Abstract
This study has examined the toughening of dicyandiamide-cured diglycidyl ether of bisphenol-A (DGEBA)-based epoxy resin with a flexible diamine. Eight different formulations were prepared by mixing DGEBA resin with a mixture of two curing agents of dicyandiamide (Dicy) and flexible polyoxypropylene diamine (Jeffamine D-400) in the presence and the absence of Monuron as an accelerator. The effect of curing agents on the curing behavior of epoxy systems was studied using differential scanning calorimetry (DSC). The fracture surfaces of the tensile test samples were examined by scanning electron microscopy (SEM). The DSC results showed that the reaction between Jeffamine and epoxy resin occurred within a much wider range of temperatures than the reaction of Dicy in the presence of accelerator. The tensile strength of the modified epoxies was much higher than the tensile strength of samples cured by one curing agent alone, due to the synergistic effect of the former. The tensile modulus of all samples was approximately equal and the Izod notched impact strength remained fairly constant up to 60 % Jeffamine and then increased beyond this amount. The fracture toughness increased as the Jeffamine content increased. All samples containing Jeffamine showed greater elongation-at-break. This behavior was a confirmation that Jeffamine increased the flexibility and toughness of Dicy-cured epoxy resin, even at 20 % Jeffamine content. The glass transition temperature (T g) for epoxy resin containing 100 % Dicy (116 °C) was higher than that containing 100 % Jeffamine (73 °C). SEM observation showed a homogeneous phase with no phase separation and that the crack area increased as the content of the flexible curing agent increased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are one of the most important classes of thermosetting polymers. These resins are used as surface coatings, adhesives and engineering composites, and in construction, automotive, and aerospace industries. They possess numerous attractive properties, including high chemical and corrosion resistance, good mechanical and thermal properties, outstanding adhesion to various substrates, low shrinkage upon curing, good electrical insulating properties, and the ability to be processed under a variety of conditions [1–3]. Their brittleness and poor resistance to crack growth, however, are major drawbacks, which significantly limit their use in applications requiring high impact and fracture strength. The inherent brittleness of cured epoxy resins can be decreased by increasing the fracture toughness of the epoxy systems with minimum sacrifice of their thermo-mechanical properties [4, 5].
Various approaches have been used to enhance the toughness of epoxy resins, including chemical modification of the epoxy backbone, lowering the cross-link density of the matrix by increasing the molecular weight of the epoxy monomers, decreasing the functionality of the curing agents, and incorporation of a dispersed toughener phase in the cured epoxy matrix or inorganic fillers into the neat resin [6, 7]. The second phase includes liquid rubber toughening agents like carboxyl, amine or epoxy-terminated butadiene-acrylonitrile rubber (CTBN, ATBN, ETBN) [8–10], thermoplastic modifiers such as polysulphones [11, 12], poly(ether imide)s [13], poly(ether sulphone)s [14, 15], polysiloxanes [16–18] and polyethylene glycol [19] and inorganic fillers/reinforcements such as silica, alumina trihydrate, zinc oxide, calcium carbonate, graphite powder, asbestos powder, and nanoparticles (carbon nanotubes) [20–22].
It is well known that a rubbery phase significantly decreases the thermo-mechanical properties in epoxy systems because of the presence of rubber particles or thermoplastic polymer. Increasing toughness by incorporating rigid particle fillers is less successful than incorporation of rubber-modified epoxy. Processing constraints and high application viscosity are significant limitations to the incorporation of rubber, thermoplastics and rigid particles into epoxy resins [23].
Toughening of epoxy resins can also be accomplished by adding flexibilizers. Jeffamine is a flexible polyether diamine that is extensively used as a hardener for the preparation of tough epoxy resins. The branched structure of Jeffamine, with its high reactivity and low viscosity, can effectively increase the toughness of epoxy resins [24]. Toughening of epoxy resins using Jeffamine to increase crack resistance has been a subject of intense research. Nograro et al. [25] studied the influence of the mixture of an aromatic amine (m-phenylenediamine, mPDA) and an amine terminated poly(propylene oxide) with Jeffamine D230 and T403 on the dynamic and mechanical properties of epoxy resin as a function of mPDA content. They observed that the toughness of the mixtures increased as the aliphatic amine content increased.
Shan et al. [26] studied the effect of three Jeffamines (D-230, D-400, and D-2000) as curing agents for epoxy resin (Epon 828). Their results showed that the difference in cross-link density of the resins was based on different molecular weights of the Jeffamines. The glass transition temperatures (T g) varied from −38 to 80 °C. The tensile strength and strain-at-break of the samples decreased as the cross-link density increased.
Shi et al. [24] studied carbon nanotubes (CNTs) grafted with Jeffamine T-403 to increase the reinforcement and toughening of an epoxy resin (DGEBA, EPON 828). The results showed that the introduction of amino-functionalized MWNTs to epoxy resin increased the tensile and flexural strength, toughness and T g of epoxy resin without sacrificing the impact strength.
Yang et al. [27] used two flexible diamines (D-230 and D-400) to modify diethyl toluene diamine (DETD)-cured diglycidyl ether of bisphenol-A (DGEBA) epoxy resin. They have studied mechanical behavior of the modified epoxy at cryogenic temperature (77 K) and room temperature (RT). The results showed that the elongation-at-break and strength at RT and 77 K increased with the addition of flexible diamines. The DSC results showed that the T g decreased as the flexible diamine contents increased.
The presence of a single T g indicated that the modified epoxy resin had a homogeneous phase structure. At 77 K, the Young’s modulus was higher than that at RT at a given content of diamine. The results showed that the addition of a specific amount of flexible diamine both strengthened and toughened DGEBA epoxy resins at room temperature and 77 K.
Flexible polyoxypropylene diamines (Jeffamine) can be simply incorporated into epoxy resins by a normal cure reaction between amine and epoxy [27]. Jeffamine as flexibilizer increases the toughness, but decreases the thermal stability of the epoxy resin because of deformation at high temperatures, which has adverse effects such as decreases in T g and Young’s modulus. One method to overcome this problem is the addition of another curing agent to the epoxy system having high thermal stability.
The curing agents are chosen based on the required physical and chemical properties, processing methods and curing conditions. Curing agents can influence the curing rate, curing chemistry, cross-link density, morphology, and affect the fracture toughness of modified epoxy resins. Dicyandiamide (Dicy) is widely used as a latent curing agent in heat-cured epoxy resins [3, 28, 29] and Dicy-cured DGEBA-based epoxy resins have wide applications, particularly in prepregs and adhesives.
It is necessary to use an accelerator to decrease the curing temperature of Dicy [30]. The curing of epoxy resin using Dicy results in a brittle structure, although no study has so far focused on decreasing the brittleness using flexible polyether diamine. In this work, the simultaneous curing of an epoxy system with two curing agents was studied. To the best of our knowledge, no systematic work has yet been reported on the effect of the simultaneous use of mixtures of the two curing agents on the curing behavior, mechanical properties, and fracture toughness of epoxy resins. We used a flexible diamine to cure epoxy resin as modifying and curing reagent of epoxy system.
The present study examined ways to toughen the epoxy resin using a mixture of the curing agents, Dicy and Jeffamine. Their effect on the mechanical and thermal properties of cured epoxy resin was studied. Different formulations of epoxy resin were prepared by varying the curing agent ratio (Jeffamine/Dicy) with and without accelerator. Curing characteristics, mechanical properties, fracture toughness, and dynamic mechanical properties of cured epoxy resin and microstructures of the fractured surfaces were also analyzed.
Experimental
Materials
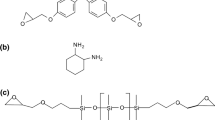
The epoxy resin used was a diglycidyl ether of bisphenol-A (DGEBA; Epikote 828, Momentive, USA) with an epoxy group content of 5.34 mmol/g. Dicyandiamide (Dicy) curing agent was supplied by Merck (Germany). Monuron (3-(4-chlorophenyl)-1,1-dimethylurea; purity 99 %) was used as received as an accelerator (Aldrich, UK). The flexible polyoxypropylene diamine, Jeffamine®D-400 (M W = 400) was obtained from Huntsman. The chemical structures of the epoxy resin, curing agents and accelerator are shown in Scheme 1.
Preparation of samples
To evaluate the effect of two curing agents on DGEBA epoxy resin, eight formulations were prepared (Table 1). Two curing agents were mixed with epoxy resin as per calculated stoichiometric ratios. As Table 1 shows, sample 1 contained only epoxy resin and Jeffamine. To prepare this sample, 100 phr of epoxy resin and 56 phr of Jeffamine were mixed for 10 min at 40 °C. No accelerator was added for this sample. Sample 2 was prepared similarly, but 2.5 phr of Monuron was added as an accelerator. Sample 8 contained epoxy resin, Dicy and Monuron without Jeffamine. Dicy curing agent needed an accelerator to cure at 120 °C [31].
Samples 3 to 7 contained epoxy resin, a mixture of the two curing agents and Monuron as an accelerator. To prepare these samples, 100 phr of the epoxy resin was first preheated at 70 °C and a specific amount of Dicy was added. The Dicy particles were well dispersed using a homogenizer and the mixture was stirred mechanically until its viscosity increased slightly to prevent sedimentation of Dicy. The mixture cooled then to 40 °C and specified amounts of Jeffamine and Monuron were added under mechanical stirring for 10 min until homogeneous mixtures were obtained.
Curing
All the samples were cured in a similar way. First, they were degassed in a vacuum oven for 10 min. The degassed resin mixtures were then poured into a preheated 80 °C waxed metal mold. The mold was placed into an oven at 80 °C for 2 h and followed by 3 h at 120 °C for full curing.
Characterization and instrumentation
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was used to investigate the curing behavior of the samples using a NETZSCH DSC 200F3 calorimeter (Germany). The specified amount of epoxy resin and curing agent mixture was placed in a shallow aluminum pan in the calorimeter. The measurements were obtained under nitrogen atmosphere at a heating rate of 10 °C/min from room temperature to 300 °C.
Tensile and impact tests
The tensile properties of dumbbell-shaped cured samples were measured using a universal testing machine (UTM, 150 kN, Santam, Iran). Tests were carried out according to ASTM D 638 standards. The crosshead speed of the machine was kept at 5 mm/min. The average of five specimens was recorded.
The Izod notched impact method was used for impact testing according to ASTM D256 standard using a Zwick impact tester (model 5102). The dimensions of the rectangular specimens were 63.5 × 12.7 × 3.5 mm3. The average of at least three specimens was recorded.
Fracture toughness test
Fracture toughness was measured using the critical stress intensity factor (K IC) in 3-point single-edge notched bending specimens (SENB) according to ASTM D5045 standard. Tests were performed using a UTM with crosshead speed of 10 mm/min. The dimensions of the rectangular specimens were 56.4 × 12.7 × 6.8 mm3. An initial crack (6.5 mm) was machined into the specimens and a natural crack was then generated by tapping on a fresh razor blade placed in the notch. K IC, was calculated as:
where P Q as the critical load for crack propagation (kN), B as the thickness of the specimen (cm); W the width of the specimen (cm); and f(x) as the non-dimensional shape factor:
where x = a/W and a, taken as the crack length (cm).
Dynamic mechanical thermal analysis
The storage modulus, loss modulus, and T g were obtained by dynamic mechanical thermal analysis (DMTA) using a Tritec 2000B. A controlled sinusoidal strain was applied to a sample and the resulting stress was determined. Dynamic mechanical spectra were determined at −100 to 200 °C at a heating rate of 5 °C/min and a constant frequency of 1.0 Hz. The dimensions of the samples were 13 × 10 × 1.5 mm3.
Scanning electron microscopy
The fracture surfaces of the tensile test samples were investigated by scanning electron microscopy (SEM) using a VEGA model SEM (Tescan) at a filament voltage of 20 kV. The surfaces of the fractured samples were sputter-coated with a thin layer of gold to provide a conductive surface for SEM.
Results and discussion
Curing process
Epoxy resins can react with curing agents at room temperature, but heat is often applied to accelerate and improve curing [32]. The type of curing agent used affects curing and cross-linking of the epoxy resins and their thermal behavior. Accelerators are usually used to decrease the temperature required by curing agents such as Dicy [31].
The present study used a mixture of Jeffamine and Dicy. Dicy is widely used as a curing agent in heat-cured epoxy resins. The effect of curing agents on the behavior of epoxy systems was studied by DSC. The results for the neat Dicy-cured epoxy sample (100 % Dicy, EJ0D100 M), the neat Jeffamine-cured epoxy sample (100 % Jeffamine, EJ100D0 M) and the sample containing 50 % of each curing agent (EJ50D50 M) are shown in Fig. 1. The curing characteristics of these formulations are summarized in Table 2. The curing data for the sample containing 100 % Jeffamine without accelerator (EJ100) are also shown in Table 2.
Figure 1 shows that the reaction between Jeffamine and epoxy resin in the presence of accelerator occurs across a much wider range of temperatures than what it is required for the Dicy curing agent. This indicates that much longer time is needed to cure the Jeffamine-epoxy sample, although the peak curing temperature of Dicy is much higher than that of Jeffamine. The area under the DSC curve for the neat Jeffamine-cured epoxy sample is higher than that for the neat Dicy-cured epoxy sample, indicating that more heat has been released during curing of epoxy systems containing Jeffamine.
Figure 1 shows two peaks for the DSC curve of the sample containing a mixture of the two curing agents. The peak 1 can be attributed to Jeffamine and peak 2 to Dicy. Figure 1 and Table 2 indicate that the peak temperature of epoxy resin cured by Jeffamine only is about 128 °C and that cured by Dicy only is about 143 °C. The mixture with 50 % Jeffamine and 50 % Dicy (EJ50D50 M) showed two peaks at around 116 and 146 °C.
With just using Jeffamine, the maximum curing temperature is higher than when the mixture is used. When Dicy is used on its own, it displays a lower maximum curing temperature than that of the mixture. In three samples EJ100D0 M, EJ50D50 M, and EJ0D100 M, the accelerator content was constant and equal to 2.5 phr, while the amount of curing agents was changed. Since the Jeffamine/accelerator ratio in a neat Jeffamine-cured epoxy sample (EJ100D0 M) is higher than that in the sample containing 50 % Jeffamine (EJ50D50 M), the maximum curing temperature for Jeffamine occurs at lower temperatures. In other words, Jeffamine uses more accelerator than does Dicy. This decreases the maximum curing temperature for Jeffamine and increases the maximum curing temperature for Dicy.
Figure 1 shows an endothermic peak at about 209 °C. This is related to the melting point of Dicy and also indicates that Dicy has not been completely consumed and some remained in the reaction mixture [33]. It should be noted that, if DSC were heated at a heating rate <10 °C/min, this peak would likely disappear because there would be sufficient time to complete the curing reaction.
Effect of Monuron on curing behavior of epoxy/Jeffamine
An accelerator is required to cure epoxy resins containing Dicy; thus, the effect of Monuron accelerator on the curing behavior of epoxy resin/Jeffamine was investigated. DSC results for neat Jeffamine-cured epoxy samples with accelerator (EJ100D0 M) and without accelerator (EJ100) were evaluated (Fig. 2; Table 2). When only Jeffamine curing agent was used to cure epoxy resin, the maximum curing temperature was 139.7 °C; when Monuron was added, the maximum curing temperature decreased to 128 °C. The initial and final curing temperatures shifted downward from 206.8 to 182 °C. This indicated that Monuron has affected the curing behavior of Jeffamine.
This behavior can be attributed to Monuron as a tertiary amine that catalyzes the reaction between Jeffamine and epoxy resin and decreases the maximum curing temperature [3]. Figure 2 and Table 2 show that the addition of accelerator did not significantly alter the initial curing temperature, which decreased from 96.2 to 93.7 °C, but significantly decreased the end curing temperature by about 25 °C. This decease can also be observed in the decrease in the temperature of the reaction for EJ100D0 M over that for EJ100.
Mechanical properties
Figure 3 shows the stress-strain curves of the samples containing 100 % Dicy (EJ0D100 M) and 100 % Jeffamine (EJ100D0 M). As shown, the sole use of Dicy as a curing agent increased the brittleness of the epoxy sample; the stress-strain curve for this sample is a straight line while the stress-strain curve for the sample containing 100 % Jeffamine shows significant curvature. The high elongation-at-break value for the Jeffamine sample also confirms that this sample is flexible. This behavior has been due to the introduction of flexible polyether groups of Jeffamine into the epoxy resin backbone, which has effectively decreased the brittleness of the cured epoxy resin [34].
The stress-strain curves for neat Jeffamine epoxy in the presence (EJ100D0 M) and absence of accelerator (EJ00) are compared in Fig. 4. As seen, the two samples show similar behavior up to strains of 1.5 % (40 MPa). After this point, they exhibit different behavior and EJ100D0 M shows higher strain and deflects faster than EJ00.
The average values for elongation-at-break, tensile strength, tensile modulus, and toughness (area under the stress-strain curve) are plotted as a function of Jeffamine content in Figs. 5, 6, 7, and 8, respectively. Figure 5 shows the variation of elongation-at-break versus Jeffamine content for all epoxy resins. As shown, all epoxy resins containing Jeffamine exhibit higher elongation-at-break values than the sample without Jeffamine (0 %). This behavior confirms that Jeffamine curing agent increases the toughness of Dicy-cured epoxy resin, even at 20 % Jeffamine.
Figure 5 shows that 20 % Jeffamine has increased elongation-at-break considerably. This behavior may be related to the result of the reaction between curing agents with epoxy resin. The reaction between epoxy resin and Jeffamine has formed flexible and longer bond lengths than the reaction between epoxy resin and Dicy. Short and rigid bonds are formed from the reaction between epoxy resin and Dicy. Increasing the percentage of Jeffamine has altered this trend; as Jeffamine content has increased, elongation-at-break has remained almost constant.
Figure 4 shows the effect of accelerator on elongation-at-break for epoxy resin/Jeffamine. As shown, elongation-at-break in the presence of accelerator (4.24 ± 0.92 %) has greater than in the absence of accelerator (3.15 ± 0.20 %). This behavior can be ascribed to the formation of intermolecular bond lengths in the epoxy resin. The addition of accelerator has led to formation of tertiary amine as a catalyst, accelerating the reaction between epoxy resin and Jeffamine. The intermolecular bonds in the epoxy resin have increased because the accelerator has provided more oxirane rings for curing, increasing the reaction rate between the epoxy resin and Jeffamine. The increase in the number of intermolecular bonds has produced samples with acceptable strength; however, Jeffamine must have been settled between the epoxy resin molecules, increasing the flexibility. This has produced both acceptable strength and good flexibility, which in turn increases the elongation-at-break.
Figure 6 shows the variation in tensile strength versus Jeffamine content for all modified epoxy resins. As seen, low percentages of Jeffamine have increased the tensile strength considerably and the effect has been intensified as the Jeffamine content is increased. At high Jeffamine content, however, tensile strength is decreased as the Jeffamine content is increased. It should be noted that the tensile strength of all cured epoxy resins is higher than that of neat Dicy-cured or neat Jeffamine-cured epoxy. This indicates a synergistic effect from the curing reaction of these two agents. It can be inferred that a combined curing agents has increased tensile strength. Figure 4 shows the effect of accelerator on tensile strength for epoxy resin/Jeffamine. The results show that tensile strength is deceased from 56.64 ± 0.48 to 49.21 ± 3.9 MPa with the addition of an accelerator.
Figure 7 shows the effect of Jeffamine on the tensile modulus of all samples. All samples show similar values for tensile modulus; this probably has been due to similar tensile modulus values for the boundary samples containing 100 % Dicy (EJ0D100 M) and 100 % Jeffamine (EJ100D0 M). The tensile modulus is 2892 ± 129 MPa for EJ0D100 M and 2643 ± 172 MPa for EJ100D0 M. These values are approximately equal in significance; the mixture of curing agents, thus, also produces similar values for tensile modulus.
Figure 4 shows that the tensile modulus of epoxy resin/Jeffamine system remains almost constant with the addition of accelerator. This means that the accelerator has had no significant effect on the tensile modulus of this system.
Figure 8 shows the area under the stress-strain curve, which is a measure of toughness. All samples containing Jeffamine give higher toughness values than a neat Dicy-cured epoxy. This indicates that the addition of small amounts of Jeffamine has considerably increased toughness. The trend has been similar to that for elongation-at-break (Fig. 5) for similar reasons.
Figure 4 shows the effect of accelerator on toughness of the epoxy resin/Jeffamine system. The results show that the toughness has increased from 58.24 ± 6.79 to 85.51 ± 15.52 kJ/m2 with the addition of accelerator. The reason for this decline is similar to that for elongation-at-break.
Figure 9 shows the notched Izod impact strength for modified epoxy samples as a function of Jeffamine content. As shown, impact strength has remained constant as the Jeffamine content is increased to 60 %; beyond this point, it is increased significantly. It is well known that the type and amount of curing agent affects the impact properties of epoxy resin. Dicy has formed short and weak bond lengths with epoxy resin molecules and produced low impact strength. Jeffamine has formed long bond lengths and the presence of ether groups in its structure has increased the impact strength of epoxy resin. The main reason for the constant impact strength up to 60 % Jeffamine content is the dominant role of Dicy in the cross-linking reaction of epoxy resin.
In samples containing 0, 20, 4, 50, and 60 % Jeffamine, the cross-link network and the impact strength are controlled by Dicy. At high percentages of Jeffamine (80 and 100 %), however, Dicy is not able to adjust the impact strength of the samples. In the sample containing 80 % Jeffamine, the Jeffamine content is high enough to increase the flexibility of the epoxy resin and slightly increases the impact strength of the sample. The neat Jeffamine-cured epoxy resin has the highest impact strength at 100 % Jeffamine because of its flexibility.
Impact strength can be used to evaluate the effect of accelerator on Jeffamine. The results show that the addition of accelerator to the epoxy resin/Jeffamine system decreases impact strength from 120.63 ± 15.87 to 21.66 ± 8.71 J/m (about sixfold). This decrease can be attributed to the short intermolecular bond lengths in the epoxy network due to the presence of accelerator. The reaction between Monuron accelerator and epoxy resin and formation of a tertiary amine, as discussed above, has accelerated the curing process between the epoxy resin and Jeffamine. The increase in curing rate has decreased the intermolecular bond lengths and decreased the impact strength.
Figure 10 shows the variation in fracture toughness (K IC) as a function of Jeffamine content. As shown, K IC is continuously increased as Jeffamine content is increased in the epoxy resins. K IC for all samples containing Jeffamine is higher than in a neat Dicy-cured epoxy resin. This demonstrates the poor crack resistance of the neat Dicy-cured epoxy resin and the high crack resistance of the samples cured by Jeffamine. As discussed before, Jeffamine leads to the formation of long bonds in the epoxy resin. As the content is increased the percentage of long bonds, the K IC of samples is also increased. Another reason for this trend is the presence of ether linkages in the Jeffamine backbone; the addition of the Jeffamine to epoxy resin has increased the toughness of samples. The toughness of samples has been increased as the Jeffamine content is increased. If we compare the data presented in Figs. 8, 9 and 10 we can evaluate the toughness of epoxy resin using the area under the tensile curves, Izod impact strength and K IC. It can be seen that there are good agreements between these data particularly for the trends in increased Izod impact strength and K IC. It is worth mentioning that the data of fracture toughness, K IC, which show the resistance of a material for crack propagation, is the best parameter for the evaluation of toughness.
Viscoelastic behavior
Figure 11 shows dynamic mechanical loss factor (tan δ) of the neat Dicy- and Jeffamine-cured epoxy resins and the sample containing 50 % of each curing agent as function of temperature. The glass transition temperatures (T g; the maximum value of the tan δ curve) of these samples are given in Table 3. Figure 11 shows that tan δ is decreased as the Jeffamine content is decreased. The high loss factor and damping is another reason for the toughening of epoxy resin with the increase in Jeffamine. Figure 11 and Table 3 indicate that the neat Dicy-cured epoxy resin has higher T g values than the neat Jeffamine-cured epoxy resin. This behavior can be attributed to the formation of long bonds in the epoxy resin and the presence of ether groups in the Jeffamine curing agent backbone. T g for the sample containing 50 % of each curing agent (EJ50D50 M) falls between the T g for neat Dicy-cured (EJ0D100 M) and that for neat Jeffamine-cured epoxy resin (EJ100D0 M). It is interesting to note that the T g values follow the rule of mixtures where the T g for a sample containing a mixture of two curing agents can be calculated as:
where W Dicy is the percentage of Dicy (wt); and W Jeffamine is the percentage of Jeffamine (wt). Equation (3) produces a T g of 94.6 °C for sample EJ50D50 M; the T g value obtained from dynamical-mechanical analysis of this sample (Table 3) is 94.4 °C, which are similar. T g for the sample containing 20 % Jeffamine has been calculated to be 107.5 °C.
Figure 12 and Table 3 can be used to investigate the effect of accelerator on tan δ and T g for the epoxy resin/Jeffamine system. As shown, tan δ has increased slightly and T g has increased by about 7.9 °C with the addition of accelerator. This is likely the result of greater cross-linking density.
Figure 13 shows the variation in the storage modulus (E′) of the neat Dicy- and Jeffamine-cured epoxy resins and the sample containing 50 % of each curing agent as a function of temperature. As shown, the three samples show similar trends with only one drop in storage modulus. Because T g for neat Jeffamine-cured epoxy resin is lower than for the other samples, where the onset of decrease in the storage modulus for the sample containing 100 % Jeffamine is faster. Figure 13 also indicates that the decrease in storage modulus for the neat Jeffamine-cured sample is higher than for the neat Dicy-cured sample, which is probably the result of the long bonds formed by Jeffamine that further degrades the curing agent and decreases the storage modulus. The higher storage modulus in the rubbery region of the Dicy-cured sample indicates the higher cross-linking density of this sample.
Figure 14 shows that the presence of Monuron accelerator has no significant effect on the storage modulus of the epoxy/Jeffamine system. The slight increase in initial storage modulus with the addition of accelerator is a result of the higher cross-linking density of the epoxy system containing accelerator.
Morphology
The microscopic structure of the samples after curing has been investigated using SEM. The fracture surfaces of the tensile samples are shown in Fig. 15 based on the increase in Jeffamine content. It can be clearly seen that the fracture surface of an unmodified epoxy resin is relatively rough and glassy, showing a typical brittle fracture mode, and the crack area has increased as the flexible curing agent content has been increased in the epoxy system. The increase in crack area of the modified epoxy resins indicates that greater energy is required to initiate fracture. It can be concluded that the epoxy resins modified with Jeffamine have higher tensile strength and toughness. Further, the morphology of the fracture surface shows a homogeneous mixture with no phase separation for epoxy resin. The transparency of all modified samples is another reason for the homogeneity of these samples.
Conclusion
Toughening Dicy-cured DGEBA-based epoxy resin with flexible diamine was investigated in the present study. Different formulations were prepared by mixing DGEBA with different percentages of the two curing agents and by varying their ratios in the presence or absence of Monuron as an accelerator. DSC results indicate that the reaction of Jeffamine with epoxy resin occurs across a wider span of temperature than the reaction of Dicy in the presence of accelerator.
The T g for neat Dicy-cured epoxy resin (116 °C) is higher than that for the neat Jeffamine-cured epoxy resin (73 °C). All samples containing Jeffamine show higher elongation-at-break than the sample without Jeffamine. The stress-strain curve for the neat Jeffamine-cured epoxy sample is not a straight line and the high elongation-at-break indicates that the sample is tough. This behavior can be attributed to the introduction of flexible polyether groups of Jeffamine into the epoxy resin backbone that has decreased the brittleness of the modified epoxy resin effectively. These results confirm that Jeffamine curing agent can increase the toughness of epoxy resin, even in small amounts.
The tensile strength of the modified epoxies has much higher value than the tensile strength of samples cured by one curing agent alone, pointing to a synergistic effect. SEM results show a homogeneous phase with no phase separation and an increase in crack area with an increase in flexible curing agent content.
References
Lee H, Neville K (1967) Handbook of epoxy resins, Ch. 5. McGraw Hill, New York
May CA (1988) Epoxy resins chemistry and technology, Ch. 5, 2nd edn. Marcel Dekker, New York
Hamerton I (1996) Recent developments in epoxy resins. Rapra Review Reports, Shawbury, vol 8
Potter WG (1975) Uses of epoxy resins, Ch. 2. Newnes-Butterworths, London
Salamon JC (1996) Polymeric materials encyclopedia. CRC, Boca Raton, pp 2233–2252
Ratna D, Banthia AK (2004) Rubber toughened epoxy. Macromol Res 12:11–21
Ikram S, Munir A (2012) Mechanical and thermal properties of chemically modified epoxy resin. Open J Synth Theory Appl 1:36–43
Akbari R, Beheshty MH, Shervin M (2013) Toughening of dicyandiamide-cured DGEBA-based epoxy resins by CTBN liquid rubber. Iran Polym J 22:313–324
Hwang JF, Manson JA, Hertzberg RW, Miller GA, Sperling LH (1989) Structure-property relationship in rubber-toughened epoxies. Polym Eng Sci 29:1466–1476
Saadati P, Baharvand H, Rahimi A, Morshedian J (2005) Effect of modified liquid rubber on increasing toughness of epoxy resins. Iran Polym J 14:637–646
Martinez I, Martin MD, Eceiza A, Oyanguren P, Mondragon I (2000) Phase separation in polysulfone-modified epoxy mixtures: relationships between curing conditions, morphology and ultimate behavior. Polymer 41:1027–1035
Giannotti MI, Bernal CR, Oyanguren PA, Galante MJ (2005) Morphology and fracture properties relationship of epoxy-diamine systems simultaneously modified with polysulfone and poly(ether imide). Polym Eng Sci 45:1312–1318
Li H, Gan W, Zhao L, Li S (2003) Studies on the phase separation of a polyetherimide modified epoxy resin. VI. Effect of surface energy on reaction-induced phase separation of epoxy resin modified with polyetherimide. J Macromol Sci Part A Pure Appl Chem A40:833–846
Andrés MA, Garmendia J, Valea A, Eceiza A, Mondragon I (1998) Fracture toughness of epoxy resins modified with polyethersulfone: influence of stoichiometry on the morphology of the mixtures. J Appl Polym Sci 69:183–191
Grishchuk S, Gryshchuk O, Weber M, Karger-Kocsis J (2012) Structure and toughness of polyethersulfone (PESU)-modified anhydride-cured tetrafunctional epoxy resin: effect of PESU molecular mass. J Appl Polym Sci 123:1193–1200
Ma SQ, Liu WQ, Hu CH, Wang ZF (2010) Modification of epoxy resins with polyether-g-polysiloxanes. Iran Polym J 19:185–196
Tong JD, Bai RK, Zou YF, Pan CY, Ichimura S (1994) Flexibility improvement of epoxy-resin by using polysiloxanes and their derivatives. J Appl Polym Sci 52:1373–1381
Zhao F, Sun QC, Fang DP, Yao KD (2000) Preparation and properties of polydimethylsiloxane-modified epoxy resins. J Appl Polym Sci 76:1683–1690
Mahnam N, Beheshty MH, Barmar M, Shervin M (2013) Modification of dicyandiamide-cured epoxy resin with different molecular weight of polyethylene glycol and its effect on epoxy/glass prepreg characteristic. High Perform Polym 25:705–713
Sprenger S (2013) Epoxy resins modified with elastomers and surface-modified silica nanoparticles. Polymer 54:4790–4797
Lee J, Yee AF (2000) Fracture of glass bead/epoxy composites: on micro-mechanical deformations. Polymer 41:8363–8373
Sahoo NG, Rana S, Cho JW, Chan SH, Li L (2010) Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci 35:837–867
Rahman MM, Hosur M, Zainuddin S, Jajam KC, Tippur HV, Jeelani S (2012) Mechanical characterization of epoxy composites modified with reactive polyol diluents and randomly-oriented amino-functionalized MWCNTs. Polym Test 31:1083–1093
Shi LF, Li G, Sui G, Yang XP (2009) Preparation and mechanical properties of epoxy resin reinforced with Jeffamine-grafted carbon nanotubes. Adv Mater Res 79–82:553–556
de Nograro FF, Llano-Ponte R, Mondragon I (1996) Dynamic and mechanical properties of epoxy networks obtained with PPO based amines/mPDA mixed curing agents. Polymer 37:1589–1600
Shan L, Verghese KNE, Robertson CG, Reifsnider KL (1999) Effect of network structure of epoxy DGEBA-poly(oxypropylene) diamines on tensile behavior. J Polym Sci, Part B: Polym Phys 37:2815–2819
Yang G, Fu SY, Yang JP (2007) Preparation and mechanical properties of modified epoxy resins with flexible diamines. Polymer 48:302–310
Fedtke M, Domaratius F, Walter K, Pfitzmann A (1993) Curing of epoxy resins with dicyandiamide. Polym Bull 31:429–435
Fedtke M, Domaratius F, Pfitzmann A (1990) Curing of epoxy resins with dicyandiamide. Polym Bull 23:381–388
Pfitzmann A, Schlothauer K, Fedtke M (1991) Epoxy resin curing by dicyandiamide using model compounds. Polym Bull 27:59–66
Hayaty M, Honarkar H, Beheshty MH (2013) Curing behavior of dicyandiamide/epoxy system using different accelerators. Iran Polym J 22:591–598
Moshirnia M, Kokabi M, Moadel H (2004) Curing kinetics study of epoxy resin at nonisothermal conditions. Iran J Polym Sci Technol (Persian) 17:135–149
Wang Q, Storm BK, Houmoller LP (2003) Study of isothermal curing of an epoxy prepreg by near-infrared spectroscopy. J Appl Polym Sci 87:2295–2305
Burton B, Alexander D, Klein H, Garibay-Vasquez A, Pekarik A, Henkee C (2005) Epoxy formulations using Jeffamine® polyetheramines, Huntsman (report)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jamshidi, H., Akbari, R. & Beheshty, M.H. Toughening of dicyandiamide-cured DGEBA-based epoxy resins using flexible diamine. Iran Polym J 24, 399–410 (2015). https://doi.org/10.1007/s13726-015-0332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-015-0332-5