Abstract
Polymethyl methacrylate (PMMA)/zinc oxide (ZnO) composites were prepared using melt mixing process. A nano ZnO-treated with aminopropyl triethoxysilane (APTS) was used as reinforcing particles. PMMA composites were obtained with different ZnO loadings of 0, 0.5, 1 and 2 parts by weight. This research was focused on determination of the influence of different loadings of silane-treated ZnO particles on the morphology, electrical, mechanical and flammable properties of the PMMA composites. Addition of ZnO to the PMMA composite was observed to enhance the thermal properties and char formation. The results from cone calorimeter showed 21% reduction in the peak heat release rate for the composite loaded with 1.5 wt% of ZnO as compared to that of neat PMMA. The surface resistivity, volume resistivity and EMI shielding properties of the composites as a function of ZnO loading were estimated. The results showed that the surface and volume resistivity of the composites reinforced with ZnO particles decreased and the EMI shielding increased almost linearly with increasing ZnO volume content. However, the tensile strength of the composites showed a slight decrease with increase in ZnO content. The SEM micrographs and AFM images showed dispersion of ZnO particles in the PMMA matrix. The nanoparticles were distributed evenly on the surface. Nevertheless the pockets of agglomerates could be seen at higher ZnO loading level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The inclusion of nanoparticles into polymers imparts and enhances the properties of the base matrix by improving mechanical, optical or fire retardant characteristics. PMMA is one such polymer, which is being extensively used for a wide variety of applications owing to its optical transparency, good mechanical strength and ease of moldability. Zinc oxide (ZnO) also has gained very much attention owing to its antibacterial properties, catalysis activity and ultraviolet/infrared absorption characteristics. The nanoparticles of ZnO have high surface energy and area and thus can be used as filler in polymer matrices [1]. It is well known that a composite comprised of an organic matrix like PMMA and inorganic nanoparticles leads to products with improved mechanical, thermal, fire retardant, electrical and optical properties [2]. Enhanced wear properties, modulus and strength values have also been obtained by incorporating ZnO nanoparticles in ABS resins [3]. An optimal loading of ZnO nanoparticles in poly (styrene-co-acrylonitrile)/PMMA has been found to increase modulus and impact strength [4]. ZnO nanoparticles were used as reinforcement in commonly used thermoplastics such as PP, PE and their binary blends with elastomers. It was found that the stiffness and strength improved while the coefficient of friction reduced due to the addition of nano-sized ZnO [5]. Improved UV and thermal stability have been obtained by adding ZnO nanoparticles in PMMA as reported by Anzlovar et al. [6]. Similar observations have been reported by Tang et al. [7] for methacrylic acid-modified ZnO nanoparticles blended with PMMA. Silane-treated ZnO/PMMA nanocomposites showed improved dispersability and thermal stability as observed by Hong et al. [8]. Laachachi et al. [9] observed that lower content of ZnO nanoparticles in PMMA had a catalytic effect while higher ZnO loading had a stability effect. The enhanced dispersability of ZnO particles in PMMA could be facilitated by supercritical carbon dioxide as reaction medium [10]. In situ suspension polymerization has been found to improve interfacial properties for ZnO/PMMA composites as suggested by Shim et al. [11].
Zinc–aluminum layered double hydroxide/PMMA/melamine composites showed improved fire retardancy as examined by cone calorimetry [12]. However, these additives have to be added in large quantities to ensure adequate fire retardancy. Hence, nanoparticles would be appropriate as they have large surface to volume ratio and thus lesser quantity can be loaded.
In the present study, ZnO nanoparticles were added to PMMA matrix. In order to enhance interfacial interactions, these nanoparticles were surface-treated with an APTS coupling agent. The mechanical, thermal, electrical and fire retardancy characteristics were assessed. No such work using a combination of PMMA and silane-treated ZnO and by melt blending could be cited from literature. Enhanced fire retardancy of composite was observed using silane-treated ZnO as nanofiller. The loading of nanofillers (i.e., nano ZnO) resulted in a linear increase in electrical properties. The fire retardancy characteristics for PMMA nanocomposites were analyzed by cone calorimetry measurements and the thermal properties were also examined. Morphology studies were carried out by atomic force microscopy (AFM) and scanning electron microscopy.
Experimental
Materials
Poly(methyl methacrylate) was obtained from Saraswati Plastics Limited (India). 3-Amino propyl-tri-ethoxy-silane (APTS) was procured from Sigma Aldrich (USA). Zinc nitrate hexahydrate (Merck; >96.0%) AR grade was used as a precursor. Commercial sugar was used as a fuel. The synthesis of nano zinc oxide was performed by gel combustion method and all other solvents were obtained from S.d. Fine Chemicals, India.
Synthesis of ZnO and grafting of silane
Zinc oxide was synthesized from Zinc nitrate (hexahydrate) as precursor and sugar as fuel by combustion technique [13]. Zinc nitrate of 15 g and sugar of 5 g were dissolved in 80 mL of double-distilled water in a liter beaker (Borosil). Sugar to nitrate mole ratio was maintained at 0.15. The mixture was introduced into a preheated muffle furnace maintained at 300 °C. Large amount of nitrates was evolved during the reaction and resulted in the formation of gel. Further exposure to heat resulted in the formation of foam-like material, indicating the completion of the reaction. When sugar was used as a fuel the material obtained was coated with a carbon film. To obtain the pure zinc oxide (white color powder) the synthesized zinc oxide was calcinated at 600 °C. The calcinated ZnO was characterized using SEM and XRD and the same was used for further studies. Grafting of silane onto ZnO particles was carried out as per our earlier work using a mixture of water/ethanol (20:80) [14].
Blend preparation
The blends of PMMA/ZnO were prepared in a Brabender mixer (Plasticorder, Keltron, MODEL-16 CME SPL Germany) by melt mixing at 200 °C with a rotor speed of 20 rpm for 5 min at different amounts 0, 0.5, 1.0, 1.5 and 2.0% of ZnO.
Compression molding
The blends from the Brabender were pressed into sheets in a compression mold (Hot pressing machine, Santec, India) at 30 MPa pressure and 200 °C. A 10-min heating time was kept in the mold, and the nanocomposite was cured for 20 min.
FTIR spectroscopy
Fourier transform infrared spectroscopy (FTIR) (Bruker Alpha-FTIR spectroscope with an ECO-ATR module) was performed for characterization of silane-treated ZnO.
Tensile properties
The tensile strength and modulus of the blends were measured by Kudale UTM (Model PC-2000, India). The tests were performed as per ASTM D-638 standards.
Volume and surface resistivity
The volume and surface resistivity of the composites were studied using a Keithley 6220/2182A Delta Mode System analyzer.
Blend morphology
Specimen morphology was studied using scanning electron microscopy (SEM) (Quanta 200 microscope). Gold sputtering of fractured specimens was done prior to microscopy.
Atomic force microscope (AFM) was used to characterize the morphology of composites. Measurements were conducted in tapping mode by a Solver Nano Scanning Probe microscope AFM (NT-MDT, Russia) at ambient temperature.
Thermogravimetric analysis (TGA)
TGA was carried out for the blends using a thermogravimetric analyzer from TA instrument (model Q500, UK). The blends were subjected to a heating rate of 10 °C/min in a heating range of 40–800 °C in nitrogen atmosphere with Al2O3 as the reference material.
Differential scanning calorimetry (DSC)
DSC for the samples was performed in a Mettler Toledo DSC 822e model. Samples were placed in sealed aluminum cells, using a quantity less than 10 mg for each sample and scanned at a heating rate of 10 °C/min over a temperature range of ambient temperature to 350 °C.
Cone calorimeter (CC) analysis
A cone calorimeter based on the ‘oxygen consumption’ principle [15] made by FTT (UK) was used. The calorimeter testing was carried out using ASTM: E 1354 standards at a heat flux of 35 kW/m2. A specimen size of 10 × 10 cm with a 0.3 cm thickness was prepared for the test.
Results and discussion
FTIR analysis
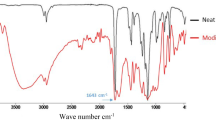
FTIR spectroscopy was used to assess the functionalization of ZnO with silane. Figure 1 shows typical FTIR spectrum of ZnO nanoparticles and surface-modified ZnO. The peaks, located at 2938 cm−1, correspond to the asymmetric methylene stretching vibration [16]. For the APTS-treated ZnO, the peaks at 1050–1100 cm−1are assigned to both the symmetric and asymmetric stretching vibrations of Si–O–Si, indicating that the silane is grafted on the surface of ZnO [17].
X-ray diffraction analysis of synthesized ZnO and composites
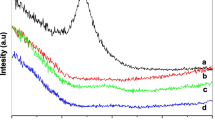
X-ray diffraction patterns of synthesized ZnO and nanocomposites are presented in Fig. 2. The calcinated ZnO was characterized by a Bruker D2 Phase diffractometer from 2θ of 5 to 60º with a step size 0.02º/0.5 s. Neat ZnO has a set of sharp crystalline peaks in the 2θ values in the range of 30–60o. The three closely placed crystalline peaks at 31.71 (100), 34.34 (002) and 36.17 (101) typically represent the hexagonal Wurtzite structure [18] and these peaks in Fig. 2 match with JCPDS standard (Powder diffraction file: card No. 36-1451) [19]. Crystal size was calculated by Scherrer relation using FWHM (Full Wave Half Medium) method. The average particle size was found to be around 45 nm.
PMMA has two broad peaks at 2θ values of 13.41o and 29.68o, indicating it is predominantly amorphous. The nanocomposites loaded with 1 and 1.5% ZnO nanoparticles show peaks corresponding to the peaks of both PMMA and ZnO. Hence, this indicates that the nanocomposite has a two-phase structure. A slight broadening of ZnO peaks due to the presence of PMMA can be observed [20] and the addition of silane coupling agent facilities the interfacial interactions to a certain extent [21].
Mechanical properties
The tensile strength for the PMMA/ZnO nanocomposites as a function of composition is shown in Fig. 3. The values of tensile strength reduced marginally by 2.5 (for 1% ZnO) and 4.3% with 2% ZnO content. This may be due to the aggregates of ZnO particles in a polymer matrix increased with increasing ZnO content. Thus, loading of zinc oxide does not improve the tensile strength of PMMA composite specimens prepared by melt mixing and compression molding methodology [4].
Figure 3 also shows the Young’s modulus of PMMA/ZnO composites. Young’s modulus increases as the ZnO content increases due to stiffness induced by addition of rigid ZnO nanoparticles. A significant increase in the Young’s modulus has been observed with 1.0% ZnO loading [22]. Silane-treated ZnO promotes good interaction and dispersion with the PMMA matrix.
The tensile fracture morphology of the blends loaded with 1.5% ZnO nanoparticles is shown in Fig. 4a, b. The composite has undergone severe layered deformation. The entire surface has both small and large elongated voids indicating effective dispersion of the filler particles. Furthermore, deformation of matrix suggests enhanced interfacial adhesion between PMMA and ZnO particles, thereby indicating that there is an effective stress sharing between the PMMA matrix and nanofiller, because there is little or no particle–matrix debonding as seen more clearly at higher magnification). The SEM image of the synthesized ZnO with magnification 1000× is shown in Fig. 4c. All the particles are regular with a flake-like morphology and large voids, thereby indicating a less dense structure when compared with normal zinc oxide. The tap density of the powder was found to be 0.987 g/cc. Atomic force microscopy (AFM) imaging technique has been used to study the morphology of PMM/ZnO nanocomposites. Due to that the soft surface of PMMA the tapping mode was used to study the morphology of composites at a scan rate of 1.2 Hz using an INTEGRA scanning probe. Figure 5a, b shows the 3D AFM images and the corresponding particle distribution images of neat PMMA and nanocomposite loaded with 1.5% ZnO nanoparticles. It can be seen that the nanoparticles are spread throughout the surface although pockets of agglomerates can be seen [23]. thus supporting the inference made by SEM for dispersion of nanofiller into the nanocomposites. The root mean square roughness and the average roughness parameters have increased considerably (Table 1), suggesting the immiscibility of synthesized nanofillers of different sizes embedded in the PMMA matrix.
Electrical properties
Figure 6 depicts the plot of volume and surface electrical resistivity of PMMA/ZnO composites versus ZnO percentage content. As the conductivity of filler particles is higher than that of PMMA, the electrical properties of the composites are expected to change with the ZnO content. For the ZnO nanoparticles modified by APTS by free radical reaction, the silane functional group interacts with ester groups of PMMA, which, in turn, facilitates anchoring of the PMMA to ZnO nanoparticle surface [24]. As it can be seen from the figure there is a significant reduction in resistivity as the ZnO content increases in the nanocomposite. The volume resistivity value is reduced from 1.66 × 1015 Ω cm for neat PMMA to 1.3 × 1012 Ω cm for the nanocomposite with just 1% nano ZnO. Further reduction of volume resistivity value with increasing the nanoparticle content to 2% can be observed in Fig. 6. The enhanced dispersion of these nanoparticles in PMMA facilitates the formation of conductive network, leading to reduced resistivity values. A similar trend can b observed for the surface resistivity values. The surface resistivity value is reduced to 1.1 × 1010 Ω/sq. for 2% ZnO nanoparticles as compared to that of neat PMMA (9.3 × 1013 Ω/sq.) [25]. This is also reflected in the EMI shielding effectiveness values at various frequencies shown in Fig. 7. The shielding effectiveness value is increased from 0.19 dB for neat PMMA to 1.92 dB for 1% ZnO nanocomposite at a frequency of 1.5 GHz. The shielding effectiveness values are further increased to values greater than 3 dB for 2% ZnO loading as shown in Fig. 7. The band gap (E g) is the value of optical energy gap between the valence band and the conduction band, which represents a range of energies that electrons do not have. The band gap of pure PMMA lies at 4.53 eV; nevertheless, the study revealed that with the increase of ZnO loading in the PMMA/ZnO composites the band gap became narrower compared to pure PMMA. A similar observation has been reported by Gaurang et al. [26].
Thermal analysis
Thermogravimetric analysis
TGA Thermograms for pure PMMA, pure ZnO and PMMA/ZnO composites are shown in Fig. 8. Thermal degradation of pure PMMA sequentially occurred as scission, depropagation and degradation into volatile compounds [27]. As Fig. 8 shows the weight loss percentage for pure ZnO is 1.96% of its starting weight and its midpoint is located at 397.2 °C. By addition of 1 and 1.5% ZnO the thermal stability was enhanced as compared to pure PMMA. Indeed, 15.1 and 17.2% chars were formed by addition of 1 and 1.5% ZnO, respectively.
Differential scanning calorimetry
Figure 9 shows DSC thermograms of neat PMMA, ZnO and the composites. The T g values of the blends obtained from the DSC thermograms are given in Table 2. The glass transition temperature of 99.8 °C given for pure PMMA in Table 2 slightly decreases with increase in filler content [28]. The addition of small amount of synthesized silane-treated ZnO nanoparticles results in polymer chain disruption and increasing the free volume of the polymer; it, therefore, slightly decreases the glass transition temperature of the composites [29]. However, the endothermic decomposition curves clearly show improved thermal stability for nanocomposites. For pure PMMA, decomposition temperature is 343.2 °C, but in case of nanocomposites it is in the range of 375–388 °C, indicating increase in thermal stability of nanocomposites.
Cone calorimetric (CC) studies
The fire retardant behavior of neat PMMA and PMMA/ZnO nanocomposites was studied by CC under an incident flux of 35 kW/m2 as per ASTM E-1354 standards. It is recognized that heat release rate (HRR) is an important factor in fire hazard analysis: it is a measure of intensity of fire growth [30]. The HRR curves of the samples are shown in Fig. 10. The HRR values reduce due to the addition of ZnO nanoparticles. This is also reflected in the reduction behavior of peak heat release rate (pHRR). The PHRR values are reduced from 528 to 463.7 kW/m2 by the addition of 1.0% ZnO and also further reduced to 417.3 kW/m2 by increasing the ZnO concentration to 1.5%. The values of the time-to-ignition (TTI), pHRR, time-of-flame-out (TOF), total heat released (THR) along with total smoke released (TSR) are presented in Table 3. TTI is one of the most important parameters to evaluate the fire resistant ability of material as longer TTI indicates enhanced flame retardancy. The combustion time corresponds to the difference between TOF and TTI and the fire performance index (FPI) is the ratio of TTI to pHRR. In this case, TTIs of PMMA/ZnO composites were 10 and 14 s for 1.0 and 1.5% ZnO loadings, respectively, as compared to TTI of pure PMMA which was around 8 s.
As expected, the residues after CC test could show the different results of heat release rates between PMMA and PMMA/ZnO nanocomposites (Fig. 11a–c). Pure PMMA at the end of the test was completely burnt and it left behind no residue, as can be seen in Fig. 11a.
The composites loaded with 1% ZnO produced heterogeneous and interconnected char. On the other hand, increasing the ZnO loading to 1.5% led to the formation of dense char, indicating intumescence to a certain extent which, in turn, provided a protective barrier layer. Metal oxides and hydroxides can help reduce the flame propagation [31, 32] through condensed phase mechanism. Figure 12 shows the CO and CO2 yields for PMMA and the nanocomposites, respectively. The blends containing 1.5% ZnO show a decrease of 30.3 and 21.9% for CO and CO2, respectively. A similar observation on the smoke generation was made by Friederich et al. for TiO2-reinforced PMMA composites [33].
Conclusion
The mechanical, electrical and flame retardancy characteristics of PMMA blends, reinforced with varied amounts of silane-treated ZnO, were investigated. The results showed a decrease in tensile strength with an increase in ZnO content. A noticeable change in modulus was observed after adding ZnO. Both volume and surface resistivity of the composites began to decrease as ZnO loading increased. The AFM and SEM micrographs indicated an effective dispersion of the filler particles, thus suggesting enhanced interfacial adhesion and effective stress sharing between PMMA and ZnO particles. The introduction of nano ZnO particles improved the thermal stability and flame retardancy of PMMA. Compared with neat PMMA the composite loaded with 1.5% ZnO exhibited a delayed TTI of 14 s, and the THR was reduced from 75.27 MJ/m2 for pure PMMA to 65.27 MJ/m2 for the composite.
References
Matei A, Cernica I, Cadar O, Roman C, Schiopu V (2008) Synthesis and characterization of ZnO–polymer nanocomposites. Int J Mater Form 1:767–770
Hussein MA, El-Shishtawy RM, Abu-Zied BM, Asiri AM (2016) The impact of cross-linking degree on the thermal and texture behavior of poly(methyl methacrylate). J Therm Anal Calorim 124:709–717
Sudeepan J, Kumar K, Barman TK, Sahoo P (2014) Study of mechanical and tribological properties of ABS/ZnO polymer composites. Procedia Mater Sci 6:391–400
Wacharawichanant S, Thongbunyoung N, Churdchoo P, Sookjai T (2010) Effect of zinc oxide on the morphology and mechanical properties of poly(styrene-co-acrylonitrile)/poly(methyl methacrylate)/zinc oxide composites. Sci J Ubu 1:21–26
Grigalovica A, Bochkov I, Merijs RMI, Zicans J, Grabis J, Kotsilkova R, Borovanska I (2012) The effect of nanosize ZnO on the properties of the selected polymer blend composites. In: IOP conf. series: materials science and engineering, vol 38, conference 1
Anzlovar A, Kogej K, Crnjak ZO, Zigon M (2011) Polyol mediated nano size zinc oxide and nanocomposites with poly(methyl methacrylate). Express Polym Lett 5:604–619
Tang E, Cheng G, Ma X (2006) Preparation of nano-ZnO/PMMA composite particles via grafting of the copolymer onto the surface of zinc oxide nanoparticles. Powder Technol 161:209–214
Hong RY, Qian JZ, Cao JX (2006) Synthesis and characterization of PMMA grafted ZnO nanoparticles. Powder Technol 163:160–168
Laachachi A, Ruch D, Addiego F, Ferriol M, Cochez M, Lopez C (2009) Effect of ZnO and organo-modified montmorillonite on thermal degradation of poly(methyl methacrylate) nanocomposites. Polym Degrad Stab 94:670–678
Matsuyama K, Maeda Y, Matsuda T, Okuyama T, Muto H (2015) Formation of poly(methyl methacrylate)-ZnO nanoparticle quantum dot composites by dispersion polymerization in supercritical CO. J Supercrit Fluids 103:83–89
Shim J, Kim J, Han S, Chang I, Kim H, Kang H, Lee O, Suh K (2002) Zinc oxide/polymethylmethacrylate composite microspheres by in situ suspension polymerization and their morphological study. Colloid Surf A207:105–111
Manzi-Nshuti C, Hossenlopp JM, Wilkie CA (2008) Fire retardancy of melamine and zinc aluminum layered double hydroxide in poly(methyl methacrylate). Polym Degrad Stab 93:1855–1863
Venkatesham V, Madhu GM, Satyanarayana SV, Preetham HS (2013) Adsorption of lead on gel combustion derived nano ZnO. Procedia Eng 51:308–313
Deepthi MV, Madan S, Sailaja RRN, Anantha P, Sampathkumaran P, Seetharamu S (2010) Mechanical and thermal characteristics of high density polyethylene–fly ash cenospheres composites. Mater Des 31:2051–2060
Babrauskas V, Hasemi Y (1993) Ten years of heat release research with the cone calorimeter in heat release and fire hazard, III-1. Building Research Institute, Tsukuba
Wang X, Xing WY, Zhang P, Song L, Yang HY, Hu Y (2012) Covalent functionalization of graphene with organosilane and its use as a reinforcement in epoxy composites. Compos Sci Technol 72:737–743
Ma J, Meng QS, Zaman I, Zhu SM, Michelmore A, Kawashima N, Wang CH, Kuan HC (2014) Development of polymer composites using modified, high-structural integrity graphene platelets. Compos Sci Technol 91:82–90
Kulyk B, Kapustianyk V, Tsybulskyy V, Krupka O, Sahraoui B (2010) Optical properties of ZnO/PMMA nanocomposite films. J Alloys Compd 502:24–27
Joint Committee on Powder Diffraction Standards, Powder Diffraction File, Card no: 36-1451
Ge J, Zeng X, Tao X, Li X, Shen Z, Yun J, Chen J (2010) Preparation and characterization of PS-PMMA/ZnO nanocomposite films with novel properties of high transparency and UV-shielding capacity. J Appl Polym Sci 118:1507–1512
Petinakis E, Yu L, Edward G, Dean K, Liu H, Scully AD (2009) Effect of matrix–particle interfacial adhesion on the mechanical properties of poly(lactic acid)/wood-flour micro-composites. J Polym Environ 17:83–94
Dorigato A, Regoretti A (2012) Fracture behaviour of LDPE–fumed silica nanocomposites. Eng Fract Mech 79:213–224
Jassim AN, Alwan RM, Kadhim QA, Nsaif AA (2016) Preparation and characterization of ZnO/polystyrene nanocomposite films using ultrasound irradiation. J Nano Sci Nanotechnol 6:17–23
Farzi GA, Tayebee R, Naghibinasab S (2015) Surface modification of ZnO nano-particles with trimetoxyvinyl silane and oleic acid and studying their dispersion in organic media. Int J Nano Dimens 6:67–75
Lee T, Jeong YG (2014) Enhanced electrical conductivity, mechanical modulus, and thermal stability of immiscible polylactide/polypropylene blends by the selective localization of multi-walled carbon nanotubes. Compos Sci Technol 103:78–84
Gaurang P, Sureshkumar MB, Purvi P (2012) Effect of TiO2 on optical properties of PMMA: an optical characterization. Adv Mater Res 383:3249–3256
Madras G, Smith JM, McCoy BJ (1996) Degradation of poly(methyl methacrylate) in solution. Ind Eng Chem Res 35:1795–1800
Teng H, Koike K, Zhou D, Satoh Z, Koike Y, Okamoto Y (2009) High glass transition temperatures of poly(methyl methacrylate) prepared by free radical initiators. J Polym Sci Part A Polym Chem 47:315–317
Chapala PP, Bermeshev MV, Starannikova LE, Shantarovich VP, Gavrilova NN, Yampolskii Y, Finkelshtein E (2015) Gas-transport properties of novel composites based on addition poly(3-trimethylsilyltricyclononene-7) and substituted cyclodextrines. Polym Compos 36:1029–1038
Filipczak R, Crowley S, Lyon RE (2005) Heat release rate measurements of thin samples in the OSU apparatus and the cone calorimeter. Fire Saf J 40:628–645
Price D, Pyrah K, Hull TR, Milnes GJ, Ebdon JR, Hunt BJ, Joseph P, Konkel CS (2001) Flame retarding poly(methyl methacrylate) with phosphorus containing compounds: comparison of an additive with a reactive approach. Polym Degrad Stab 74:441–447
Sun QF, Lu Y, Xia YZ, Yang DJ, Li J, Liu YX (2012) Flame retardancy of wood treated by TiO2/ZnO coating. Surf Eng 28:555–559
Friederich B, Laachachi A, Ferriol M, Cochez M, Sonnier R, Toniazzo V, Ruch D (2012) Investigation of fire-resistance mechanisms of the ternary system (APP/MPP/TiO2) in PMMA. Polym Degrad Stab 97:2154–2161
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, M.A., Madhu, G.M. & Sailaja, R.R.N. Reinforcement of polymethyl methacrylate with silane-treated zinc oxide nanoparticles: fire retardancy, electrical and mechanical properties. Iran Polym J 26, 765–773 (2017). https://doi.org/10.1007/s13726-017-0562-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-017-0562-9