Abstract
This study investigates the microstructural, thermal, and mechanical behavior of high density polyethylene (HDPE)-based composites prepared using compression molding technique. HDPE was mixed with either micro-size zinc oxide (bulk ZnO) or zinc oxide nanoparticles (nano-ZnO) as fillers’ contents at 0, 10, 20, 30, and 40 wt%. The structural, morphological, and thermal properties of the composites were identified using X-ray diffraction (XRD), scanning electron microscope (SEM), Fourier transform infrared spectrophotometer (FTIR), and thermal gravimetric analysis (TGA). The results showed good dispersion and interaction mechanisms between HDPE and the fillers at low weight percentage. The thermal stability of HDPE was enhanced by adding both bulk and nano-ZnO, especially for higher filler loading. Tensile tests at different speeds and Vickers microhardness tests conducted at different indentation loads (0.25–5 N) at t = 60 s were performed to realize how the mechanical properties of the composites were influenced. The values of stiffness, ultimate tensile strength, and yield stress increased by increasing the filler loading to 20 wt% of either bulk ZnO or nano-ZnO. The values of ultimate tensile strain and ductility were deteriorated by increasing the filler loading. Nano-ZnO, at 20 wt% content in composite, showed higher mechanical properties than bulk composite, so it has been recommended for a better tensile performance at higher strain rates. Vickers microhardness measurements showed that the tested samples exhibited reverse indentation size effect (RISE) behavior. The obtained results were analyzed using Meyer’s law which was a preferred approach for analysis of HDPE/ZnO composite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prompt technological advancements in materials and research disciplines have made ways to enhanced findings and manufacturing new composite materials for various applications. Polymer and metal nanocomposites are considered as top most interesting options that are merits in material engineering due to their properties and advantages such as, flexibility, good functionality, easy processing, durability, large surface-to-volume ratio, and thermo-mechanical properties [1]. The extended fields of applications of polymer nanocomposites, compelled to focus on improving their properties for a better performance, included biomedical applications [2, 3], food processing [4], radiation shielding [5], tissue engineering [6], and purifying water from waste [7, 8]. The nanocomposites can be, consequently, distinguished upon several properties that strongly influence their applications. The thermal, structural, and mechanical properties (Young’s modulus, strength, and toughness) of polymers can be upgraded by incorporating nanoparticles as fillers within the polymer matrix to form the nanocomposites. The properties of obtained nanocomposites depend on various parameters including the production technique and nanostructure design [9]. When the filler, mainly at nanometer scale, is merged into the polymer matrix, the composite properties are ameliorated. High density polyethylene (HDPE) is a frequently used polymer due to its superior properties, lightness, and low manufacturing cost. However, its use is constrained due to its limited thermal stability, and mechanical and structural failure. Therefore, introducing specific amounts of nanoparticles may overcome these limitations. Metal oxide nanoparticles such as TiO2, MgO, ZnO, and Al2O3 are selected as nanofillers due to their photocatalytic, antibacterial, functional, and mechanical effect [10, 11]. Among these fillers, ZnO is one of the metal oxides that has gained much interest during the last decade. ZnO is a semiconductor material [12, 13] which has a broad, direct band gap close to UV region and large binding energy [14,15,16,17]. Other important features of ZnO that have been reported recently include: its antibacterial activity against wide range of bacteria, which enables its use in clothing, food packaging, and automotive and solar energy fields [18, 19]. Also, ZnO is known to have “amenability to wet chemical etching”, i.e., its performance against corrosion in different acidic and alkaline media. Furthermore, it has been reported that ZnO has an interesting radiation resistance mainly at high altitude or in space [20, 21].
Many innovative research studies have reported the incorporation of ZnO nanoparticles with several polymers. Lee et al. [22] prepared ZnO nanoparticles in poly(ethylene oxide) (PEO), and they found that ZnO nanoparticles preserved their crystallite stability and improved their structure within the polymer matrix. Yang et al. [23] studied the photo-degradation of linear low-density polyethylene through adding different nanoparticles, such as Al2O3, SiO2, and ZnO. The results showed that ZnO had the best effect as a nanofiller. Gi Jeong et al. [24] prepared ZnO nanorods and nanoplates by solvothermal synthesis to investigate the effect of zinc oxide morphology on the UV absorbance and antimicrobial activity of nylon 6/zinc oxide composite filaments. The composite containing nanoplates revealed a better UV shielding than those containing nanorods. Also, an interesting antimicrobial activity was noticed even by adding only 0.5 wt% of ZnO nanoparticles. Kiaei et al. [25] investigated the effect of ZnO nanoparticles on the morphological, mechanical, and flammability properties of wood flour/HDPE composites. The flexural strength and modulus of the samples containing higher amount of ZnO nanoparticles increased, while the heat release and burning rate were decreased. Chang et al. [26] recorded the response of ZnO nanoparticles reinforcements into ultra-high-molecular-weight polyethylene. The Vickers micro-hardness, tensile modulus, and compressive modulus increased with increasing ZnO nanoparticles filler. Fekri et al. [27] tested the influence of ZnO nanoparticles loading on the corrosion protection of polypyrrole coatings of Al alloy2024. The results showed that a solution containing 0.025% of ZnO nanoparticles showed an optimum anti-corrosion coating of Al alloy2024 in NaCl solution. Ansari et al. [28] prepared nanocomposites containing PANI, nylon 6,6, and zinc oxide nanoparticles through solution mixing. They found that the stability of electrical conductivity increased with increase in zinc oxide nanoparticle content. Ameen Khan et al. [29] prepared poly(methyl methacrylate) (PMMA)/zinc oxide (ZnO) composites using melt mixing process by adding 0, 0.5, 1, and 2 wt% of ZnO. Compared to neat PMMA, cone calorimeter results of the composite loaded with 1.5 wt% of ZnO showed 21% reduction in the peak heat release rate. Also, a slight decrease in the tensile strength of the composites with increase in ZnO content was observed. In a recent study, Junlapong et al. [30] synthesized ZnO nanoparticles with different morphologies through a hydrothermal method which were then compounded with natural rubber (NR) latex at loading contents of 0.1–0.6 phr. The obtained results confirmed that the tensile strength, tear strength, and modulus of all vulcanizates increased with increased loadings of ZnO.
There are a few systematic reviews on the mechanical and thermal characteristics of zinc oxide (nano-ZnO or bulk ZnO filler) HDPE-based composite. This work introduces a new composite by exploring the effect of adding bulk and nano-ZnO with various percentages (0, 10, 20, 30, and 40 wt%) as fillers on HDPE polymer matrix. Since HDPE is insoluble in common solvents, this technique allows the mixing of HDPE through molding at specific temperature and adding the required amounts of filler to obtain the composite. A large amount of material has been used, since the obtained composites were tested as radiation shielding materials against gamma radiation [31]. The structural, surface area functionalization, TGA, and mechanical properties of these composites were investigated. The proposed synthetic technique of HDPE/ZnO composite is simple, formable, and cost-effective compared to other techniques. The tensile test was performed at different speeds (7, 14, and 70 mm/min) to highlight the strain rate effect on the behavior of the fabricated composite which has not been reported in any literature before. Also, Vickers microhardness test was performed at different loads (0.25–5 N) with indentation time t = 60 s. This detailed study about the new synthesized HDPE/ZnO composite nominates them as good candidates in many applications such as textiles, barriers, and automotive industries as alternatives to metal based materials. Also, due to their thermal stability and good mechanical properties, these new composite materials can be used in aerospace industries and radiation facilities.
Experimental
Materials
The polymer used in this study was in the form of pellets named “Egyptene HD5301FB” and supplied by Sidi Kerir Petrochemicals Co. (SIDPEC, Alexandria, Egypt). HDPE possessing the following properties: melt flow rate of 0.75 g min−1 and density of 960 kg m−3 was dried at 90 C and smashed into little balls by a mechanical grinder (HRPM-13, Jiangsu, China). Bulk ZnO (molar weight: 81.39 g mol−1, purity 98–100.5%, relative density: 5610 g cm−3), purchased from Sigma-Aldrich (St. Louis, Missouri, USA), was used without additional purification. ZnCl2 (molar weight: 136.3 g mol−1, purity > 99%) and NaOH (molar weight: 40 g mol−1, purity > 96%) used in the synthesis of ZnO nanoparticles were supplied by Sigma-Aldrich (St. Louis, Missouri, USA).
ZnO nanoparticle synthesis
ZnO nanoparticles were synthesized by co-precipitation technique using 1 M ZnCl2 and distilled water as precursors. An amount of 30 g of ZnCl2 was dissolved in 100 mL distilled water. Homogeneous and constant stirring was applied for 30 min to maintain a uniform mixture, to which 4 M NaOH was added dropwise at room temperature to adjust pH 13. This was followed by constant stirring of the mixture at 80 C for 2 h. The resultant solution was washed by distilled water to adjust pH 7 and then dried at 100 C for 18 h. The obtained powder was subsequently annealed at 550 C for 5 h to achieve the desired nanoparticles. Then, all the obtained samples were submitted to ball milling with a weight ratio of 1:5 (powder: balls) at 250 rev/min for 10 min.
HDPE/(bulk and nano) composite synthesis
Using the compression molding technique, different weight fractions of ZnO filler (10, 20, 30, and 40 wt%) were fabricated. After weighing the needed amount of HDPE, it was melted in a two-roll mill mixer (XK400, Shandong, China) at 170 C for 10 min with speed of 50 rev/min. With successive blending for 15 min, a proper ratio of either bulk ZnO or nano-ZnO was progressively introduced into the mixture. To ensure the uniform distribution of the filler within the polymer matrix, the obtained mixtures were milled for 10 min. Subsequently, the obtained samples were smashed and spewed into a rectangular stainless steel mold of dimensions (25 × 25 × 0.3 cm) and sandwiched between Teflon layers to achieve a refined sheet. In the next stage, the sheet was hot pressed with 20 MPa hydraulic compression molding (Shanghai, China) at 170 C for 15 min with capacity of molding, tooling, and heating. The sample was sintered and cooled under compression at a rate of 20 C min−1. Standard test specimens for the Vickers microhardness testing were obtained by cutting the prepared sheet into square specimens (3 × 3 mm).
Characterization techniques
XRD analysis
The synthesized samples were characterized using X-ray diffraction XRD (Model: D8 Focus Bruker, Lebanon) to determine their structure by employing CuKα radiation source of wavelength (λ = 0.154 nm) in a range of 10 ≤ 2θ ≤ 70 with scan speed of 3°/min. Nearly 0.7 g of each sample was crushed very well to form fine powder. This powder was put in a plate-like shape holder and placed inside the diffractometer to obtain the XRD pattern.
SEM analysis
The surface morphology was observed by a Tescan scanning electron microscope (SEM) (MIRA3, Lebanon) operated at 20 kV. An electron beam incident was focused upon the investigated sample, thus scattering electrons and emitting an X-ray beam. Each specimen was coated with gold to reduce the charge resulting from electron bombardment, so that a clear image was obtained from the reflected beam of electrons.
FTIR analysis
FTIR (Nicolet iS5, Lebanon) was used to obtain the FTIR spectra of the synthesized composite and to identify the functional groups and the chemical interactions in a wavenumber range of 350–3150 cm−1. An amount of 100 mg KBr powder was mixed with 10 mg of sample to be analyzed and then finely pulverized into a pellet-forming die. The resultant powder was then pressed at 2000 psi to form 13 mm-diameter pellets ready for FTIR measurements.
Thermal properties
Thermal degradation and stability were detected by TGA (TG–DTA/DSC Setaram-Labsys, Lebanon). The changes in the samples’ weight were sealed in aluminum pan and detected from room temperature to 600 C in a heating rate of 10 C min−1 in pure nitrogen atmosphere. The mass of each sample was taken in the range of 7–12 mg.
Mechanical properties
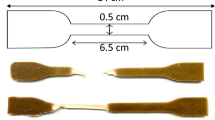
The mechanical tensile tests were performed using ESM303 Tension/Compression Force Tester (Mark-10 Force measurements, Lebanon). According to the international standard (ASTM: D638), the samples were cut into dumbbell shape, as illustrated in Fig. 1. To perform the tensile test at different speeds (7 mm/min, 14 mm/min, and 70 mm/min), three specimens of the same wt% were used. The samples at different weight percentages after the tensile test are shown in Fig. 1.
Microhardness measurements on various specimens were carried out using Vickers hardness tester (SIOMM model MHVD-1000IS, Lebanon) with a Vickers diamond pyramidal indenter attached to it, at room temperature. The applied forces ranged between 0.25 and 5 N. The average of five Hv readings was taken at different locations of the sample surface for the same load to ensure accuracy. Vickers microhardness was calculated using Eq. 1 [32]:
where F is the load (kg) and d is diagonal of indentation (mm). The duration of the load application was 60 s for each test.
Results and discussion
XRD
Figure 2 represents the XRD patterns of 10 wt%, 20 wt%, 30 wt%, and 40 wt% of HDPE/nano-ZnO composite. The inset of Fig. 2 displays the XRD profile of ZnO nanoparticles, having the planes (100), (002), (101), (102), (110), (103), (200), (112), and (201) which are in accordance to hexagonal (wurtzite) structure. Three major strong peaks at 2θ = 31.86, 34.52, and 35.74 appear sharply; implying the high degree of crystallinity of ZnO. The crystallite size (D) of the synthesized ZnO nanoparticles was calculated using Scherrer’s equation:
where λ = 0.154 nm, β is the full width at half maximum height (FWHM) in radian, 0.9 is the shape coefficient for the reciprocal lattice point, and θ is the diffraction angle in the range of 10 ≤ 2θ ≤ 70. The step size of 2θ is 0.02. The average crystallite size of ZnO nanoparticles was 27 nm. Figure S1 represents the XRD patterns of 10 wt%, 20 wt%, 30 wt%, and 40 wt% of HDPE/bulk ZnO composite. The inset of Fig. S1 shows the XRD patterns of HDPE revealing an orthorhombic, hexagonal crystal structure with an intense reflection peak at 2θ = 21.61, followed by less intensive peaks displayed at 24.02 and ~ 40, having their reflection planes, (110), (200), and (020), respectively [33].
The incorporation of both bulk and nano-ZnO into HDPE matrix resulted in the appearance of characteristic sharp peaks of the composite, revealing an amorphous and crystallite nature. Changes in the peaks intensities are observed for all samples with no shift in their positions. The peaks of bulk and nano-ZnO are detected at 2θ = 31.86, 34.52, and 35.74, and those corresponding to HDPE are discerned at 2θ = 21.61, 24.02, and 30.04. In spite of the fact that the peak positions of HDPE have not changed; the intensity of HDPE peaks was attenuated, along with an increase in the intensity of ZnO peaks by increasing the filler concentrations from 10 to 40 wt%. Thus, illustrating the good dispersion of ZnO into the polymer matrix and the successful mixing process. Similar attenuation of peaks was observed [34] by increasing the amount of multi-walled carbon nanotube filler from 0.1 to 10 wt% added to polyethylene. Also, the peaks of r-HDPE were attenuated with increasing both bulk and nanofiller as reported by another study [35].
SEM
Figure 3a, b reveals the SEM photographs of 20 wt% and 40 wt% HDPE/nano-ZnO composite, illustrating a reasonably good dispersion of ZnO nanoparticles within the polymer matrix with slight tendency of agglomeration at 40 wt% filler loading. Due to the effect of van der Waals forces, the nanoparticles tend to agglomerate. This could be avoided by introducing high-intensity ultrasonic homogenizer for mixing and dispersing of nanoparticles during the casting technique as described by Mula et al. [36].
Figure S2a illustrates the representative SEM photograph of ZnO nanoparticles. The obtained ZnO nanoparticles show a rod-like irregular hexagonal morphology [37]. The SEM photograph of HDPE is revealed in Fig. S2b with rough compact dense clusters and aggregates appearing on the surface. Figures S3a and S3b show the representative SEM photographs of 20 wt% and 40 wt% HDPE/bulk ZnO composite. SEM photographs show that most parts of bulk and nano-ZnO fillers were interlaced with the polymer matrix. For the low weight percentage of both fillers, the surfaces of the polymer were typically smooth and sheet like. By increasing the filler weight percentage from 20 to 40 wt%, the surface roughness increased. For HDPE/nano-ZnO composite, nano-ZnO filler appeared to be deeply implanted within the polymer matrix by forming an interlocking structures which indicates good interfacial adhesion and interaction between ZnO nanoparticles and the chains of polymeric matrix. However, bulk ZnO seems to be pulled out from the polymer matrix and clearly appears on its surface at high filler loading due to low interfacial adhesion which results in de-bonding between the filler and matrix. Typically, de-bonding occurs most likely with large particles and results in formation of void which may merge into large cracks leading to mechanical failure. This confirms a poor adhesion between the filler and the polymer chain in contrary to the ZnO nanoparticles which are uniformly embedded within HDPE matrix. The same behavior was reported by Mahmoud et al. [38], who observed the improved interfacial adhesion between PbO nanoparticles and HDPE matrix. An important fact can explain the filler–matrix interaction which occurs at nanometric scale, and the interphase region between the surface of the particle and the matrix itself attains a significant role, so it can be considered as a secondary phase in the material. Thus, the resulting interphase fills a large part of the volume of the composite, since the nanoparticles have large surface-to-volume ratio. Moreover, at low filler loadings, the produced interfaces maintain the absorption of the whole amount of the polymer on the inorganic filler surface [39].
FTIR
To better understand the interaction and binding mechanisms between HDPE and ZnO filler, FTIR analysis was performed between 350 and 3150 cm−1 at room temperature. The FTIR spectra of ZnO nanoparticles in Fig. S4a show a sharp peak at 434 and 564.6 cm−1 followed by less intense peak at 1635 cm−1 corresponding to Zn–O-stretching mode. Meanwhile, the peak at 1116 cm−1 attributed to O–H-stretching vibrations is mainly due to water adsorption at the metal surface [40]. The FTIR spectra of HDPE illustrated in Fig. S4b show that HDPE exhibits mainly two overlapped peaks at 2919 and 2850 cm−1, corresponding to the C-H stretching vibrations. The other two peaks at 1467 and 723 cm−1 were assigned to the polymer chain characterizing the polymer corresponding to methylene CH2 group [41]. Figure 4a shows the FTIR spectra of HDPE/nano-ZnO composite for 10, 20, 30, and 40 wt% of nano-ZnO filler. The peaks corresponding to the functional group of nano-ZnO filler are clearly embedded within the peaks corresponding to HDPE, verifying the chemical interaction between the polymer chain and the filler. For low content of ZnO, the peak corresponding to Zn–O stretching mode is shown at 415 cm−1, and this peak appears stronger for higher content of ZnO. The FTIR spectra of HDPE/bulk ZnO composite for 10, 20, 30, and 40 wt% of bulk ZnO filler are illustrated in Fig. 4b. New peaks appeared at 434 and 564.6 cm−1, corresponding to the bulk ZnO filler functional group with the existence of HDPE peaks confirm the chemical bonding between bulk ZnO and HDPE polymer matrix. The mechanism of interaction between the ZnO filler and HDPE can be explained by the formation of an interphase between the filler and polymer matrix. ZnO is a reactive filler, the functional groups created during synthesis favor the development of an ordered structure when dispersed within the polymer matrix. A covalent bond is formed through cross-linking mechanism [42] which increases with the filler concentration. The FTIR spectra of HDPE/ZnO composite verified the chemical interaction between HDPE and ZnO which was attributed to the appearance of HDPE and ZnO unaltered characteristic peaks.
TGA
The detailed thermal degradation steps of HDPE/bulk ZnO and HDPE/nano-ZnO composites are listed in Table 1. Two main thermal degradation temperatures were considered: initial thermal degradation temperature, Tin, corresponding to the temperature at 5% weight loss, and, T50%, corresponding to the temperature for half-weight loss of each examined sample. Figure 5a shows the TGA curves of HDPE/bulk ZnO composite. The decomposition of HDPE was established at 448.78 C till reaching full decomposition mainly at 599.32 C with a residual mass of 1.53%. The addition of 10–40 wt% of bulk ZnO increased the values of Tin from 459.87 to 460.23 C and the values of T50% from 482.43 to 483.49 C, respectively. The residual mass percentage at 600 C increased to 28.33% for 10 wt% sample and to 47.95% for 30 wt% sample. On the other hand, the residual mass decreased abruptly to 30.55% for the sample containing 40 wt% bulk ZnO. This may be attributed to the inhomogeneous distribution of bulk ZnO within the polymer matrix. Figure 5b illustrates the TGA analysis of HDPE/nano-ZnO composite. The incorporation of 10 wt% to 40 wt% nano-ZnO within HDPE increased the values of Tin from 459.71 to 460.85 C and T50% from 482.38 to 486.03 C, respectively. Furthermore, a systematic increase in the residual mass percentage from 17.05% to 41.74% at 600 C was observed by increasing the filler loading of nano-ZnO from 10 to 40 wt%. Although, the addition of various weight percentages of both bulk and nano-ZnO to HDPE enhanced its thermal stability and worked as degradation retardants by shifting Tin and T50% to higher values. HDPE/nano-ZnO composite exhibited better thermal stability and residual mass conservation than bulk composite even at high filler loading, which reflects the state of good dispersion of nano-ZnO within the polymer matrix. The interfacial adhesion between the filler and the polymer matrix decreases substantially after heat exposure which is performed at high temperature. Above the glass transition temperature, Tg, HDPE is transformed to a rubbery viscous state due to its amorphous nature. As the temperature rises, the polymer expands, since it is a solid. The increase in glass transition temperature is attributed to the loss of the polymer chain mobility caused by interaction, whereas Tg is reduced in the presence of filler aggregates due to greater difficulty of packing the macromolecules which constitute the matrix phase [43].
Mechanical properties
Tensile test at different speeds
To describe the mechanical properties of HDPE/ZnO composite at different strain rates (7, 14, and 70 mm/min), the values of Young’s modulus (stiffness), ultimate tensile strength, ultimate tensile strain, yield stress, and elongation-at-break were determined and are listed in Table 2. The standard deviations are represented by error bars. The stress–strain behavior of HDPE at different strain rates (7, 14 and 70 mm/min) is illustrated in Fig. 6. HDPE is mainly considered as a super ductile material with yield stress and Young’s modulus values of 7.58 MPa and 675.7 MPa attained, respectively, at 7 mm/min.
These values increased to 20.05 MPa and 772.9 MPa, respectively, by increasing the strain rate to 70 mm/min. HDPE exhibited a strain hardening region corresponding to the movement of atomic dislocations [44]. This was followed by a large neck behavior within the gauge length described by extended elongation from 209 to 236% at speeds of 7 and 14 mm/min, respectively. However, this zone was narrowed at 70 mm/min indicating the rupture and the loss of ductility to which elongation-at-break percentage declined to 37.46%. The improvement of Young's modulus and tensile strength at 70 mm/min is quite significant. This may be attributed to the fact that high strain rates support the elastic behavior of materials which is associated with the load bearing performance of the specimen by making it stiffer and stronger [45].
Figures 7 and S5 show the stress strain behavior of HDPE/nano-ZnO and HDPE/bulk ZnO composites at 7 mm/min, respectively. It is obvious from the figures and the data listed in Table 3 that the incorporation of 10 and 20 wt% of either nano or bulk ZnO induced an increment in the values of Young’s modulus (stiffness), ultimate tensile strength, and yield stress compared to HDPE. Both composites achieved their highest values of Young’s modulus of 883.8 MPa for nano-ZnO and 790.67 MPa for bulk ZnO at 20 wt%, whereas these values deteriorated for 30 and 40 wt% of filler loadings. The improvement in the values of Young’s modulus is mainly attributed to the addition of inorganic fillers to the polymer matrix which are typically stiffer than the polymer itself. This important mechanical property strongly depends on the filler loading, since the deformation capacity is restricted by fillers within the elastic zone [46]. By increasing the amount of filler loading to a certain value (30 wt%), the mechanical properties of the composites were reduced due to the agglomeration of both nano- and micro-ZnO particles even with further increase in weight percentage. With their big particle size, these agglomerated particles acted as defects and areas of stress concentrations which led to crack of the specimen and resulted in poor composite strength [47]. It is also noted that HDPE/nano-ZnO composite showed a better improvement in the mechanical properties more than HDPE/bulk ZnO composite which indicated good dispersion and better interfacial interaction between the filler and the polymer matrix, especially at low filler loading which confirms SEM results. The composite strength was enhanced by well-bonded ZnO nanoparticles which received the tensile force through the filler–matrix interface.
However, the values of ultimate tensile strain and percentage of elongation-at-break decreased with the increase of filler loading from 10 to 40 wt% for both composites. The reduction in the ductility of the samples with high filler loading was mainly attributed to the barrier effect of the filler that induced slipping, extending, and direction adjustment of the lamellae [48]. By increasing the filler content, the specimens that were submitted to tensile loading revealed a failure mode due to the delamination failure in the matrix. As a result, the filler/matrix bonding decreased which was attributed to the delamination damage. Many studies investigated the reason behind these mechanisms. Some of them suggested that the pinning of filler particles induced the crack bowing effect which may increase the energy at the crack line. Others attributed the increase in fracture energy to the plastic deformation of the matrix around the filler. Also, it has been reported that the filler addition induces crack branching [49]. Although, the samples become stiffer by increasing the filler loading, but they were ruptured easily losing their flexibility during the stress test. This behavior was ascribed to the formation of irregular micro-voids due to the presence of large micro-particles of bulk ZnO that were stuck to the surface of the polymer thus hindering its movement [50].
The stress–strain behaviors of the samples containing 20 wt% of nano-ZnO and bulk ZnO composites at different strain rates (7, 14, and 70 mm/min) are demonstrated, respectively, in Figs. S6a and S6b. The values of Young’s modulus and yield stress were noticeably increased with the increase of strain rates revealing their maximums at 70 mm/min (1282 MPa and 20.02 MPa) for nano-ZnO and (848.86 MPa and 17.28 MPa) for bulk ZnO composites, respectively. The strength of the composite relies on the effective stress transfer between filler and polymer matrix [45]. It is quite significant to notice that the 20 wt% nano-ZnO composite exhibited higher values of Young’s modulus and yield stress compared to bulk composite. The ultimate tensile strain and elongation-at-break percentage were reduced for both composites with the increment in strain rate. Bulk ZnO composite was fractured rapidly showing a brittle behavior with an extreme decrease in ductility from 76.5% to 35.9%, indicating that the sample was submitted to failure rather than elongation. However, nano-ZnO composite preserved its mechanical properties even at high strain rate, which confirmed proper dispersion and interfacial adhesion between the nanofiller and the polymer matrix.
Vickers microhardness
Figure 8 illustrates the average Hv values versus F for HDPE/nano-ZnO composite at t = 60 s and those for HDPE/bulk ZnO composite are shown in Fig. S7, and their values calculated using Eq. 1 are listed in Tables 3 and 4. Both figures showed a non-linear increasing behavior of Hv for F ≤ 3 N associated with strain hardening of the composite. However, the existence of plateau saturation region for F > 3 N demonstrated the reverse indentation size effect (RISE) depending on the applied load and directly associated with the indentation-induced specimen cracking. As investigated by Li et al. [51], the RISE behavior is directly accompanied with the indentation induced specimen cracking caused by continuous series of indenter penetrations or indenter sink-ins. Another study described by Baitsch et al. [52] showed that the indentation size effect originated from dislocations that accumulated when the shear stress on slippery planes exceeded a crucial threshold limit. So that the succeeding piled up dislocations against the indenter’s contact area and dislocation network strongly depended on the indentation depth. Since there are several bonding forces between atoms and molecules in polymers, the deformation mechanisms occurring within their matrix are complex.
The variations in average Hv versus wt% for nano and bulk ZnO composite are illustrated in Fig. 9. The standard deviations are represented by error bars. The values of Hv for both were noticeably decreased with the increase of filler from 10 to 20 wt% compared to pure HDPE. The reason behind this variation may be due to the absence of filler reinforcement at the point of indentation on the surface layer of the specimens considering the fact that the load-bearing capacity of a filler composite depends on the strength of the weakest path throughout the microstructure [47]. Also, at low filler loading ZnO nanoparticles were well dispersed within the polymer matrix. Whereas, a remarkable increase in Hv was noticed for 30 and 40 wt% filler loadings in both composites. The fluctuation of Hv values for HDPE/nano-ZnO composite can be explained by the agglomeration of nanoparticles at high filler loadings which was revealed by SEM. During the synthesis process and with their small sizes, nanoparticles have high energy and high relative surface area compared to micro-sized particles. Thus, nanoparticles show strong tendency to agglomerate by minimizing their excess surface energy to achieve lower energy state [53]. These agglomerates increase the mass density and the rigidity of the composite, so the hardness increases. Moreover, HDPE/nano-ZnO composite had higher values of Hv compared to HDPE/bulk ZnO composite except for 40 wt% filler content confirming the dependence of Vickers microhardness on good dispersion of the filler and proper amount of wt%.
In their literature, Elmustafa et al. [54], explained the relation between indentation size and hardness values, revealing that small hardness values were attributed to small indentations. To investigate the behavior of HDPE/ZnO composite, Meyer’s law (Eq. 3) is used to explain ISE (indentation size effect) or RISE (reverse indentation size effect) behaviors. Where A is the standard hardness constant and n is the Meyer’s number used to characterize the behavior. For n < 2, normal ISE behavior is established, whereas for n > 2, reverse ISE behavior is prevailed [55]:
The values of A and n are listed in Table 5. The values of n for the composite containing either nano or bulk ZnO are greater than 2, thus confirming that HDPE/ZnO composite exhibited RISE behavior.
Conclusion
HDPE/ZnO composite was successfully prepared through compression molding technique by adding both nano and bulk ZnO as fillers with different weight percentages. The SEM photographs revealed the filler–matrix surface morphology with better adhesion and dispersion of nano-ZnO within the polymer matrix. XRD patterns revealed the amorphous crystallite nature of HDPE, the hexagonal (wurtzite) structure of ZnO nanoparticles and confirmed the formation of HDPE/ZnO composite. The FTIR spectra showed the interaction and binding mechanisms between HDPE and ZnO filler. HDPE/nano-ZnO composite exhibited higher thermal stability than bulk composite with relatively lower weight loss especially at high contents of ZnO nanoparticles. The values of stiffness, ultimate tensile strength, and yield stress increased by increasing the filler loading to 20 wt% of either bulk ZnO or nano-ZnO. Whereas, the values of ultimate tensile strain and ductility were deteriorated by increasing the filler loading. The stress–strain results confirmed that adding nano and bulk ZnO enhanced the mechanical properties of the composite. Vickers microhardness measurements were conducted at different indentation loads and investigated using Meyer's law. The tested samples exhibited RISE behavior with increasing applied load. Nevertheless, Hv values for both nano and bulk ZnO decreased with increase of filler from 10 to 20 wt% and increased for 30 and 40 wt%. Thus, HDPE/nano-ZnO composite obtained by a simple and low manufacturing technique can be used widely in many applications such as the fields of industry, fabrics, textiles, vehicles, food packaging, space technology, and radiation shielding. It is recommended to use an optimum amount of ZnO nanofiller (20 wt%) to obtain better mechanical performance, good dispersion, and interfacial filler–matrix adhesion mainly at high strain rates. An important issue to be figured out is to improve molding and mixing technique by controlling some process parameters such as particle size and large volume fraction of fillers to prevent agglomeration of nanoparticles and improve the properties of the fabricated composite with low cost and large volume production.
References
Mohammadi M, Yousefi AA, Ehsani M (2012) Study of the thermal and mechanical properties of blown films of high-and low-density polyethylene blends. J Appl Polym Sci 125:755–767
Owens GJ, Singh RK, Foroutan F, Alqaysi M, Han CM, Mahapatra C, Kim HW, Knowles JC (2016) Sol–gel based materials for biomedical applications. Prog Mater Sci 77:1–79
Chaudhary GR, Singh P, Kaur G, Mehta SK, Kumar S, Dilbaghi N (2015) Multifaceted approach for the fabrication of metallomicelles and metallic nanoparticles using solvophobic bisdodecylaminepalladium (II) chloride as precursor. Inorg Chem 54:9002–9012
Maisanaba S, Pichardo S, Jordá-Beneyto M, Aucejo S, Cameán AM, Jos Á (2014) Cytotoxicity and mutagenicity studies on migration extracts from nanocomposites with potential use in food packaging. Food Chem Toxicol 66:366–372
Mahmoud ME, El-Khatib AM, Badawi MS, Rashad AR, El-Sharkawy RM, Thabet AA (2018) Fabrication, characterization and gamma rays shielding properties of nano and micro lead oxide-dispersed-high density polyethylene composites. Rad Phys Chem 145:160–173
Okamoto M, John B (2013) Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog Polym Sci 38:1487–1503
Heydarifard S, Taneja K, Bhanjana G, Dilbaghi N, Nazhad MM, Kim KH, Kumar S (2018) Modification of cellulose foam paper for use as a high-quality biocide disinfectant filter for drinking water. Carbohydr Polym 181:1086–1092
Kumar S, Ahlawat W, Bhanjana G, Heydarifard S, Nazhad MM, Dilbaghi N (2014) Nanotechnology-based water treatment strategies. J Nanosci Nanotechnol 14:1838–1858
Thomas S, Shanks R, Chandran S (2015) Design and applications of nanostructured polymer blends and nanocomposite systems, 1st edn. William Andrew. Hardcover ISBN: 9780323394086, eBook ISBN: 9780323394543
Li SC, Li YN (2010) Mechanical and antibacterial properties of modified nano-ZnO/high-density polyethylene composite films with a low doped content of nano-ZnO. Appl Polym Sci 116:2965–2969
Mao Q, Wyatt TP, Chen J, Wang J (2015) Insert injection molding of high-density polyethylene single-polymer composites. Polym Eng Sci 55:2448–2456
Farhat S, Rekaby M, Awad R (2018) Synthesis and characterization of Er-doped nano ZnO samples. J Supercond Nov Magn 31:3051–3061
Janotti A, Van de Walle CG (2009) Fundamentals of zinc oxide as a semiconductor. Rep Prog Phys 72:126501
Lang J, Wang J, Zhang Q, Xu S, Han D, Yang J, Han Q, Yang L, Sui Y, Li X, Liu X (2016) Synthesis and photoluminescence characterizations of the Er3+-doped ZnO nanosheets with irregular porous microstructure. Mater Sci Semicond Proc 41:32–37
Musa I, Qamhieh N (2019) Study of optical energy gap and quantum confinement effects in zinc oxide nanoparticles and nanorods. Dig J Nanomater Biostruct 14:119–125
Samouco A, Marques AC, Pimentel A, Martins R, Fortunato E (2018) Laser-induced electrodes towards low-cost flexible UV ZnO sensors. Flex Print Electron 3:044002
Sun X, Azad F, Wang S, Zhao L, Su S (2018) Low-cost flexible ZnO microwires array ultraviolet photodetector embedded in PAVL substrate. Nanoscale Res Lett 13:277
Liu S, Yao K, Wang B, Ma MG (2017) Microwave-assisted hydrothermal synthesis of cellulose/ZnO composites and its thermal transformation to ZnO/carbon composites. Iran Polym J 26:681–691
Vittal R, Ho KC (2017) Zinc oxide based dye-sensitized solar cells: a review. Renew Sust Energ Rev 70:920–935
Hariharan C (2006) Photocatalytic degradation of organic contaminants in water by ZnO nanoparticles: revisited. Appl Catal 304:55–61
Geetha N, Sivaranjani S, Ayeshamariam A, Suthan Kissinger J, Valan Arasu M, Jayachandran M (2016) ZnO doped oxide materials: mini review. Fluid Mech Open Acc 3:141
Lee J, Bhattacharyya D, Easteal AJ, Metson JB (2008) Properties of nano-ZnO/poly(vinyl alcohol)/poly(ethylene oxide) composite thin films. Curr Appl Phys 8:42–47
Yang R, Li Y, Yu J (2005) Photo-stabilization of linear low densitypolyethylene by inorganic nano-particles. Polym Degrad Stab 88:168–174
Gi Jeong M, Min Im Y, Won Son T, Hwan OhT (2019) Effect of zinc oxide morphology on the antimicrobial activity and UV absorbance of nylon 6/zinc oxide composite filaments. Fiber Polym 20:1624–1630
Kiaei M, Kord B, Samariha A, Moghdam YR, Farsi M (2017) Mechanical, flammability, and morphological properties of nano-composite plastic based on hardwood flour high-density polyethylene embedding by nano-zinc oxide. BioResources 12:6518–6528
Chang BP, Akil HM, Nasir RB, Bandara IM, Rajapakse S (2014) The effect of ZnO nanoparticles on the mechanical, tribological and antibacterial properties of ultra-high molecular weight polyethylene. J Reinf Plast Compos 33:674–686
Fekri F, Zandi M, Foroughi M (2019) Polypyrrole coatings for corrosion protection of Al alloy2024: influence of electrodeposition methods, solvents, and ZnO nanoparticle concentrations. Iran Polym J 28:577–585
Ansari SH, Mohammad F (2016) Conducting nanocomposites of polyaniline/nylon 6,6/zinc oxide nanoparticles: preparation, characterization and electrical conductivity studies. Iran Polym J 25:363–371
Ameen Khan M, Madhu GM, Sailaja RRN (2017) Reinforcement of polymethyl methacrylate with silane-treated zinc oxide nanoparticles: fire retardancy, electrical and mechanical properties. Iran Polym J 26:765–773
Junlapong K, Suwanboon S, Khaokong S (2019) Effects of zinc oxide particle shape on properties of a prevulcanized latex. Iran Polym J 28:325–335
Alsayed Z, Badawi MS, Awad R, Thabet AA, El-Khatib AM (2019) Study of some γ-ray attenuation parameters for new shielding materials composed of nano ZnO blended with high density polyethylene. Nucl Technol Rad Protect 2019:33
Callister WD, Jr D, Rethwisch G (2009) Materials science and engineering an introduction, 8th edn. Wiley, Hoboken
Jafarzadeh Y, Yegani R, Sedaghat M (2015) Preparation, characterization and fouling analysis of ZnO/polyethylene hybrid membranes for collagen separation. Chem Eng Res Des 94:417–427
McNally T, Pötschke P, Halley P, Murphy M, Martin D, Bell SE, Brennan GP, Bein D, Lemoine P, Quinn JP (2005) Polyethylene multiwalled carbon nanotube composites. Polymer 46:8222–8232
Mahmoud ME, El-Khatib AM, Badawi MS, Rashad AR, El-Sharkawy RM, Thabet AA (2018) Recycled high-density polyethylene plastics added with lead oxide nanoparticles as sustainable radiation shielding materials. J Clean Prod 176:276–287
Mula S, Padhi P, Panigrahi SK, Pabi SK, Ghosh S (2009) On structure and mechanical properties of ultrasonically cast Al-2% Al2O3 nano-composite. Mater Res Bull 44:1154–1160
Mansour SA, Elsad RA, Izzularab MA (2016) Dielectric investigation of high density polyethylene loaded by ZnO nanoparticles synthesized by sol-gel route. J Sci Technol 80:333–341
Mahmoud ME, El-Khatib A, El-Sharkawy R, Rashad A, Badawi M, Gepree MA (2019) Design and testing of high-density polyethylene nanocomposites filled with lead oxide micro- and nano-particles: mechanical, thermal, and morphological properties. J Appl Polym Sci 136:47812
Puglia D, Kenny JM (2018) Structure-property relationships of thermoset nanocomposites. Thermosets pp 231–276
Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG (1991) The handbook of infrared and Raman characteristic frequencies of organic molecules. Elsevier, New York
Uhm YR, Kim J, Lee S, Jeon J, Rhee CK (2011) In situ fabrication of surface modified lead monoxide nanopowder and its HDPE nanocomposite. Ind Eng Chem Res 50:4478–4483
Kaltenegger-Uray A, Rieß G, Lucyshyn T, Holzer C, Kern W (2019) Physical foaming and crosslinking of polyethylene with modified talcum. Polymers (Basel) 11:1472
Mansoori Y, Masooleh TM (2015) Polyimide/organo-montmorillonite nanocomposites: a comparative study of the organo clays modified with aromatic diamines. Polym Compos 36:613–622
Ovid’ko IA, Valiev RZ, Zhu YT (2018) Review on superior strength and enhanced ductility of metallic nanomaterials. Prog Mater Sci 94:462–540
Sutar H, Maharana HS, Dutta C, Murmu R, Patra S (2019) Strain rate effects on tensile properties of HDPE-PP composite prepared by extrusion and injection moulding method. Mater Sci Appl 10:205–215
Erdem E, Kiraz K, Somer M, Eichel RA (2010) Sol-gel based synthesis of nano-sized Fe3+-doped PbTiO3 powders. J Eur Ceram Soc 30:289–293
Fu SY, Feng XQ, Lauke B, Mai YW (2008) Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Comp Part B 39:933–961
Kahar AWM, Sarifuddin N, Ismail H (2017) Structural, thermal and physico-chemical properties of high density polyethylene/natural rubber/modified cassava starch blends. Iran Polym J 26:149–159
Kim KH, Ong JL, Okuno O (2002) The effect of filler loading and morphology on the mechanical properties of contemporary composites. J Prosthet Dent 87:642–649
Sepet H, Tarakcioglu N, Misra RD (2016) Investigation of mechanical, thermal and surface properties of nanoclay/HDPE nanocomposites produced industrially by melt mixing approach. J Compos Mater 50:3105–3116
Li H, Bradt RC (1996) The effect of indentation-induced cracking on the apparent microhardness. J Mater Sci 31:1065–1070
Baitsch M, Le KC, Tran TM (2015) Dislocation structure during microindentation. Int J Eng Sci 94:195–211
Tsuda A, Venkata NK (2016) The role of natural processes and surface energy of inhaled engineered nanoparticles on aggregation and corona formation. NanoImpact 2:38–44
Elmustafa AA, Stone DS (2003) Nanoindentation and the indentation size effect: kinetics of deformation and strain gradient plasticity. J Mech Phys Solid 51:357–381
Sangwal K (2000) On the reverse indentation size effect and microhardness measurement of solids. Mater Chem Phys 63:145–152
Acknowledgements
This work was done in the frame of the scientific collaboration between Physics Department, Faculty of Science, Beirut Arab University, Beirut, Lebanon, Physics Department, Faculty of Science, Lebanese University, Beirut, Lebanon, and National Council for Scientific Research (CNRS), Beirut, Lebanon.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alsayed, Z., Awad, R. & Badawi, M.S. Thermo-mechanical properties of high density polyethylene with zinc oxide as a filler. Iran Polym J 29, 309–320 (2020). https://doi.org/10.1007/s13726-020-00796-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-020-00796-7