Abstract
Using epoxy-terminated hyperbranched polymer (E-HBP) to modify epoxy resin (EP) is an effective way to improve the toughness of EP. In the present study, two different epoxy resin systems with E-HBP are researched: a commercial diglycidyl ether bisphenol A (DGEBA) resin with anhydride as curing agent and a tetraglycidyl diaminodiphenyl methane (TGDDM) resin with diamine as curing agent. Characterization results show that the addition of E-HBP could improve the mechanical properties of the two epoxy resin systems, such as tensile strength, elongation, and modulus of elasticity. Meanwhile, the glass transition temperature (T g ) of the two systems does not decrease. However, the morphology of the tensile fracture surfaces of the two modified systems shows different behaviors. Significant plastic deformation could be observed in the fracture surfaces of the modified DGEBA/anhydride system, and particle cavitations are clearly shown in the fracture surfaces of the modified TGDDM/diamine systems. The analysis of the tensile fracture surfaces suggests that firstly E-HBP participates in the curing process of the modified resin systems, followed by the chemical-induced phase separation; finally, a gradient transition interface layer (GTIL) is formed. Apart from these, during the external loading process, the mechanical behaviors (deformation or cavitation) of the separated E-HBP particles in the modified epoxy resins are affected by the properties of the epoxy matrix itself.

A gradient transition interface layer (GTIL) between E-HBP and the epoxy matrix can significantly enhance their interfacial interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Most of epoxy resins have superior mechanical properties and excellent processibility, so that they are widely used in coatings, adhesives, molding compounds, and polymer composites. Epoxy resins are a kind of thermosetting material, while the application is often limited by the toughness properties which include impact resistance, fatigue behavior, damage tolerance, etc. Therefore, tougheners are often used in many epoxy resins, such as carboxyl-terminated butadiene acrylonitrile (CTBN), thermoplastic polymers, inorganic nanoparticles, etc. [1,2,3,4]. However, these approaches may seriously affect the modulus and thermal properties of the epoxy resins, while the toughness moderately increases. So, finding new tougheners with fine thermo-mechanical properties and good processing property is still a main task in the development of epoxy resins.
E-HBPs (epoxy-functionalized hyperbranched polymers) are a new kind of toughening, and its use as modifiers for epoxy resins is proposed by Boogh et al. [5]. They have been proven to have unique and promising characteristics, such as high functionality, high molecular weight, and low viscosity-to-molecular weight ratio. Moreover, they also have been proven to be a kind of relatively efficient toughener for epoxy resins [6], which attracted widespread attention in the toughening mechanisms of E-HBP-modified epoxy resins. The controlled chemically induced phase separation (CIPS), which has been reported elsewhere, is considered to be the key mechanism, significantly affecting the morphology of E-HBP-modified epoxy resins [7,8,9]. The other toughening mechanism observed in epoxy resins toughened by E-HBP is particle cavitation [10, 11], which needs CIPS to get the particles. Under the load conditions, the particles formed in the CIPS process can induce large stress concentrations, which lead to extensive shear deformation and a high energy absorption, thereby improving the toughness of the materials. Tests on E-HBP-toughened DGEBA-IPD (isophorone diamine) resin systems can clearly show the cavitated particles and extensive shear which is in the form of shear bands emanating from the cavitated particles [12]. However, whether the particle cavitation is necessary or not for the toughening mechanism in E-HBP-toughened epoxy resins is still unexplained, and the influencing factors of particle cavitation are not studied until now.
Based on commercial diglycidyl ether bisphenol A (DGEBA) resin with anhydride as curing agent system and tetraglycidyl diaminodiphenyl methane (TGDDM) resin with diamine as curing agent system, the mechanical behaviors of the phase separated particles in E-HBP-toughened epoxy resins and the influencing factors causing particle cavitation were studied in this paper.
2 Experiment
2.1 Materials
Diglycidyl ether bisphenol A (DGEBA, the epoxy equivalent weight of it is 196.1 g/equivalent) (E-51, Jiangsu Sanmu Chemical Co. Ltd., China), with methyl tetrahydrophthalic anhydride (MeTHPA, Zhejiang Qingming Chemical Co. Ltd., China) used as the cross-linking agent, was selected as one thermosetting resin. This resin had a glass transition temperature of 103.76 °C and a Young’s modulus of 2.74 GPa after cure. Tetraglycidyl diaminodiphenyl methane (TGDDM, Shanghai Institute of Synthetic Resins, China), with diaminodiphenyl sulfone (DDS, Shanghai Institute of Synthetic Resins, China) used as the cross-linking agent, was selected as the other thermosetting resin. This resin had a glass transition temperature of 196.6 °C and a Young’s modulus of 3.75 GPa after cure. The molecular structures of the above materials are all shown in Fig. 1.
A 3-generation epoxy-functionalized hyperbranched polyester was synthesized with dimethylolpropionic acid (DMPA) as monomer, trimethylolpropane (TMP) as initiator and epichlorohydrin as epoxidation reagent according to the patent of Petterson [13]. The synthesis diagram of the ideal structure of 3-generation E-HBP is shown in Fig. 2. This synthesized product was used as E-HBP modifier, with concentrations of 5, 10, and 20 parts per hundred parts of resin (phr). The epoxy equivalent weight (EEW, HBP molecular weight divided by the number of epoxy groups grafted onto its shell) of synthesized 3-generation E-HBP was 327 g/equivalent, as obtained directly by titration [14].
In order to compare with the traditional modification methods, a carboxyl-terminated butadiene acrylonitrile rubber (CTBN, Lanzhou Petrochemical Co., China) with acrylonitrile content of 25% and molecular weight of 2500 and a poly(ether sulfones) (PES, BASF Corp., Germany) were used as the modifiers to prepare the modified DGEBA/MeTHPA resins and TGDDM/DDS resins, respectively.
2.2 Procedures
The samples of DGEBA/MeTHPA resins containing E-HBP were prepared by direct mixing of DGEBA, MeTHPA, and E-HBP according to the equivalent stoichiometric ratio of anhydride-to-epoxy groups, and then degassed for 10 min before being poured into a preheated mold, followed by being cured at 120 °C for 3 h and post-cured for 1 h at 160 °C. In the preparation of samples of TGDDM/DDS resins containing E-HBP, a stoichiometric DDS dissolved in acetone was added to TGDDM under stirring and heating until a homogeneous and clear solution was obtained. The acetone was then removed under reduced pressure, and E-HBP was added under stirring conditions until a homogeneous solution was obtained. The mixture was degassed for 15 min, and then poured into a preheated mold, followed by being cured for 4 h at 150 °C and post-cured at 220 °C for 1 h. They were then all allowed to cool to room temperature gradually. The stoichiometric ratios of amine-to-epoxy groups were equivalent in all samples in order to achieve 100% conversion.
The samples of DGEBA/MeTHPA resins and TGDDM/DDS resins, modified by CTBN and PES, respectively, were also prepared according to the above procedures, just replacing E-HPB with CTBN and PES. Because of the high melting temperature of PES, it was dissolved in dichloromethane first and then added into the epoxy resins.
Infrared spectroscopy (FTIR) used the Nicolet 6700 Fourier transform infrared spectrometer (Thermo Scientific, USA). The wave number range is 400~4000 cm−1. The glass transition temperature of the epoxy systems was determined by the DSC method, and the apparatus was a TA-60WS differential scanning calorimeter manufactured by Shimadzu Corp. (Japan). The mechanical properties, including Young’s modulus, of the samples were measured by standard tensile tests by using a universal material testing system of type AGS-J produced by Shimadzu Corp. (Japan) at a crosshead displacement rate of 2 mm/min. Scanning electron microscopy (SEM, Hitachi S-4800, Japan) was used for morphological characterization of the samples. Transmission electron microscopy (TEM, Hitachi HE-800, Japan) was used for phase structure characterization of the cured, frozen, sectioned samples stained by osmium tetroxide (OsO4).
In order to clearly observe the phase structure, a method of chemical etching was used as well. The etching agent was a 60 wt% aqueous solution of sulfuric acid. Before chemical etching, the cured epoxy samples were placed into liquid nitrogen for several minutes and broken quickly, and then the etching agent was coated on the fracture surfaces and allowed to stand for 24 h. After that, the etched surface was rinsed with distilled water.
3 Results and discussion
3.1 FTIR and T g analysis of E-HBP
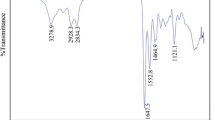
Figure 3 shows the FTIR spectra of aliphatic hyperbranched polyester (HBPE) and epoxy-terminated hyperbranched polyester (E-HBP). It is found that the characteristic absorption peaks of epoxy groups appeared at 910 and 847 cm−1 in the E-HBP spectrum but did not appear in the spectrum of HBPE, indicating that a hyperbranched polyester with epoxy groups at the end is obtained. The appearance of a hydroxyl peak at 3450 cm−1 in the E-HBP spectrum is because the space hindrance effect causes the hydroxyl group not to be completely replaced, yet the intensity of the hydroxyl peak is significantly lower than that of the HBPE system.
The glass transformation temperatures (Tg/°C) of different curing systems are shown in Fig. 4, and the changes of T g with different E-HBP contents are shown in Table 1.
Due to the different chemical nature of the hydroxyl and epoxy groups, different tougheners have different effects on the structure of a cross-linked network after participating in the curing reaction of the resin matrix. The addition of CTBN and PES may reduce the cross-link density of the network and introduce more flexible segments relatively [15,16,17], while the addition of E-HBP may take an effect in increasing the cross-linking density and thus have a higher glass transition temperature, as is shown in Fig. 4 and Table 1.
3.2 Mechanical properties of E-HBP-modified epoxy resins
The stress-strain curves of the two epoxy resins are shown in Fig. 5, and the effects of E-HBP on the tensile properties of the two epoxy resins are shown in Table 2, including tensile strength, elongation, and Young’s modulus. They indicate that the tensile properties are improved significantly by the addition of E-HBP. At a high content of 20 phr, the elongation of the two epoxy resins still increased while tensile strength and Young’s modulus decreased. It can be understood from two main aspects. Firstly, the higher the content of E-HBP, the more E-HBP dissolved in the continuous phase of the epoxy matrix, thence the capability of the matrix to undergo plastic deformation is improved since E-HBP cured with a hardener has been characterized by a rubbery behavior [18]. Secondly, during the phase separations, a high content of E-HBP could cause massive particle coalescence of E-HBP [19]. That would be expected to be harmful to the continuity of the epoxy matrix phase and cause the decreasing of tensile strength and Young’s modulus, which are still higher than those of the non-E-HBP content.
3.3 SEM analysis of fracture surfaces
With the addition of E-HBP, the tensile fracture surfaces of the two epoxy resins were different, as is shown in Figs. 6 and 7. For the DGEBA-MeTHPA resins, the tensile fracture surfaces become rougher after the addition of E-HBP (comparison between Fig. 6a, c, corresponding to the plastic deformation of the epoxy matrix phase). Compared with Fig. 6b and d shows several divergent crack propagation regions with relatively smooth areas close to the centers of initiation regions. On the lines of crack propagation, many protrusions (identified by arrows in Fig. 6d) with a gradual increase in their numbers and sizes can be clearly observed. These protrusions were formed in the process of tensile fracture and caused by the addition of E-HBP which induced the deformation of the epoxy matrix. Moreover, it should be noted that no obvious particle cavitations were observed in the fracture surface. These indicate that the toughening mechanisms of E-HBP in DGEBA/anhydride epoxy resins should be the particle deformation, which is similar with epoxy resins toughened by rubber materials and thermoplastics. However, as is shown in Table 3, the toughness effects of E-HBP seem to be more effective than those of PES and CTBN because of special characteristics of E-HBP. With multiple epoxy functionality and good miscibility between E-HBP and the epoxy matrix, E-HBP can participate in the curing reaction and increase the cross-linking density of epoxy systems, which can increase the strength and modulus simultaneously.
The tensile fracture surface of the TGDDM/DDS system is different from that of the DGEBA/MeTHPA system. After the additions of the E-HBP modifier, fracture surfaces with many cavities are formed which indicates that particle cavitation of E-HBP (Fig. 7d) occurs in the TGDDM/diamine systems during the fracture process. From Fig. 7d, e, it can be seen that most of cavities exist on or close to the crack propagation lines, and the sizes of the cavities are about 1 to 1.5 μm. In E-HBP-modified epoxy systems, the cavities were particles which have been fully cavitated, i.e., a void has grown within the particles during the process of particle deformations [10]. The process of particle cavitations can absorb the energy of external load, and the cavitated particles on the propagation lines (as is shown in Fig. 7e) can also play a role in preventing crack propagations. These should be the main reasons for the improvement of the fracture toughness of E-HPB-modified TGDDM/diamine epoxy resins.
3.4 The phase structure after CIPS
Epoxy groups on the E-HBP shell are expected to increase the initial solubility of E-HBP in the epoxy resins. The magnitude of this effect can be seen from the comparison between E-HBP and epoxy resins in the solubility parameters. By using the group contribution approach [20], the calculated solubility parameters of E-HBP, epoxy matrixes, and the curing agents are shown in Table 3. It can be found that the calculated solubility parameter of E-HBP match well with those of DGEBA and MeTHPA, and is close to those of TGDDM and DDS. This indicates that E-HBP is more soluble in the DGEBA resin than in the TGDDM resin. However, with increasing miscibility between E-HBP and the resins, the greater the solubility of E-HBP in the epoxy resins, the smaller the expected size of the separated phases [8, 11]. Figure 8 shows TEM pictures of the fracture surfaces of the E-HBP-modified DGEBA/MeTHPA system and TGDDM/DDS system. From the comparison between two pictures, it can be seen that the sizes of the separated E-HBP particle phases in the DGEBA/MeTHPA system are smaller than those in the TGDDM/DDS system. In both systems, the boundaries between the separation phases and the matrix are indistinct, especially in the DGEBA/MeTHPA system. However, in the TGDDM/DDS system, the separated phases are more spherical.
Figure 9 shows the fracture surfaces of the DGEBA/MeTHPA systems with and without E-HBP after the chemical etching. The main chains of E-HBP contain large numbers of ester groups which can be hydrolyzed by a strong acid. Thus, many etched holes could be seen in the fracture surfaces of the DGEBA/MeTHPA system containing 10 wt% E-HBP. As is shown in Fig. 9b, the sizes of these holes are not uniform, several to a dozen microns in diameter, and most holes are not spherical. Furthermore, the sizes of some holes are close to, even larger than, those of the initiation sites shown in Fig. 6d.
Because of the random growth of branched chains, the molecular shape of E-HBP is not like that of dendrites, i.e., it is not spherical. Thus, not all of the termination epoxy groups of E-HBP are in the outermost layers of the molecules, and some termination epoxy groups could be embedded in adjacent branched chains (as is shown in Fig. 2). When these embedded epoxy groups participated in the curing reaction, an interesting interface structure—gradient transition interface layer (GTIL)—may be formed between the separated E-HBP particles and the epoxy matrix during both processes of the curing reaction and the CIPS (Fig. 10). This led to the concentration of the DGEBA/MeTHPA resin increasing gradually from the interior of separated E-HBP particles to the DGEBA/MeTHPA matrix, and the phase interface also became indistinct. During the process of chemical etching, all of the molecules with ester groups including branched chains of E-HBP in the interface layer and separated E-HBP particles could be etched. These were the main reasons for the larger size of some etched holes than the initiation sites, as is shown in Fig. 6d.
Actually, in the fracture surface pictures of the DGEBA/MeTHPA system (containing E-HBP), three main different deformation zones can be identified, including the particle deformation zone, the transition layer deformation zone, and the matrix deformation zone, as is shown in Fig. 11. The particle deformation zone with semi-diameter of 2 to 2.5 μm is smooth, corresponding to the deformation of the separated E-HBP particle phase, and several cracks developed from the deformation center can be observed. The transition layer deformation zone surrounded the particle deformation zone, with the width of which is about 2 μm. In this zone, under the load condition, both the matrix and E-HBP phases would deform. Because of the concentration gradient of E-HBP and different deformation sizes between the two phases, the transition layer deformation zone gradually becomes rough along the radial direction of separated particles. Outside the transition layer deformation zone is the matrix deformation zone, corresponding to the bulk deformation of the epoxy matrix. Apart from the intrinsic nature of the DGEBA/MeTHPA matrix, the deformation of the separated E-HBP particle phase is another reason which induces a sufficient deformation of the matrix. From the features of the fracture surfaces, it can also be seen that the external load was transferred along the radial direction of separated E-HBP particles in the DGEBA/MeTHPA systems.
Due to the much higher modulus than that of the DGEBA/MeTHPA resins, the TGDDM/DDS resins with different E-HBP contents are not easy to deform under external loading, and no significant GTIL structure could be observed on the fracture surfaces except for fully cavitated E-HBP particles, as is shown in Fig. 7d. However, there are some other circumstantial evidences which indicate the existence of the GTIL structure in E-HBP-modified TGDDM/DDS resins. We occasionally posted cured samples of the TGDDM/DDS systems containing E-HBP at a temperature higher than 300 °C for 30 min, and then the cured samples were put into liquid nitrogen, became brittle, and were fractured. The fracture surface is shown in Fig. 12. Due to the better thermal stability of the TGDDM/DDS resin matrix [21], many obvious holes can be seen in the fractured surface as a result of thermal degradation of separated E-HBP molecular chains. From Fig. 12, it can be seen that the area, which was composed of numerous holes, is not spherical and larger in size than that of the separated particle in Fig. 9b. These effects should be the results of the coalescing of several E-HBP particles. However, the size distribution of the holes shows a gradient from the interior to the edge of the area. The gradient size distribution of the holes and the indistinct boundaries between the separation phases and the matrix both indicate a gradient concentration distribution of E-HBP molecular chains within this region and the existence of the GTIL structure in TGDDM/DDS systems containing E-HBP.
3.5 The effect of epoxy matrix on the particle cavitations
Through CIPS in the curing process, phase-separated E-HBP particles were formed in the epoxy matrix with a GITR structure; thence, the mechanical properties of the modified system were significantly improved. However, the mechanical behaviors (deformation or cavitation) of E-HBP particles in the loading process would be affected by the properties of the epoxy matrix itself.
Because of the high average functionality, the TGDDM/DDS resins (the average number of epoxy groups in TGDDM molecules is 4, while that in DGEBA molecules is 2, as is shown in Fig. 1) have much higher cross-linking density than the DGEBA/MeTHPA resins after the curing reaction. In addition, the number of benzene rings along the molecular chains between the two cross-linking points in the TGDDM/DDS systems is higher than that in DGEBA/MeTHPA systems because of more benzene rings in the molecules of TGDDM and DDS than those in the molecules of DGEBA and MeTHPA. Thus, the rigidity of the cross-linked molecular chains in the TGDDM/DDS resins was greater than that in the DGEBA/MeTHPA resins. Studies on the visco-elasticity of polymers have shown that great rigidity and high cross-linking density of molecular chains will cause a low hysteresis effect of the polymers [22]. If this kind of polymers was subjected to the external load, the loading could be quickly spread in the cross-linked network and the polymers only deformed with instantaneous elastic deformation. Comparatively speaking, the plastic deformation of the epoxy matrix, as is shown in Fig. 6b, implies that the hysteresis effect of the DGEBA/MeTHPA matrix was obvious because of low rigidity of cross-linked molecular chains, which would cause the energy loss in the process of load transmission. Thus, the deformation of separated E-HBP particles only occurs in DGEBA/MeTHPA resins and could lead to a greater plastic deformation of the matrix, thereby improving the toughness of the epoxy resins. While, because of the great rigidity of the cross-linked molecular chains, the separated E-HBP particles in TGDDM/DDS resins could be cavitated quickly and fully before the fracture of the epoxy matrix under the external load. In this way, the load energy can be absorbed, the crack propagation can be stopped, and the toughness of the modified epoxy resin can be improved.
4 Conclusions
In the E-HBP-modified DGEBA/MeTHPA systems and TGDDM/DDS systems, E-HBP participated in the curing process, and then underwent chemical induced phase separation, and finally formed the GTIL structure. The GTIL structure (about 2 μm thick) between E-HBP and the epoxy matrix can significantly enhance the inter-facial interactions between the phase-separated particles (E-HBP) and the epoxy matrix. It is also advantageous to the load transmission between the two phases and the full playing of the role of modification of E-HBP. This may be the key reason why the properties of E-HBP-modified epoxy resins are better than those of other modified epoxy resins. However, the properties of the epoxy matrix itself will affect the mechanical behaviors (deformation or cavities) of separated E-HBP particles in the modified epoxy resins during the external loading process, thereby influencing the modification mechanism of E-HBP-modified epoxy resins and resulting in the different morphology of fracture surfaces between the two modified epoxy systems. In order to obtain a better modification effect, it can be seen from the above findings that we need to learn how to control the thickness of the inter-facial layer and the concentration gradient, which will be the next focus of our study.
References
Marouf BT, Mai YW, Bagheri R, Pearson RA (2016) Toughening of epoxy nanocomposites: nano and hybrid effects[J]. Polym Rev 56(1):70–112
Gu H, Ma C, Gu J et al (2016) An overview of multifunctional epoxy nanocomposites[J]. J Mater Chem C 4(25):5890–5906
Gu J, Liang C, Zhao X et al (2017) Highly thermally conductive epoxy nanocomposites with superior outstanding flame retardances and excellent electrical conductivities[J]. Compos Sci Technol 139:83–89
Zhang DH, Liang EB, Li TC et al (2013) The effect of molecular weight of hyperbranched epoxy resins with silicone skeleton on performance[J]. RSC Adv 3(24):9522–9529
Boogh L, Pettersson B, Månson JAE (1999) Dendritic hyperbranched polymers as tougheners for epoxy resins. Polymer 40:2249–2261
Jian YL, Yan M, Li FH et al (2013) Novel epoxidized hyperbranched poly(phenylene oxide): synthesis and application as a modifier for diglycidyl ether of bisphenol A. J Appl Polym Sci 128:907–914
Ratna D, Simon GP (2010) Epoxy and hyperbranched polymer blends: morphology and free volume. J Appl Polym Sci 117:557–564
Mezzenga R, Luciani A, Månson JAE (2002) Phase separation and gelation of epoxy resins-hyperbanched polymer blends. Polym Eng Sci 42:249–257
Li J, Xiang Y, Zheng S (2016) Hyperbranched block copolymer from AB2 macromonomer: synthesis and its reaction-induced microphase separation in epoxy thermosets. J Polym Sci A Polym Chem 54:368–380
Liu YH (2012) Polymerization-induced phase separation and resulting thermomechanical properties of thermosetting/reactive nonlinear polymer blends: a review. J Appl Polym Sci 127:3279–3292
Varley RJ, Tian W (2004) Toughening of an epoxy anhydride resin system using an epoxidized hyperbranched polymer. Polym Int 53:69–77
Zhang J, Guo QP, Fox B (2010) Thermal and mechanical properties of a dendritic hydroxyl-functional hyperbranched polymer and tetrafunctional epoxy resin blends. J Polym Sci POLY PHYS 48:417–424
Petterson B (1994) A thermosetting materials, U.S. Patent, SE 94/04440
Plastic-Epoxy compound-Determination of epoxy equivalent. National standard of China GB/T 4612–2008
Xia M, Luo YJ, Wang XY (2006) Study on curing behaviors and mechanical performances of hyperbranched polymers/epoxy curing systems[C]// Proceeding of 4th Annual Conference for Ph. D. Students of China Association for Science and Technology. 3(1):12–16
Van Velthem P, Ballout W, Horion J et al (2016) Morphology and fracture properties of toughened highly crosslinked epoxy composites: a comparative study between high and low T-g tougheners. Compos Part B Eng 101:14–20
Liu WS, Wang J, Miao RZ (2016) Molecular dynamics simulations of the cross-linked epoxy resin TGDDM/DDS[J]. Polym Mater Sci Eng 32:109–114
Mezzenga R, Boogh L, Pettersson B et al (2000) Chemically induced phase separated morphologies in epoxy resin-hyperbranched polymer blends. Macromol Symp 149:17–22
Cicala G, Recca A, Restuccia C (2005) Influence of hydroxyl functionalized hyperbranched polymers on the thermomechanical and morphological properties of epoxy resins. Polym Eng Sci 45:225–237
Hansen C (1967) M. The three dimensional solubility parameter—key to paint component affinities I.—solvents, plasticizers, polymers, and resins. J Pain Tecnol 39:104–117
Nagendiran S, Alagar M, Hamerton I (2010) Octasilsesquioxane-reinforced DGEBA and TGDDM epoxy nanocomposites: characterization of thermal, dielectric and morphological properties. Acta Material 58:3345–3356
van Krevelen DW (2010) Properties of polymers. Science Press, Beijing
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xia, M., Yang, H., Ling, J. et al. The mechanical behaviors of epoxy-terminated hyperbranched polyester (E-HBP) as toughener in different epoxy resins. Adv Compos Hybrid Mater 1, 310–319 (2018). https://doi.org/10.1007/s42114-018-0027-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-018-0027-4