Abstract
The aim of this present work was to study the effect of surface treatment methods, i.e., silane and permanganate on curing characteristics, dynamic mechanical, mechanical morphological and thermal properties of hemp fiber filled natural rubber composites. Hemp fiber surfaces were pretreated with an alkali solution and followed by a KMnO4 solution or (3-triethoxysilylpropyl) tetrasulfide (Si69). After that, the chemical, physical and morphological properties of untreated and treated hemp fiber samples were investigated by attenuated total reflectance-Fourier transform infrared spectroscopy, X-ray diffraction and scanning electron microscopy. It was found that the KMnO4 and silane (Si69) treatments affected the surface characteristics of hemp fibers. The curing characteristics of the rubber composites, i.e., scorch and curing times increased with increased fiber loading, except for 15 parts per hundred of rubber (phr) fiber level. The curing characteristics improved with the addition of KMnO4 or silane treated hemp fiber compared to unfilled natural rubber composites. At 5 phr fiber loading, it was found that silane treated hemp fiber filled natural rubber composite showed greater tensile strength and crosslink density in comparison with KMnO4 treated and untreated hemp fiber filled rubber composites. Good interface interaction between the silane treated hemp fiber and rubber matrix was observed in SEM images. Additionally, the thermal stability of rubber composites was unaltered with the incorporation of KMnO4 or silane treated hemp fibers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, natural fiber-reinforced polymer composite materials are rapidly growing both in terms of their industrial application and fundamental research. In general, the utilization of plant-based fibers as fillers for polymer composites is attractive because they are renewable, available in commercial quantities at relatively low cost, and can be added to polymers to form a biodegradable matrix (Osabohien and Egboh 2008). Therefore, natural fiber-reinforced polymer composites are used as alternative low-cost/sustainable materials for structural and nonstructural application such as building products, automotive applications, packing, furniture and consumer goods (Lopattananon et al. 2011). Additionally, the advantages of natural fiber are their availability, lightweight, low density, ease of processing, high toughness, good specific mechanical properties, environmental friendliness, better electrical resistance, good thermal properties and higher resistance to fracture (Wang et al. 2011; Väisänen et al. 2018; Masłowski et al. 2019). Use of jute, palm, cotton, rice husk, bamboo, sisal, coir, banana, cereal straw, hemp and pineapple leaves in polymer composites have been studied (Chaudhary et al. 2010).
Hemp fiber is obtained from the plant Cannabis sativa L., which shows excellent mechanical properties and Young’s modulus. It consists of cellulose, hemicellulose, lignin, wax and minerals. The chemical composition and the physical properties of hemp fiber are presented in Table 1 (Dayo et al. 2017). Hemp fibers are used in fabrics and textiles, yarns and spun fibers, paper, carpeting, home furnishings, construction and insulation materials, auto parts, and composites (Johnson 2018). Additionally, the biomass yield of hemp is high and it improves the soil structure. The tall plant stems of hemp suppress weeds effectively while diseases and pets have been rarely recorded (Manaila et al. 2015). In Thailand, planting hemp is recommended for a crop cycle before planting a primary crop such as rice, sugar cane, corn and cassava. This is done to increase the nitrogen level in soil (Rice Department 2016). Above all, it is of interest to determine the potential applications of hemp fiber for use in fiber-reinforced polymers. However, the compatibility between hemp fiber and polymers is poor due to polarity differences (Ismail et al. 2011; Wongsorat et al. 2014). Polymers are common organic materials. They show hydrophobic properties whereas hemp fibers (natural fibers) exhibit hydrophilic surface properties. This difference of surface properties between polymer and natural fiber needs to be modified to improve the compatibility of the polymer/natural fiber interface, which can enhance the mechanical properties of these composites (Conzatti et al. 2018)
Natural rubber (NR) is harvested from Hevea brasiliensis. It contains cis-1,4-polyisoprene as the main component (94% hydrocarbon) and non-rubber components (6%) (Utara and Saengsila 2015; Nawamawat et al. 2011). NR gives excellent mechanical properties, including high tensile strength, high elasticity, flexibility, good crack growth resistance and low heat build-up (Lake et al. 1991). As a result, NR has been used for producing many products such as tires, window profiles, hoses, belts, matting, flooring and dampeners for the automotive industry (Rodgers and Waddell 2005). Many researchers reported the uses of hemp as a filler for reinforcement of rubber composites. Osabohien and Egboh (2008) studied the effect of bowstring hemp fiber (BHF) on the properties of natural rubber vulcanizates. The authors demonstrated that the elongation at breakage and rebound resilience decreased, whereas the modulus, specific gravity, abrasion resistance and hardness increased with the filler content. Interestingly, hemp fiber/natural rubber had higher hardness in comparison with carbon black/natural rubber. Wang et al. (2011) used bis-(3-triethoxysiylpropyl) tetrasulfide (Si69) to treat hemp hurd powder (HP) in an investigation of the loading effect on a styrene-butadiene rubber (SBR) and Ethylene Propylene Diene Monomers (EPDM) composite with a silica filler. They found that addition of modified HP enhanced the vulcanization process as well as producing good stiffness and toughness in the composites. However, higher HP loading gave poor mechanical properties since HP tends to form agglomerates in a rubber matrix. In a peroxide curing system, it was found that the values of hardness and modulus at 100% elongation increased with hemp fiber content due to a better interaction between fiber and rubber (Manaila et al. 2014). Additionally, the mechanical properties of hemp/natural rubber using a sulfur-curing system also improved with fiber loading (Manaila et al. 2015). Stelescu et al. (2014) found that adding hemp fibers to natural rubber and using electron beam irradiation for crosslinking improved the mechanical properties of the resulting composites. The crosslinking rates of samples also increased with the amount of hemp in blends and the electron beam irradiation dose.
Many methods have been reported in the literature to improve the compatibility between natural fibers and rubber matrices such as acylation, peroxide, permanganate, alkaline and silane treatments (Rohit and Dixit 2016). Silane and permanganate treatments have been used for modifying the surface of natural fiber (Lopattananon et al. 2011). In silanization processes, a silane coupling agent reacts with the hydroxyl groups of the fibers resulting in the reduction of cellulose hydroxyl groups at the fiber-matrix interface (Rohit and Dixit 2016; Masłowski et al. 2019). In permanganate treatment, a decreased hydrophilic nature of the natural fiber is observed while the compatibility between the fiber and polymer matrix is improved (Sheng et al. 2014). However, few reports have been published on the effect of silane and permanganate treatments on the properties of hemp fibers/natural rubber composites (Osabohien and Egboh 2007; Wang et al. 2011; Manaila et al. 2014, 2015).
The aim of this work was to study the effect of treated hemp fiber using (3-triethoxysilylpropyl) tetrasulfide (Si69) as a silane coupling agent and permanganate treatment on the properties of hemp fibers/natural rubber composites. The effect of hemp fiber loading on the curing characteristics, mechanical properties, dynamic mechanical properties, morphology and thermal properties was also studied. Additionally, the characteristics of untreated hemp and treated hemp fiber were investigated using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR) and scanning electron microscopy (SEM).
Experimental
Materials
Rubber and chemicals
Natural rubber latex (NRL) with 60 wt% dry rubber content (60% DRC) was purchased from the Thai Rubber Latex Co., Ltd. and preserved with a 0.6% ammonia (NH3) solution in the presence of 0.025% tetramethylthiuram disulphide (TMTD)/ZnO, yielding a final pH between 10.0 and 10.5. Zinc oxide (white seal) and stearic acid, used as activators, were manufactured by Global Chemical Co., Ltd., (Samutprakarn, Thailand) and Imperial Industry Chemical Co., Ltd., (Pathumthani, Thailand), respectively. Mercaptobenzothiazole (MBT), used as an accelerator, was supplied by Flexsys (Termoli, Italy). TMTD, also used as an accelerator, was supplied by Sigma-Aldrich. The sulfur used as a vulcanizing agent was obtained from Ajax Chemical Co., Ltd., (Samutprakarn, Thailand). Sodium hydroxide (NaOH, 98.6%, Ajax-Finechem) pellets were used for the alkali treatment of fibers. Potassium permanganate (97%) from Sigma-Aldrich was reagent grade. Silane coupling agent (3-triethoxysilylpropyl) tetrasulfide (Si69) was obtained from Sigma-Aldrich. All chemicals were utilized as received.
Hemp fiber
Raw hemp fibers were harvested from cultivation areas in Ban Chai Ja learn District, Amphor Vung Mam More, Udon Thani Province, Thailand, during the period of January–February 2018. The hemp fiber had a length of 1.5–2 m and 20–70 μm. These fibers were rinsed with detergent followed by clean water to remove dirt from the fiber and then dried at room temperature for a week (Chaudhary et al. 2010). Then, the fibers were cut into small pieces about 2–3 cm long and fed into a grinding machine (WF-04, Thai Grinder). After that, the hemp fibers were sieved through a 400 ± 100 μm sieve using ASTM Standard sieves. Finally, the hemp fibers were dried in a convection oven at 70 °C for 24 h. The resulting sample was stored in a desiccator before further analysis.
Methods
Pretreatment
Hemp fibers were immersed in a NaOH (5% w/v) solution for 4 h with continuous stirring at room temperature and a fiber:solution weight ratio of 1:15 (Mohanta and Acharya 2016). An alkali treatment was used for removing hemicellulose, lignin and pectin as well as to roughen the fiber surfaces (Roy et al. 2018; Sepe et al. 2018). After that, the hemp fibers were washed thoroughly with distilled water until the water dripping from the fibers had a neutral pH (7). Finally, the fibers were filtered and dried in hot air oven (UF110, Memmert) at 70 °C for 24 h.
Permanganate treatment
Permanganate treatments of alkali pretreated hemp fibers were conducted using a KMnO4 solution 1% w/v (in acetone) with a soaking time of 15 min at room temperature (Sheng et al. 2014). After that, they were filtered and washed several times with distilled water to eliminate excess potassium permanganate and dried in a hot air oven (UF110, Memmert) at 70 °C for 24 h.
Silane (Si69) treatment
The alkali pretreated hemp fibers were allowed to react with a silane coupling agent by immersing them in a 2% w/w of filler content (Lopattananon et al. 2011). A 2 g mass of Si69 was diluted in acetone (500 mL). The Si69 solution was added to pretreated hemp fibers (100 g). Then, the mixture was stirred for 1 h at 30 °C. After that, the fibers were filtered and washed several times with acetone to remove unreacted silane coupling agent. Finally, the fibers were dried at 70 °C for 24 h.
Characterization of hemp fibers
The surface characteristics of untreated and treated hemp fibers were analyzed using Attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy (Perkin Elmer Model Spectrum Two). The spectra were recorded over the range of 4000–400 cm−1 with a resolution of 4 cm−1. X-ray diffraction (XRD, PW3710, the Netherlands) was used to determine the crystalline structure and identify crystallographic phases of untreated and treated hemp fibers. The diffraction intensities were recorded between 5° and 60° (2θ). The crystallinity indices (CrI) of the fibers were calculated using the following equation (Segal et al.1959):
where I200 is the maximum intensity of diffraction of the (200) lattice and Iam is the intensity at the minimum at 2θ between 18° and 19°. The morphology of the untreated and treated fibers was observed using scanning electron microscopy (HITACHI MODEL S-3000 N) with an accelerating voltage of 5 kV. The fibers were coated with a thin layer of a gold to improve conductivity prior to SEM observations.
Preparation of hemp fiber/NR master batches
The procedure for preparation of hemp fiber/NR was as follows: NRL (60%DRC) was first mechanically stirred at 150 rpm for 10 min and then hemp fiber was added into the latex such that the dried NR would contain 0, 5, 10 and 15 (parts per hundred of rubber) phr of fiber. The mixture was continuously stirred at room temperature for 30 min. Finally, the latex was cooled to room temperature and then cast on a 13 cm × 13 cm × 3 mm raised glass plate. The cast sheet was dried at 70 °C for 5 h and then kept in a desiccator for further preparation (Utara et al. 2017).
Preparation of hemp fiber/NR compounds
The formulation of hemp fiber/NR compounds was based upon a sulfur vulcanization process following the procedure of Manaila et al. (2015), as shown in Table 2. In this formulation, no antioxidant agent was used. Furthermore, two accelerators such as MBT and TMTD were employed for rubber compounding. The compounding of fibers, rubber additives and natural rubber was carried out with a laboratory two-roll mill (MLD150L350, Chareon Tut Co., Ltd, Thailand) according to ASTM D3184. After that, the sheeted rubber compound was kept at 25 °C for 24 h in a closed container before assessment using a Monsanto Moving Die Rheometer (MDR2000, Alpha Technology) according to ASTM D2084-9. The curing characteristic parameters, scorch time (ts2) and cure time (t90) were obtained. Measurements of rheological properties were performed in triplicate for each sample. The rubber vulcanizates were prepared by compressing the molds (PR1D, Chareon Tut Co., Ltd, Thailand) at 150 °C with an applied force of 10 MPa in a hot press using their respective curing times, as determined from MDR 2000 data. Molded sheets (2.0 mm thick) were conditioned in desiccators for 24 h prior to further testing. According to Table 2, the molded sheets of untreated hemp fiber/NR composites were designated according to their various filler contents, UN0, UN5 UN10 and UN15. Permanganate treated hemp fiber/NR composites were designated as MODK5 MODK10 and MODK15. Silane treated hemp fiber/NR composites were designated as MODS5 and MODS10 and MODS15.
Characterization of hemp fiber/NR vulcanizates
The curing characteristics of the composites were studied using a Monsanto Moving Die Rheometer (MDR2000, Alpha Technology) at 150 °C. Three replicate specimens were tested and the mean values of scorch time, cure time, maximum torque (MH) and minimum torque (ML) were reported. The crosslink density (Ve) of vulcanizate natural rubber composites was determined according to ASTM D6814. The composites were swollen in toluene at room temperature for 72 h until equilibrium swelling was reached. After that, the swollen rubber was taken from the solvent and excess solvent removed from the surface of the rubber before being weighed to an accuracy of 0.1 mg (Utara et al. 2017). The crosslink density was evaluated using the Flory–Rehner relationship following (Wongsorat et al. 2014; Manaila et al. 2015; Datta and Wloch 2017):
where \({{\upchi }}_{ 1}\) is polymer–solvent interaction parameter (0.393 for toluene). V1 is molecular volume of the solvent (106.2 for toluene). Vr is the volume fraction of polymer in the swollen network in equilibrium with pure solvent and evaluated as follows (Wongsorat et al. 2014; Datta and Wloch 2017):
where the density of toluene is 0.87 g/cm3 and the density of natural rubber 0.92 g/cm3 (Utara et al. 2017; Datta and Wloch 2017). Averaged values of three replicate specimens are reported.
Dynamic mechanical analysis (DMA) was done, in which the thermal properties of the rubber vulcanizates were determined using a dynamic mechanical analyzer (Model GABO, EPLEXOR QC 25) at a frequency of 10 Hz in tension mode. The temperature employed ranged from − 80 to 100 °C at a heating rate of 3 °C/min (Utara and Saengsila 2015; Utara et al. 2017). Tensile strength tests were carried out with an Instron Universal Testing Machine (Model 3366) using a crosshead speed of 500 mm/min and an initial clamp separation 65 mm according to ASTM D412. An average of five replicate tests for each sample was recorded.
The hardness was measured using with a Shore A durometer (Frank GmbH, Hamburg, Germany), according to ASTM D2240. An average value from six positions was recorded for each specimen.
Thermogravimetric-differential thermal analysis (TG–DTA, Shimadzu TGA-50) was used to examine the decomposition step of the composites. TG–DTA was carried out between 30 °C and 500 °C in nitrogen gas at a heating rate of 10 °C/min.
The morphology of the hemp fiber and the dispersion of the composites surface were evaluated by means of scanning electron microscopy (SEM; HITACHI MODEL S-3000 N) with an accelerating voltage of 5 kV. Tensile-fracture samples were observed at the fractured surface. Before the SEM measurement, the fractured surfaces were coated with a 20 nm thick layer of gold.
Results and discussion
Characteristics of hemp fiber
FTIR analysis
FTIR analysis can confirm the chemical composition as well as the functional groups of hemp fibers. A comparison of FTIR spectrum for hemp fiber before and after chemical treatments is shown in Fig. 1. The respective band positions of the various samples are tabulated in Table 3. In the spectrum of the untreated hemp fiber, signals of their main components, i.e., cellulose, hemicellulose and lignin, are clearly observed. In all spectra, a peak at 3332 cm−1 and ca 2910 cm−1 are attributed to OH stretching in cellulose and C-H stretching in cellulose-hemicellulose, respectively. After chemical treatment with NaOH (alkali), KMnO4 (permanganate) and silane, these peaks remain in the same position. However, reductions of those peaks are clearly observed after NaOH and silane treatment. The reduction of 3332 cm−1 peak is due to the reduction of hydrogen bonding on the surface of hemp fiber (Mwaikambo and Ansell 2002). The reduction of ca 2910 cm−1 may have been due to the decrease of cellulose and hemicellulose at the fiber surfaces. These findings suggest that alkali treatment removes of some portion of cellulose and hemicellulose from the hemp fiber. The lack of a peak at 1735 cm−1 is ascribed to a substantial number acetyl ester groups becoming attached to hemicellulose. These results confirm that hemicellulose is also removed from the hemp fiber surfaces after alkali pre-treatment (Sepe et al. 2018; Väisänen et al. 2018; Kabir et al. 2013). The peak at 1595 cm−1 is due to the presence of an aromatic ring in lignin. The position of this peak remained unchanged for alkali, permanganate and salinized treated fibers. The intensity of this peaks decreased after alkalized (pre-treated) and salanized fibers. This suggests that the lignin can be partially removed by alkali and silane treatments. However, for permanganate treated fibers, a similar peak to that of the untreated fibers appears at this region. This indicates that there is no reaction between lignin and permanganate. Interestingly, permanganate treated fibers show a Mn–O bending peak at 557 cm−1 (Parikh and Chorover 2005; Zhang et al. 2015). This indicates that the interaction between hydrogen atoms on the surfaces of hemp fibers with permanganates occurs. Additionally, the increase in the peak at around 3600–300 cm−1 is possibly due to increased hydrogen bonding on the surfaces of hemp fibers. The probable reaction mechanism of KMnO4 and cellulose is shown in Fig. 2. Additionally, Sheng et al. (2014) suggested that the hydroxyl groups on the cellulose surface are oxidized to aldehyde groups at low KMnO4 concentrations. Silane treatments did not show any reactivity with the hemp fiber, as there are no changes in the FTIR spectra compared to untreated and NaOH pre-treated fibers. This finding is in agreement with a previous study by Kabir and coworkers (Kabir et al. 2013). They concluded that alkali treatments have a degree of reactivity in removing hemicellulose and lignin from hemp fiber. However, the reactivity with hemp fiber is unaltered by silane treatment. Additionally, after silane treatment, two peaks should be present at 766 and 847 cm−1 (Sgriccia et al. 2008; Khan and Drzal 2004). The possible explanation for this is that the concentration of silane at the fiber surfaces is too small to detect by FTIR (Sgriccia et al. 2008).
A schematic reaction of KMnO4 and cellulose (Sheng et al. 2014)
Morphological analysis
Figures 3, 4, 5 and 6 show SEM micrographs of untreated, alkali pre-treated, permanganate and silane treated fibers. Figure 3a, b reveal that the surfaces of hemp fiber have waxes, hemicellulose, lignin and other constituents and are therefore much rougher (Väisänen et al. 2018; Sgriccia et al. 2008). The waxy/gummy substances result in poor fiber-matrix adhesion (Mohanta and Acharya 2016). SEM micrographs of untreated hemp fiber reveal that fiber structure consists of cellulose micro-fibrils covered with hemicellulose and lignin.
Figure 4a, b show alkalized fiber surfaces. It appears that gummy and waxy substances are removed away and the fiber structure was packed together. This finding indicates that waxes, hemicellulose and lignin covering the surfaces of hemp fibers are removed by alkali pre-treated when compared with untreated fibers (Kabir et al. 2013). This is substantially consistent with the FTIR spectra of untreated and NaOH pre-treated fibers.
The KMnO4 treated fibers in Fig. 5a, b exhibit a partitioning of cellulose micro-fibrils and cleaner surfaces in relation to the untreated fibers. The fiber surfaces of hemp exhibit more fibrillation and roughness. This result is due to the elimination of impurities, which cover the surfaces of the fibers. Additionally, KMnO4 can hydrogen bond with cellulose (Sheng et al. 2014) as shown in Fig. 2. This is in agreement with FTIR analysis, which confirms the presence of Mn–O bond and increased O–H stretching intensity (peaks) compared with untreated fibers. Therefore, the KMnO4 treatment is believed to change the hydrophilic properties of hemp fiber through increased hydrogen bonding.
The silanised fibers in Fig. 6a, b show small microfibers that seem to be composed of several micro-fibrils. Each fiber has a more compact structure compared to both NaOH pre-treated and KMnO4 treated fibers. These findings suggest that silanised fibers have more micro-fibrils and smaller diameters than untreated, NaOH pre-treated and KMnO4 treated fibers. A possible explanation may be the formation of a silane-coupling agent on the surfaces of hemp fibers. However, Si–O–C linkages were not observed in the FTIR results. This is in agreement with the results of Sgriccia et al. (2008), who suggested that the concentration of silane is lower that the detection limit.
X-ray diffraction analysis
The X-ray diffraction patterns of untreated and treated hemp fibers are shown in Fig. 7. The major peaks observed in all fiber samples are at 2θ diffraction angles of 15.30°, 16.05°, 22.44°, and 34.30° representing (1–10), (110), (200) and (004) planes indicating the presence of Type I cellulose (French 2014). No structural transformation from cellulose I to cellulose II is observed. The fiber crystallinity indices (CrI) of these fibers are summarized in the Table 4. The CrI values of untreated, NaOH pre-treated, KMnO4 treated and silane treated fibers are 45.50%, 60.17%, 61.15% and 58.79%, respectively. An increased crystallinity index is observed for alkali pre-treated fibers, and to a lesser degree for KMnO4 and silane treated fibers. This may been due to the better packing and stress relaxation of cellulose after removal of the amorphous constituents and pectin from the fiber (Mohanta and Acharya 2016). This explanation is supported by FTIR results. These results confirm that the alkali pre-treatment removes amorphous (non-cellulosic or amorphous cellulose compound) components of the fiber and the fiber contains more crystalline cellulose after treatment (Mwaikambo and Ansell 2002; Borchani et al. 2015). The crystallinity index of KMnO4 treated fibers was the highest among all samples. Patra et al. (2012) reported a similar observation working with potassium permanganate treated short sisal fibers. They suggested that KMnO4 treatment helps in removing non-crystalline material from the fibers, making the inter-fibrilar region less dense and less rigid, allowing cellulose micro-fibrils to rearrange in a more compact manner. Interestingly, silane treated fiber shows a lower crystallinity index in comparison with KMnO4 treated and NaOH pre-treated fibers. A possible explanation for this is the introduction of a silane coupling agent into the polycrystalline domains. The size of silane molecules is larger than that of –OH groups, which means the distance between the polymer chains increased after modification. Therefore, the energy of intermolecular attraction decreases with increasing distance between the polymer chains (Sheltami et al. 2015; Seki et al. 2018). According to SEM results, silane treated fibers (Fig. 6) show more micro-fibrils than other chemically treated samples.
Curing characteristics of the composites
Figure 8a–c show the curing cures of the hemp fiber/rubber composites. The scorch time (ts2), cure time (t90), maximum torque (MH), minimum torque (ML) and delta torque or ∆M (MH − ML) are shown in Table 5. In the case of untreated hemp fiber filled composite, the minimum and maximum torque slightly increased with increasing amounts of hemp fiber. The increase in the maximum torque with higher contents of untreated hemp fiber indicates that the presence of fibers in the polymer matrix reduces the mobility of polymer chains. These observed trends are in agreement with results reported by Wongsorat et al. (2014) and Roy et al. (2018) using sisal fiber and short jute fiber as fillers in natural rubber composites, respectively. Delta torque values of untreated hemp filled composites tended to increase with hemp fiber loading. The delta torque indicates the rigidity of a rubber vulcanizate arising from the level of crosslinking (Utara and Saengsila 2015; Sriring et al. 2018). Therefore, the increase of delta torque of UN5, UN10 and UN15 suggests that the crosslink density increases with hemp fiber content. However, delta torque of the composites exhibits a declining trend with increasing untreated hemp fiber content (UN15). With increased untreated hemp fiber loading, the scorch and cure times of UN5 and UN10 slightly increase compared with the UN0 sample (without filler). The increased scorch and cure times of the untreated fiber filled composite may be relate to the larger particle sizes and lower surface area of the filler as revealed in Fig. 3a, b. This hinders the formation of sulfur crosslinks during vulcanization. A similar result has been found using kernel shell in a rubber matrix (Daud et al. 2017). Additionally, hemp cellulose consists of about 88.3% α-cellulose (Janjic et al. 2007). Haghighat et al. (2005) suggested that the increased scorch and curing times might be associated with the more acidic properties of the filler (α-cellulose). In vulcanization, chemical reactions take place in a base media (accelerators). Therefore, the addition of any material that increases the acidity of system may cause the initiation time of the curing reactions (e.g., scorch time) to increase. However, scorch and curing times of UN15 are lower than those of UN0, UN5 and UN10. There may also be an agglomeration effect of the filler at higher contents. Ismail et al. (2002) suggested that decreased scorch and curing times with increasing fiber loading are possible with increased mixing times. This results in more head build-up, which then leads to a decreased curing time. Interestingly, the ts2 and t90 of untreated hemp fiber filled composites are very short. This might have been because of a combined effect of TMTD and MBT accelerators. TMTD is an ultra-fast accelerator. It produces intermediate sulphurating reagents (chelators) that are more active (Sadequl 2000). MBT is a semi-fast accelerator. Its amine and metal salts have an exposed thiol functional group. They easily react, providing an acceleration effect even at low temperatures. However, compounded rubber has poor scorch safety (short ts2) including limited stability in processing and storage (Ohnuki 2014). This suggests that formulation of rubber compounds used for this study are suitable for molding thin sheets due to their short scorch and curing times. Sae-oui et al. (2007) reported that mercapto accelerators (MBT and MBTS) provide an unsatisfactory curing in NR/EPDM blends. Based on our knowledge, Manaila et al. (2015) did not study the curing characteristics of this formulation of rubber compounds. Therefore, for the first time, the present study attempts to elucidate the curing characteristics of this formulation. In the case of KMnO4 treated hemp fiber filled composites, MH, ∆M, ts2 and t90 values are higher than untreated hemp fiber filled composites with similar filler contents. The increase in ML a higher hemp fiber contents indicates that the presence of hemp fiber has reduced the mobility of polymer chains. As expected, both untreated and KMnO4 treated fibers show an increasing trend with fiber content. However, the ML values of KMnO4 treated hemp fiber are lower than untreated hemp fiber filled composites. These results suggest that KMnO4 treatment affects the curing characteristics of these composites. According to Saramolee et al. (2010), ML is related to the viscosity of the natural rubber compound before vulcanization and is influenced by mixing time and filler properties. The lower ML of KMnO4 treated fiber composites may be due to the reduced stiffness of hemp fibers after pre-treatment with NaOH and KMnO4 treated. As shown in FTIR and SEM results, NaOH and KMnO4 treatment can remove waxes, hemicellulose and lignin on the surfaces of hemp, which can decrease the rigidity of hemp fibers. It is established that the presence of a filler in an elastomer matrix is an indirect measure of the hydrodynamic effect, which is related to the change in ∆M values. A higher ∆M value results from an incrementally greater bio-composite crosslinking density (Masłowski et al. 2019). These results indicate that KMnO4 treatment may improve the interaction between hemp fibers and the rubber matrix. The shorter ts2 and t90 values of untreated hemp fiber filled composites compared with KMnO4 treated hemp fiber filled composites possibly results when accelerators are susceptible to chemical attack by water (Butler and Freakley 1992). Moisture is easily adsorbed on the surface of untreated hemp fiber due its content of cellulose, hemicellulose, pectin and lignin (Célino et al. 2014). Similar results were found by Osabohien and Egboh (2007). They reported that a faster (shorter) curing time was obtained with fillers having higher moisture content. Additionally, the longer curing time of KMnO4 treated hemp fiber filled composites might have been due to the adsorption of accelerator molecules with the Mn–O and O–H bonds observed on the surface of hemp fibers. ML values of silane treated hemp fiber filled rubber composites exhibit no noticeable change with greater fiber loading compared with untreated hemp fiber filled rubber composites, except for the MODS15 sample. The MH value of MODS15 is slightly higher than UN15. However, the MH value of the MODS5 sample was lower than UN5. In general, the MH value is considered a measure of the modulus for cured rubber composites (Lopattananon et al. 2011). This means that the MODS15 sample shows a higher modulus than UN15. In the other words, MODS15 probably exhibits a greater crosslink density than the UN10 sample. Additionally, the ∆M values of silane treated hemp composites are not significantly different from untreated hemp fiber composites. However, the MODS15 sample shows a greater ∆M value compared with UN15. It can be seen that ts2 and t90 values of silane treated hemp fiber composites are higher than untreated hemp fiber filled composites and composites with no fiber (UN0). The increase of ts2 and t90 values is possibly due to improved filler dispersion and increased steric hindrance for crosslinking due to the presence of bulky groups (triethoxysilylpropyl groups) in the long-chain Si69 (Lopattananon et al. 2011; Poh and Ng 1998). The increased ts2 and t90 of the filled rubber composite has been previously reported in natural rubber samples reinforced with short cellulose fibers (Lopattananon et al. 2011) and glass fiber filled rubber composites (De et al. 2004). These results indicate that natural rubber (NR) compounds filled with silane treated hemp fiber possesses better processability and scorch safety. At similar filler loadings, the ML values of silane treated fiber composites are higher than those of KMnO4 treated fiber composites. These results indicate that silane treated fiber composites exhibit a higher viscosity compared to KMnO4 treated fiber composites. The ts2 and t90 values of silane treated fiber composites are also higher than those of KMnO4 treated fiber composites.
Dynamic mechanical thermal analysis of the composites
The dynamic mechanical properties of unfilled rubber (UN0), chemical treatment with KMnO4 and chemical treatment with silane hemp fiber filled rubber composites are displayed in Figs. 9 and 10, respectively. The corresponding DMA parameters are tabulated in Table 6. Figure 9 is a plot of the storage modulus (E′) as a function of temperature. The E′ values of all samples decrease with increasing temperature. Theoretically, below the glass transition temperature (Tg), the filler network (filler–filler interaction) is the main contributor to the storage modulus since particle movements are restricted in the polymer matrix. However, as the temperature increases to ambient levels or above, the rubber-filler interaction and rubber network structure dominates the storage modulus (Utara et al. 2017; Li et al. 2014). From Fig. 9a, at low temperature in the glassy region, the UN0 has the lowest storage modulus, while MODK15 has the highest storage modulus. The addition of untreated fiber or KMnO4 treated fiber increases the storage modulus of all composites. The storage modulus for both cases tends increases with increased fiber loading. This is probably due to a combination of the hydrodynamic effect of hemp fiber embedded in the rubber matrix and the mechanical restraint imposed by the fillers at high contents, which reduces the motion of the polymer chains (Wang et al. 2011). At similar filler loadings, KMnO4 treated fiber filled composites have higher storage moduli compared to those systems containing untreated fiber filled composites. This may be due to stronger filler network in a KMnO4 treated fiber polymer matrix compared to untreated fibers. As temperature is increased, the moduli remained roughly constant to the glass to rubber transition temperature. Above this transition, the unfilled rubber sample (UN0) has the lowest values and the transition starts at about − 44 °C for a natural rubber matrix. Moreover, in the case of composites containing a KMnO4 treated fiber, the moduli have a greater value compared with untreated fiber composites at the same filler loading. This may be related to stronger rubber-filler interactions and a higher elasticity modulus of KMnO4 treated hemp fibers compared to raw hemp fiber. Figure 9b represents the effect of silane treated fiber on the storage modulus compared to an untreated fiber system. It can be seen that the moduli of silane treat fibers are also higher than that of untreated fiber. MODS15 has the highest storage modulus. Interestingly, in Fig. 9c, the E′ values of KMnO4 treated fiber filled composites are higher than those of silane treated fiber composites. A possible explanation is that the dispersed KMnO4 fibers in a rubber matrix enhances the stiffness of the systems compared with silane treated fiber composites.
Figure 10 is the plot of loss factor (tanδ) as a function of temperature for natural rubber composites with untreated, KMnO4 treated and silane treated fibers. The tanδmax value is the peak height of a tanδ versus temperature plot. It is not only used to evaluate the glass transition temperature (Tg), but also to determine the extent of reinforcement of rubber matrix in the presence of a particular filler (Utara et al. 2017; Roy et al. 2018). From the Figs. 10a–c, the Tg values of natural rubber composites are unaltered by the chemical treatment and filler loading (Table 6). However, the MODK15 and MDS15 samples show slightly increased Tg values (− 42.21 and − 42.14 °C, respectively) compared to unfilled composites (− 44.3 °C). A higher Tg value is obtained for composites containing chemically treated fibers (KMnO4 and silane), which may be due to good interaction between the rubber and filler surfaces. The tan δmax is also known as the damping peak factor. It depends on the fiber-matrix adhesion (Dayo et al. 2017). Therefore, reinforcement of rubber vulcanizates with filler-rubber interaction can be assessed either from a decreased tan δmax value or from an increase in the Tg value (Utara et al. 2017). From Fig. 10a, b, the damping peaks decrease with increased filler loading. This is due to the limitation of the movement of polymer macromolecules because of the introduction of a rigid solid phase. Interestingly, at similar filler loadings (10 and 15 phr), KMnO4 treated and silane treated fiber filled composites show lower damping peaks compared to untreated fiber composite systems. These results indicate stronger interfacial interaction of elastomers and filler surfaces. From Table 6, it can be seen that the lowest damping peaks are for the MODK15 sample. This might have been because of strong interaction between the filler and the rubbery matrix in the KMnO4 treated fiber networks. This is supported by the observed E′ values at temperatures around − 40 to − 10 °C, which are higher for MODK15 than other composite systems as, seen in Fig. 9c.
Mechanical properties and crosslinking of the composites
Mechanical properties such as hardness, tensile strength, 100% modulus and elongation at break of the unfilled and filled rubber composites are shown in Fig. 11. According to Fig. 11a, the hardness of hemp fiber filled rubber composites shows an increasing trend with hemp loading compared to unfilled rubber composites. This result is in good agreement with that of Osabohien and Egboh (2007), who found that addition of hemp fiber to rubber composites enhances their hardness. Ismail et al. (2002) suggested that addition of bamboo fiber in rubber composites enhances composite stiffness. As expected, KMnO4 and silane treated fiber filled composites show higher hardness than untreated fiber composites. This finding indicates that chemical treatment (KMnO4 or silane) of hemp fiber filled rubber composite improves the hardness of rubber composites. At a similar filler loading, the hardness of silane treated fiber filled composites is slightly higher than KMnO4 treated fiber filled composite, except for MODK15. As shown in Fig. 11b, the modulus at 100% strain values of rubber composites improves after incorporation of hemp fiber, either KMnO4 or silane treated fiber. According to Lopattananon et al. (2011), greater fiber loading increases the rubber modulus because of its effect on fiber stiffness. In other words, a filler with a higher stiffness than the matrix increases the modulus of the composite. A higher modulus is observed in the KMnO4 treated fiber filled composites versus those filled with silane treated fibers. This may be due to improved fiber/rubber molecular interactions (Osabohien and Egboh 2007). These results are supported by the lower tanδ max value of KMnO4 treated fiber filled composites compared to those filled with silane treated fibers (Table 6). At a filler loading of 15 phr, the modulus of MODK composites is almost twice that of the untreated fiber composites. This indicates that KMnO4 treated fibers impart much more material stiffness to the composites. This finding is supported by the highest \({\text{E}}^{ '}\) and Tg values of KMnO4 treated fiber composites (MODK15) in Fig. 9c and Table 6, respectively. Tensile strength for hemp fiber filled rubber composites with and without chemical treatments is presented in Fig. 11c. The tensile strength of hemp fiber with and without chemical treatments is comparatively lower than that of the unfilled vulcanized natural rubber (23.1 MPa). This is likely due strain-induced crystallization of natural rubber (Jacob et al. 2004). Addition of hemp fiber into natural rubber may interrupt the arrangement of rubber chains, resulting in less strain-induced crystallization of the rubber leading to reduced tensile strength of natural rubber composites (Jacob et al. 2004; Srisuwan et al. 2018). At 5 phr filler loading, the silane treated hemp fiber filled rubber composites exhibited a greater tensile strength than KMnO4 treated fiber and unfilled rubber composites. The improved tensile strength is due to enhanced filler-rubber interaction after treating the hemp fiber surface with a silane coupling agent (Si69). Several studies reported similar observations (Ismail et al. 2011, 2012; Lopattananon et al. 2011; Srisuwan et al. 2018; Masłowski et al. 2019). Lopattananon et al. (2011) also proposed a reaction between Si69 and cellulose fiber. Additionally, it is established that the tensile strength of filled rubber depends on the filler size and surface area. Therefore, small silane treated fibers (Fig. 6) exhibit good dispersion in a rubber matrix compared with that of large KMnO4 treated fibers (Fig. 5). However, above 5 phr loading, silane treated fiber is not very effective in reinforcing the tensile properties of rubber composites. Elongations at breaks of the rubber composites are presented in Fig. 11d. The elongation at break decreases with increasing in fiber content. This reduction indicates that ductility diminishes when hemp is added to rubber composites. This is due to restriction of molecular chain movement by hemp (Manaila et al. 2015). Both natural rubbers filled with KMnO4 and silane treated hemp fibers have a lower elongation at break that those of untreated hemp fiber composites. However, at 5 phr fiber loading, the elongation of silane treated fiber composites is slightly lower than that of an untreated fiber composite. Based on mechanical properties such as hardness, modulus at 100% strain, tensile strength and elongation at break, the optimal filler loading and chemical treatment methods for hemp fiber filled natural rubber composites are 5 phr and silane treated hemp fiber.
The crosslink density of the rubber composites is shown in Fig. 12. The slightly increased crosslink density has been taken into account with an increase of filler content From Fig. 11c, tensile strength decreased with increased filler loading. The tensile strength values of untreated fiber, filled rubber composites (10 and 15 phr filler) were greater that than those of filled fiber rubber composites, except for 5 phr of filler. As a result, the crosslink density is likely not the main factor governing the mechanical properties at high deformation (Hariwongsanupab et al. 2017). Therefore, the morphology of tensile-fractured rubber composites and their thermal properties are discussed in the next section.
Morphology of tensile-fractured composites
In an early part of the current study, it was found that 5 phr is the optimum amount of hemp fiber filler for natural rubber composites. Figure 13 presents SEM images of tensile fractures of UN0, UN5, MODK5 and MODS5. SEM images support our previous results for the modulus 100% at strain, tensile strength, elongation at break and crosslink density. From Fig. 13b, it can be seen that the surface of untreated hemp fiber filled rubber became rougher with hemp fiber pullouts from the rubber matrix, which produced cavities on the surface of the composite. This indicates that the level of adhesion between untreated hemp fiber and the rubber matrix is poor. Similar findings were found by Wang et al. (2011) and Ismail et al. (2011). In Fig. 13c, after KMnO4 treatment, a much rougher surface and fractured layers are clearly observed. This finding confirms weak adhesion between KMnO4 treated hemp fiber and the rubber matrix. A possible explanation for this is that the OH and Mn–O groups on the surface of KMnO4 treated hemp fiber (seen in Fig. 1) have high moisture absorption properties, which cause poor compatibility between hemp fiber and the rubber matrix. Broken hemp fibers projecting out of rubber matrix are seen at the fractured surface of the silane treated fiber filler rubber composite in Fig. 13d. When stress is applied, hemp fiber likely broke due to strong adhesion between fiber and matrix. Therefore, silane treated hemp fiber enhances adhesion between fiber and a rubber matrix (Wang et al. 2011; Ismail et al. 2011).
Thermodynamic study
Thermodynamics is successfully utilized to explain the interaction between a filler and polymer matrix. Thermodynamic properties of natural rubber, such as its elastic Gibbs free energy (ΔG) and conformational entropy (ΔS) are evaluated by an equilibrium swelling experiment and calculated using the Flory–Huggins equation (Bindu and Thomas 2013; Roy et al. 2018; Roy and Potiyaraj 2018):
where R is the universal gas constant, T is the absolute temperature, Vr is the volume fraction of swollen rubber, χ is the rubber solvent interaction parameter. According to the statistical theory of rubber elasticity, ΔS can be calculated from the equation, \({{\Delta \text{G} }}= - {\text{T}}\Delta {\text{S}}\), where it is assumed that there is no change in the internal energy of the rubber network during stretching (Roy et al. 2018; Roy and Potiyaraj 2018). Variation of the thermodynamic parameters of UN0, UN5, MODK5 and MODS5 are tabulated in Table 7. The value of ΔS was higher for MODS5 than for other samples. This may be due to the more uniform dispersion of silane treated hemp fiber within the natural rubber matrix compared to other composites (Roy et al. 2018). It is established that the ΔG value is closely linked to the elastic behavior of the materials (Bindu and Thomas 2013). Therefore, a higher ΔG indicates greater elastic properties. The value of ΔG for MODS5 is higher than those of UN5 and MODK5. These results can be attributed to better compatibility between silane treated hemp fiber and the rubber matrix, which is supported by the mechanical properties and the morphological behavior of the composites.
Thermal properties of the composites
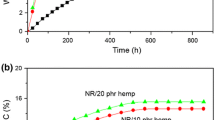
The thermal properties of selected natural rubber composite, such as UN5, MODK5 and MODS5, were investigated using TGA. Figure 14 shows the TGA curves of rubber composites. These TGA cure curves indicate a single-stage degradation with well-defined initial and final degradation temperatures. Within the temperature range of 100–150 °C, there are no differences in the % mass loss among the rubber composites. The initial degradation temperatures of UN5 and MODK5 were 170 and 175 °C. Interestingly, the initial degradation temperature of MODS5 was clearly observed at 230 °C. Within the temperature range of 365–413 °C, there are also no differences in the % mass loss among the rubber composites. These findings suggested that the thermal stability of hemp fiber filled rubber composites is not improved by chemical treatment of hemp fiber. After 450 °C, UN5 shows higher ash content compared to MODS5 and MODK5. The explanation may be due to the hemicellulose, lignin and other constituents found on the surface of untreated hemp fibers.
Conclusions
In this study, the effects of chemical treatments (potassium permanganate and silane) on the surfaces of hemp fibers used in filled natural rubber composites on their curing characteristics, dynamic mechanical, mechanical, morphological and thermal properties were investigated. Additionally, the effects of hemp fiber loading on the properties of the composites were also studied. It was found that the scorch and curing time values of rubber composites increase with increased hemp loading, except at the 15 phr hemp level. At similar levels of fiber loading, maximum torque, delta torque and cure time values for both silane treated fiber and KMnO4 treated fiber filled rubber composites are higher than for untreated hemp fiber filled rubber composites. According to DMA results, it can been be concluded that incorporation of surface treated hemp fiber in natural rubber composites promotes a better interfacial interaction between the fiber and rubber matrix compared to untreated hemp fiber filled rubber composites. For best mechanical properties, the optimal chemical treatment and fiber loading was silane treated fiber and a 5 phr fiber level, respectively. This finding is supported by the increased tensile strength of silane treated fiber composites compared with untreated and KMnO4 treated hemp fiber filled rubber composites. At 5 phr of fiber loading, SEM images indicated better interfacial interaction between silane treated fibers and the rubber matrix compared to untreated hemp fiber and KMnO4 treated hemp fiber filled rubber composites. Based on this study, silane is a more effective treatment method than KMnO4 for improved compatibility between hemp fiber and natural rubber. Additionally, the thermal stability of fiber filled rubber composites was unaltered by the chemical treatment of hemp fibers.
References
Alvarez VA, Vazquez A (2006) Influence of fiber chemical modification procedure on the mechanical properties and water absorption of MaterBi-Y/sisal fiber composites. Compos Part A 37:1672–1680. https://doi.org/10.1016/j.compositesa.2005.10.005
Bindu P, Thomas S (2013) Viscoelastic behavior and reinforcement mechanism in rubber nanocomposites in the vicinity of spherical nanoparticles. J Phys Chem B 117:12632–12648. https://doi.org/10.1021/jp4039489
Borchani KE, Carrot C, Jaziri M (2015) Untreated and alkali fibers from alfa stem: effect of alkali treatment on structural, morphogical and thermal features. Cellulose 22:1577–1589. https://doi.org/10.1007/s10570-015-0583-5
Butler J, Freakley PK (1992) Effect of humidity and water content on the cure behavior of a natural-rubber accelerated sulfur compound. Rubber Chem Technol 65:374–384. https://doi.org/10.5254/1.3538618
Célino A, Goncalves O, Jacquemin F, Fréour S (2014) Qualitative and quantitative assessment of water sorption in naturalfibres using ATR-FTIR spectroscopy. Carbohydr Polym 101:163–170. https://doi.org/10.1016/j.carbpol.2013.09.023
Chaudhary SN, Borkar SP, Mantha SS (2010) Sunnhemp fiber-reinforced waste polyethylene bag composites. J Reinf Plast Compos 29:2241–2252. https://doi.org/10.1177/0731684409345615
Conzatti L, Brunengo E, Utzeri R, Castellano M, Hodge P, Stagnaro P (2018) Macrocyclic oligomers as compatibilizing agent for hemp fibres/biodegradable polyester eco- composites. Polymer 146:396–406. https://doi.org/10.1016/j.polymer.2018.05.053
Datta J, Wloch M (2017) Preparation, morphology and properties of natural rubber composites filled with untreated short jute fibers. Polym Bull 74:763–782. https://doi.org/10.1007/s00289-016-1744-x
Daud S, Ismail H, Bakar AA (2017) A study on the curing characteristics, tensile, fatigue, and morphological properties of alkali-treated palm kernel shell-filled natural rubber composites. BioRes 12:1273–1287
Dayo AQ, Gao BC, Wang J, Liu W, Derradji M, Shah AH, Babar AA (2017) Natural hemp fiber reinforced polybenzoxazine composites: curing behavior, mechanical and thermal properties. Compos Sci Technol 144:114–124. https://doi.org/10.1016/j.compscitech.2017.03.024
De Rosa IM, Kenny JM, Puglia D, Santulli C, Sarasini F (2010) Morphological, thermal and mechanical characterization of okra (Abelmoschus esculentus) fibres as potential reinforcement in polymer composites. Compos Sci Technol 70:116–122. https://doi.org/10.1016/j.compscitech.2009.09.013
De D, De D, Adhikari B (2004) The effect of grass fiber filler on curing characteristics and mechanical properties of natural rubber. Polym Adv Technol 15:708–715. https://doi.org/10.1002/pat.530
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Haghighat M, Zadhoush A, Khorasani SN (2005) Physicomechanical properties of cellulose-filled styrene-butadiene rubber composites. J Appl Polym Sci 96:2203–2211. https://doi.org/10.1002/app.21691
Hariwongsanupab N, Thanawan S, Amornsakchai T, Vallat MF, Mougin K (2017) Improving the mechanical properties of short pineapple leaf fiber reinforced natural rubber by blending with acrylonitrile butadiene rubber. Polym Test 57:94–100. https://doi.org/10.1016/j.polymertesting.2016.11.019
Ismail H, Edyham MR, Wirjosentono B (2002) Bamboo fibre filled natural rubber composites: the effects of filler loading and bonding agent. Polym Test 22:139–144. https://doi.org/10.1016/S0142-9418(01)00060-5
Ismail H, Mahir NA, Ahmad Z (2011) The effect of bis-(3-triethoxysilylpropyl) tetrasulphide (Si-69) as a coupling agent on the properties of natural rubber/knenaf fiber composites. Polym Plast Technol Eng 50:893–897. https://doi.org/10.1080/03602559.2011.551980
Ismail H, Othman N, Komethi M (2012) Curing characteristics and mechanical properties of rattan-powder-filled natural rubber composites as a function of filler loading and silane coupling agent. J Appl Polym Sci 123:2805–2811. https://doi.org/10.1002/app.34730
Jacob M, Thomas S, Varughes KT (2004) Mechanical properties of sisal/oil palm hybrid fiber reinforced natural rubber composites. Compos Sci Technol 64:955–965. https://doi.org/10.1016/S0266-3538(03)00261-6
Janjic S, Kostic M, Skundric P (2007) Direct hemp cellulose dissolution in N-methylmorpoline- N-oxide. J Nat Fiber 4:23–36. https://doi.org/10.1300/J395v04n03_02
Johnson R (2018) Hemp as an agricultural commodity. Congressional research service. https://fas.org/sgp/crs/misc/RL32725.pdf Accessed 20 Dec 2018
Kabir MM, Wang H, Lau KT, Cardona F (2013) Effect of chemical treatments on hemp fiber structure. Appl Surf Sci 276:13–23. https://doi.org/10.1016/j.apsusc.2013.02.086
Khan MA, Drzal LT (2004) Characterization of 2-hydroxyethyl methacrylate (HEMA)-treated jute surface cured by UV radiation. J Adhesion SciTechnol 18:381–393. https://doi.org/10.1163/156856104773635481
Lake GE, Samuri A, Teo SC, Vaja J (1991) Time-dependent fracture in vulcanized elastomers. Polymer 32:2963–2975. https://doi.org/10.1016/0032-3861(91)90194-N
Li Y, Han B, Wen S, Lu Y, Yang H, Zhang L, Liu L (2014) Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos Part A 62:52–59
Lopattananon N, Jitkalong D, Seadan M (2011) Hybridized reinforcement of natural rubber with silane modified short cellulose fibers and silica. J Appl Polym Sci 120:3242–3254. https://doi.org/10.1002/app.33374
Manaila E, Stelescu MD, Cracium G, Surdu L (2014) Effect of benzoyl peroxide on some properties of composites based on hemp and natural rubber. Polym Bull 71:2001–2022. https://doi.org/10.1007/s00289-014-1168-4
Manaila E, Stelescu MD, Doroftei F (2015) Polymeric composites based on natural rubber and hemp fibers. Iran Polym J 24:135–148. https://doi.org/10.1007/s13726-015-0307-6
Masłowski M, Miedzianowska J, Strzelec K (2019) Silanized cereal straw as a novel, functional filler of natural rubber biocomposites. Cellulose 26:1025–1040. https://doi.org/10.1007/s10570-018-2093-8
Mohanta N, Acharya SK (2016) Fiber surface treatment: its effect on structural, thermal, and mechanical properties of Luffa cylindrica fiber and its composite. J Compos Mater 50:3117–3131. https://doi.org/10.1177/0021998315615654
Mwaikambo LY, Ansell MP (2002) Chemical modification of hemp, sisal and kapok fibers by alkalization. J Appl Polym Sci 84:2222–2234. https://doi.org/10.1002/app.10460
Nawamawat K, Sakdapipanich JT, Ho CC, Ma Y, Song J, Vancso JG (2011) Surface nanostructure of Hevea brasiliensis natural rubber latex particles. Colloid Surf A 390:157–166. https://doi.org/10.1016/j.colsurfa.2011.09.021
Oh SY, Yoo DI, Shin Y, Kim HC, Kim HY, Chung YS, Park WH, Youke JH (2005) Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr Res 340:2376–2391. https://doi.org/10.1016/j.carres.2005.08.007
Ohnuki T (2014) The vulcanizing system of diene rubber. Nippon Gomu Kyokaishi 87:467–472
Osabohien E, Egboh SHO (2007) Cure characteristics and physico-mechanical properties of natural rubber filled with the seed shells of cherry (Chrysophyllum albidum). J Appl Sci Environ Manag 11:43–48. https://doi.org/10.4314/jasem.v11i2.54983
Osabohien E, Egboh SHO (2008) Utilization of bowstring hemp fiber as a filler in natural rubber compounds. J Appl Polym Sci 107:210–214. https://doi.org/10.1002/app.27012
Paiva MC, Ammar I, Campos AR, Cheikh RB, Cunha AM (2007) Alfa fibres: mechanical, morphological and interfacial characterization. Compos Sci Technol 67:1132–1138. https://doi.org/10.1016/j.compscitech.2006.05.019
Pandey KK (1999) A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J Appl Polym Sci 71:1969–1975. https://doi.org/10.1002/(SICI)1097-4628(19990321)71:12%3c1969:AID-APP6%3e3.0.CO;2-D
Parikh SJ, Chorover J (2005) FTIR spectroscopic study of biogenic Mn-oxide formation by pseudomonas putida GB-1. Geomicrobiol J 22:207–218. https://doi.org/10.1080/01490450590947724
Patra A, Bisoyi DK, Manda PK, Singh AK (2012) Electrical and mechanical properties of the potassium permanganate treated short sisal fiber reinforced epoxy composite in correlation to the macromolecular structure of the reinforced fiber. J Appl Polym Sci 128:1011–1019. https://doi.org/10.1002/app.38195
Poh BT, Ng CC (1998) Effect of silane coupling agents on the mooney scorch time of silica-filled natural rubber compound. Eur Polym J 34:975–979. https://doi.org/10.1016/S0014-3057(97)00211-5
Popescua CM, Larsson PT, Olaru N, Vasile C (2012) Spectroscopic study of acetylated kraft pulp fibers. Carbohydr Polym 88:530–536. https://doi.org/10.1016/j.carbpol.2011.12.046
Rachini A, Troedec ML, Peyratout C, Smith A (2008) Comparison of the thermal degradation of natural, Alkali-treated and silane-treated hemp fibers under air and an inert atmosphere. J Appl Polym Sci 112:226–234. https://doi.org/10.1002/app.29412
Rice Department, Ministry of Agriculture and Cooperatives, Thailand (2016) Rice knowledge bank (Thai Version). http://www.ricethailand.go.th/rkb3/title-index.php-file=content.php&id=0733.htm. Accessed 20 Dec 2018
Rodgers B, Waddell W (2005) The science of rubber compounding. In: Mark JE, Erman B, Eirich FR (eds) Science and technology of rubber, 3rd edn. Elsevier Academic, Burlington, pp 401–453
Rohit K, Dixit S (2016) A review-future aspect of natural fiber reinforced composite. Polym Renew Resour 7:43–57. https://doi.org/10.1177/204124791600700202
Roy K, Potiyaraj P (2018) Development of high performance microcrystalline cellulose based natural rubber composites using maleated naturalrubber as compatibilizer. Cellulose 25:1077–1087. https://doi.org/10.1007/s10570-017-1613-2
Roy K, Debnath SC, Das A, Heinrich G, Potiyaraj P (2018) Exploring the synergistic effect of short jute fiber and nanoclay on the mechanical, dynamic mechanical and thermal properties of natural rubber composites. Polym Test 67:487–493. https://doi.org/10.1016/j.polymertesting.2018.03.032
Sadequl AM (2000) The effect of accelerator/sulphur ratio on the cure time and torque maximum of epoxidized natural rubber. Intern J Polymeric Mater 46:597–615. https://doi.org/10.1080/00914030008033899
Sae-oui P, Sirisinha C, Thepsuwan U, Thapthong P (2007) Influence of accelerator type on properties of NR/EPDM blends. Polym Test 26:1062–1067. https://doi.org/10.1016/j.polymertesting.2007.07.004
Saramolee P, Lertsuriwat P, Hunyek A, Sirisathitkul C (2010) Cure and mechanical properties of recycled NdFeB-natural rubber composites. Bull Mater Sci 33:597–601. https://doi.org/10.1007/s12034-010-0091-z
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29(10):786–794. https://doi.org/10.1177/004051755902901003
Seki K, Çağrı Kılınç A, Dalmis R, Atagür M, Köktaş S, Göktaş AA, Çelik E, Özgür Seydibeyoğlu M, Bülent Önay A (2018) Surface modification of new cellulose fiber extracted from conium maculatum plant: a comparative study. Cellulose 25:3267–3280. https://doi.org/10.1007/s10570-018-1797-0
Sepe R, Bollino F, Boccarusso L, Caputo F (2018) Influence of chemical treatments on mechanical properties of hemp fiber reinforced composites. Compos Part B: Eng 133:210–217. https://doi.org/10.1016/j.compositesb.2017.09.030
Sgriccia N, Hawley MC, Misra M (2008) Characterization of natural fiber surfaces and natural fiber composites. Compos Part A Appl Sci Manuf 39:1632–1637. https://doi.org/10.1016/j.compositesa.2008.07.007
Sheltami RM, Kargarzadeh H, Abdullah I (2015) Effects of silane surface treatment of cellulose nanocrystals on the tensile properties of cellulose-polyvinyl chloride nanocomposite. Sains Malays 44:801–810. https://doi.org/10.17576/jsm-2015-4406-05
Sheng K, Qian S, Wang H (2014) Influence of permanganate pretreatment on mechanical properties and thermal behavior of moso bamboo particle reinforced PVC composites. Polym Compos 35:1460–1465. https://doi.org/10.1002/pc.22799
Silva MC, Lopes OR, Colodette JL, Porto AO, Rieumont J, Chaussy D, Belgacem MN, Silva GG (2008) Characterization of three non-product materials from a bleached eucalyptus kraft pulp mill, in view of valorizing them as a source of cellulose fibers. Ind Crops Prod 27:288–295. https://doi.org/10.1016/j.indcrop.2007.11.005
Široká B, Široký J, Bechtold T (2011) Application of ATR-FT-IR single-fiber analysis for the identification of a foreign polymer in textile matrix. Int J Polym Anal Charact 16:259–268. https://doi.org/10.1080/1023666X.2011.570066
Sriring M, Nimpaiboon A, Kumarn S, Sirisinha C, Sakdapipanich J, Toki S (2018) Viscoelastic and mechanical properties of large- and small-particle natural rubber before and after vulcanization. Polym Test 70:127–134. https://doi.org/10.1016/j.polymertesting.2018.06.026
Srisuwan L, Jarukumjorn K, Suppakarn N (2018) Effect of silane treatment methods on physical properties of rice husk flour/natural rubber composites. Adv Mater Sci Eng 4583974:1–14. https://doi.org/10.1155/2018/4583974
Stelescu MD, Manaila M, Craciun G, Dumitrascu M (2014) New green polymeric composites based on hemp and natural rubber processed by electron beam irradiation. Sci World J 10:15–20. https://doi.org/10.1155/2014/684047
Utara S, Saengsila P (2015) Effect of divalent metal ions on curing characteristics and dynamic mechanical properties of natural rubber. Macromol Symp 354:287–293. https://doi.org/10.1002/masy.201400050
Utara S, Jantachum P, Sukkaneewat B (2017) Effect of surface modification of silicon carbide nanoparticles on the properties of nanocomposites based on epoxidized natural rubber/natural rubber blends. J Appl Polym Sci 134:45289. https://doi.org/10.1002/app.45289
Väisänen T, Batello P, Lappalainen R, Tomppo L (2018) Modification of hemp fibers (Cannabis sativa L.) for composite applications. Ind Crops Prod 111:422–429. https://doi.org/10.1016/j.indcrop.2017.10.049
Wang J, Wu W, Wang W, Zhang J (2011) Preparation and characterization of hemp hurd powder filled SBR and EPDM elastomers. J Polym Res 18:1023–1032. https://doi.org/10.1007/s10965-010-9503-4
Wongsorat W, Suppakran N, Jarukumjorn K (2014) Effect of compatibilizer type and fiber loading on the mechanical properties and cure characteristics of sisal fiber/natural rubber composites. J Compos Mater 48:2401–2411. https://doi.org/10.1177/0021998313498790
Zhang Y, Zhang C, Huang G, Xing B, Duan Y (2015) Synthesis and capacitive properties of manganese oxide nanoparticles dispersed on hierarchical porous carbons. Electrochim Acta 166:107–116. https://doi.org/10.1016/j.electacta.2015.03.073
Acknowledgments
The authors are grateful to the Division of Chemistry, Faculty of Science, Udon Thani Rajabhat University for financial support of this project and the Department of Physics, Faculty of Science, Udon Thani Rajabhat University for providing the spectroscopy measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moonart, U., Utara, S. Effect of surface treatments and filler loading on the properties of hemp fiber/natural rubber composites. Cellulose 26, 7271–7295 (2019). https://doi.org/10.1007/s10570-019-02611-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02611-w