 = 1.5 Hz, Ar), 7.95 (dd, 1H, J 1 = 7.9, J 2 = 1.3 Hz, Ar), 10.82 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 48.3, 56.8, 101.2, 111.3, 113.1, 117.2, 117.4, 123.2, 125.1, 125.5, 126.5, 129.3, 134.2, 135.5, 141.6, 152.6, 155.9, 158.9, 159.0, 177.5.
2′-Amino-5-nitro-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carbonitrile (5c) (Table 3, entry 3)
mp > 270 °C; 1H-NMR (400 MHz, DMSO-d6): δ 7.09 (d, 1H, J = 8.8 Hz, Ar), 7.52 (d, 1H, J = 7.8 Hz, Ar), 7.57 (dt, 1H, J 1 = 7.7, J 2 = 1.0 Hz, Ar), 7.79 (dt, 1H, J 1 = 7.4, J 2 = 1.5 Hz, Ar), 7.86 (s, 2H, NH2), 7.96 (dd, 1H, J 1 = 8.0, J 2 = 1.5 Hz, Ar), 8.22 (dd, 1H, J 1 = 8.5, J 2 = 2.5 Hz, Ar), 8.37 (d, 1H, J = 2.5 Hz, Ar), 11.43 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 48.2, 56.1, 100.7, 110.2, 113.2, 117.2, 117.3, 121.1, 123.3, 125.5, 126.8, 134.3, 134.5, 143.2, 149.1, 152.6, 156.3, 159.2, 159.3, 178.4. Anal calcd for C20H10N4O6: C, 59.71; H, 2.51; N, 13.93. Found: C, 59.42; H, 2.57; N, 13.80.
Methyl-2′-amino-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′- carboxylate (5d) (Table 3, entry 4)
mp > 270 °C, (Ref. [11] 275–277 °C); 1H-NMR (400 MHz, DMSO-d6): δ 3.33 (s, 3H, OCH3), 6.74–6.85 (m, 2H, Ar), 7.02 (d, 1H, J = 7.3 Hz, Ar), 7.11 (t, 1H, J = 7.5 Hz, Ar), 7.44 (d, 1H, J = 8.0 Hz, Ar), 7.51 (t, 1H, J = 7.5 Hz, Ar), 7.73 (t, 1H, J = 7.4 Hz, Ar), 8.04 (d, 1H, J = 7.8 Hz, Ar), 8.09 (s, 2H, NH2), 10.45 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 47.9, 50.9, 76.3, 104.3, 108.9, 112.9, 116.8, 121.5, 123.3, 123.7, 125.3, 128.5, 133.9, 134.9, 144.4, 152.3, 154.3, 158.2, 158.9, 167.6, 179.4.
Methyl-2′-amino-5-chloro-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carboxylate (5e) (Table 3, entry 5)
mp > 270 °C, (Ref. [20] 279–281 °C); 1H-NMR (400 MHz, DMSO-d6): δ 3.35 (s, 3H, OCH3), 6.76 (d, 1H, J = 8.3 Hz, Ar), 7.16 (dd, 1H, J 1 = 8.1, J 2 = 2.2 Hz, Ar), 7.20 (d, 1H, J = 2.2 Hz, Ar), 7.46 (d, 1H, J = 8.3 Hz, Ar), 7.53 (t, 1H, J = 7.7 Hz, Ar), 7.75 (dt, 1H, J 1 = 7.8, J 2 = 1.5 Hz, Ar), 8.04 (dd, 1H, J 1 = 7.9, J 2 = 1.5 Hz, Ar), 8.13 (s, 2H, NH2), 10.58 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 48.2, 50.9, 75.8, 103.5, 110.1, 113.1, 116.8, 123.5, 124.1, 125.3, 125.4, 128.2, 133.9, 137.0, 143.4, 152.4, 154.7, 158.4, 158.4, 159.0, 167.5, 179.2.
Methyl-2′-amino-5-bromo-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carboxylate (5f) (Table 3, entry 6)
mp > 270 °C, (Ref. [20] 288–290 °C); 1H-NMR (400 MHz, DMSO-d6): δ 3.35 (s, 3H, OCH3), 6.72 (d, 1H, J = 8.0 Hz, Ar), 7.26–7.33 (m, 2H, Ar), 7.46 (d, 1H, J = 8.3 Hz, Ar), 7.53 (t, 1H, J = 7.6 Hz, Ar), 7.75 (t, 1H, J = 7.9 Hz, Ar), 8.04 (dd, 1H, J 1 = 8.0, J 2 = 1.2 Hz, Ar), 8.13 (s, 2H, NH2), 10.59 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 48.1, 51.0, 75.8, 103.5, 110.7, 113.1, 116.8, 123.5, 125.3, 126.7, 131.1, 133.9, 137.4, 143.8, 152.4, 154.7, 158.4, 159.0, 167.5, 179.1.
Methyl-2′-amino-5-nitro-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carboxylate (5 g) (Table 3, entry 7)
mp > 270 °C; 1H-NMR (400 MHz, DMSO-d6): δ 3.36 (s, 3H, OCH3), 6.95 (d, 1H, J = 8.5 Hz, Ar), 7.46 (d, 1H, J = 8.3 Hz, Ar), 7.54 (t, 1H, J = 7.7 Hz, Ar), 7.76 (t, 1H, J = 7.9 Hz, Ar), 8.06 (dd, 1H, J 1 = 8.0, J 2 = 1.5 Hz, Ar), 8.09 (d, 1H, J = 2.2 Hz, Ar), 8.13 (dd, 1H, J 1 = 8.5, J 2 = 2.2 Hz, Ar), 8.20 (s, 2H, NH2), 11.21 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 48.0, 51.0, 75.3, 102.9, 108.8, 113.1, 116.9, 119.8, 123.5, 125.3, 126.1, 134.1, 136.0, 142.4, 151.1, 152.4, 155.1, 158.7, 159.1, 167.3, 180.2. Anal calcd for C21H13N3O8: C, 57.94; H, 3.01; N, 9.65. Found: C, 57.65; H, 3.07; N, 9.38.
Ethyl-2′-amino-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carboxylate (5 h) (Table 3, entry 8)
mp 253–255 °C, (Ref. [18] 252–254 °C); 1H-NMR (400 MHz, DMSO-d6): δ 0.83 (t, 3H, J = 7.2 Hz, CH3), 3.71–3.83 (m, 2H, OCH2), 6.75 (d, 1H, J = 7.8 Hz, Ar), 6.81 (dt, 1H, J 1 = 7.5, J 2 = 0.8 Hz, Ar), 7.02 (d, 1H, J = 6.8 Hz, Ar), 7.13 dt, 1H, J 1 = 7.7, J 2 = 1.2 Hz, Ar), 7.45 (d, 1H, J = 8.3 Hz, Ar), 7.53 (dt, 1H, J 1 = 7.7, J 2 = 1.0 Hz, Ar), 7.75 (dt, 1H, J 1 = 7.9, J 2 = 1.5 Hz, Ar), 8.04 (dd, 1H, J 1 = 8.0, J 2 = 1.2 Hz, Ar), 8.15 (s, 2H, NH2), 10.43 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 13.6, 47.8, 59.6, 76.0, 104.3, 108.9, 112.9, 116.8, 121.5, 123.3, 123.7, 125.3, 128.4, 133.9, 135.1, 144.6, 152.3, 154.2, 158.2, 159.0, 167.6, 179.3.
Ethyl-2′-amino-5-chloro-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carboxylate (5i) (Table 3, entry 9)
mp > 270 °C, (Ref. [20] 275–277 °C); 1H-NMR (400 MHz, DMSO-d6): δ 0.86 (t, 3H, J = 7.2 Hz, CH3), 3.74–3.86 (m, 2H, OCH2), 6.75 (d, 1H, J = 8.0 Hz, Ar), 7.15–7.22 (m, 2H, Ar), 7.46 (d, 1H, J = 8.3 Hz, Ar), 7.53 (t, 1H, J = 7.7 Hz, Ar), 7.75 (dt, 1H, J 1 = 7.8, J 2 = 1.3 Hz, Ar), 8.03 (dd, 1H, J 1 = 7.8, J 2 = 1.3 Hz, Ar), 8.20 (s, 2H, NH2), 10.58 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 13.7, 48.1, 59.7, 75.5, 103.6, 110.1, 113.1, 116.8, 123.4, 124.1, 125.3, 125.4, 128.1, 133.9, 137.2, 143.6, 152.4, 154.6, 158.4, 159.1, 167.5, 179.2.
Ethyl-2′-amino-5-bromo-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carboxylate (5j) (Table 3, entry 10)
mp > 270 °C, (Ref. [20] 280–282 °C); 1H-NMR (400 MHz, DMSO-d6): δ 0.87 (t, 3H, J = 7.2 Hz, CH3), 3.74–3.86 (m, 2H, OCH2), 6.71 (d, 1H, J = 8.0 Hz, Ar), 7.27–7.33 (m, 2H, Ar), 7.46 (d, 1H, J = 8.5 Hz, Ar), 7.53 (t, 1H, J = 7.5 Hz, Ar), 7.75 (dt, 1H, J 1 = 7.8, J 2 = 1.3 Hz, Ar), 8.03 (d, 1H, J = 8.0 Hz, Ar), 8.20 (s, 2H, NH2), 10.59 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 13.7, 48.0, 59.7, 75.5, 103.6, 110.7, 113.1, 116.8, 123.4, 125.3, 126.8, 131.0, 133.9, 137.6, 144.0, 152.4, 154.6, 158.4, 159.1, 167.5, 179.1.
Ethyl-2′-amino-5-nitro-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[2,3-c]chromene]-3′-carboxylate (5k) (Table 3, entry 11)
mp 267–268 °C; 1H-NMR (400 MHz, DMSO-d6): δ 0.85 (t, 3H, J = 7.1 Hz, CH3), 3.80 (q, 2H, J = 7.1 Hz, OCH2), 6.96 (d, 1H, J = 8.5 Hz, Ar), 7.46 (d, 1H, J = 8.3 Hz, Ar), 7.55 (t, 1H, J = 7.6 Hz, Ar), 7.76 (dt, 1H, J 1 = 7.8, J 2 = 1.0 Hz, Ar), 8.05 (dd, 1H, J 1 = 7.9, J 2 = 1.7 Hz, Ar), 8.11 (d, 1H, J = 2.5 Hz, Ar), 8.15 (dd, 1H, J 1 = 8.5, J 2 = 2.3 Hz, Ar), 8.28 (s, 2H, NH2), 11.21 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6): δ 13.7, 47.9, 59.8, 74.9, 103.0, 108.9, 113.1, 116.9, 119.9, 123.5, 125.3, 126.1, 134.1, 136.2, 142.4, 151.3, 152.4, 155.1, 158.7, 159.3, 167.2, 180.2. Anal calcd for C22H15N3O8: C, 58.80; H, 3.36; N, 9.35. Found: C, 58.49; H, 3.39; N, 9.11.
Results and discussion
The catalytic activity of silica-bonded ionic liquids such as [Sipim]HSO4, [Sipim]H2PO4, [Sipim]OTf, and [Sipim]Cl was investigated as a heterogeneous catalysts in one-pot synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazoles] through three-component reaction of isatin, malononitrile, and 3-methyl-l-phenyl-5-pyrazolone and the results are summarized in Table 1.
For this purpose, the reaction between isatin (1 mmol), malononitrile (1 mmol), and 3-methyl-l-phenyl-5-pyrazolone (1 mmol) was selected as a model reaction in aqueous media in order to establish the feasibility of the strategy and optimize the reaction conditions. All of these silica immobilized propyl imidazolium salts were accomplished this three-component condensation reaction in aqueous media at refluxing conditions. The model reaction was converted into corresponding product (4a) in a higher yield using solid [Sipim]Cl (0.05 g) as catalyst (Table 1, entry 5). The lower amounts of [Sipim]Cl (0.03 g) was converted the model reaction in longer reaction time and lower yield (Table 1, entry 4) and using higher amounts of the catalyst (0.07 g) did not improve the result to an appreciable extent (Table 1, entry 6). The model reaction was examined at 120 °C and solvent-free conditions (Table 1, entry 9) and lower temperature 45 and 80 °C in water at solvent (Table 1, entries 7 and 8) gave the corresponding product in longer reaction time and lower yields. The model reaction was treated with available ionic liquids such as imidazolium chloride, imidazolium bromide, imidazolium hydrogen sulfate, and imidazolium hydrogen phosphate and the corresponding product (4a) was obtained in the presence of 0.20 mmol of these ionic liquids after 15 min in 75–80 % yields (Table 1, entries 10–13). The optimum conditions was isatin (1 mmol), methylene active compound (1 mmol), 3-methyl-l-phenyl-5-pyrazolone (1 mmol), and [Sipim]Cl (0.05 g) in refluxing aqueous media.
The reaction can tolerate isatin and some of its derivatives carrying both electron-withdrawing group such as NO2 and halogen such as Cl and the results were reasonable (Table 2). Beside malonitrile, other methylene active compound such as methyl acetoacetate and ethyl acetoacetate reacted under optimized conditions and gave the corresponding products in high yields (Table 2, entries 4–8).
In addition, 4-hydroxy coumarin as 1,3-dicarbonyl compound (enol form) was treated with isatin and methylene active compounds under optimized conditions and the corresponding spiro[indoline-3,4′-pyrano[2,3-c]chromene derivatives were obtained in high yields (Table 3).
Proposed mechanism for the synthesis of spirooxindole derivatives 4 and 5 was described in Scheme 2 [11, 20]. The process represents a typical cascade reaction in which the isatin 1 first condenses with methylene active compound 2 to afford isatylidene malononitrile derivative 6 in the presence of [Sipim]Cl in water. This step was regarded as a fast Knoevenagel condensation. Then, 6 is attacked via Michael addition of 1,3-dicarbonyl compound 3 to give the intermediate 7 followed by the cycloaddition of hydroxyl group to the cyano moiety to form the desired product 4 or 5 (Scheme 3).
The possibility of recycling the catalyst [Sipim]Cl was examined using the reaction of isatin, malononitrile, and 3-methyl-l-phenyl-5-pyrazolone under the optimized conditions. Upon completion, the reaction mixture was washed with warm ethanol (3 × 5 cm3). The recovered catalyst was dried and reused for subsequent runs. The recycled catalyst was reused four times without any additional treatment. No observation of any appreciable loss in the catalytic activity of [Sipim]Cl was observed (Fig. 2).
Conclusions
In conclusion, this work shows that [Sipim]Cl which can be prepared by simple operation from commercially available and relative cheap starting materials, efficiently catalyzed the synthesis of spirooxindoles. It could also be recovered and reused for several times without noticeable loss of reactivity.
References
C.C. Galliford, K.A. Scheidt, Angew. Chem. 119, 8902–8912 (2007) [C.C. Galliford, K.A. Scheidt, Angew. Chem. Int. Ed. 46, 8748–8758 (2007)]
K. Ding, Y.P. Lu, N. Coleska, J. Med. Chem. 49, 3432–3435 (2006)
B. Yu, D.Q. Yu, H.M. Liu, Eur. J. Med. Chem. 97, 673–698 (2015)
J.P. Nandy, M. Prakesch, S. Khadem, P.T. Reddy, U. Sharma, P. Arya, Chem. Rev. 109, 1999–2060 (2009)
M. Pettersson, D. Knueppel, S.F. Martin, Org. Lett. 9, 4623–4626 (2007)
C. Marti, E.M. Carreira, Eur. J. Org. Chem. (12), 2209–2219 (2003)
C.V. Galliford, J.A. Martenson, C. Stern, K.A. Scheidt, Chem. Commun. (6), 631–633 (2007)
B. Liang, S. Kalidindi, J.A. Porco Jr, C.R.J. Stephenson, Org. Lett. 12, 572–575 (2010)
R.G. Redkin, L.A. Shemchuk, V.P. Chernykh, O.V. Shishkin, S.V. Shishkin, Tetrahedron 63, 11444–11450 (2007)
A. Dandia, R. Singh, P. Sarawgi, S. Khaturia, Chin. J. Chem. 24, 950–954 (2006)
S.L. Zhu, S.J. Ji, Y. Zhang, Tetrahedron 63, 9365–9372 (2007)
M.N. Elinson, A.S. Dorofeev, F.M. Miloserdov, G.I. Nikishin, Mol. Divers. 13, 47–52 (2009)
R. Sridhar, B. Srinivas, B. Madhav, V.P. Reddy, Y.V.D. Nageswar, K.R. Rao, Can. J. Chem. 87, 1704–1707 (2009)
A. Dandia, A.K. Jain, D.S. Bhati, Synth. Commun. 41, 2905–2919 (2011)
A. Dandia, V. Parewa, A.K. Jain, K.S. Rathore, Green Chem. 13, 2135–2145 (2011)
Y. Zou, Y. Hu, H. Liu, D. Shi, ACS Comb. Sci. 14, 38–43 (2012)
H.R. Safaei, M. Shekouhy, A. Shirinfeshan, S. Rahmanpur, Mol. Divers. 16, 669–683 (2012)
P. Saluja, K. Aggarwal, J.M. Khurana, Synth. Commun. 43, 3239–3246 (2013)
A. Thakur, M. Tripathi, U.C. Rajesh, D.S. Rawat, RSC Adv. 3, 18142–18148 (2013)
G. Mohammadi Ziarani, N. Hosseini Mohtasham, N. Lashgari, A. Badiei, M. Amanlou, R. Bazl, JNS 2, 489–500 (2013)
S.P. Satasia, P.N. Kalaria, J.R. Avalani, D.K. Raval, Tetrahedron 70, 5763–5767 (2014)
W. Liju, K. Ablajan, F. Jun, Ultrason. Sonochem. 22, 113–118 (2015)
K. Qiao, H. Hagiwara, C. Yokoyama, J. Mol. Catal. A: Chem. 246, 65–69 (2006)
R. Sugimura, K. Qiao, D. Tomida, C. Yokoyama, Catal. Commun. 8, 770–772 (2007)
A. Chrobok, S. Baj, W. Pudlo, A. Jarzebski, Appl. Catal. A Gen. 366, 22–28 (2009)
M.N. Sefat, D. Saberi, K. Niknam, Catal. Lett. 141, 1713–1720 (2011)
M. Baghernejad, K. Niknam, Int. J. Chem. 4, 52–60 (2012)
K. Niknam, A. Piran, Green Sustain. Chem. 3(2A), 1–8 (2013)
F. Shirini, M. Seddighi, M. Mazloumi, M. Makhsous, M. Abedini, J. Mol. Liq. 208, 291–297 (2015)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Zarei, A. Zare, V. Khakyzadeh, J. Mol. Liq. 211, 373–380 (2015)
M. Seddighi, F. Shirini, M. Mamaghani, C. R. Chim. 18, 573–580 (2015)
K. Niknam, P. Abolpour, Monatsh. Chem. 146, 683–690 (2015)
K. Niknam, A. Jamali, M. Tajaddod, A. Deris, Chin. J. Catal. 33, 1312–1317 (2012)
K. Niknam, A. Jamali, Chin. J. Catal. 33, 1840–1849 (2012)
S.M.G. Ahmadi-Ana, M. Baghernejad, K. Niknam, Chin. J. Chem. 30, 517–521 (2012)
K. Niknam, N. Borazjani, R. Rashidian, A. Jamali, Chin. J. Catal. 34, 2245–2254 (2013)
K. Niknam, R. Rashidian, A. Jamali, Sci. Iran. C 20, 1863–1870 (2013)
K. Niknam, M. Sadeghi Habibabad, A. Deris, N. Aeinjamshid, Monatsh. Chem. 144, 987–992 (2013)
K. Niknam, S. Mojikhalifeh, Mol. Diver. 18, 111–117 (2014)
K. Niknam, N. Borazjani, Monatsh. Chem. (2015). doi:10.1007/s00706-015-1552-2
Acknowledgments
We are thankful to Persian Gulf University Research Council for partial support of this work. Also, we are thankful to the School of Chemistry, Manchester University for running NMRs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niknam, K., Piran, A. & Karimi, Z. Synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] and spiro[indoline-3,4′-pyrano[2,3-c]chromene] derivatives using silica-bonded ionic liquids as a recyclable catalyst in aqueous medium. J IRAN CHEM SOC 13, 859–871 (2016). https://doi.org/10.1007/s13738-015-0801-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0801-y