Abstract
Toughening of a diglycidyl ether of bisphenol-A (DGEBA)-based epoxy resin with liquid carboxyl-terminated butadiene acrylonitrile (CTBN) copolymer has been investigated. For this purpose six blend samples were prepared by mixing DGEBA with different concentrations of CTBN from 0 to 25 phr with an increment of 5 phr. The samples were cured with dicyandiamide curing agent accelerated by Monuron. The reactions between oxirane groups of DGEBA and carboxyl groups of CTBN were followed by Fourier-transform infrared (FTIR) spectroscopy. Tensile, impact, fracture toughness and dynamic mechanical analysis of neat as well as the modified epoxies have been studied to observe the effect of CTBN modification. The tensile strength of the blend systems increased by 26 % when 5 phr CTBN was added, and it remained almost unchanged up to 15 phr of CTBN. The elongation-at-break and Izod notched impact strength increased significantly, whereas tensile modulus decreased gradually upon the addition of CTBN. The maximum toughness of the prepared samples was achieved at optimum concentration of 15 phr of CTBN, whereas the fracture toughness (K IC) remained stable for all blend compositions of more than 10 phr of CTBN. The glass transition temperature (T g) of the epoxy resin significantly increased (11.3 °C) upon the inclusion of 25 phr of CTBN. Fractured surfaces of tensile test samples have been studied by scanning electron microscopic analysis. This latter test showed a two-phase morphology where the rubber particles were distributed in the epoxy resin with a tendency towards co-continuous phase upon the inclusion of 25 phr of CTBN.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are widely used as coatings, structural adhesives, electronic and electrical materials [1], automotive, construction, aerospace industries [2] and advanced composite materials in many applications [3, 4], due to their appropriate material properties including excellent mechanical and electrical properties [1], thermal and chemical stability [5], high strength, and resistance to creep [6]. They can be formulated to perform adequately in extreme environments that include solvents, temperature extremes, and ultraviolet radiation and are relatively easy to process and apply [7].
However, one of the most undesirable properties of epoxy resins is their very poor fracture toughness. The cured neat epoxy matrices are often very brittle and show poor resistance to crack propagation and this adversely affects most physical and mechanical properties [1, 8]. Therefore, much research [9–11] has been carried out to enhance the toughness of the cured epoxy resins without a significant loss in other properties [12]. One of the most well-known methods is to incorporate various amounts of a reactive rubber into the epoxy matrix [3, 5]. When the curing reactions of epoxy and a liquid rubber proceed under controlled sets of conditions, phase separation occurs between the epoxy matrix and rubber particles [6, 13].
Among the liquid rubbers, carboxyl-terminated copolymer of acrylonitrile and butadiene (CTBN) has been largely used as a modifier for epoxy resins cured with Jeffamine [14], polyamine [15, 16] and diaminodiphenyl sulfone [17]. The effects of CTBN addition on mechanical and dielectric properties of NiZn ferrite powders in CTBN-modified epoxy resin coatings were also investigated [18]. The enhancement in toughness was achieved due to the phase separation of elastomeric phase, at some stages of the curing process, into particles of very small sizes.
Many applications benefit from elastomer-modified epoxy resins including structural automotive composite parts, prepregs, aerospace adhesives, coatings, marine, construction, etc.
Verchere et al. [19] have studied the influence of several formulation parameters, i.e., molecular weight of the diglycidyl ether of bisphenol-A (DGEBA) monomer and type of CTBN on the miscibility gap of the system. They used two kinds of diamine (ethylenediamine and isophoronediamine) for curing an epoxy resin. They have shown that the miscibility of epoxy monomers with a particular rubber is very sensitive towards the molar mass of the epoxy molecule. Wise et al. [20] investigated the toughening of DGEBA with three types of chain-end functionalized liquid rubber (CTBN X13, CTBN X8 and ATBN X16). They used diaminodiphenylmethane (DDM) and aniline as curing agents.
Ramos et al. [4] prepared a series of different blends such as CTBN-modified epoxy, in proportions ranging between 5 and 20 phr; epoxy modified with hydroxyl-terminated polybutadiene (HTPB), in proportions between 5 and 10 phr and piperidine was chosen as the curing agent. Their results showed that the impact resistance of CTBN-modified epoxy was superior to that of the pure epoxy resin and for the blends with HTPB, the impact resistance increased with elastomer concentration up to three parts per hundred parts of resin.
Tripathi and Srivastava [13] have modified DGEBA with CTBN and, in another report [5] they toughened the blend of DGEBA and cycloaliphatic epoxy resin with varying weight ratios (0–25 wt%) of CTBN. They used an aromatic amine hardener (4,4′-diaminodiphenyl sulfone) as a curing agent. They achieved better-toughened properties by incorporation of the elastomer of about 20 phr.
The effect of nanoclay and CTBN rubber on the reaction-induced phase separation and cure kinetics of an epoxy/cyclic anhydride system have been recently investigated [21]. The results showed that the phase-separated CTBN rubber hindered the cure reaction and had 3 % higher activation energy for epoxy/CTBN than an unfilled system and the accelerating effect of clay on the cure process was enhanced.
The shape memory behavior of CTBN–epoxy resin system cured with a cycloaliphatic amine hardener has been recently investigated by Kavitha et al. [22]. They showed that the CTBN-modified epoxy shape memory polymers exhibited a significant increase in the number of shape memory cycles, which is attributed to the toughening effect of the CTBN phase.
One of the conventional epoxy-curing agents is dicyandiamide (dicy) which is widely used as a latent curing agent in heat-cured epoxy resins for laminates or prepreg fabrications, coatings, and adhesives [23–25]. The curing reactions of various epoxy/dicyandiamide systems have been investigated by many investigators [26–28], but there are no significant reports on using this curing agent in CTBN-modified epoxy resins.
In this study, toughening of a DGEBA epoxy resin with CTBN has been studied. The mixture of DGEBA/CTBN is cured with dicyandiamide which is novel and has not been yet studied well. A major problem in utilization of dicyandiamide is its poor solubility in epoxy resins and tendency to separate and form a white precipitate after the curing process. Large particle size and poor mixing adversely affect the mechanical properties of dicyandiamide-cured resins and the sedimentation of this curing reagent’s crystals affects the degree of cross-linking [29]. In this paper, we used simultaneity, a way to overcome this problem in dicyandiamide curing of CTBN-toughened epoxy resin.
Experimental
Materials
The epoxy resin used was a DGEBA (Epikote 828, supplied by Momentive, USA) with its epoxy group content of 5.34 mmol/g. Dicyandiamide curing agent was from Merck and Monuron (3-(4-chlorophenyl)-1-1-dimethylurea) (99 %) was used as an accelerator, purchased from Aldrich and used as received.
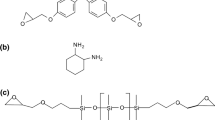
The liquid elastomer used was a CTBN copolymer and was purchased from Zibo Qilong Chemical Industry Co. of China. It contained 27 % acrylonitrile and 0.40 mmol/g carboxyl and had the viscosity of 60 Pa s at 70 °C. Scheme 1 shows the structures of the materials used in the study.
Samples preparation
In order to investigate the effect of CTBN on dicyandiamide-cured DGEBA, six formulations were prepared (Table 1). Epoxy resin, a required amount of CTBN and 7 phr of dicyandiamide were mixed and pre-cured at 140 °C for 2.5 h. The solubility of the curing reagent in epoxy resin increased at high temperature of 140 °C and both components reacted with DGEBA [27]. The viscosity of the mixture increased and prevented the dicyandiamide from sedimentation. The temperature was then reduced and by addition of 2.5 phr of Monuron and stirring of the mixture at low temperature for 5 min, a clear homogeneous mixture was obtained. The whole mixture was degassed in a vacuum oven for 10 min and it was then poured into a preheated desired iron mold which was greased and the mixture was cured in a hot air oven at 120 °C for 2 h.
Characterization and instruments
Fourier-transform infrared spectroscopy
Fourier-transform infrared (FTIR) spectroscopy was used to show the extent of reaction of epoxy resin with liquid rubber (CTBN). For this test the spectra were obtained with a Bruker (Equinox 55) FTIR.
Epoxide equivalent weight (EEW) analysis
The commercial epoxides were titrated as per ASTM D 1652 to determine their respective EEW values [30]. An amount of 100 g tetraethylammonium bromide was dissolved in 400 mL glacial acetic acid. About 0.4 g of each of the samples was dissolved in 10 mL methylene chloride in a flask. Ten millimeter of the above tetraethylammonium bromide solution was then added to the flask containing blend samples. Six to eight drops of crystal violet indicator were added into the same flask, stirred well and titrated against 0.16 N perchloric acid solution to an end point transition from sharp violet to green. This test was performed on 12 samples after pre-curing at 140 °C for 2.5 h (6 samples containing epoxy/CTBN/dicyandiamide and 6 samples containing epoxy/CTBN).
Tensile and impact tests
Dumbbell-shaped cured samples were used for the determination of tensile properties according to the ASTM D 638 standard on a universal testing machine (UTM) (150 kN, Santam, Iran). The crosshead speed of the machine was kept at 5 mm/min. The reported values were taken from an average of five specimens.
A minimum of three specimens, each with a dimension of 63.5 × 12.7 × 3.5 mm3, were prepared and tested by the Izod notched impact method, following the ASTM D 256 procedure, by using Zwick impact tester model 5102.
Fracture toughness test
Three-point single-edge notch bend (SENB) specimens were chosen for the fracture toughness measurements. According to ASTM D 5045 requirements, the rectangular specimens, each with 56.4 × 12.7 × 6.8 mm3 dimension, were in such a size to assure plane strain conditions. An initial crack (6.5 mm) was machined in specimens and a natural crack was generated by tapping on a fresh razor blade placed in the notch. The samples were tested using 150 kN Santam UTM machine in the flexure mode at a crosshead rate of 10 mm/min. All tests were carried out at ambient temperature. The following relationship was used to determine fracture toughness in terms of plane strain critical-stress-intensity factor, K IC:
where P Q is the critical load for crack propagation (kN); B is the specimen thickness (cm); W is the specimen width (cm); and f(x) is a non-dimensional shape factor given by;
where x = a/W and a is the crack length (cm) [31].
Dynamic mechanical thermal analysis
The glass transition temperatures of cured samples were determined by dynamic mechanical analysis (DMA) with a Tritec 2000B DMA, Triton which is a well-known method for determining viscoelastic properties by applying a controlled sinusoidal strain to a sample and measuring the resulting stress. DMA gives both storage modulus and loss modulus characteristics as a function of temperature. The measurements were carried out at a heating rate of 5 °C/min from −100 to 200 °C at fixed frequency of 1 Hz. The samples were rectangular bars of sizes 13 × 10 × 1.5 mm3.
Scanning electron microscopic analysis
The fractured tensile test samples were sputter coated with gold prior to scanning electron microscopy (SEM) study. A VEGA model SEM made by Tescan Company was used to examine the fractured surface of specimens and the SEM filament voltage was set at 20 kV and several micrographs were obtained for each sample.
Results and discussion
Fourier-transform infrared spectroscopic analysis
Fourier-transform infrared spectra of neat CTBN, neat epoxy and epoxy resin reacted with 15 phr CTBN (without curing agent) is demonstrated in Fig. 1a–c. For better understanding, the schematic diagram for the reaction between oxirane groups of DGEBA and carboxyl groups of CTBN is illustrated in Scheme 2.
Figure 1a (neat CTBN) shows two peaks at 1,738 and 1,708 cm−1 related to the carboxylic group of CTBN, but these absorption bands are not observed in the FTIR spectra of epoxy resin reaction with 15 phr CTBN (Fig. 1c). On the other hand, the peak at 1,732 cm−1 (Fig. 1c) might be indicative of the formation of ester functional group [13]. As depicted in Fig. 1a the presence of an absorption band near 3,200 cm−1 and another one at 2,237 cm−1 can be ascribed to –OH and –COOH groups and –C≡N of CTBN, respectively.
Figure 1b (neat epoxy) shows two peaks at 914 and 830 cm−1 which can be ascribed to the oxirane group of epoxy resin [13]. When CTBN is added to epoxy resin, generally it is expected that the oxirane ring is opened by the carboxyl functional group of the CTBN as it is shown in Scheme 2, but as can be seen in Fig. 1b (neat epoxy), c (epoxy resin reaction with 15 phr CTBN), no significant differences could be observed between those peaks (914 and 830 cm−1). This observation could be the result of either the incomplete reaction between the epoxy ring and carboxyl group or it can be said that the amount of epoxy ring reacted with carboxyl functional group was not high enough to be observed in FTIR. To verify whether the reaction was complete or not, the acid number of the mixture was examined. Thus, we understood that there were no unreacted carboxyl groups remaining in the reaction mixture; therefore, the second reason could be responsible of this situation. It can be said that since the end groups of CTBN is less than that of epoxy groups, so the end groups of epoxy that reacted with CTBN is low and for this reason we cannot see a significant change in oxirane peak. To support this reasoning, the following formula is given:
The values of epoxy group content in epoxy resin and carboxyl group content in CTBN are 5.34 and 0.40 mmol/g, respectively. For sample with 15 phr of CTBN (EC15), the value of epoxy/carboxyl ratio is calculated and it is observed that epoxy group content value is much larger than the carboxyl group content (Eq. 5)
In other words, if by assuming that all carboxyl groups have reacted with the epoxy groups, then it is just 1.1 % of the epoxy groups that are used in reaction and for this reason we cannot see a significant change in oxirane peak in FTIR.
Epoxide equivalent weight analysis
The EEWs of 12 samples (6 samples containing epoxy/CTBN/dicyandiamide and 6 samples containing epoxy/CTBN) were determined via the standard titration method and the results are shown in Fig. 2. Epoxy/CTBN without dicyandiamide samples were prepared just for comparison with those containing this curing agent in order to make sure that in the mixing of epoxy with CTBN in the presence of dicyandiamide, no reaction takes place between the epoxy and the curing agent. Any change in EEW of epoxy/CTBN blends is related to the reaction of epoxy resin with CTBN.
According to Fig. 2, while the concentration of CTBN is increased, the samples containing epoxy and CTBN without dicyandiamide after reaction at 140 °C for 2.5 h have equal amounts of EEW. This observation is in accordance with the justifications made for FTIR analysis. On the other hand, as it is evident from Fig. 2, in the samples containing dicyandiamide, when its concentration remains unchanged, the values of EEWs are increased slightly; but this behavior is not observed for the samples without dicyandiamide. The increase in the amount of EEWs is meant to reduce the epoxy groups, therefore it can be concluded that the presence of liquid rubber (CTBN) in the system of epoxy/dicyandiamide can increase the rate of epoxy curing.
Mechanical properties
Figure 3 shows the stress–strain curves of one sample as representative of each CTBN-modified epoxy resins as well as neat epoxy sample. Brittle fracture, i.e., absence of yielding and non-linear viscoelastic behavior are confirmations for the neat epoxy resin; whereas, the CTBN-modified epoxy resins exhibit large deformation and higher strain-at-break. It can be observed from Fig. 3 that CTBN improved the toughening of epoxy resin.
Tensile strength, modulus, elongation-at-break and toughness (the area under the stress–strain curve) and impact strength are plotted with the variation of CTBN content in the blend of epoxy/rubber samples and have been illustrated in Figs. 4, 5, 6, 7, 8, respectively. Figure 4 shows the tensile strength initially increased from 42 to 57 MPa by about 26 % compared with neat epoxy as 5 phr CTBN was added in the blend of epoxy and CTBN. An increase in tensile strength up to 15 phr might have demonstrated some types of interaction between the rubber particles (CTBN) with epoxy resin, which might have been mechanically stronger than the unmodified blend system [12]. Beyond 15 phr, the reduction in the values of modified blends may be ascribed to the lowering in cross-linking density of the epoxy network as the modifier occupies the reaction centers during modification. For the decrease in the tensile strength, different discussions were given by Huang and Kinloch [32] and Shukla and Srivastava [12]. Kinloch et al. concluded that the value of tensile strength is appreciably reduced by the presence of microvoids, but Srivastava et al. concluded that the lowered tensile strength can be interpreted with the stress concentration effect of the rubber particles.
A continuous decrease in modulus was observed as the concentration of the CTBN phase increased (Fig. 5). The modulus decreased from 2.89 to about 0.89 GPa with increasing CTBN content. In other words, when 25 phr CTBN was added in the blend, the value of modulus decreased by about 225 % compared with the neat epoxy resin. It is indeed important to note that the modulus of neat dicyandiamide-cured epoxy was higher than the values reported for other systems [5, 13]. The decrease in modulus can be due to lower modulus of elastomer phase than the modulus of the epoxy phase that this phenomenon may be due to the increase in the relative amount of dissolved rubber as rubber content increases. Generally, when a rubber modifier (like CTBN) is added to the epoxy resin, its elastomeric character enforces a decrease in modulus, significantly. In agreement with many studies [13, 33], the tensile modulus decreases with increases in soft rubber content.
The elongation-at-break of the modified epoxies increased continuously with increasing rubber phase content in the blend samples (Fig. 6). The elongation-at-break increases from about 1.60 to 3.87 % with increasing CTBN content from 0 to 25 phr. This increasing could be a reason for a good adhesion via a chemical reaction between the rubber and epoxy phase.
The area under the stress–strain curves, which is a measure of toughness, is indicated in Fig. 7 as a function of CTBN content. From Fig. 7, it can be observed that all modified epoxies have higher toughness than the neat epoxy resin. The blend network containing a 15 phr CTBN has a maximum toughness value of 45.77 kJ/m2 which is about 58 % higher than that of neat epoxy. The increase in toughness may be due to the amount of elastic energy stored in the rubber particles during stretching [13]. Beyond 15 phr, the reduction in the values of toughness can be seen and this behavior is in agreement with the reported results [5, 13]. It is worth mentioning that the toughness reported in Fig. 7 is calculated from the area under the stress–strain curves. Since the modulus and strength decrease following the addition of CTBN beyond 15 phr, it is expected to see that the toughness would be decreased as well. But, the better evaluation of true toughness of a system is to measure the fracture toughness, K IC, the results of which will be explained further and we will see that the fracture toughness is not reduced beyond 15 phr of CTBN addition. Overall, the results of tensile properties indicate that the dicyandiamide-cured epoxy resin containing CTBN particles ranging between 10 and 15 phr showed well-balanced properties.
Figure 8 illustrates the Izod impact strength of the notched modified epoxy samples as function of CTBN content. It is evident from Fig. 8 that the impact strength of the modified networks is significantly greater than that of the neat resin. The impact strength values ranged from 7.19 J/m for the neat epoxy to 19.20 J/m for 15 phr CTBN content. In other words, at 25 phr rubber, a threefold increase in the impact strength was observed. The impact behavior of the blend samples may be explained according to the two-phase nature of the system [5]. These might dissipate at the crack tip by these multiple deformations, which reflect an increase in the values of impact strength [12]. Thus, the rubber particles are able to prevent the crack growing to a catastrophic size [5]. The deformation of the rubber particles in the epoxy resin seemed to be responsible for the stress transfer. Shear yielding of the matrix is another reasonable mechanism that could have happened.
Fracture toughness
The variation of fracture toughness (K IC) as a function of CTBN concentration is displayed in Fig. 9. This figure reveals that the values of K IC for the CTBN-modified epoxy resins are much higher than K IC of the unmodified epoxy which reflects the poor crack resistance of the neat epoxy resin systems. It is also revealed that the values of K IC increased up to 15 phr of CTBN added in the blend system and then a slight reduction in the values of K IC was observed as it is evident in Fig. 9. In general, above 10 phr CTBN content, all blend samples have shown approximately the same values of K IC. This may indicate that in all blend samples the increase in K IC values can be attributed to the percolation of rubber particles around the polymer chains of the epoxy resin [5]. Huang and Kinloch [32] concluded that the increasing of fracture toughness (K IC) can be interpreted by the presence of the microvoids.
The differences between Izod impact strength test and fracture toughness test might be attributed to the fact that the tests were performed under different conditions. K IC was realized at a relatively low speed, whereas the impact was tested at a high speed. From these two tests it may be realized that this system shows different responses in high-speed (impact) and slow-speed conditions. In other words, when blend samples are tested at a high speed, the rubber particles would not have had time to respond to this type of loading.
Dynamic mechanical thermal analysis
Dynamic mechanical loss factor (tan δ) and storage modulus (E′) of neat epoxy resin as well as 15 and 25 phr of CTBN-modified epoxies as a function of temperature are depicted in Figs. 10 and 11, respectively. The glass transition temperature (at maximum point of tan δ curve at 1 Hz) is 116.1 °C for the neat epoxy, which is lower than the T g of the modified epoxy. The addition of 15 and 25 phr CTBN in the epoxy increased the T g of the epoxy phase by about 6.3 and 11.3 °C, respectively. In other words, the addition of CTBN in epoxy system causes a significant increase in the glass transition temperature. Different effects have been reported before [13]. The full width at half maximum (FWHM) of the tan δ peak of 25 phr of CTBN-modified epoxy is much higher than that of the neat epoxy. This suggests that this sample has a wider distribution of the local cross-link density than the neat epoxy resin [34].
Figure 11 shows that the neat epoxy has higher initial storage modulus. In all samples, the values of E′ decrease with increases in temperature. A significant drop in modified epoxies at about −30 °C may be attributed to the T g of CTBN. Also, at any given temperature the gradual drop of storage modulus with the addition of CTBN denotes the increase in flexibility of modified epoxy which could be due to lower modulus of liquid rubber or plasticizing effect of CTBN liquid rubber. The neat epoxy exhibits higher rubbery plateau values of storage modulus and 25 phr modified epoxy resin showed much lower values. This means that the modified resins displayed lower equivalent average cross-link density and this might be the reason of their lower tensile modulus.
Scanning electron microscopic analysis
The fractured surfaces of blend samples from tensile test were studied using SEM. Generic fracture surfaces of the unmodified and modified epoxy resin containing CTBN ranging between 5 and 25 phr, with an increment of 5 phr CTBN, are shown in Fig. 12. The pattern of morphology observed for the unmodified epoxy matrix (Fig. 12a) shows smooth and glassy fractured surfaces with ripples. The fractured surfaces of the CTBN-modified epoxy matrix showed two-phase morphology with a scattered rubber phase (CTBN spherical particles) and a rigid continuous phase (epoxy matrix). The soft elastomeric phase was phase separated from the hard epoxy matrix during the early stage of cure [13].
The size of rubber particles in blend sample containing 15 phr of CTBN (Fig. 10d) was larger than rubber particles in blend samples containing 5 and 10 phr of CTBN (Fig. 12b, c). This increase in the size of the scattered rubber particles could be associated with the coalescence of the dispersed rubber particles.
From Fig. 12f (systems with 25 phr of CTBN) it can be observed that with the amount of rubber higher than the optimum, the second rubbery phase became more and more aggregated leading to a phase of less distinct epoxy matrix [13] with a tendency towards co-continuous phase. This condition led to the flexibility of the blend resulting in reduced mechanical properties like tensile strength, tensile modulus and toughness.
Different mechanisms like crazing, shear banding and elastic deformation of the rubber particles have been proposed and these mechanisms were thought to act alone or in combination, to produce the toughening effect in rubber-modified epoxies [13].
Conclusion
Toughening of dicyandiamide-cured DGEBA with liquid CTBN copolymer has been investigated by varying the composition of CTBN from 0 to 25 phr. The samples were cured with dicyandiamide and Monuron accelerator. To avoid the sedimentation of dicyandiamide, the mixture of epoxy/dicyandiamide/CTBN was heated for a certain period of time. FTIR spectra and EEWs of samples showed the reaction between oxirane groups of DGEBA and carboxyl groups of CTBN. The results showed that the tensile strength of the modified epoxy increased by about 26 % compared with the neat epoxy resin, when 5 phr of CTBN was used. Tensile modulus decreased gradually by adding CTBN but, the elongation-at-break increased by 59 % and Izod impact strength increased by 63 % with increases in CTBN content compared with the neat epoxy resin. The maximum toughness was achieved at optimum concentration of 15 phr of CTBN, whereas the fracture toughness (K IC) increased up to 10 phr of CTBN and it more or less remained constant (1.5 MPa m1/2) for higher CTBN content. The glass transition temperature (T g) of the modified epoxy was significantly increased upon the inclusion of CTBN. The SEM analysis showed a two-phase morphology where the rubber particles were dispersed in the epoxy matrix and a tendency towards co-continuous phase was observed upon the inclusion of 25 phr of CTBN.
References
Thomas R, Boudenne A, Ibos L, Candau Y, Thomas S (2010) Thermophysical properties of CTBN and HTPB liquid rubber modified epoxy blends. J Appl Polym Sci 116:3232–3241
Kaynak C, Arikan A, Tincer T (2003) Flexibility improvement of short glass fiber reinforced epoxy by using a liquid elastomer. Polymer 44:2433–2439
Tripathi G, Srivastava D (2009) Cure kinetics of ternary blends of epoxy resins studied by nonisothermal DSC data. J Appl Polym Sci 112:3119–3126
Ramos VD, Costa HM, Soares VLP, Nascimento RSV (2005) Modification of epoxy resin: a comparison of different types of elastomer. Polym Test 24:387–394
Tripathi G, Srivastava D (2008) Studies on the physico-mechanical and thermal characteristics of blends of DGEBA epoxy, 3,4 epoxy cyclohexylmethyl, 3′,4′-epoxycylohexane carboxylate and carboxyl terminated butadiene co-acrylonitrile (CTBN). Mater Sci Eng A 496:483–493
Liu K, Lu S, Li S, Huang B, Wei C (2012) Mechanical and thermal properties of POSS-g-GO reinforced epoxy composites. Iran Polym J 21:497–503
Tripathi G, Srivastava D (2009) Toughened cycloaliphatic epoxy resin for demanding thermal applications and surface coatings. J Appl Polym Sci 114:2769–2776
Thomas R, Durix S, Sinturel C, Omonov T, Goossens S, Groeninckx G, Moldenaers P, Thomas S (2007) Cure kinetics, morphology and miscibility of modified DGEBA-based epoxy resin—Effects of a liquid rubber inclusion. Polymer 48:1695–1710
Lu S, Ban J, Yu C, Deng W (2010) Properties of epoxy resins modified with liquid crystalline polyurethane. Iran Polym J 19:669–678
Thomas R, Yumei D, Yuelong H, Le Y, Moldenaers P, Weimin Y, Czigany T, Thomas S (2008) Miscibility, morphology, thermal, and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 49:278–294
Ma SQ, Liu WQ, Hu CH, Wang ZF (2010) Modification of epoxy resins with polyether-g-polysiloxanes. Iran Polym J 19:185–196
Shukla SK, Srivastava D (2006) Blends of modified epoxy resin and carboxyl-terminated polybutadiene. J Appl Polym Sci 100:1802–1808
Tripathi G, Srivastava D (2007) Effect of carboxyl-terminated poly(butadiene-co-acrylonitrile) (CTBN) concentration on thermal and mechanical properties of binary blends of diglycidyl ether of bisphenol-A (DGEBA) epoxy resin. Mater Sci Eng A 443:262–269
Lee HB, Kim HG, Yoon KB, Lee DH, Min KE (2009) Preparation and properties of a carboxyl-terminated butadiene acrylonitrile toughened epoxy/montmorillonite nanocomposite. J Appl Polym Sci 113:685–692
Yadav R, Srivastava D (2009) The effect of CTBN concentrations on the kinetic parameters of decomposition of blends of cardanol-based epoxidized novolac resin modified with carboxyl-terminated liquid copolymer. J Appl Polym Sci 114:1694–1701
Yadav R, Srivastava D (2009) Synthesis and properties of cardanol-based epoxidized novolac resins modified with carboxyl-terminated butadiene–acrylonitrile copolymer. J Appl Polym Sci 114:1670–1681
Yadav R, Srivastava D (2009) Toughened cycloaliphatic epoxy resin for demanding thermal applications and surface coatings. J Appl Polym Sci 114:2769–2776
Chenab WS, Changa YL, Hsianga HI, Hsua FC, Shena YH, Yena FS (2011) Mechanical and dielectric properties of NiZn ferrite powders-CTBN modified epoxy resin coatings. Polym-Plast Technol Eng 50:568–572
Verchere D, Sautereau H, Pascault JP (1990) Rubber-modified epoxies: I. Influence of carboxyl-terminated butadiene-acrylonitrile random copolymers (CTBN) on the polymerization and phase separation processes. J Appl Polym Sci 41:467–485
Wise CW, Cook WD, Goodwin AA (2000) CTBN rubber phase precipitation in model epoxy resins. Polymer 41:4625–4633
Poornima Vijayan P, Puglia D, Jyotishkumar P, Kenny JM, Thomas S (2012) Effect of nanoclay and carboxyl-terminated (butadiene-co-acrylonitrile) (CTBN) rubber on the reaction induced phase separation and cure kinetics of an epoxy/cyclic anhydride system. J Mater Sci 47:5241–5253
Kavitha, Revathi A, Rao S, Srihari S, Dayananda GN (2012) Characterization of shape memory behaviour of CTBN-epoxy resin system. J Polym Res 19:9894–9901
Poisson N, Maazouz A, Sautereau H, Taha M, Gambert X (1998) Curing of dicyandiamide epoxy resins accelerated with substituted ureas. J Appl Polym Sci 69:2487–2497
Hong SG, Lin JJ (1996) Effects of DICY content and metal oxide on the curing behavior of a brominated epoxy resin. J Appl Polym Sci 59:1597–1605
Ivanova KI, Pethrick RA, Affrossman S (2001) Hygrothermal aging of rubber-modified and mineral-filled dicyandiamide-cured DGEBA epoxy resin: II. Dynamic mechanical thermal analysis. J Appl Polym Sci 82:3477–3485
Jackson ML, Love BJ (2004) Dicyandiamide precipitation in epoxy solutions and latex dispersions: threshold concentration analysis using a two-stage drying model. Polymer 45:7229–7238
Wang Q, Storm BK, Houmøller LP (2003) Study of the isothermal curing of an epoxy prepreg by near-infrared spectroscopy. J Appl Polym Sci 87:2295–2305
Gaukler JC, Müller U, Krüger JK, Possart W (2011) Functional nano fillers in epoxy-dicyandiamide adhesives for prolonged shelf life and efficient cure. e-Polymers 10:1–15
Hagnauer GL, Dunn DA (1981) Dicyandiamide analysis and solubility in epoxy resins. J Appl Polym Sci 26:1837–1846
Dreerman E, Narkis M, Siegmann A, Joseph R, Dodiuk H, Dibenedetto AT (1999) Mechanical behavior and structure of rubber modified vinyl ester resins. J Appl Polym Sci 72:647–657
Pramanik M, Mendon SK, Rawlins JW (2012) Determination of epoxy equivalent weight of glycidyl ether based epoxides via near infrared spectroscopy. Polym Test 31:716–721
Huang Y, Kinloch AJ (1992) The toughness of epoxy polymers containing microvoids. Polymer 33:1330–1332
Saadati P, Baharvand H, Rahimi A, Morshedian J (2005) Effect of modified liquid rubber on increasing toughness of epoxy resins. Iran Polym J 14:637–646
Kishi H, Naitou T, Matsuda S, Murakami A, Muraji Y, Nakagaw Y (2007) Mechanical properties and inhomogeneous nanostructures of dicyandiamide-cured epoxy resins. J Appl Polym Sci 45:1425–1434
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akbari, R., Beheshty, M.H. & Shervin, M. Toughening of dicyandiamide-cured DGEBA-based epoxy resins by CTBN liquid rubber. Iran Polym J 22, 313–324 (2013). https://doi.org/10.1007/s13726-013-0130-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-013-0130-x