Abstract
The purpose of this work is to present the results of preparing a polymeric composite with enhanced properties based on natural rubber and hemp. Amounts of 10 and 20 phr hemp were used to obtain the composites. The samples have been processed by sulfur vulcanization and characterized by several methods. The mechanical characteristics, gel fraction, cross-link density, rubber-fiber interactions and water uptake have been investigated depending on the hemp content. Fourier transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) techniques were also employed for characterization. The values of hardness, tensile strength and tearing strength have increased with the fiber content increasing due to the interaction between the fibers and natural rubber. Also, good adhesion between hemp fibers and rubber matrix was observed in SEM micrographs. The gel fraction value was over 95 % for all composites and increased with the increasing of hemp content. The cross-link density was determined on the basis of equilibrium solvent-swelling measurements applying the modified Flory–Rehner equation. It was observed that cross-linking density of composites increased slightly with the increase of amount of hemp but still was lower than that of the natural rubber without hemp. The extent of interaction between rubber and fiber was determined using the Kraus equation. Results of water absorption tests showed that water uptake increased with the increase of fiber content and temperature. The physical and chemical investigations have shown the reinforcing effect of hemp on sulfur vulcanized natural rubber, as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The reinforcement of rubber compounds with fibers has become necessary in many products, especially in the tire, hose and belt industries [1]. Currently, the most viable way toward eco-friendly composites is the use of natural fibers as reinforcement. Natural fibers represent a traditional class of renewable materials which, nowadays, are experiencing a great revival [2]. On the other hand, natural fibers exhibit many advantageous properties which promote the replacement of synthetic fibers in polymer composites. They are low-density materials yielding relatively lightweight composites with high-specific properties, therefore, natural fibers offer a high potential for an outstanding reinforcement in lightweight structures.
Natural fibers are derived from renewable resources and do not require a large amount of energy to process them and are biodegradable, as well [3, 4].These fibers also offer significant cost advantages and therefore the utilization of lightweight, lower cost natural fibers such as jute, flax, hemp, sisal, abaca, coir offer the potential to replace a large segment of the synthetic fibers in numerous applications [5, 6]. Production of natural fibers causes less severe environmental impacts as compared to those of synthetic fibers. Natural fibers cultivation depends mainly on solar energy and for the fiber production, processing and extractions, a relatively small amount of fossil fuel energy is required. On the other hand, the production of synthetic fiber depends mainly on fossil fuels and needs nearly ten times more energy as compared to natural fiber. As a result, the pollutant gas emissions to the environment from synthetic fiber production are significantly higher than those from the natural fiber production [3, 7].
In our work, hemp fibers obtained from the bast of the Cannabis sativa L. were used for natural rubber reinforcement. Hemp has been cultivated for at least 6,000 years and it may be one of the oldest non-food crops. Growing practice shows that biomass yield of hemp is high, and hemp improves the soil structure, the tall plant stems of hemp suppress weeds effectively, and diseases and pests are rarely recorded. Thereby, addition of pesticides is not needed. It has also been reported that hemp produces several times more of the important cellulose source, fiber component, than other crops such as corn, kenaf, and cotton. Therefore, it is of interest to determine the potential for hemp fibers to find appropriate solutions and sustainable systems. The most usual purpose of hemp cultivation is to isolate the fibers present in the bark on the hemp stem surface, for production of ropes, textiles and paper. Some newer industrial uses of plant cellulose have been developed and are found to be promising; one of them is cellulose nanoparticles usage as fillers to improve mechanical and barrier properties of biocomposites that are a rapidly developing branch of biotechnology [4, 8].
In this work, some composites based on natural rubber and hemp were analyzed in which the elastomer was cross-linked using accelerator and sulfur. The sulfur vulcanization process requires the presence of carbon–carbon double bonds in the polymer chains which leads to a three-dimensional rubber network, where the polymer chains are linked to each other by sulfur bridges. As a result, sulfur-cured articles have good tensile and tear strength, good dynamic properties, but poor high-temperature properties like aging, for instance [9, 10]. Sulfur vulcanization reactions can be broadly classified into two types: unaccelerated and accelerated ones [11, 12]. Unaccelerated sulfur formulation consists of rubber and sulfur while the accelerated systems contain rubber, sulfur, accelerators and activators (ZnO, PbO, MgO etc.) [11]. Zinc oxide is the most important activator. Usually an activator system, a combination of zinc oxide and a long-chain fatty acid such as stearic acid is used. Fatty acids, e.g., stearic acid, are used to solubilize zinc ions into the system and set them free to form complexes with accelerators [12]. Generally, it can be stated that increasing the pH leads to activation of the vulcanization. The basic activators mentioned lead to improved strength properties of the vulcanizates and reduced vulcanization time [11]. In our experiments, we used two accelerators, zinc oxide and stearic acid (Table 1).
Vulcanization with sulfur and accelerators of NR is done generally by ionic mechanism and leads to the formation of sulfur bridges between (C–S x –C) macromolecules or cyclic combination of sulfur. At high temperatures, desulphuration takes place, determining the formation of shorter sulfur bridges. The initial step in vulcanization (Scheme 1) seems to be the reaction of sulfur with the zinc salt of the accelerator to give a perthio-salt, XS x ZnS x X where X is a group derived from the accelerator. This salt reacts with the rubber hydrocarbon RH, to give a rubber-bound intermediate and a perthio-accelerator group which, with further zinc oxide will form a zinc perthio-salt of lower sulfur content; this may again be an active sulfurating agent, forming intermediates XS x−1R. In this way, each molecule of accelerator gives rise to a series of intermediates of varying degrees of polysulfidity [13].
The intermediate XS x R then reacts with a molecule of rubber hydrocarbon RH to give a cross-link, and more accelerator is regenerated (Scheme 2).
Scheme 3 shows the reaction route which may be written for sulfur vulcanization. In the reaction, intramolecular bridges (cyclic structure) can be formed as showed in Scheme 4.
The objective of this research is to obtain a new elastomeric material based on NR with good characteristics and compatibility with environment, by replacing active fillers of rubber blends such as carbon black or silica (which has serious harmful effects on health) with natural fibers. Silica is known to have adverse effects on health, causing silicosis, cancer (Group 1 according to IARC—the International Agency for Research on Cancer) tuberculosis, autoimmune and kidney diseases. In 1995, the IARC rated carbon black as IARC classification 2B—possibly carcinogenic to humans and definitely carcinogenic to animals [14–16]. The novelty of the present work consisted in the use of hemp fibers as organic fillers in the natural rubber mixtures to obtain composites with enhanced properties. There are many research works in which composites were obtained based on synthetic rubber and natural fibers [17–19], but only a few composite materials were based on natural rubber and hemp fibers that were analyzed [20–22]. Two of these studies were conducted by us, where the cross-linking has been achieved with benzoyl peroxide or by electron beam irradiation [21, 22]. Studies on the use of natural fibers to replace inorganic fillers in rubber mixtures started to grow during the last 10 years due to their advantages and for these reasons, this research can contribute to developing and consolidating new knowledge in the rubber field.
Experimental
Materials
The following materials were used to prepare the above polymer composites: natural rubber (NR) Crep 1X (74 ML1 + 4 Mooney viscosity at 100 °C, 0.32 % volatile materials, 0.38 % nitrogen, 0.22 % ash, 0.021 % impurities) from Almar Trading Co (Pte) Ltd, Sri Lanka and ground hemp (thread length of max 3 mm). Cross-linking was done using: 2.5 phr sulfur, 0.5 phr 2-mercaptanobenzothiazole (MBT) and 1 phr tetramethylthiuram disulphides (TMTD) (assay 97 %, autoignition temperature 316 F), Sigma-Aldrich, as accelerators, the 5 phr zinc oxide (97.1 % active ingredient) Werco Metal, Zlatna Romania and 1 phr stearic acid (0.025 % of ask) Cevo Industry Co., China as activator. The main properties and chemical structure of the reactants used (accelerators and activators) are presented in Table 1.
Sample preparation
The blends were prepared on an electrically heated laboratory roller mill. For preparation of the polymeric composites, the blend constituents were added in the following sequences: natural rubber (NR) roll binding (2 min), embedding ZnO and stearic acid (1 min), adding ground hemp (2–3 min), cooling the mixture and adding sulfur and vulcanization accelerators (1 min), homogenization of blends and removing from the roll in the form of sheet (4 min). The process variables were: temperature 25-50 ± 5 °C, ratio 1/1.1 and total blending time 9–10 min. The plates required for physico-mechanical tests with sizes of 150 × 150 × 2 mm were obtained by pressing in a hydraulic press at 160 °C and 150 MPa; the curing time was 19 min. The recipe according to which the rubber samples have been prepared is shown in Table 2.
Mechanical characteristics
Tensile strength tests were carried out with a Schopper strength tester with testing speed of 460 mm/min, using dumb-bell shaped specimens according to ISO 37/2012. Residual elongation is the elongation of a specimen and was calculated using the following equation:
where, L 0 is the initial length between two marks and L is the length between the marks 1 min after the sample broke in a tensile test.
The hardness was measured using a hardener tester according to ISO 7619-1/2011 on samples having the thickness of 6 mm. The elasticity (rebound resilience) was evaluated with a Schob type test machine also on samples of 6 mm thick, according to ISO 4662/2009.
The sol–gel analysis was performed on cross-linked NR rubber (with and without hemp) to determine the mass fraction of insoluble NR (the network material resulting from network-forming cross-linking process) from samples (gel fraction). The samples were swollen in toluene and extracted after 72 h to remove any scissioned fragments and unreacted materials. The networks thus treated were dried in air for 6 days, after that in a laboratory oven at 80 °C for 3 h and finally were reweighed. The gel fraction was calculated as follows:
Where, m s and m i are the weight of the dried sample after extraction and the weight of the sample before extraction, respectively [23, 24].
The cross-link density (ν) of the samples was determined on the basis of equilibrium solvent-swelling measurements (in toluene and xylene at 23−25 °C) by applying the well-known modified Flory–Rehner equation for tetrafunctional networks. The samples (2 mm thick) were initially weighed (m i) and immersed in solvent for 72 h. The swollen samples were removed and cautiously dried to remove the excess solvent before being weighed (m g) and, during this operation, the samples were covered to avoid solvent evaporation during weighing. The solvent traces and other small molecules were then eliminated by drying in air for 6 days and in a laboratory oven at 80 °C for 3 h. Finally, the samples were weighed for the last time (m s) and volume fractions of polymer in the samples at equilibrium swelling ν 2m were determined from swelling ratio G as follows:
where,
and ρ r and ρ s are the densities of rubber samples and solvent (0.942, 0.865 and 0.864 g/cm3 for rubber, toluene and xylene, respectively). The densities of elastomer samples were determined by hydrostatic weighing method, according to SR ISO 2781/2010. By this method, the volume of a solid sample is determined by comparing the weight of the sample in air with the weight of the sample immersed in a liquid of a known density. The volume of the sample is equal to the difference in the two weights divided by the density of the liquid. The cross-link densities of the samples, ν, were determined from measurements in a solvent, using the Flory–Rehner relationship as follows
where, V 1 is the molar volume of solvent (106.52 and 122.88 cm3/mol for toluene and xylene, respectively), ν 2m is the volume fraction of polymer in the sample at equilibrium swelling, and χ 12 is the Flory–Huggins polymer–solvent interaction term.
Rubber–fiber interactions
The extent of interaction between rubber and fiber can be analyzed using Kraus equation. The Kraus theory and Kraus equation [25] have been successfully used by some researchers to assess the interfacial interaction in fiber-reinforced rubber composites [26–28]. The Kraus equation is as follows:
where, V ro and V rf are the volume fractions of rubber in the vulcanized gum and in fiber-filled swollen sample, respectively, f is the volume fraction of fiber and m is the fiber polymer interaction parameter. The volume fraction of rubber in the swollen sample, V rf, was calculated by the following expression:
where, ρ r and ρ s are the densities of rubber samples and solvent (0.94 g/cm3 for natural rubber and 0.866 g/cm3 for toluene), respectively, D is the deswollen weight of the test specimen (dry weight), F is the weight fraction of the insoluble components, T is the weight of the specimen and A 0 is the weight of the absorbed solvent at swelling equilibrium.
Water uptake test
Effect of water absorption on fiber reinforced natural rubber composites are investigated in accordance with SR EN ISO 20344/2004. The samples were dried in an oven at 80 °C for 2 h and then were allowed to cool at room temperature in desiccators before weighing. Water absorption tests were conducted by immersing the samples in distilled water in bottles and keeping at room temperature 23 ± 2 °C and in a laboratory oven at 70 ± 1 °C. Samples were removed from the bottles at periodic intervals and the wet surfaces were quickly wiped using a clean dry cloth or tissue paper and weights of the specimen after swelling were determined at regular intervals until no further increase in solvent uptake was detected. The moisture absorption was calculated by the weight difference. The percentage weight gain of the samples was measured at different time intervals. The water uptake was calculated as follows:
where, m S is the weight of the sample saturated with water, determined at periodic intervals and m 1 is the initial weight of the oven-dried sample [29, 30].
FTIR spectroscopy
Changes of the chemical structure of natural rubber/hemp fibre composites were highlighted using a FTIR spectrophotometer—JASCO FT/IR 4200, by ATR measurement method. Samples spectra are the average of 30 scans realized in absorption in the range of 4,000–600 cm−1, with a resolution of 4 cm−1.
SEM
The surface texture of the rubber/hemp composites was examined using the Quanta 200 scanning electron microscope (FEI Co., USA). All the surfaces were examined after sputter coating with gold to avoid electrostatic charging and a poor image resolution.
Results and discussion
Mechanical characteristics
The variations of mechanical properties are shown in Table 3. It can be seen from Table 3 that mechanical properties of the NR/hemp composites vulcanizates were affected by increasing the fiber concentration, compared with NR sample. The results illustrate that the properties, excepting the elasticity, are improved by the addition of hemp. The elasticity decreased with the increase of fiber amount in blends. According to Morton [31], elasticity (rebound resilience) is the ratio of energy given up on recovery from deformation to the energy required to produce the deformation [32]. The decrease in resilience is explained by the hemp particles that introduce a mechanism by which the strain energy diminishes. Since rebound resilience is directly proportional to the degree of elasticity and segment mobility, the presence of hemp reduces elasticity and segment mobility of the cured NR composites. The increase of the amounts of filler leads to the increase of hardness and decreased resilience [31–33].
The mobility of hemp particles and slippage of chains attributed to applied stresses on cured composite increases the hysteretic behavior of the cured composite [33, 34]. Therefore, the resilience decreased with increasing load of hemp. This decrease may be due to the change in the morphology of the sample by vulcanization. The hardness exhibited an increase of about 40 % for 10 phr hemp and 47 % for 20 phr hemp while the tensile strength exhibited an increase of 92 and 200 % with the same fiber loading. Therefore, this can be attributed to a strong interface as well as a close packing arrangement in the composite.
As expected, incorporation of hemp in matrix has improved tensile strength. This improvement is due to the adequate interaction between natural rubber and fiber [28]. The increase in tensile strength is due to the good adhesion of the filler in matrix and to the agglomeration of filler particles. The elongation-at-break decreased with the increase of fiber content in composites. The decrease of elongation-at-break with the increasing of fiber content in the composite is the result of the high cross-linking. On the other hand, its reduction indicates that ductility became worse when hemp was added to NR composites. Thus, the hemp addition to composites restricts the molecular chains movement [35]. The decrease in the elongation-at-break is also due to the striking forces between the filler and the polymer molecules leading to the development of a cross-linked structure which limited the free mobility of the polymer chains, hence increased the resistance to accelerate upon the execution of tension [8, 35]. The elongation-at-break values showed a decrease of 28 % for both concentrations of hemp. The tearing strength has followed the same trends as the hardness and tensile strength: it has increased with the increase of fiber content in composite. The tearing strength has shown an increase of about 75 % for 10 phr hemp and with 287 % for 20 phr hemp content in composite. The values of hardness, tensile strength and tearing strength have increased with the fiber content increasing in the composite due to a better interaction of fiber with NR. These results indicate that the hemp has a reinforcing effect on natural rubber.
Gel fraction and cross-link density of blends
To determine the cross-linking density, it is necessary to know the parameter χ, that is the Flory–Huggins interaction parameter between solvent and polymer, which can be determined according to Blanks and Prausnitz [36, 37] by applying Eq. (3).
where:χ S is the entropic contribution of this parameter (usually beings 0.34, according to Blanks and Prausnitz);χ H is the enthalpic contribution, obtained from the molar volume of a solvent, V mS,, universal gas constant R, absolute temperature T (K), the Hildebrand solubility parameters of the polymer (δ P) and solvent (δ S).
The Hildebrand solubility parameters of the solvents (δ S ) are 18.3 (MPa)1/2 and 18.2 (MPa)1/2 for toluene and xylene, respectively [38].
The solubility parameter of a polymer can be estimated by use of one of several group-contribution methods, such as those given by Small, Hoy and Van Krevelen [37]. Calculation of δ P by a group-contribution method requires the value of a molar attraction constant F i , for each chemical group in the polymer repeating unit. The solubility parameter of a polymer is then calculated from molar attraction constants and the molar volume of the polymer, V (cm3 mol−1), as follows:
The molar volume of the natural rubber is of 72.468 cm3 mol−1. A listing of the molar attraction constants, F i for chemical group in natural rubber repeating-unit and the molar attraction constant for the repeating unit of NR, \(\sum {F_{i} }\), by Small, Hoy and Krevelen is given in Table 4.
In Table 5 are given the results obtained for the solubility parameter (by Small, Hoy and Krevelen) and for the Flory–Huggins interaction parameter between solvent and polymer, χ.
Table 6 shows the gel fraction (mass fraction of the network material resulting from a network-forming polymerization or cross-linking process; the gel fraction comprises a single molecule spanning the entire volume of the material sample) and cross-link density (number of cross-links per unit volume in a polymer network) of the samples vulcanized as a function of the hemp content. The cross-link density was calculated using the values of χ 12 as being 0.3900 and 0.3875 for toluene and xylene, respectively.
The gel fraction values are over 95 % for all blends and this increase depended on the amount of hemp in the composites. The cross-link density (ν) of the samples changed slightly as the amount of hemp in blends increased. Our experimental results, confirmed by others [39–41], show that with the increase in cross-link density, the hardness and tensile strength have increased, whereas the elongation-at-break decreased. Thus, it can be considered once again that hemp acted as filler in natural rubber blends and leads to reinforcement of them.
Rubber-fiber interactions
The extent of interaction between rubber and fiber was analyzed using Kraus equation and the results are listed in Table 7. Samples of NR with 10 and 20 phr content of hemp fiber were swollen in toluene and xylene for 72 h. From the results it is observed that for both solvents, the equilibrium solvent uptake of the samples has increased when the fiber content increased, fact which caused a decrease in V rf. The ratio V ro/V rf increased because V r0 is constant. This is due to the decreased hindrance exerted by the hemp fibers at higher loadings.
The diffusion mechanism in the composite in strongly connected with the ability of rubber to provide pathways for the solvent to progress in the form of randomly generated voids. As the void formation increased with fiber content, the solvent uptake also increased. The ratio V ro/V rf is the degree of restriction of swelling of the rubber matrix due to the presence of fibers [28]. The ratio V ro/V rf for composites with 10 phr and 20 phr hemp was found to be 1.0881 and 1.0952 in toluene, respectively. Similar values of 1.0779 and 1.0865 were also obtained in xylene for composites with 10 and 20 phr hemp, respectively. Because the increases up to 1 % (0.65 and 0.79 % in the case of toluene and xylene, respectively) for composites having 20 phr hemp compared with those having 10 phr hemp are extremely small, we can say that we have a good adhesion between natural rubber and hemp fibers.
Interactions between fillers and rubbers have a significant effect on reinforcement properties of a filled rubber. Chemical and physical properties of rubber and filler as well as their amount in a compound affected on these interactions [42]. Rubber–rubber interactions mainly occur when blends of rubber are used in compounds and are considered to be not as significant as filler–rubber and filler–filler interactions. Filler–rubber interactions are described by the compatibility of the filler with the rubber, while filler–filler interactions are described by the attraction of filler to itself and the ability to form a network. Filler–filler interactions are a primary mechanism in reinforcement, especially at high-filler loading. These attractions depend on chemical interactions between the filler particle surfaces (filler–filler, filler–rubber), physical interactions (van der Waals forces, hydrogen bonding), morphology of the filler network, and filler volume fraction [42, 43].The mechanism proposed was that sulfur forms bond between NR–NR rubber. From this perspective it was quite expected that the presented results indicate a rather poor adhesion between NR–hemp filler and sulfur cure. In another study [6, 21, 22], elastomer cross-linking was performed using benzoyl peroxide or electron beam irradiation to obtain also a natural rubber/fiber composite. In addition to elastomer cross-linking, peroxide and electron beam can perform chemical surface modification of fibers [6, 21, 22].
Water uptake
The water uptake of samples is presented in Fig. 1 depending on immersion time (in hours): (Fig. 1a) at 23 ± 2 °C and (Fig. 1b) at 70 ± 1 °C. It can be observed that water uptake increased with the increase of fiber content and temperature. Thus: (a) after 24 h in water at room temperature, water uptake values were: 0.38 % for the sample without hemp, 2.15 % for samples having 10 phr hemp and 2.57 % for samples having 20 phr hemp; (b) after 24 h in water at 70 °C water uptake values were: 2.78 % for the sample without hemp, 7.38 % for samples having 10 phr hemp and 8.38 % for samples having 20 phr hemp; (c) after 192 h in water at room temperature water uptake values were: 3.12 % for the sample without hemp, 9.63 % for samples having 10 phr hemp and 11.29 % for samples having 20 phr hemp; (d) after 192 h in water at 70 °C water uptake values were: 6.43 % for the sample without hemp, 14.15 % for samples having 10 phr hemp and 15.16 % for samples having 20 phr hemp.
The water absorption at 23 ± 2 °C stops after 456 h for the sample without hemp and after 600 h for sample with hemp (Fig. 1a). Temperature of 70 °C seems to accelerate the moisture water uptake behavior. At this temperature the water absorption stops after 216 h for the sample without hemp and after 264 h for sample with hemp (Fig. 1b). It can be seen that the composites absorb water very fast initially (in the first 24 h) but after that the amount of water absorbed increased slower (after 192 h). In these composites, water is absorbed mainly by the hemp. By increasing the amount of hemp in composite, the water absorption increased, as well. This can be explained by the fact that the diffusion of water in elastomers is not straight forward by presence of hydrophilic materials (such as the hemp). When the NR/hemp composite is exposed to moisture, the hemp fibers undergo swelling. The high cellulose content in hemp fiber (approximately 75 %) further contributes to more water penetrating into the interface of composites materials [30].The natural rubber is a hydrophobic material and its water absorbability can be neglected [17]. For the same absorption time, composites having high-fiber content exhibit high-water absorption because one of the properties of these natural fibers (hemp) is hydrophilic characteristic.
FTIR analysis
The natural rubber/hemp fiber composites were analyzed by FTIR in order to know their various chemicals constituents. The main component of NR is cis-1,4-polyisoprene with a high degree of long chain branching generally associated with the presence of non-hydrocarbon groups distributed along the chains. The natural rubber is composed of hydrocarbons 89.3–92.4 wt%, protein 2.5–3.5 wt% and other ingredients (fatty acids, resins, and inorganic materials) 4.1–8.2 wt%). Natural fibers can be considered as naturally occurring composites consisting mainly of cellulose fibrils embedded in lignin matrix. The cellulose fibrils are aligned along the length of the fiber, which render maximum tensile and flexural strengths, in addition to providing rigidity. The reinforcing efficiency of natural fiber is related to the nature of cellulose and its crystallinity. The main components of natural fibers are cellulose (α-cellulose), hemicellulose, lignin, pectins and waxes [44].
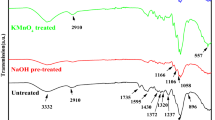
Figures 2, 3, 4 and 5 shows the infrared spectra and the characteristic bands of natural rubber, hemp fibers and natural rubber/hemp fiber composites vulcanized with sulfur, in the range of 4,000–650 cm−1. The broad band in the region of 3,300–3,265 cm−1, which can be due to the OH-stretching vibration, gives information concerning the hydrogen bonds from the amorphous celluloses [45], but at the same time the absorption bands at 3,280–3,290 cm−1 were identified to be attributed to the proteins and both monopeptides and dipeptides present in natural rubber [46]. This band is specific for NR and cellulose, lignin and hemicellulose from the hemp fibers existing into the mixture [47]. Absorption bands with maxima at 3,040–3,033 cm−1 corresponding to CH stretching in the –CH=CH2 group, are observed. Vulcanization of the polymeric compositions results in consumption of the double bonds in NR molecules, thus the intensities of these absorption bands decreased. The characteristic bands of the saturated aliphatic sp 3 C–H bonds are observed in the region 2,970–2,850 cm−1 which are assigned to ν as (CH3), ν as (CH2), and ν s (CH2), respectively (as three corresponding bends) [48].
On the other hand, the presence of amorphous cellulosic samples can be further confirmed by the band from 2,925 to 2,917 cm−1, corresponding to the C–H stretching vibration [45]. The absorption located in the region 1,742–1,734 cm−1 corresponds to the C=O stretch in non-conjugated ketones, carbonyls and in ester groups [49] and the absorption bands in the region of 1,635–1,623 cm−1 were due to absorbed water in cellulose [50] or are caused by lignin (aromatic skeletal vibrations) [49]. Still in the 1,740–1,727 cm−1 range, the absorption band was identified to the fatty acid ester groups of NR [51] and the presence of absorption bands in the spectral region located between 1,664 and 1,658 cm−1, is due to valence vibration of homogeneous double bonds (C=C) in the NR structure. Their intensity decreased for vulcanized samples compared with non-vulcanized samples.
In addition, strong bands were observed for all vulcanizates at 1,450–1,375 cm−1 and can be attributed to chain scission of the C–H groups or to amide groups (–NH2) produced by the accelerated sulfur curing systems. The bands in the range of 1,100–950 cm−1 indicated the formation of a high content of C–S stretches in this system [52]. Natural rubber cis-isomer configurations can be observed at 850–740 cm−1 [52]. At the same time absorption bands in this region can be due to the hemp presence in the composite material. Thus, the absorption band in the region 1,450–1,430 cm−1, is assigned to a symmetric CH2 bending vibration and this band is also known as the “crystallinity band” [45]. The band which appeared in the range of 1,320–1,305 cm−1 is assigned to the cellulose component: bending vibration of C–H and C–O groups of aromatic rings [49, 50]. The absorption located in the region 1,165–1,150 cm−1 corresponds to the anti-symmetrical deformation of the C–O–C bond and the absorption located in the region 1,070–1,020 cm−1 is due to the C–O, alcohol, O–H or aliphatic ethers [49, 50].
SEM analysis
To correlate the influence of fibers hemp content on the mechanical properties and rubber–fiber interactions, the morphological aspect of the natural rubber/hemp composites was necessary to be evaluated by SEM technique. For SEM analysis, the samples were initially immersed in solvent (toluene and xylene) for 72 h to remove unvulcanized natural rubber. SEM micrographs of the natural rubber and natural rubber/hemp composites with 10 and 20 phr hemp content are shown in Figs. 6, 7 and 8.
Fiber alignment factors play a crucial role in the overall properties of composites. There is always a chance of fiber entanglement with randomly oriented fibers reinforced composites. The random orientation of fibers produces lower mechanical properties compared to long unidirectionally oriented fibrers. This fiber entanglement can create elastomeric rich areas, which can contribute to the formation of voids and porosity. Voids and porosity can act as stress concentrators leading to failure of composite samples [53]. The SEM micrographs obtained showed fibers dispersion within the matrix.
Figures 7 and 8 indicate that the hemp fibers present striations, parallel to the fiber axis. The SEM micrographs also revealed that the hemp fibers are uniformly distributed in the natural rubber matrix. This distribution plays a positive role in improving the properties of the composite, especially the tensile strength, as observed in this study. Also, it is observed that with the increase of hemp amount in composites, fibers appear not individually but forming large bundles. Micrographs also show the effect of the fracture on the fibers. As expected for efficiently reinforced composites, during the fracture process fibers suffered rupture instead of being pulled out. This observation is indicative of a good adhesion of the fibers to matrix. Materials with high fiber load presented some small voids in its matrix structure. The nature of these voids is not clear. Absence of pulled-out fibers suggests that these voids may be formed at the conformation of the material [54].
The good adhesion showed in the SEM micrographs, was transferred to the mechanical properties of the composites prepared, which were considerably enhanced by the presence of fibers, as can be seen from the data summarized in Table 3. In addition, a good adhesion between the hemp fibers and the rubber matrix was observed from the SEM micrographs.
Conclusion
The objective of this research was to obtain a new elastomeric material based on NR, with enhanced properties, by replacing active fillers from rubber blends such as carbon black or silica with natural hemp fibers. The values of hardness, tensile strength and tearing strength have increased with the fiber content increasing in the composite due to a good interaction of fiber with NR and these results indicate that hemp has a reinforcing effect on natural rubber. The gel fraction value was over 95 % for all blends and increased with the amount of hemp in the composites. The cross-linking density (ν) of samples changed slightly as the amount of hemp in blends increased. It can be observed that water uptake increased with increasing fiber content and temperature because the diffusion of water in elastomers was not straightforward by the presence of hydrophilic materials (such as the hemp). The water absorption tests indicate that for the temperature of 23 ± 2 °C the saturation appeared after 456 h for the sample without hemp and after 600 h for sample containing hemp, while the high temperature of 70 °C seems to accelerated the water uptake behavior and the saturation appeared after 216 h for the sample without hemp and after 264 h for sample with hemp. To investigate the reinforcement efficiency of natural fibers, the infrared spectra of natural rubber, hemp fibers and natural rubber/hemp fiber composites vulcanized with sulfur have been achieved in the 4,000–650 cm−1 range. The main components of natural fibers (cellulose, hemicellulose, and lignin), NR-specific proteins and fatty acid ester groups and the amide groups produced by the accelerated sulfur curing systems were identified in the investigated mixture. Also, to correlate the influence of fibers hemp content on the mechanical properties and rubber-fiber interaction, the morphological aspect of the natural rubber/hemp composites was evaluated by SEM technique. The micrographs obtained showed the fiber dispersion within the matrix and a good adhesion of fibers with the matrix. SEM micrographs also revealed that the hemp fibers are uniformly distributed in the natural rubber matrix, fact which plays a very important role in improving the properties of the composite, especially the tensile strength. Our investigations concerning the basic mechanical properties, some physical and chemical parameters, the infrared spectroscopy and scanning by electron microscopy depending on hemp content in NR vulcanized with sulfur proved the reinforcing effect of the hemp fibers.
References
Ashida M (1996) In: De SK, White JR (eds) Short fiber-polymer composites. Woodhead Publishing Limited, Cambridge
Cristaldi G, Latteri A, Recca G, Cicala G (2010) In: Dubrovski PD (ed) Woven fabric engineering. Sciyo, Rijeka, pp 318–342 (published online 18 Aug 2010)
Begum K, Islam MA (2013) Natural fiber as a substitute to synthetic fiber in polymer composites: a review. Res J Eng Sci 2:46–53
Suddell BC, Evans WJ (2003) The increasing use and application of natural fiber composite materials within the automotive industry. In: Proceedings of 7th international conference wood–fiber–plastic composites (ICWFPC), forest products society, Madison, pp 7–14
Pamuk G, Ceken F (2013) Comparison of the mechanical behavior spacer knit cotton and flax fabric reinforced composites. Ind Textila 64:3–7
Manaila E, Craciun G, Stelescu MD, Dinca CL, Surdu L, Gurau D (2014) Polymeric composites based on flax wastes and natural rubber. Ind Textila 65:53–60
Corbiere-Nicollier T, Laban BG, Lundquist L, Leterrier Y, Manson JAE, Jolliet O (2001) Life cycle assessment of biofibers replacing glass fibers as reinforcement in plastics. Resour Conserv Recy 33:267–287
Kukle S, Gravitis J, Putnina A, Stikute A (2011) The effect of steam explosion treatment on technical hemp fibres. In: Proceedings of 8th international scientific and practical conference, vol 1, pp 230–237
Dluzneski PR (2001) Peroxide vulcanization of elastomers. Rubber Chem Technol 74:451–492
Alvarez Grima MM (2007) Novel co-agents for improved properties in peroxide cure of saturated elastomers. PhD thesis, University of Twente, Enschede
Susamma AP (2002) Studies on new binary accelerator systems in rubber vulcanization. PhD thesis, Cochin University of Science and Technology
Heideman G (2004) Reduced zinc oxide levels in sulphur vulcanisation of rubber compounds. Ph.D thesis, Twente, Enschede
Niyogi UK (2007) Polymer science: polymer additives and compounding—additives for rubbers. pp 1–30
Chaiear N (2001) Health and safety in the rubber industry. Rapra review reports, Report 138, vol 12, RAPRA Technology LTD, England
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (1997) Silica, some silicates, coal dust and para—aramid fibrils. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 68. World Health Organization, Lyon. http://monographs.iarc.fr/ENG/Monographs/vol68/index.php. Accessed 18 Sep 2014
Beliczky LS, Fajen L (1998) In: Stellman JM (ed) Encyclopaedia of occupational health and safety, 4th edn. Switzerland, International Labor Office, Geneva
Chaudhuri S, Chakraborty R, Bhattacharya P (2013) Optimization of biodegradation of natural fiber (Chorchorus capsularis): HDPE composite using response surface methodology. Iran Polym J 22:865–875
Harle SM (2014) The performance of natural fiber reinforced polymer composites: review. Int J Civ Eng Res 5:285–288
Singha AS, Thakur VK (2010) Physico-chemical and mechanical characterization of natural fiber reinforced polymer composites. Iran Polym J 19:3–16
Ismail H, Edyham MR, Wirjosentono B (2001) Dynamic properties and swelling behaviour of bamboo filled natural rubber composites: the effect of bonding agent. Iran Polym J 10:377–383
Stelescu MD, Manaila E, Craciun G, Dumitrascu M (2014) New green polymeric composites based on hemp and natural rubber processed by electron beam irradiation. Sci World J, Article ID 684047:1–13
Manaila E, Stelescu MD, Craciun G, Surdu L (2014) Effects of benzoyl peroxide on some properties of composites based on hemp and natural rubber. Polym Bull 71:2001–2022
Lopez-Manchado MA, Herrero B, Arroyo M (2003) Preparation and characterization of organoclay nanocomposites based on natural rubber. Polym Int 52:1070–1077
Chenal JM, Chazeau L, Guy L, Bomal Y, Gauthier C (2007) Molecular weight between physical entanglements in natural rubber: a critical parameter during strain-induced crystallization. Polymer 48:1042–1046
Kraus G (1963) Swelling of filler-reinforced vulcanizates. J Appl Polym Sci 7:861–871
Mathew L, Ulahannan J, Joseph R (2006) Effect of curing temperature, fibre loading and bonding agent on the equilibrium swelling of isora-natural rubber composites. Compos Interfac 3:391–401
Jacob M, Thomas S, Varughese KT (2004) Mechanical properties of sisal/oil palm hybrid fiber reinforced natural rubber composites. Compos Sci Technol 64:955–965
Dong Z, Liu M, Jia D, Zhou Y (2013) Synthesis of natural rubber-g-maleic anhydride and its use as a compatibilizer in natural rubber/short nylon fiber composites. Chinese J Polym Sci 31:1127–1138
Samsuri A (2014) In: Thomas S, Chan CH, Pothen LA, Joy J, Maria JH (eds) Natural Rubber Materials: Composites and Nanocomposites. The Royal Society of Chemistry, Cambridge
Dhakal HN, Zhang ZY, Richardson MOW (2007) Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos Sci Technol 67:1674–1683
Conant FS (1973) In: Morton M (ed) Rubber Technology, 2nd edn. Van Nostrand Reinhold, New York
Ahmed K, Nizami SS, Raza NZ, Mahmood K (2013) Effect of micro-sized marble sludge on physical properties of natural rubber composites. Chem Ind Chem Eng Q 19:281–293
Siriwardena S, Ismail H, Ishiaku US (2001) A comparison of white rice husk ash and silica as fillers in ethylene–propylene–diene terpolymer vulcanizates. Polym Int 50:707–712
Zhao J, Ghebremeskel GN (2001) A review of some of the factors affecting fracture and fatigue in SBR and BR vulcanizates. Rubb Chem Technol 74:409–427
Ahmed K (2013) Hybrid composites prepared from industrial waste: mechanical and swelling behavior. J Adv Res. http://www.sciencedirect.com/science/article/pii/S209012321300146X. Accessed 11 Dec 2013
Diez E, Camacho J, Diaz I, Ovejero G (2014) Turbidimetric and intrinsic viscosity study of EVA copolymer–solvent systems. Polym Bull 71:193–206
Fried RJ (2003) Polymer science and technology, 2nd edn. Prentice Hall, New Jersey
Das S (2014) Solvents: properties, solubility parameter, solvation, toxicity, safety. http://www.engineering.ucsb.edu/~saurabh/Presentations/Solvents.pdf. Accessed 15 May 2014
Stelescu MD, Manaila E, Craciun G (2013) Vulcanization of ethylene–propylene–terpolymer-based rubber mixtures by radiation processing. J Appl Polym Sci 128:2325–2336
Stelescu MD, Manaila E, Craciun G, Zuga N (2012) Crosslinking and grafting ethylene vinyl acetate copolymer with accelerated electrons in the presence of polyfunctional monomers. Polym Bull 68:263–285
Manaila E, Craciun G, Stelescu MD, Ighigeanu D, Ficai M (2014) Radiation vulcanization of natural rubber with polyfunctional monomers. Polym Bull 71:57–82
Haghighat M, Nouri Khorasani S, Zadhoush A (2007) Filler–rubber interactions in α_cellulose-filled styrene butadiene rubber composites. Polym Compos 28:748–754
Kohls DJ, Beaucage G (2002) Rational design of reinforced rubber. Curr Opin Solid St M 6:183–194
John MJ, Anandjiwala RD, Thomas S (2009) In: Thomas S, Pothan LA (eds) Natural fibre reinforced polymer composites: From macro to nanoscale. Old City Publishing Inc, Philadelphia
Ciolacu D, Ciolacu F, Popa VI (2011) Amorphous cellulose – structure and characterization. Cellulose Chem Technol 45:13–21
Eng AH, Tanaka Y, Gan SN (1992) FTIR studies on amino groups in purified Hevea rubber. J Nat Rubb Res 7:152–155
Liang CY, Marchessault RH (1959) Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native celluloses. J Polym Sci Pol Chem 37:385–395
Ali AMM, Subban RHY, Bahron H, Winie T, Latif F, Yahya MZA (2008) Grafted natural rubber based polymer electrolytes: ATR-FTIR and conductivity studies. Ionics 14:491–500
Bodirlau R, Teaca CA (2009) Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom J Phys 54:93–104
Pang AL, Ismail H (2013) Tensile properties, water uptake and thermal properties of polypropylene/waste pulverized tire/kenaf (PP/WPT/KNF) composites. Bioresources 8:806–817
Chaikumpollert O, Yamamoto Y, Suchiva K, Kawahara S (2012) Protein-free natural rubber. Colloid Polym Sci 290:331–338
Rohana Yahya YS, Azura AR, Ahmad Z (2011) Effect of curing systems on thermal degradation behaviour of natural rubber (SMR CV 60). J Phys Sci 22:1–14
Dhakal HN, Zhang ZY, Richardson MOW (2006) Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos Sci Technol 67:1674–1683
Girones J, Lopez JP, Mutje P, Carvalho AJF, Curvelo AAS, Vilaseca F (2012) Natural fiber-reinforced thermoplastic starch composites obtained by melt processing. Compos Sci Technol 72:858–863
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manaila, E., Stelescu, M.D. & Doroftei, F. Polymeric composites based on natural rubber and hemp fibers. Iran Polym J 24, 135–148 (2015). https://doi.org/10.1007/s13726-015-0307-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-015-0307-6