Abstract
Silica-bonded N-propyltriethylenetetramine was prepared via the reaction between 3-silicapropyl chloride and triethylenetetramine. This heterogeneous solid base was used as a catalyst for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) by a one-pot condensation of aromatic aldehydes with 3-methyl-1-phenyl-5-pyrazolone in 72–93 % yields. The catalyst could be recycled several times without any additional treatment.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Based-catalyzed condensation and addition reactions are important in the industrial production of drugs, fragrances, and chemical intermediates [1–6]. In addition, solid base catalysts are used not only for condensation reactions but also in particular for asymmetric organic syntheses [7, 8], other organic syntheses [9–12], and characterization of active centers [13]. The potential use of microporous and mesoporous base catalysts in fine chemical production is enormous [14]. These heterogeneous catalysts are known to suppress side reactions, which include self-condensation and oligomerization, resulting in better selectivity and product yield. It also avoids the complex neutralization and separation steps needed to recover the homogeneous base catalysts from the reaction mixture. The recovered solid catalysts can be readily regenerated for further use.
Pyrazoles are an important class of bioactive drug target in the pharmaceutical industry, as they are the core structure of numerous biologically active compounds [15, 16]. For example, they exhibit antianxiety, antipyretic, analgesic, and anti-inflammatory properties. 2,4-Dihydro-3H-pyrazol-3-one derivatives including 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) have a broad spectrum of biological activity, being used as anti-inflammatory [17], antipyretic [18], gastric secretion stimulatory [19], antidepressant [20], antibacterial [21], and antifilarial agents [22]. Moreover, the corresponding 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) are applied as fungicides [23], pesticides [24], insecticides [25], dyestuffs [26], and as chelating and extracting reagents for different metal ions [27].
In spite of the extensive application of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) [16], few methods have been reported for the preparation of these interesting compounds. These methods include application of piperidine in ethanolic solution [28], tandem Knoevenagel–Michael reaction in benzene solutions [29–31], application of sodium dodecyl sulfate in aqueous media [32], and electrocatalytic synthesis [33]. Nevertheless, most of these methods suffer from limitations such as moderate yields, long reaction times, harsh reaction conditions, application of hazardous solvents, and/or tedious workup procedures. Therefore, finding an efficient and a practicable protocol for the preparation of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) is of obvious importance.
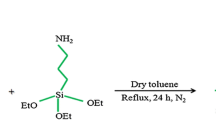
Recently, ceric ammonium nitrate (CAN) [34], ionic liquid under ultrasonic irradiation [35], silica-bonded S-sulfonic acid [36], silica sulfuric acid [37], and sulfuric acid [[3-(3-silicapropyl)sulfanyl]propyl]ester [38] were reported for the synthesis of 4,4′-(arylmethylene)bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols). Continuing our studies on the application of heterogeneous catalysts in chemical transformations [36–43], we herein describe the preparation of silica-bonded N-propyltriethylenetetramine (SBNPTT) as illustrated in Scheme 1 and its use as a catalyst for the synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols).

Results and discussion
The thermogravimetric analysis (TGA) curves of SBNPTT show the mass loss of organic materials as they decompose upon heating (Fig. 1). The initial weight loss from SBNPTT up to 100 °C is due to removal of physically adsorbed solvent and surface hydroxyl groups. The weight loss of about 11.43 % between 250 and 340 °C and 8.93 % between 370 and 450 °C is contributed to the thermal decomposition of organic groups. The covalent chemical bonds between the linker and surface imparted high thermal stability to the catalyst.
Figure 2 shows Fourier transform infrared (FT-IR) spectra for SBNPTT. The major peaks for silica (SiO2) are broad anti-symmetric Si–O–Si stretching from 1,240 to 1,000 cm−1 and symmetric Si–O–Si stretching near 800–785 cm−1. The band assigned to silanol groups is located at 3,757 cm−1; its intensity is small, indicating that most of the silanols had reacted. For the amine functional group, the FT-IR absorption range of the H–N–H asymmetric and symmetric stretching modes lie at 3,500 to 3,300 cm−1, overlapped with broad OH stretching bands. The N–H bending mode lies around 1,643 cm−1.
The confirmation of the presence of a mesoporous structure was performed by SEM and N2 adsorption–desorption measurements. The microscopic features of the catalyst were observed with SEM (Fig. 3). In this figure we can see the morphology of the silica substrate.
Additionally, the N2 adsorption–desorption isotherm showed a characteristic type IV isotherm, indicating the presence of mesopores. The average pore diameter was determined to be 8 nm by the Barret–Joyner–Halenda (BJH) method based on the isotherm. These observations revealed that the mesoporous structure was successfully formed. The Brunauer–Emmett–Teller (BET) surface area using nitrogen adsorption isotherms at the temperature of liquid nitrogen gave the results of a s,BET = 83.75 m2 g−1 and a total pore volume of 19.23 cm3 g−1 (see Supplementary Material).
To study the effect of catalyst loading on the condensation reaction of aromatic aldehydes with 3-methyl-1-phenyl-5-pyrazolone the reaction of 4-chlorobenzaldehyde and 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one was chosen as a model reaction (Table 1). The results show clearly that SBNPTT is an effective catalyst for this condensation and in the absence of SBNPTT the condensation reaction gave very low yield after 24 h. The optimal amount of SBNPTT was 0.03 g (2.77 mol %) per 1 mmol of aldehyde in refluxing ethanol. In addition, the result of this condensation in the presence of commercial amines such as triethylamine, morpholine, piperazine, and triethylenetetramine and sodium acetate is shown in Table 1.
Therefore, we employed the optimized conditions (0.03 g mmol−1 of SBNPTT in ethanol under reflux conditions) for the condensation reaction of various aryl aldehydes with 3-methyl-1-phenyl-5-pyrazolone into the corresponding 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) (Scheme 2).

As shown in Table 2, both aromatic and heteroaromatic aldehydes reacted with 3-methyl-1-phenyl-5-pyrazolone to afford 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) in excellent yields. On the other hand, benzaldehydes with electron-donating or electron-withdrawing groups, i.e., methyl-, methylthio-, and 3,4,5-trimethoxybenzaldehyde (Table 2, entries 2–4) or 4-nitro-, 3-nitro-, and 4-cyanobenzaldehyde (Table 2, entries 10–12), were condensed into the corresponding 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) 3b–3d and 3j–3l in high yields. 3-Pyridinecarbaldehyde, 2-thiophenecarbaldehyde, and furfural (Table 2, entries 14–16) were converted into the corresponding products 3n, 3o, and 3p in 83, 72, and 76 % yield, respectively. 2-Naphthalinecarbaldehyde was converted into corresponding product 3m in 85 % yield (Table 2, entry 13). It should be mentioned that in the case of lower isolated yields, the conversion was not 100 % and small amounts of starting materials remained. There were not any by-products.
The practical synthetic efficiency of this reaction was highlighted by the reaction of terephthalaldehyde and isophthalaldehyde with 3-methyl-1-phenyl-5-pyrazolone to give structurally complex pyrazol-5-ol derivatives 4a, 4b (Scheme 3).

The possibility of recycling the catalyst SBNPTT was examined using the reaction of 4-chlorobenzaldehyde and 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one under the optimized conditions. Upon completion, the reaction mixture was washed with warm ethanol (3 × 30 cm3). The recovered catalyst was washed with diethyl ether, dried, and reused for subsequent runs. The recycled catalyst was reused six times without any additional treatment. No appreciable loss in the catalytic activity of SBNPTT was observed (Fig. 4).
In conclusion, we have prepared some 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) by a tandem condensation reaction of aromatic aldehydes with two equivalents of 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one in the presence of SBNPTT as a solid base catalyst in refluxing ethanol.
Experimental
Chemicals were purchased from Fluka, Merck, and Aldrich. All the products are known compounds and were characterized by comparison of their IR, 1H NMR, and 13C NMR spectroscopic data and their melting points with reported values [29–38].
Preparation of silica-bonded N-propyltriethylenetetramine (SBNPTT)
Chloropropyl silica was prepared by a known procedure as previously reported [46]. To a mixture of 25 g chloropropyl silica in 250 cm3 anhydrous xylene, 25 cm3 of triethylenetetramine was added and the mixture was refluxed with stirring for 24 h. The reaction was then stopped and the modified silica was cooled to room temperature, transferred to a vacuum glass filter, and washed with xylene and a large excess of ethanol in turn. The resulting SBNPTT was dried under vacuum overnight at 80 °C and 26.4 g was obtained. The pH of 0.1 g of this solid base at 25 °C was determined by a pH-ISE conductivity titration controller (Denver Instrument Model 270) to be 9.7.
General procedure for the synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols
Aromatic aldehyde (1 mmol) was added to a flask containing a mixture of 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (2 mmol) and 0.03 g SBNPTT (2.77 mol %) in 10 cm3 warm ethanol and heated under reflux for an appropriate time. After completion of the reaction, as indicated by TLC, the reaction mixture was washed with warm ethanol (3 × 30 cm3). After cooling the crude products were precipitated and purified by recrystallization from ethanol (95 %). The recovered catalyst was washed with diethyl ether, dried, and reused for subsequent runs.
References
Kelly GJ, King F, Kett M (2002) Gr Chem 4:392
Ertl G, Knözinger H, Weitkamp J (eds) (1997) Handbook of heterogeneous catalysis. VCH, Weinheim
Dörwald FZ (ed) (2002) Organic synthesis on solid phase. Wiley, Weinheim
Toy PH, Lam Y (eds) (2012) Solid-phase organic synthesis: concepts, strategies, and applications. Wiley, Weinheim
Sheldon RA, van Bekkum H (eds) (2001) Fine chemicals through heterogeneous catalysis: Solid base catalysts, chap 7. Wiley-VCH, Weinheim, pp 309–350
Sheldon RA, Arends I, van Rantwijk F (eds) (2006) Green chemistry and catalysis. Wiley, Weinheim
Bartók M (2010) Chem Rev 110:1663
Ding K, Uozumi Y (eds) (2008) Handbook of asymmetric heterogeneous catalysis. Wiley-VCH, Weinheim
Yokoi T, Kubota Y, Tatsumi T (2012) Appl Catal A Gen 421:14
Tang Y, Chen G, Lu Y (2012) Res Chem Intermed 38:937
Szöllösi G, Bartók M (1999) J Mol Struct 482:13
Szöllösi G, Bartók M (1999) Catal Lett 59:179
Tsuchiya S, Morishige H, Imamura H (1991) J Catal 129:303
Weitkamp J, Hunger M, Rymsa U (2001) Micropor Mesopor Mater 48:255
McDonald E, Jones K, Brough PA, Drysdale MJ, Workman P (2006) Curr Top Med Chem 6:1193
Elderfield RC (ed) (1957) Heterocyclic compounds, vol 5. Wiley, New York
Elguero J (1996) In: Katritzky AR, Rees CW, Scriven EFV (eds) Comprehensive heterocyclic chemistry, vol 5. Pergamon, Oxford
Elguero J, Goya P, Jagerovic N, Silva AMS (2002) Targets Heterocycl Syst 6:52
Sugiura S, Ohno S, Ohtani O, Izumi K, Kitamikado T, Asai T, Kato K (1977) J Med Chem 20:80
Behr LC, Fusco R, Jarboe CH (1967) In: Weissberger A (ed) The chemistry of heterocyclic compounds: pyrazoles, pyrazolines, pyrazolidines, indazoles and condensed rings. Interscience, New York
Rosiere CE, Grossman MI (1951) Science 113:651
Bailey DM, Hansen PE, Hlavac AG, Baizman ER, Pearl J, Defelice AF, Feigenson ME (1985) J Med Chem 28:256
Mahajan RN, Havaldar FH, Fernandes PS (1991) J Indian Chem Soc 68:245
Chauhan PMS, Singh S, Chatterjee RK (1993) Indian J Chem Sect B 32:858
Singh D, Singh D (1991) J Indian Chem Soc 68:165
Uzoukwu AB (1993) Polyhedron 12:2719
Garnovskii AD, Uraev AI, Minkin VI (2004) Arkivoc iii:29
Singh D, Singh D (1984) J Chem Eng Data 29:355
Li XL, Wang YM, Tian B, Matsuura T, Meng JB (1998) J Heterocycl Chem 35:129
Pavlov PT, Goleneva AF, Lesnov AE, Prokhorova TS (1998) Pharm Chem J (Engl Trans) 32:370
Sivaprasad G, Perumal PT, Prabavathy VR, Mathivanan N (2006) Bioorg Med Chem Lett 16:6302
Wang W, Wang SX, Qin XY, Li JT (2005) Synth Commun 35:1263
Elinson MN, Dorofeev AS, Nasybullin RF, Nikishin GI (2008) Synthesis 1933
Sujatha K, Shanthi G, Selvam NP, Manoharan S, Perumal PT, Rajendran M (2009) Bioorg Med Chem Lett 19:4501
Zang H, Su Q, Mo Y, Cheng B (2011) Ultrason Sonochem 18:68
Niknam K, Saberi D, Sadegheyan M, Deris A (2010) Tetrahedron Lett 51:962
Niknam K, Mirzaee S (2011) Synth Commun 41:2403
Tayebi S, Baghernejad M, Saberi D, Niknam K (2011) Chin J Catal 32:1477
Niknam K, Zolfigol MA, Khorramabadi-Zad A, Zare R, Shayegh M (2006) Catal Commun 7:494
Niknam K, Karami B, Zolfigol MA (2007) Catal Commun 8:1427
Niknam K, Saberi D, Nouri Sefat M (2009) Tetrahedron Lett 50:4058
Niknam K, Saberi D (2009) Appl Catal A Gen 366:220
Niknam K, Saberi D (2009) Tetrahedron Lett 50:5210
Niknam K, Deris A, Naeimi F, Majleci F (2011) Tetrahedron Lett 52:4642
Reiner K, Richter R, Hauptmann S, Becher J, Hennig L (1995) Tetrahedron 51:13291
Baghernejad M, Niknam K (2012) Int J Chem 4:52
Acknowledgments
We are thankful to the Persian Gulf University Research Council for the partial support of this work. Also, we are thankful to the School of Chemistry, Manchester University for running NMR, BET, CHN, TGA, and SEM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niknam, K., Habibabad, M.S., Deris, A. et al. Preparation of silica-bonded N-propyltriethylenetetramine as a recyclable solid base catalyst for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) . Monatsh Chem 144, 987–992 (2013). https://doi.org/10.1007/s00706-012-0910-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0910-6