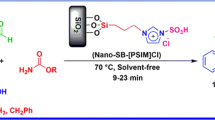
Abstract
Preparation of N-(3-silicapropyl) imidazolium hydrogen sulfate ([Sipim]HSO4) as a heterogeneous acidic ionic liquid is described. This heterogeneous ionic liquid was used as catalyst for the synthesis of α-aminonitriles by a one-pot condensation of aldehydes, amines, and trimethylsilyl cyanide at room temperature. Catalyst could be recycled for several times without any additional treatment.
Graphical Abstract
A simple and efficient procedure for the preparation of silica-based acidic ionic liquid ([Sipim]HSO4) is described. This heterogeneous solid acid is employed as a catalyst for the synthesis of a-amino nitriles at room temperature.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the recent years, ionic liquids have become a powerful alternative to conventional molecular organic solvents due to their particular properties, such as undetectable vapor pressure and the ability to dissolve many organic and inorganic substances [1]. One type is Brønsted acidic task-specific ionic liquids (BAILs). Among these ionic liquids possessing HSO4 − as a counteranion find a broad application in organic synthesis, acting as both solvents and catalysts [2, 3]. The acidic nature of these ionic liquids as catalysts has been exploited for many other important organic reactions and chemical production [4–12]. Recently, immobilisation processes involving acidic ionic liquids on solids supports have been designed. The heterogenisation of catalysts and reagents can offer important advantages in handling, separation and reuse procedures. Based on economic criteria, it is desirable to minimize the amount of ionic liquid utilized in a potential process. Immobilised acidic ionic liquids have been used as novel solid catalysts, e.g., for esterification, nitration reactions, acetal formation and Baeyer–Villiger reaction [13–15].
The addition of cyanide anion to imines (the Strecker reaction) [16] provides one of the most important and straightforward method for the synthesis of α-aminonitriles, which are useful intermediates for the synthesis of amino acids [17, 18] and nitrogen containing heterocycles such as thiadiazoles and imidazoles, etc. [19, 20]. The classical Strecker reaction usually is carried out in aqueous solution and the work-up procedure is also tedious. Thus, several modifications of Strecker reaction have been reported using a variety of cyanide reagents [21, 22], such as diethyl phosphorocyanidate and α-trimethylsiloxy nitriles, as well as catalysts such as montmorillonite KSF clay [23], silica-based scandium(III) [24], SO4 2−/ZrO2 [25], Fe(Cp)2PF6 [26], InCl3 [27], I2 [28], Fe3O4 [29], xanthan sulfuric acid [30], [bmim]BF4 [31], silica sulfuric acid [32], hydrophobic sulfonic acid based nanoreactors [33], silica-bonded S-sulfonic acid [34], sulfamic acid-functionalized magnetic Fe3O4 nanoparticles [35] under various reaction conditions. The use of trimethylsilyl cyanide is a safer and more effective cyanide anion source for the nucleophillic addition reactions of imines under mild conditions [36, 37]. Furthermore many of these catalysts are deactivated or sometimes decomposed by amines and water that exist during imine formation. In order to overcome these problems, recently one-pot procedures have been developed for this transformation [38].
2 Results and Discussion
Along the line of our studies in application of ionic liquids as a catalyst in chemical transformations [9–12] herein, we wish to describe the preparation of N-(3-silicapropyl) imidazolium hydrogen sulfate ([Sipim]HSO4) as illustrated in Scheme 1, and then used as catalyst for the synthesis of α-amino nitriles.
The thermogravimetric analysis (TGA) curves of [Sipim]HSO4 show the mass loss of organic materials as they decompose upon heating (Fig. 1). The initial weight loss from the [Sipim]HSO4 up to 126 °C is due to removal of physically adsorbed solvent and surface hydroxyl groups. The weight loss of about 4% between 200 and 450 °C is contributed to the thermal decomposition of organic groups. The covalent chemical bonds connection made the catalyst owned high thermal stability.
Figure 2 shows Fourier transform infrared (FT-IR) spectra for [Sipim]HSO4. The major peaks for silica (SiO2) are broad anti symmetric Si–O–Si stretching from 1300 to 1010 cm−1 and symmetric Si–O–Si stretching near 820–780 cm−1. For sulfuric acid functional group, the FT-IR absorption range of the O=S=O asymmetric and symmetric stretching modes lie in 1170 and 1060 cm−1, respectively, the S–O stretching mode lies around 590 cm−1. FT-IR spectrum shows the overlap asymmetric and symmetric stretching bands of SO2 with Si–O–Si stretching bands in the silica functionalized alkyl-sulfuric acid. The spectrum also shows a broad OH stretching absorption around 3600 to 2600 cm−1. The NH stretching is shown around 3150 cm−1 and NH bending 1650 cm−1.
The BET surface area using nitrogen adsorption isotherms at the temperature of liquid nitrogen which gave the results of a s,BET 1.11 m2 g−1 and the total pore volume 0.1737 cm3 g−1 (see supplementary material).
At first, we investigated the reaction of benzaldehyde with aniline and trimethylsilyl cyanide in the presence of different ionic liquid catalysts. As shown in Table 1, ionic liquids and silica-based ionic liquids accomplished this condensation at room temperature. [Sipim]HSO4 and N-(3-silicapropyl)-N-methyl imidazolium hydrogen sulfate [Sipmim]HSO4 [15] gave the best results with respect to catalytic loading, reaction time and yields (Table 1, enties 1,5). These results demonstrate that Brönsted acid counter ions perform better than other counter ions. The role of the counter ions was explored when ILs were used as catalysts. Ionic liquids with the HSO4 −counter ion give better results than H2PO4 − with respect to time and yield. This is due to the acidic power of HSO4 − over H2PO4 −. When −OTf was used as the counter ion there was no product formation even after 24 h (Table 1, entry 13), and very low yield (10%) in the case of N-methylimidazolium triflate (Table 1, entry 11). While in the case of [Sipim]OTf or [Sipmim]OTf as catalysts, this condensation was accomplished in 180 and 110 min with 70% and 85% yields, respectively (Table 1, entries 3 and 7). These results clearly demonstrate the role of counter ions in the catalytic activity of their corresponding supported and non-supported one.
To study the effect of catalyst loading of [Sipim]HSO4 on the condensation reaction of aldehydes, amines, and trimethylsilyl cyanide as the corresponding α-amino nitriles the reaction of benzaldehyde and aniline with TMSCN was chosen as a model reaction (Table 2). To illustrate the need of [Sipim]HSO4 for these reactions we examined the strecker reaction of benzaldehyde with aniline and TMSCN in the absence of this catalyst. In this case the reaction did not proceed even after 24 h (Table 2, entry 1). Obviously, [Sipim]HSO4 is an important component of the reaction.
The model reaction was also examined in various solvents as well as under solvent-free conditions in the presence of 0.2 g mmol−1 of [Sipim]HSO4 (Table 3).
The results showed that the efficiency and the yield of the reaction in EtOH were higher than those obtained in other solvents or under solvent-free conditions. Therefore, we employed the optimized conditions {aldehyde (1 mmol), amine (1.2 mmol), TMSCN (1.2 mmol), [Sipim]HSO4 (0.2 g) and EtOH (2 mL)} for the synthesis of α-amino nitriles in this one-pot three-component condensation (Scheme 2).
Next, we prepared a range of α-amino nitriles under the optimized conditions (Table 4). Both aromatic and aliphatic aldehydes reacted with amines and TMSCN in the presence of [Sipim]HSO4 and [Sipmim]HSO4 in this one-pot condensation to afford excellent yields of corresponding α-amino nitriles. Moreover, aldehydes with electron-withdrawing groups or electron-donating, i.e., 3-nitrobenzaldehyde or 4-methoxybenzaldehyde, and 3,4,5-trimethoxy-benzaldehyde were converted into the corresponding α-amino nitriles 1f–1k in high yields (Table 4, entries 6–11). The acid sensitive substrate thiophene-2-carbaldehyde gave the expected α-amino nitrile 1e in very good yield (Table 4, entry 5). Aliphatic aldehydes such as 2-methylpropanal and pentanal gave the corresponding products 1n and 1o in 82 and 80% yields, respectively (Table 4, enties 14, 15). Secondary amine such as morpholine treated with benzaldehyde under optimized conditions gave corresponding product 1p in 80% yield (Table 4, entry 16). Bis-amino nitrile was obtained by the reaction of benzaldehyde (2 mmol) with piperazine (1.5 mmol) and TMSCN (2.4 mmol) in the presence of [Sipim]HSO4 (0.4 g) gave corresponding product 1q in 60% yield (Table 4, entry 17). Also, we investigated the reaction of ketones such as acetophenone and 4-methylacetophenone with aniline under optimized conditions. In these cases the condensation of ketones proceeded only about 45–50% conversion (Table 4, entries 18, 19).
The possibility of recycling the catalyst was examined using the reaction of benzaldehyde and aniline with TMSCN under the optimized conditions. Upon completion, as indicated by TLC, the reaction mixture was filtered and the remaining washed with warm ethanol (3 × 5 mL). After cooling, the corresponding α-amino nitrile products were obtained which were purified by recrystallization from hot ethanol. The recovered catalyst was dried and reused for subsequent runs. Table 5 shows the catalyst after being recycled four times. The catalyst was recovered with high yield (90–91).
Finally, a comparative study of [Sipim]HSO4 with other recently reported catalysts for condensation of benzaldehyde and aniline with TMSCN as a model compound was made which revealed that [Sipim]HSO4 is an equally efficient and reusable catalyst (Table 6).
In conclusion, it has shown that silica propyl imidazolium hydrogen sulfate which can be prepared from commercially available and cheap starting materials, catalyzed efficiently the synthesis of α-amino nitriles by a one-pot three-component condensation of aldehydes, amines, and trimethylsilyl cyanide. The catalyst shows high thermal stability and was recovered and reused without any noticeable loss of activity. The mild reaction conditions and simplicity of the procedure offers improvements over many existing methods.
3 Experimental Section
Chemicals were purchased from Fluka, Merck and Aldrich Chemical Companies. For recorded NMR spectra we using Bruker (400 MHz) and Bruker (500 MHz) Avance DRX in pure deuterated CDCl3 solvent with tetramethylsilane (TMS) as internal standards. All the products were characterized by comparison of their IR, 1H NMR and 13C NMR spectroscopic data and their melting points with reported values [16–35].
4 Preparation of Catalyst
Chloropropyl silica was prepared by a known procedure as previously reported [15].
4.1 Preparation of Silica Propyl Imidazolium Chloride [Sipim]Cl
Silicapropyl chloride (10.0 g) was added to a flask containing 100 mL of anhydrous toluene and a large excess of imidazole (10.0 g). The reaction mixture was refluxed with stirring for 24 h. Then, the reaction mixture was cooled to room temperature, transferred to a vacuum glass filter, and washed with toluene, ethanol, and methanol in turn. The silica chemically bonded with imidazolium ([Sipim]Cl) was dried under vacuum at 50 °C for 4 h [15].
4.2 Preparation of Silica Propyl Imidazolium Hydrogen Sulfate [Sipim]HSO4
Into the three-necked round bottom flask equipped with stirrer, ice bath condenser, and thermometer [Sipim]Cl (3.0 g) was suspended in dry CH2Cl2 (20 mL). During vigorous stirring, concentrated H2SO4 (97%) (2.9 mmol) was added drop by drop at 0 °C. Then the mixture was warm up to the room temperature, and was refluxing for 48 h. When the formed HCl was completely distilled of the condenser the solution was cooled and the CH2Cl2 was removed under vacuum. To remove any water from the reaction mixture 10 mL of benzene was added to the crude ionic liquid and stirred for 3 h with magnetic stirrer at 50 °C. Formed azeotrope was distilled of yielding [Sipim]HSO4. Elemental analysis showed the S content to be 2.57%; C, 10.90%; H, 2.10%; N, 3.1%. According to S content the number of H+ sites of [Sipim]HSO4 is 0.8 mmol g−1. In addition, when [Sipim]HSO4 (0.2 g) was placed in an aqueous NaCl solution (25 mL), the solution pH dropped virtually instantaneously to pH 1.43, as ion exchange occurred between protons and sodium ions.
4.2.1 General Procedure
A mixture of aldehyde (1 mmol), amine (1.5 mmol), trimethylsilyl cyanid (1.2 mmol), and [Sipim]HSO4 (0.2 g, 0.16 mmol of H+) in EtOH (2 mL) was stirred at room temperature for appropriate time (Table 4). After completion of the reaction, as indicated by TLC, the reaction mixture was filtered and the remaining washed with warm ethanol (3 × 5 mL). After cooling, the corresponding α-amino nitrile products were obtained which were purified by recrystallization from hot ethanol. The recovered catalyst was dried and reused for subsequent runs.
4.2.2 Spectral Data of New Compounds
2-[N-(4-Methylanilino)]-2-(3,4,5-trimethoxyphenyl)acetonitrile 1j: IR (KBr): 3360, 3320, 2990, 2943, 2843, 2220, 1600, 1520, 1130, 820 cm−1. 1H NMR (500 MHz, CDCl3): δ (ppm) 2.32 (s, 3H), 3.78 (s, 1H), 3.90 (s, 9H), 5.37 (s, 1H), 6.75 (d, 2H, J = 7.3 Hz), 6.84 (s, 2H), 7.12 (d, 2H, J = 7.3 Hz). 13C NMR (125 MHz, CDCl3): δ (ppm) 20.94, 51.39, 56.72, 61.32, 104.74, 115.02, 118.71, 130.51, 139.32, 154.22. Anal. Calcd for C18H20N2O3: C, 69.21; H, 6.45; N, 8.97. Found: C, 69.02; H, 6.49; N, 8.70.
2-[N-(4-Methylanilino)]-2-(2,4-dichlorophenyl)acetonitrile 1m: IR (KBr): 3340, 3025, 2240, 1620, 1585, 1515, 1468, 800 cm−1. 1H NMR (500 MHz, CDCl3): δ (ppm) 2.32 (s, 3H), 3.95 (brs, 1H), 5.67 (s, 1H), 6.73 (d, 2H, J = 8.2 Hz), 7.11 (d, 2H, J = 8.0 Hz), 7.38 (dd, 1H, J1 = 8.2 Hz, J2 = 1.6 Hz), 7.53 (d, 1H, J = 1.6 Hz), 7.69 (d, 1H, J = 8.3 Hz). 13C NMR (125 MHz, CDCl3): δ (ppm) 20.96, 48.45, 115.21, 117.95, 128.49, 130.30, 130.55, 130.71, 130.76, 130.99, 134.73, 136.82, 142.39. Anal calcd for C15H12Cl2N2: C, 61.87; H, 4.15; N, 9.62. Found: C, 6.68; H, 4.20; N, 9.44.
[4-(Cyano-phenyl-methyl)-piperazin-1yl]-phenylacetonitrile 1q: IR (KBr): 3355, 3050, 2810, 2230 cm−1. 1H NMR (500 MHz, CDCl3): δ (ppm) 2.52–2.60 (m, 8H), 4.88 (s, 2H), 7.32–7.38 (m, 6H), 7.46–7.50 (m, 4H). 13C NMR (125 MHz, CDCl3): δ (ppm) 48.00, 62.12, 115.47, 128.29, 129.25, 129.54, 132.75. Anal. Calcd for C20H20N4: C, 75.92; H, 6.37; N, 17.71. Found: C, 75.73; H, 6.41; N, 17.60.
References
Wasserscheid P, Welton T (2007) Ionic liquids in synthesis, 2nd edn. Wiley-VCH, Weinheim
Keim W, Korth W, Wasserscheid P (2000) WO 016,902 Al, March 30
Wasserscheid P, Sesing M, Korth W (2002) Green Chem 4:134
Gupta N, Sonu GL, Kad J, Singh (2007) Catal Commun 8:1323
Xu DQ, Yang WL, Luo SP, Wang BT, Wu M, Xu ZY (2007) Eur J Org Chem 6:1007–1012
Khosropour AR (2008) Can J Chem 86:264
Wang W, Cheng W, Shao L, Yang J (2008) Catal Lett 121:77
Hajipour AR, Rajaei A, Ruoho AE (2009) Tetrahedron Lett 50:708
Tajik H, Niknam K, Parsa F (2009) J Iran Chem Soc 6:159
Niknam K, Damya M (2009) J Chin Chem Soc 56:659
Niknam K, Zolfigol MA, Saberi D, Khonbazi M (2009) Chin J Chem 27:1548
Tajik H, Niknam K, Sarrafan M (2011) Synth Commun 41:2103
Qiao K, Hagiwara H, Yokoyama C (2006) J Mol Catal A 246:65
Sugimura R, Qiao K, Tomida D, Yokoyama C (2007) Catal Commun 8:770
Chrobok A, Baj S, Pudlo W, Jarzebski A (2009) Appl Catal A 366:22
Strecker A (1850) Ann Chem Pharm 75:27
Shafran YM, Bakulev VS, Mokrushin VS (1989) Russ Chem Rev 58:148
March J (1995) Advanced organic chemistry, 4th edn. Wiley, New York, p 965
Weinstock LM, Davis P, Handelsman B, Tull R (1967) J Org Chem 32:2823
Matier WL, Owens DA, Comer WT, Dietchman D, Ferguson HC, Seidehamel RJ, Young JR (1973) J Med Chem 16:901
Mai K, Patil G (1984) Tetrahedron Lett 25:4583
El-Ahl AAS (2003) Synth Commun 33:989
Yadav JS, Reddy BVS, Eswaraiah B, Srinivas M (2004) Tetrahedron 60:1767
Karimi B, Safari AA (2008) J Organomet Chem 693:2967
Reddy BM, Thirupathi B, Patil MK (2009) J Mol Catal A 307:154
Khan NH, Agrawal S, Kureshy RI, Abdi SHR, Singh S, Suresh E, Jasra RV (2008) Tetrahedron Lett 49:640
Ranu BC, Dey SS, Hajra S (2002) Tetrahedron 58:2529
Wang SH, Zhao LF, Du ZM (2006) Chin J Chem 24:135
Mojtahedi MM, Abaee S, Alishiri T (2009) Tetrahedron Lett 50:2322
Shaabani A, Maleki A, Soudi MR, Mofakham H (2009) Catal Commun 10:945
Yadav JS, Reddy BVS, Eshwaraiah B, Srinivas M, Vishnumurthy P (2003) New J Chem 27:462
Chen WY, Lu J (2005) Synlett 2293–2296
Karimi B, Zareyee D (2009) J Mater Chem 19:8665
Niknam K, Saberi D, Nouri Sefat M (2010) Tetrahedron Lett 51:2959
Kassaee MZ, Masrouri H, Movahedi F (2011) Appl Catal A 395:28
Leblanc J, Gibson HW (1992) Tetrahedron Lett 33:6295
Heydari A, Fatemi P, Alizadeh AA (1998) Tetrahedron Lett 39:3049
Das B, Ramu R, Ravikanth B, Reddy KR (2006) Synthesis 1419–1422
Acknowledgment
The authors are thankful to Persian Gulf University Research Council for partial support of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nouri Sefat, M., Saberi, D. & Niknam, K. Preparation of Silica-Based Ionic Liquid an Efficient and Recyclable Catalyst for One-Pot Synthesis of α-Aminonitriles. Catal Lett 141, 1713–1720 (2011). https://doi.org/10.1007/s10562-011-0696-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0696-x