Abstract
Various amounts of dicyandiamide (Dicy), two grades of epoxy resins, i.e. Epiran 06 and Epikote 828, and three different accelerators including benzyl dimethyl amine (BDMA), 3-(4-chlorophenyl)-1,1-dimethyl urea (Monuron) and 2-methyl imidazole (Im) were used in curing of Dicy/epoxy resin system. Both of the used epoxy resins were based on diglycidyl ether of bisphenol A (DGEBA). The effects of type and concentration of accelerators on curing behavior were studied by differential scanning calorimetry (DSC) method in dynamic or non-isothermal mode. The optimum concentration of Dicy for curing of epoxy resins was obtained based on the glass transition temperature of the cured epoxy/Dicy formulations. The maximum glass transition temperature of 139 °C was obtained at the stoichiometric ratio of Dicy to epoxy of 0.65. The results showed that BDMA has a broader curing peak in DSC and starts the cure reaction earlier than the others. However, Monuron has a narrow curing reaction peak with good cure latency. The tensile properties of Dicy-cured Epiran 06 and Epikote 828 epoxy resins reinforced with chopped strand mat showed that these two epoxy resins have similar mechanical properties. For composites based on the Epiran 06 and Epikote 828 reinforced with 40 wt % glass chopped strand mat, tensile strength and modulus were 156 and 153.4 MPa and 11.6 and 12.4 GPa, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are widely used in industrial applications due to their very attractive properties, such as excellent chemical and solvent resistance, high mechanical strength and modulus, good adhesion, good electrical properties, low shrinkage and good processability [1–4]. For structural applications, epoxy resins represent a number of important organic matrices, generally reinforced with glass or carbon fiber, because of significant adhesion properties [5]. It is important to investigate the cure behavior as well as to establish the correlation among the degrees of curing, thermal and mechanical properties owing to design the optimum curing conditions [6].
Differential scanning calorimetry (DSC) has been applied considerably to characterize the curing kinetics of thermosetting systems. It is the most widely used method for monitoring curing process as the rate of heat evolved is directly proportional to the rate of reaction [7, 8]. It is found that the physical properties of the cured epoxy resins depend on their structure, extend of curing and its condition and time and temperature of curing [9]. Sbirrazzuoli et al. studied [10] epoxy curing mechanisms via DSC data. In another work, Kim et al. investigated [11] the curing kinetics of epoxy resin systems by changing of the hardeners. The results showed that the rate of reaction would increase with molecular size of the curing agents.
Galy and his coworkers [12] have studied the cure behavior of different epoxy systems by DSC method. Their results showed that in the non-catalyzed systems, the value of the heat of reaction and the glass transition temperatures are related to the hardener structure. To reduce the curing temperature, accelerators are usually used for some curing agents such as dicyandiamide (Dicy) and anhydrides. Some of these accelerators are the aryl dimethylurea compounds, e.g., Monuron, Diuron and Fenuron. It is important to notice that the grade of Urone to be chosen as the accelerator depends on the application of epoxy system. Curing of epoxy resins with Dicy in the presence of several Urone accelerators has been studied by Guthner et al. [13]. The results show there are complex multistage curing processes. Urones sharply decreased the curing temperature. It means that the addition of any amount of Urone accelerator to an epoxy/Dicy formulation reduces the glass transition temperature (T g).
In another research works, curing of epoxy resin with Dicy has been investigated [14, 15]. BDMA, Imidazole and Monuron have been used as accelerators. There was a good relationship between the low T g of samples and the high content of dimethylformamide (DMF) as a solvent. The DMF can be evaporated in a wide range of temperature above its boiling point. Therefore, a post-curing process will be required and post-cured samples being nearly free from DMF and showed thermal stability.
Accelerators in curing of epoxy resins decrease the curing temperature and time and enhance its rate. Benzimidazoles and their derivatives have been used as accelerators [16]. In another work, Reddy et al. [17] used cobalt (III) acetylacetonate as accelerator for anhydride curable epoxy resin system. Furthermore, the acetylacetonates of nickel (II) and copper (II) have been used as accelerators for epoxy systems by Reddy et al. [18]. They observed that these accelerators considerably lowered the gel time and significantly increased the curing rate.
In this work, we studied the effects of Dicy concentration, type and amount of three different accelerators on curing behavior of Dicy/epoxy systems by DSC method and determined the curing kinetic parameters. Two kinds of epoxy resins based on diglycidyl ether of bisphenol A (DGEBA) were used. Although Dicy-cured epoxy resins have wide applications, but there are not much published works on the optimum amount of Dicy and the effect of accelerators on this system.
Experimental
Materials
Two kinds of epoxy resins have been used in this work, i.e., Epikote 828 (EEW 190-200, Momentive Co., USA) and Epiran 06 (EEW 230, Khuzestan Petrochemical Co., Iran). These two liquid epoxy resins are based on diglycidyl ether of bisphenol A. They have the viscosity of 12,000–14,000 cp at 25 °C.
Benzyl dimethyl amine (BDMA) and 2-methyl imidazole (Im) and dicyandiamide (Dicy) were supplied by Merck (Gemany). Dicy was milled to a size of <30 micron with a ball mill. 3-(4-chlorophenyl)-1,1-dimethylurea (Monuron) (purity 99 %) was obtained from Aldrich (UK). All of materials were used as received without any purification. The structures of these accelerators are shown in Scheme 1. Chopped strand mat (CSM) glass fiber from Cam Elyaf Co. (Turkey) with 450 g/m2 was used as reinforcement.
Measurements
DSC measurements were carried out with a Netzsch DSC 200 F3 Maia (Germany) instrument. Dynamic-heating experiments were conducted in flowing nitrogen environment (50 mL/min) using epoxy mixed samples of 12 ± 2 mg sizes. For dynamic or non-isothermal experiments, the uncured sample was placed in an aluminum pan with a sealed lid and placed opposite to the empty reference pan in the oven chamber. The instrument was then heated with a 5 °C/min heating rate from 25 to 250 °C. After cooling, the T g was measured in the third run by heating at 10 °C/min. The area under the peak of the first scan (cure reaction) and a linear baseline was considered to be the total heat of the curing reaction. It is important to note that the particle size of Dicy may have a great impact on the phase transition due to the increased diffusion rate and the homogeneity achieved by the finer particle size [19]. Thus, for curing of epoxy resin, at first Dicy was milled to make its size <30 micron with a ball mill. Different amounts of Dicy and three different accelerators were used as shown in Table 1.
To evaluate the mechanical properties and the kind of epoxy resin, the optimum formula of epoxy/Dicy/Monuron was stirred for 1 h with a mechanical stirrer. Three layers of CSM glass fiber with this mixture were laminated via hand lay-up and hot pressed for 15 min at 90 °C followed by 30 min at 125 °C. After curing, tensile test specimens were prepared. The dimensions of the samples were 20 × 200 × 2 mm. Tensile tests were performed at cross-head speed of 2 mm/min with Santam instrument, STM 150 (Iran). Five samples were tested and the average of the results was reported.
Results and discussion
Optimizing the amount of hardener
The necessary amount of hardener can be determined stoichiometrically. For this calculation, the equivalent weight of epoxy resin, functionality of hardener and its molecular weight were required. By regarding of equivalent stoichiometric amounts, the necessary amount of hardener can be determined as follows:
The amine H equivalent of Dicy can be calculated by dividing its molecular weight, 84, to the number of its active hydrogen namely 4 which yields 21. According to the Eq. (1) and by substituting the appropriate EEW of used epoxy resin, the stoichiometric amounts of 9 and 11 phr were obtained for Dicy for curing of Epiran 06 and Epikote 828, respectively. It is based on the number of amine hydrogen of Dicy which is equal to four. As the research works have shown, the cyanide groups (C≡N) in Dicy would be entered in curing process at high temperature and reacted with hydroxyl groups. Therefore, the researchers knew that the actual functionality of Dicy was greater than four. Tests on various formulations with different stoichiometries showed that the stoichiometry ratio of amine/epoxy of 0.6–0.7 was suitable for complete curing [20]. These results showed that in this ratio, the glass transition temperature of the cured compound (which relates with appropriate curing and maximum mechanical property of the compound) had the highest value.
Different amounts of Dicy in the range of 5–11 phr according to the different amine to epoxy ratios were considered to optimize the amount of the Dicy in the formulations as described in Table 1. After curing of these formulations in the first run of DSC with the heating rate of 5 °C/min, T g of the cured samples was determined in the second run at 10 °C/min
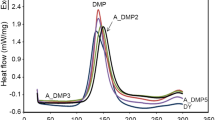
Figure 1 shows the results which obtained from the DSC experiments in dynamic or non-isothermal mode of five formulations with different Dicy contents and constant amount of 0.4 phr BDMA as accelerator. Curing characteristics of these formulations are shown in Table 2. Curing initiation temperature was about 82–89 °C and the final temperature was around 182–194 °C. In addition, the peak temperature of curing was in the range of 136–145 °C. As it is seen, EDB-7 formulation with 7 phr of Dicy shows the maximum T g of 139 °C. At the higher and lower concentrations of Dicy, T g was reduced.
DSC heat flow curves for different formulations of Table 1
Figure 2 shows the variation of T g with the changes in the ratio of amine to epoxy contents. The results show that the usage of 7 phr Dicy, which is equal to stoichiometric ratio of amine/epoxy of 0.65, gives the maximum T g value. Guthner et al. [13] showed that the optimum stoichiometry is as 7 epoxy units per 1 molecule of Dicy. Additional epoxy groups can be incorporated in the network, simultaneously reducing the overall reaction exothermic and the rigidity of the network. Most of properties of the cured resins maximized at this ratio, gave minimum concentration of residual reactive groups. In addition, Poisson et al. [20] in their work found that Dicy content clearly influenced the T g of network and the maximum T g value was obtained for an amine to epoxy molar ratio of 0.6.
As it is observed in Fig. 2, the glass transition temperature has a maximum value that does not correspond with the ratio of Dicy/epoxy which is equal to one. It has found that higher glass transition temperatures are obtained when the stoichiometric ratio of Dicy/epoxy is equal to 0.65. Thus, the optimum amount of Dicy for the next formulations is 7 phr which will be used for the investigation of type and the amount of accelerators. It is worth mentioning that the optimum concentration of Dicy or any other curing agents for curing of epoxy resins is based on the desired properties. In some applications mechanical properties are important while in other applications chemical resistance or thermal resistance might be important. Different optimum concentration of curing agent will results the maximum or optimum values for different properties. The T g of a system is very important from the heat resistance point of view. Therefore, this condition of curing was important and the optimum amount of Dicy for achieving the maximum value of T g was investigated.
Selection of type and amount of accelerator
Epoxy resins, in the absence of accelerators, usually start to react with Dicy at about 170–180 °C and show two exothermic peaks at 200 and 211 °C. Furthermore, the combination of accelerators and Dicy with epoxy resin exhibited a faster curing at lower temperatures than either system alone [13].
In this research, three accelerators usually used for Dicy-cured epoxy resins were applied. These accelerators were BDMA, Monuron and 2-methyl imidazole. BDMA and imidazoles are useful tertiary amines accelerators. Tertiary amines are the general class of anionic initiators most accepted commercially as accelerators for epoxy resins. BDMA is a basic catalyst and in the curing process the rate of reaction depends on the initial hydroxyl concentration and particularly on the proportion of BDMA. BDMA is a co-initiator and its hydroxyl functions have an initiator effect [12].
The first step in the curing of epoxy resins with imidazoles was the reaction with the NH groups of the imidazole molecules and that the other nitrogen in the 3-position of the imidazole was attacked by another epoxy group. The pyrrol-type (N–H) nitrogen in the 2-methyl imidazole takes part in the etherification reaction. Curing behavior of 2-methyl imidazole was complex and multiple exothermic peaks were seen [21].
By heating, Monuron dissociates into isocyanate and dimethylamine. Both fragments are active components: dimethylamine reacts with an epoxy group to obtain a tertiary amine that catalyzes the reaction of Dicy with epoxy group and the isocyanate reacts with other epoxy unit [13].
For the investigation of the effect of type and the amount of accelerator, nine compounds according to Table 3 were considered. In these compounds, six compositions with Epiran 06 and the rest with Epikote 828 were made. The cure behavior of these compounds was studied by non-isothermal DSC method.
Figure 3 shows the curing behavior for three formulations prepared with different accelerators at the heating rate of 5 °C/min. In these compounds Epiran 06 and constant amount of Dicy (7 phr) and accelerator (2 phr) have been used. As can be seen BDMA and Im have broad peak and start the curing reaction at about 80–90 °C and terminate it at about 185 °C. The peak of curing reaction with BDMA was broader than that of the other two accelerators namely, Im and Monuron. The broadness of this peak was at the order: BDMA > Im > Monuron. In other words when the epoxy resin was cured with Dicy and Monuron, the peak of cure reaction was narrow and started at higher temperature about 120 °C, but finished at the same temperature about 185 °C.
Cure characteristics of all formulations are shown in Table 3. It can be seen that the exact cure characteristic of initial, peak and final temperatures depend on the concentration of accelerator. Furthermore, when BDMA was used as accelerator, higher glass transition temperature was achieved. Lower glass transition temperatures are obtained with Monuron and Im depending on their concentrations. Specially, the T g of the blends containing Monuron depends on its concentration in the formula. By increasing the Monuron concentration, it can be seen that the T g was decreased.
To evaluate the effect of epoxy resin type on cure characteristics, the data given in the last three rows of Table 3 can be compared with their counter parts. The comparison of data for EDB-0.4 and KDB-0.4 (or EDM-2 and KDM-2) show that the Epikote 828 resin has higher reactivity and higher T g value can be achieved for this kind of resin. This might be due to the lower EEW of Epikote 828 in comparison with Epiran 06.
Figure 4 shows the conversion of three formulations with different accelerators and the same amounts of Dicy-cured dynamically to compare curing behavior of three different accelerators. As can be seen, for samples EDI and EDB, curing started earlier at about 80 and 90 °C, respectively. But EDM starts curing at about 135 °C. By differentiating the degree of curing with respect to time, the relationship between the curing rate and time or temperature can be determined. These data would be used as the source data to simulate the dynamic curing process.
Figure 5 shows curing rate versus temperature for three different formulas shown in Fig. 4. It can be seen that the EDM sample cured very rapidly although EDM starts the curing at higher temperature than the other two samples with EDB and EDI. It can be concluded that the control of cure reaction of EDM sample is difficult after initiation particularly in controlling B-staging in making for example prepregs or molding compounds.
Curing rate versus temperature for three different formulas shown in Fig. 4
In this section, the whole curing process of these three selected formula is studied with a method based on Borchardt and Daniels, in which all kinetic parameters can be obtained simultaneously. In the dynamic curing process, the curing rate is not only a function of degree of curing, but also a function of temperature. The curing reaction rate can be expressed as a function of conversion and temperature, as follows:
where, dα/dt is the reaction rate, k(T) is the reaction rate constant and f(α) is a conversion-dependent function. The reaction rate constant has been described by the Arrhenius expression, as follows:
where, A is the pre-exponential factor and E a is the activation energy and R is the gas constant. As it was mentioned before, the cure kinetics can be followed by using two DSC methods: dynamic or non-isothermal and isothermal. The isothermal method can identify two types of reactions: n-order and autocatalytic reactions. If the maximum peak of the isotherm is close to t = 0, the system obeys kinetics of n-order (\( f(\alpha ) = (1 - \alpha )^{n} \)) and it can be studied either by dynamic or isothermal methods. In the case when the maximum peak is formed in between 30 and 40 % of the total time of the analysis, the curing is autocatalytic. The autocatalytic model considers independent reaction orders: m and n. In autocatalytic model with initial curing rate of zero, the term of f(α) can be determined by Eq. 4 [22] as follows:
Combining Eqs. (2) and (3) yields:
Equation 5 can be solved by multiple nonlinear regressions. Since the curing rate is an exponential function of the reciprocal of the absolute temperature, it is difficult to get a good solution and the fitting results show significant errors for A and E a determined by this method. Therefore, it is necessary to re-determine the apparent pre-exponential factor, A, and activation energy, E a, to reduce the magnitudes of the standard errors. Once the orders of the curing reaction, m and n were determined, A and E a can be determined with small standard errors by the Barett method [23]. Eq. 5 can be rearranged to yield as follows:
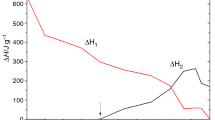
Eq. 6 shows that there is a linear relationship between the logarithm of (dα/dt)/[α m (1-α) n ] and 1/T, therefore the apparent pre-exponential factor, A, and activation energy, E a , could be determined from the intercept and slope of the plots of Eq. (6), respevtively that are shown in Fig. 6. It can be seen that a main portion of the curve in the range of α = 5−95 % exhibits a linear behavior. By fitting to the linear part of the curve, the apparent pre-exponential factor, A, and activation energy, E a , have been determined which are given in Table 4.
Mechanical properties
In order to evaluate the mechanical properties of epoxy resins, based on the results of previous sections, compounds of epoxy/Dicy/Monuron with the proportion of 100/7/2 were prepared with two kinds of epoxy resins Epiran 06 and Epikote 828. It is worth mentioning that molding or casting these kinds of epoxy systems on their own is too difficult due to the sedimentation of Dicy during curing period. Therefore, CSM glass fiber reinforced epoxy resin laminates were prepared. The fiber weight percentage of these laminates was 40 %. CSM is a random fiber and its laminates are resin dominated and isotropic. Tensile properties of these resin-reach composites are shown in Table 5. Also, Figs. 7 and 8 show the stress–strain behavior of CSM reinforced epoxy resins. It can be seen that the tensile strength, modulus and elongation-at-break values of both resins are almost the same. Therefore, both Epiran 06 and Epikote 828 epoxy resins have similar mechanical properties. Tensile strength and modulus for composites based on Epiran 06 and Epikote 828 reinforced with 40 wt % chopped strand mat are 156 and 153.4 MPa and 11.6 and 12.4 GPa, respectively.
Figs. 7 and 8 show that the both systems are strong and tough epoxy systems and have similar mechanical behavior. Since they have similar curing behavior and glass transition temperatures, it can be concluded that both of these resins have similar properties and can be used instead of each other.
Conclusion
An experimental investigation was carried out to evaluate the effect of various accelerators on curing behavior of dicyandiamide/epoxy resin system. These accelerators were benzyl dimethyl amine (BDMA), Monuron and 2-methyl imidazole (Im). The results showed that the best curing characteristic and glass transition temperature are obtained at the stoichiometric ratio of Dicy to epoxy of 0.65. BDMA had a broader peak in DSC and starts earlier the curing reaction than other two accelators, but Monuron showed narrow curing reaction peak and good curing latency due to its higher cure initiation temperature. Also, the effect of two type of epoxy resin on the mechanical properties of optimum formulation was investigated. The results of tensile strength, modulus and elongation-at-break measurements showed that the type of resin (Epiran 06 and Epikote 828) has no significant effect on tensile behavior of the cured resins and these two types of epoxy resins showed similar mechanical properties.
References
Lee H, Neville K (1982) Handbook of epoxy resins. McGraw Hill, New York
Abuali Galledari N, Beheshty MH, Barmar M (2012) Effect of NBR on epoxy/glass prepregs properties. J Appl Polym Sci 123:1597–1603
Lu S, Ban J, Yu C, Deng W (2010) Properties of epoxy resins modified with liquid crystalline polyurethane. Iran Polym J 19:669–678
Liu K, Lu S, Li S, Huang B, Wei C (2012) Mechanical and thermal properties of POSS-g-GO reinforced epoxy composites. Iran Polym J 21:497–503
Régnier N, Fayos M, Moreau P, Lafantaine E, Mortaigne B (1999) Cure behaviour and thermal degradation mechanisms of epoxy and epoxy-cyanate resins. Polym Adv Technol 10:637–646
Turi EA (1981) Thermal characterization of polymeric materials. Academic Press, New York
Gonis J, Simon GP, Cook WD (1999) Cure properties of epoxies with varying chain length as studied by DSC. J Appl Polym Sci 72:1479–1488
Hayaty M, Beheshty MH, Esfandeh M (2011) Isothermal differential scanning calorimetry study of a glass/epoxy prepreg. Polym Adv Technol 22:1001–1005
Roşu D, Caşcaval CN, Mustată F, Ciobanu C (2002) Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim Acta 383:119–127
Sbirrazzuoli N, Vyazovkin S (2002) Learning about epoxy cure mechanisms from isoconversional analysis of DSC data. Thermochim Acta 388:289–298
Kim WG, Lee JY (2002) Contributions of the network structure to the cure kinetics of epoxy resin systems according to the change of hardeners. Polymer 43:5713–5722
Galy J, Sabra A, Pascault J-P (1986) Characterization of epoxy thermosetting systems by differential scanning calorimetry. Polym Eng Sci 26:1514–1523
Güthner T, Hammer B (1993) Curing of epoxy resins with dicyandiamide and urones. J Appl Polym Sci 50:1453–1459
Pfitzmann A, Fischer A, Fryauf K, Fedtke M (1992) Curing of epoxy resins by dicyandiamide. Polym Bull 27:557–564
Hong S-G, Chan C-K (2004) The curing behaviors of the epoxy/dicyandiamide system modified with epoxidized natural rubber. Thermochim Acta 417:99–106
Reddy PV, Samuelson S, Nanje Gowda NM, Sathyanarayana DN (2008) Benzimidazole derivatives and their complexes as accelerators for curing of epoxy resin. J Appl Polym Sci 108:2101–2106
Reddy PV, Thiagarajan R, Ratra MC, Nanje Gowda NM (1990) Transition metal chelates as accelerators for epoxy resin systems, studies with cobalt (III) acetylacetonate. J Appl Polym Sci 41:319–328
Reddy PV, Nanje Gowda NM (1994) Acetylacetonates of nickel (II) and copper (II) as accelerators for the epoxy resin system. J Appl Polym Sci 53:1307–1314
Holbery JD, Bordia RK (2001) Accelerated cure of thermoset fiber composites utilizing latent cure agents. J Mater Sci 36:5301–5308
Poisson N, Maazouz A, Sautereau H, Taha M, Gambert X (1998) Curing of dicyandiamide epoxy resins accelerated with substituted ureas. J Appl Polym Sci 69:2487–2497
Ooi SK, Cook WD, Simon GP, Such CH (2000) DSC studies of the curing mechanisms and kinetics of DGEBA using imidazole curing agents. Polymer 41:3639–3649
Yang FL, Yao KD, Koh W (1999) Kinetics analysis of the curing reaction of fast cure epoxy prepregs. J Appl Polym Sci 73:1501–1508
Riccardi CC, Adabbo HE, Williams RJJ (1984) Curing reaction of epoxy-resins with diamines. J Appl Polym Sci 29:2481–2492
Acknowledgments
The authors are gratitude to Iran Polymer and Petrochemical Institute for the financial support of this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayaty, M., Honarkar, H. & Beheshty, M.H. Curing behavior of dicyandiamide/epoxy resin system using different accelerators. Iran Polym J 22, 591–598 (2013). https://doi.org/10.1007/s13726-013-0158-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-013-0158-y