Abstract
The obtaining and characterization of polymer composites based on natural rubber and hemp, in which the elastomer crosslinking has been achieved with benzoyl peroxide, are presented. The mechanical characteristics, gel fraction, crosslink density, water uptake swelling parameters and FTIR of the composites based on natural rubber and hemp fiber vulcanized by dibenzoyl peroxide have been investigated as a function of the hemp content. The hardness, modulus at 100 % elongation, tearing strength, tensile strength and elongation at break have been improving with the increasing of fiber content in composites materials due to the better interaction of fiber in natural rubber composites. These results indicate that hemp has a reinforcing effect on natural rubber. Gel fraction value is over 95 % for all blends and varies irregularly depending on the amount of hemp in the composites. The crosslinking density (ν) of samples increases as the amount of hemp in blends increases, because hemp act as a filler in natural rubber blends and leads to reinforcement of the blends. The water uptake and swelling parameters also increases with the increasing of the amount of fiber content, because of the hemp hydrophilic characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rubber–fiber composites are used in rubber industry due to the advantages they impart in processing and low cost coupled with high strength. Normally, a rubber–fiber composite can offer a set of properties that may give it the potential of entering in application areas where it is not possible with either of the components in the composite [1]. These composites combine the elastic behavior of rubber with the strength and stiffness of fiber. The aim of the present work is to study the potential of using hemp fiber as reinforcement agent in natural rubber. Hemp fiber is obtained from the bast of the plant Cannabis sativa L. It grows easily—to a height of 4 m—without agrochemicals and captures large quantities of carbon. Long, strong and durable, hemp fibers are about 70 % cellulose and contain low levels of lignin (around 8–10 %). The fiber diameter ranges from 16 to 50 microns. Shorter, woody core fibers (tow) contain higher levels of lignin. Hemp has been used for centuries to make rope, canvas and paper. Long hemp fibers can be spun and woven to make crisp, linen-like fabric used in clothing, home furnishing textiles and floor coverings. Hemp fibers are also used to reinforce molded thermoplastics in the automobile industry. The short core fibers go into insulation products, fiberboard and erosion control mats, while the fibrous core can be blended with lime to make strong, lightweight concrete [2]. There are few studies regarding the use and applications of hemp fibers in the development of composites based on rubber and hemp. Osabohien and Egboh [3] performed a study regarding the utilization of bowstring hemp fiber as filler in natural rubber compounds, but elastomer crosslinking was carried out using the classical method based on sulphur and vulcanization accelerators. Vulcanization with sulphur and accelerators of NR is done in general by ionic mechanism and leads to the formation of sulphur bridges between (C–S x –C) macromolecules or cyclic combination of sulphur. At high temperatures, desulphuration takes place, determining the formation of shorter sulphur bridges. As a consequence of thermal instability of sulphur, NR vulcanized with sulphur can be devulcanized in the presence of disulphur diaryls or amines, at temperatures of over 300 °C [4]. Physical–mechanical properties of samples containing C–S x –C crosslinking bridges exhibit better tensile strength values than those containing C–C bonds. Although vulcanization with sulphur and vulcanization accelerators leads to obtaining products with better characteristics, it also has disadvantages, such as the fact that during the process of vulcanization nitrosamines (carcinogen products) are formed and the obtained finite products are toxic, contain heavy metals (Zn), have an unpleasant odor and exudate [5, 6].
In this paper, some composites based on natural rubber and hemp, in which the elastomer crosslinking was carried out using benzoyl peroxide, were analyzed. This method of crosslinking results in a better composite resistance to aging than those obtained by the classical method of crosslinking (with sulphur and vulcanization accelerators) [7]. Vulcanisation by organic peroxides is a free radical process. The effective crosslinking can take place in the complete absence of oxygen, otherwise peroxy radicals would be formed leading to the oxidation of the rubber. Peroxide vulcanisation is, therefore, limited to products made by press molding. The structure of peroxide vulcanisate is relatively simple compared with that of a sulphur vulcanisate, as rubber chains are crosslinked by simple carbon–carbon linkages, which are highly stable towards heat. Therefore, these vulcanisates possess extremely good thermal aging characteristics, superior to those of even EV systems, and also lower compression set at elevated temperature. However, fatigue resistance and resistance to low temperature crystallization are low [8]. The mechanism of NR crosslinking by peroxide is described in Scheme 1. Vulcanization/crosslinking with peroxides [4, 9–14] is done by radicalic mechanism when bonds between C–C macromolecules are formed. Crosslinking is initiated by the thermal decomposition of a peroxide into primary radicals, R ·1 and R ·2 (Scheme 1a). This operation is carried out at high temperatures. Free radicals formed are very reactive chemical species which initiate ‘‘propagation’’ reactions. The primary radicals (R ·1 and R ·2 ) form stable species (acetone and diacetylbenzene) by scission, and the second radical (CH ·3 =R ·3 ) continues the propagation in the presence of NR. The primary radicals formed, react with NR (NR–H) under H-abstraction and leads to the formation of NR radicals (NR·) and stable species (tert-butanol and diisopropanolbenzene). The recombination of radicals from natural rubber (NR·) and the reaction of NR· with peroxide radicals (R·–R1, R2 or R3) represent “the end” of the radical polymerization reaction (Scheme 1b, c). Simultaneously with natural rubber crosslinking, may occur also the chemical modification of hemp fibers (Scheme 2). Therefore, free radicals may react with the hydrogen groups of cellulose or lignin fibers of hemp [15–17]. Benzoyl peroxide (BP) and dicumyl peroxide (DCP) are chemicals from the organic peroxide family that are used for the natural fiber surface modifications. In treatments with peroxides, fibers are coated with BP or DCP in acetone solution for about 30 min after an alkali pre-treatment [18–20]. As a result of peroxide treatment, the hydrophilicity of the fibers has decreased [18] and tensile properties have increased [19].
In our study, hemp fibers having high cellulose content are used in the form of filler in a natural rubber matrix. The interface between both components (fiber and rubber matrix) influences the mechanical properties of reinforced composites. A low adhesion between both layers causes a decrease in the mechanical properties of the material, and consequently, is necessary a good adhesion to obtain a composite with high performance (good mechanical properties). The hydrophilic nature of natural fibers, due to the existence of many hydroxyl groups in cellulose, presents a major problem for their use in composites, because it results in low compatibility with hydrophobic polyolefin matrices [21, 22].
Experimental
Materials
For preparing the above polymer composites, the following materials were used: natural rubber (NR) Crep 1× (74 ML1 + 4 Mooney viscosity at 100 °C, 0.32 % volatile materials, 0.38 % nitrogen, 0.22 % ash, 0.021 % impurities) from Almar Trading Co(Pte) Ltd, Sri Lanka, antioxidant pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate Irganox 1010 from CIBA-BASF, Germania, polyethylene glycol PEG 4000 from Bayer, Germania, (1,128 g/cm3 density, 4–8 °C melting point range), ground hemp (thread length of max 3 mm) and dibenzoyl peroxide Perkadox 14-40B (1,60 g/cm3 density, 3.8 % active oxygen, 40 % peroxide, pH 7) from AkzoNobel Polymer Chemicals, USA, as vulcanizing agent.
Sample preparation
The blends were prepared in an electrically heated laboratory roller. For preparation of the polymeric composites, the blend constituents were added in the following sequences and amounts: 100 parts natural rubber (NR) roll binding (2′), embedding 3 phr (parts to 100 parts rubber) PEG 4000 and 1 phr Irganox 1010 antioxidant (2′), adding 5, 10, 15 and 20 phr ground hemp (2-4′), respectively, 8 phr of dibenzoyl peroxide Perkadox 14-4B (2′) as vulcanizing agent was added, homogenisation of blends and removing from the roll in the form of sheet (4′). The process variables were temperature 25–50 ± 5 °C, friction 1:1.1 and total blending time 8–14′. The plates required for physico-mechanical tests with sizes of 150 × 150 × 2 mm3 were obtained by pressing in a hydraulic press at 160 °C and 150 MPa; the curing time was 19′.
Laboratory tests
Mechanical characteristics
Tensile strength tests were carried out with a Schopper strength tester with testing speed of 460 mm/min, using dumb-bell shaped specimens according to ISO 37/2012. The hardness was measured using a hardener tester according to ISO 7619-1/2011 on samples having the thickness of 6 mm. The elasticity was evaluated with a Schob type test machine also on samples of 6 mm thick, according to ISO 4662/2009.
The sol–gel analysis
The sol–gel analysis was performed on crosslinked NR rubber (with and without hemp) to determine the mass fraction of insoluble NR (the network material resulting from network-forming crosslinking process) from samples (gel fraction). The samples were swollen in toluene and extracted after 72 h to remove any scissioned fragments and unreacted materials. The networks were then dried in air for 6 days and in a laboratory oven at 80 °C for 3 h, and reweighed. The gel fraction was calculated as:
where \(m_{\text{s}}\) and \(m_{\text{i}}\) are the weight of the dried sample after extraction and the weight of the sample before extraction, respectively [23, 24].
The crosslink density
The crosslink density (\(\nu\)) of the samples was determined on the basis of equilibrium solvent-swelling measurements (in toluene at 23–25 °C) by application of the well-known modified Flory–Rehner equation for tetra functional networks. The samples (2 mm thick) were initially weighed (m i) and immersed in toluene for 72 h. The swollen samples were removed and cautiously dried to remove the excess solvent before being weighed (m g) and, during this operation, the samples were covered to avoid toluene evaporation during weighing. The solvent traces and other small molecules were then eliminated by drying in air for 6 days and in a laboratory oven at 80 °C for 3 h. Finally, the samples were weighed for the last time (m s), and volume fractions of polymer in the samples at equilibrium swelling ν 2m were determined from swelling ratio G as follows:
where
\(\rho_{\text{r}}\) and \(\rho_{\text{s}}\) are the densities of rubber samples and solvent (0.866 g/cm3 for toluene), respectively. The densities of elastomer samples were determined by hydrostatic weighing method, according to the SR ISO 2781/2010. By this method, the volume of a solid sample is determined by comparing the weight of the sample in air with the weight of the sample immersed in a liquid of a known density. The volume of the sample is equal to the difference in the two weights divided by the density of the liquid. The crosslink densities of the samples, ν, were determined from measurements in a solvent, using the Flory–Rehner relationship:
where \(V_{1}\) is the molar volume of solvent (106.5 cm3/mol for toluene), \(\nu_{2m}\) is the volume fraction of polymer in the sample at equilibrium swelling, and \(\chi_{12}\) is the Flory–Huggins polymer–solvent interaction term (the values of and \(\chi_{12}\) are 0.393 for toluene [23, 24]).
Rubber–fiber interactions
The extent of interaction between rubber and fiber can be analyzed using Kraus equation. The Kraus theory and Kraus equation [25] have been successfully used by some researchers to assess the interfacial interaction in fiber-reinforced rubber composites [26–30]. The Kraus equation is as follows:
where V ro and V rf are the volume fractions of rubber in the gum vulcanizate and in fiber-filled swollen sample, respectively; f is the volume fraction of fiber, and m is the fiber polymer interaction parameter. The volume fraction of rubber in the swollen sample V rf, was calculated by the expression:
where \(\rho_{r}\) and \(\rho_{s}\) are the densities of rubber samples and solvent (0.98 g/cm3 for natural rubber and 0.866 g/cm3 for toluene), respectively; D is the deswollen weight of the test specimen (dry weight), F is the weight fraction of the insoluble components, T is the weight of the specimen and A 0 is the weight of the absorbed solvent at equilibrium swelling.
Water uptake test
The effect of water absorption on fiber-reinforced natural rubber composites is investigated in accordance with SR EN ISO 20344/2004. The samples were dried in an oven at 80 °C for 2 h and then are allowed to cool at room temperature in desiccators before weighing. Water absorption tests were conducted by immersing the samples in distilled water in bottles and kept at room temperature 23 ± 2 °C and in a laboratory oven at 70 ± 1 °C. Samples were removed from the bottles at periodic intervals, and the wet surfaces were quickly wiped using a clean dry cloth or tissue paper and weights of the specimen after swelling were determined at regular intervals until no further increase in solvent uptake was detected. The moisture absorption was calculated by the weight difference. The percentage weight gain of the samples was measured at different time intervals. The water uptake was calculated as:
where, m S is the weight of the sample saturated with water, determined at periodic intervals and m 1 is the initial weight of the oven-dried sample.
Swelling parameters
The swelling studies provide information about the strength of interface, dispersion degree of fibers, and their alignment in the elastomer matrix. Swelling behavior was determined by the change in mass using the following method. For cured rubber blends, the test pieces of known weight (m 1) were immersed in water in diffusion test bottles and kept at room temperature for 49 days (until no further increase in water uptake was detected). After immersion, the samples were taken out from the solvents and the wet surfaces were quickly dried using a tissue paper and reweighted (m 2). The test samples of the blends were further dried in air for 6 days and in a laboratory oven at 100 °C for 6 h, cooled in a desiccator and immediately weighed (m 3). The swelling parameters [30] of blends were calculated as follows:
(a) Calculation of Qt (mol% uptake of the solvent): the mol% uptake of the solvent, Qt, for the composite samples was determined using the equation:
(b) Swelling index (SI %) was calculated by the equation:
(c) Soluble fraction (SF %) was determined by the following relation:
Fourier transform infrared (FTIR) spectroscopy
Changes of the chemical structure of natural rubber/hemp fiber composites vulcanized with dibenzoyl peroxide were highlighted using a FTIR spectrophotometer—JASCO FT/IR 4200, by ATR measurement method. Samples spectra are the average of 30 scans realized in absorption in the range of 4,000–600 cm−1 with a resolution of 4 cm−1.
Results and discussion
Physical–mechanical characteristics
The variations of mechanical properties with the increasing of the fiber amount in composites based on NR/fiber are provided in Figs. 1, 2, 3, 4 and 5. The hardness (Fig. 1) and modulus at 100 % elongation (Fig. 2) were increased with the increasing of fiber amount in blends. The hardness exhibits an increase of about 27 % for 5 phr hemp and 60 % for 20 phr hemp, while modulus at 100 % elongation exhibited an increase of 5.5 and 105 % with the same fiber loading. It can be seen from the Fig. 3 that the tensile strength decreased with the increasing of filler (hemp) loading. The decrease in tensile strength is due to the poor adhesion of the filler–matrix and the agglomeration of filler particles. It has been shown that for irregularly shaped fillers, the strength of the composites can decrease due to the inability of the filler to support stresses transferred from polymer matrix [31]. On the other hand, poor interfacial bonding causes partially separated micro-spaces between the filler particles and the polymer matrix [32]. The elongation at break (Fig. 4) decreased with increasing of fiber content in composites. The decreasing of elongation at break with the fiber content increasing in the composite is the result of the high crosslinking. The elongation at break shows a decrease of 16 % for 5 phr hemp and of 33.4 % for blend with 15-phr fiber content. The tearing strength (Fig. 5) follows the same trend, as the hardness and modulus at 100 % elongation have increased with increase of fiber content in composite. The tearing strength shows an increase of about 26 % for 10 phr hemp and with 60 % for 20 phr hemp content in composite. The values of hardness and modulus at 100 % elongation have increased due to a better interaction of fiber with NR. These results indicate that hemp has a reinforcing effect on natural rubber.
Gel fraction and crosslink density of the blends
Table 1 shows the gel fraction (mass fraction of the network material resulting from a network-forming polymerization or crosslinking process; the gel fraction comprises a single molecule spanning the entire volume of the material sample) and crosslink density (number of crosslinks per unit volume in a polymer network) of the samples vulcanized with dibenzoyl peroxide as a function of the hemp content. Gel fraction value is over 95 % for all blends and varies irregularly depending on the amount of hemp in the composites. The crosslinking density (ν) of samples increases as the amount of hemp in blends increases, because hemp acts as a filler in natural rubber blends and leads to its reinforcement of blends. The experimental results confirmed by other works [24, 33] showed that with the increasing in crosslink density, hardness and modulus at 100 % elongation were increased, whereas the elongation at break decreased. Analyzing the results presented in Figs. 1, 2, 3, 4 and 5, it can be noticed that hardness and modulus at 100 % elongation of blends have higher values and elongation at break has lower values, compared to the control sample. Those results are in accordance with the values of crosslink density in Table 1 and literature data [4, 16, 17].
Rubber–fiber interactions
The extent of interaction between rubber and fiber was analyzed using Kraus equation, and the results are listed in Table 2 and Fig. 6. Samples of NR with 5, 10, 15 and 20 phr content of hemp fiber were swollen in toluene for 72 h. The results of V r0 and V rf calculated according to Eqs. 5 and 6 are presented in Table 2 and Fig. 6. From the results in Table 2, it is observed that the equilibrium solvent uptake of the samples decreased as the fiber content increased, which caused an increase in V rf. Therefore, the ratio V r0/V rf decreases since V r0 is a constant. This is due to the increased hindrance exerted by the hemp fibers at higher loadings. The diffusion mechanism in the composite is strongly connected with the ability of rubber to provide pathways for the solvent to progress in the form of randomly generated voids. As the void formation decreases with fiber content, the solvent uptake also decreases. The ratio V r0/V rf is the degree of restriction of swelling of the rubber matrix due to the presence of fibers [29]. The more and more reduced values of V r0/V rf ratio are associated with the enhanced adhesion between fiber and rubber, according to the Kraus theory and Kraus equation. The decreased values of V r0/V rf values at higher loadings indicate the reinforcement effect of the fibers. The compatibilized composites based on NR and hemp fiber have a negative slope as shown in Fig. 6 [29].
Swelling parameters
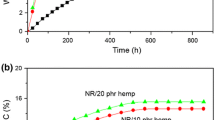
The swelling parameters and water uptake of samples are presented in Tables 3, 4, 5, 6 and Figs. 7 and 8. Figure 7 shows the water uptake according to the amount of hemp in composites: (a) at 25 °C and (b) at 70 °C. It can be observed that water uptake increased with increasing fiber content and temperature. Thus, (a) after 24 h in water at room temperature water uptake values were: 0.8 % for the sample without hemp, 1.75 % for 5 phr hemp, 2.56 % for 10 phr hemp, 3.2 % for 15 phr hemp and 3.92 % for 20 phr hemp; (b) after 24 h in water at 70 °C water uptake values were: 3.13 % for the sample without hemp, 4.41 % for 5 phr hemp, 5.31 % for 10 phr hemp, 6.57 % for 15 phr hemp and 6.88 % for 20 phr hemp; (c) after 216 h in water at room temperature water uptake values were: 1.8 % for the sample without hemp, 5.11 % for 5 phr hemp, 8.35 % for 10 phr hemp, 9.27 % for 15 phr hemp and 10.97 % for 20 phr hemp; (d) after 216 h in water at 70 °C water uptake values were: 7.78 % for the sample without hemp, 10.06 % for 5 phr hemp, 11.34 % for 10 phr hemp, 11.9 % for 15 phr hemp and 12.76 % for 20 phr hemp. It can be seen that the composites absorb water very fast initially (in the first 24 h), the amount of water absorbed then being increasingly smaller (after 216 h). The temperature of 70 °C seems to accelerate the moisture water uptake behavior. By increasing the amount of hemp in composite the water absorption increases also. This can be explained by the fact that the diffusion of water in elastomers is not straightforward due to the presence of hydrophilic materials (such as the hemp). In the case of NR it is mainly proteinaceous. When water diffuses into the rubber, the water-soluble hydrophilic materials dissolve, forming droplets of solution within the rubber. An osmotic pressure gradient would exist between watery domains in the rubber and that of the external solution immersing the rubber. This results in more water diffusing into the internal solution droplet. Water absorption reaches equilibrium when the elastic stresses acting on the droplets balance the osmotic pressure difference [34].
The water absorption at 23 ± 2 °C becomes constant after 1,176 h (Fig. 8). In these composites, water is absorbed mainly by the hemp. The maximum percentages of water uptake for NR with 5, 10, 15 and 20 phr content of hemp fiber, immersed at room temperature for 1,176 h were 8.09, 12.70, 13.70 and 15.11 %, respectively. The water uptake for all blends, except NR without other additions (which absorbed a low percentage of water, 1.85 % after 1,176 h), is linear at the beginning, then slows and reaches saturation after prolonged time. Both the initial rate of water absorption and the maximum water uptake grow for all composites samples because of the fiber volume fraction growth. This phenomenon can be explained by considering the water uptake characteristics of the hemp fibers. When the NR/hemp composite is exposed to moisture, the hemp fibers suffer swelling. The high cellulose content in hemp fiber (approximately 75 %) further contributes to more water penetrating into the interface of composites materials [35]. The natural rubber is a hydrophobic material and its water absorbability can be neglected [36]. For the same absorption time, composites having a high fiber content exhibit high water absorption because one of the properties of these natural fibers (hemp) is the hydrophilic characteristic. The obtained composites can be used in manufacturing products with applications in the automotive industry, in the furniture industry, etc.
FTIR study
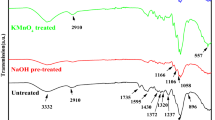
Fourier transform infrared spectroscopy is one of the most widely employed techniques for analyzing polymeric materials. The natural rubber/hemp fiber composites vulcanized with dibenzoyl are analyzed by FTIR to know the various chemicals constituents present in them. The natural rubber is composed of hydrocarbons (89.3–92.4 wt%), proteins (2.5–3.5 wt%), and other ingredients (4.1–8.2 wt%). The main component of NR is cis-1, 4-polyisoprene with a high degree of long chain branching generally associated with the presence of non-hydrocarbon groups distributed along the chains. Natural fibers can be considered as naturally occurring composites consisting mainly of cellulose fibrils embedded in lignin matrix. The cellulose fibrils are aligned along the length of the fiber, which render maximum tensile and flexural strengths, in addition to providing rigidity. The reinforcing efficiency of natural fiber is related to the nature of cellulose and its crystallinity. The main components of natural fibers are cellulose (α-cellulose), hemicellulose, lignin, pectins and waxes [37].
Figures 9, 10a–c and Table 7 show the infrared spectra and the characteristic bands observed one by one in natural rubber, hemp fibers and natural rubber/hemp fiber composites vulcanized with dibenzoyl peroxide, in the range of the 4,000–650 cm−1. The broadband in the 3,340–3,334 cm−1 region, which can be due to the OH-stretching vibration, gives considerable information concerning the hydrogen bonds from the amorphous celluloses [38]. The presence of amorphous cellulosic samples can be confirmed by the band from 2,940 to 2,840 cm−1, corresponding to the C–H stretching vibration in methyl and methylene groups [39]. The absorption located in the region 1,740–1,730 cm−1 corresponds to the C=O stretch in non-conjugated ketones, carbonyls and in ester groups [39]. Absorption bands in the region 1,635–1,623 cm−1 were due to absorbed water in cellulose [40]. or are caused by lignin (aromatic skeletal vibrations) [39]. In addition, the absorption band in the region 1,432–1,430 cm−1 is assigned to a symmetric CH2-bending vibration. This band is also known as the “crystallinity band” [38]. The characteristic peak which appeared in the range of 1,320–1,305 cm−1 is assigned to the cellulose component: bending vibration of C–H and C–O groups of aromatic rings [39, 40]. The absorption located in the region 1,165–1,150 cm−1 corresponds to the anti-symmetrical deformation of the C–O–C bond, and absorption located in the region 1,070–1,020 cm−1 is due to the C–O, alcohol, O–H or aliphatic ethers [39, 40].
The presence of absorption bands in the spectrum of natural rubber in the spectral region located between 1,664 and 1,658 cm−1 is due to valence vibration of homogeneous double bonds (C=C) in the NR structure. Their intensity decreases for vulcanized samples compared with non-vulcanized samples. The specific absorption bands of R2C=CH–R group are observed at 836–838 cm−1. These changes occur as a result of elastomer crosslinking and the consumption of double bonds. The absorption band of CH2 deformation occurs at 1,430–1,460 cm−1 and of CH3 asymmetric stretching at 1,360–1,380 cm−1 [16, 17, 41]. It is known that the NR contains also other compounds such as lipids, neutral glycolipids and phospholipids, etc. Absorption bands with maxima at 3,035–3,036 cm−1 corresponding to CH stretching in the –CH=CH2 group are observed. Vulcanization of the polymeric compositions results in consumption of the double bonds in NR molecules, so that the intensities of these absorption bands decrease. The characteristic bands of the saturated aliphatic sp3 C–H bonds are observed in the region 2,970–2,830 cm−1 which are assigned to ν as (CH3), ν as (CH2), and ν s (CH2), respectively (as three corresponding bends) [42]. These bands are specific to natural rubber and cellulose, lignin or hemicellulose, from the hemp fibers existing in the mixture [43]. It can be noticed that with the hemp amount increasing in the mixture, the intensity bands vary out of uniformity. The absorption bands at 3,280–3,290 cm−1 were identified in the proteins and in both monopeptides and dipeptides present in natural rubber [44]. This band is specific for NR and cellulose, lignin and hemicellulose from the hemp fibers existing in the mixture [43]. Band intensity significantly decreases for vulcanized samples with the amount of fiber hemp increasing in the mixture. These are the consequences of proteins and peptides degradation. The absorption band around 1,740–1,727 cm−1 was identified to the fatty acid ester groups in NR [45]. In fingerprint region, there are some specific single bends for cellulose, lignin and hemicellulose from hemp fibers but also for NR, some of them mentioned above. With the hemp fiber amount increasing, significant changes occur in the specific absorption bands of hemp fiber fingerprint.
Conclusions
Our investigation of the basic mechanical and some physical chemical parameters as a function of hemp content in peroxide-vulcanized NR proved the reinforcing effect of the hemp fibers. The mechanical characteristics, gel fraction, crosslink density, water uptake and swelling parameters of composites based on natural rubber and hemp vulcanized by dibenzoyl peroxide, have been investigated as a function of the hemp content. The mechanical characteristics (hardness, modulus at 100 % elongation, tearing strength, tensile strength and elongation at break) were improved depending on the level of filling by hemp fibers in composite. These results indicate that hemp has a reinforcing effect on natural rubber. Gel fraction value is over 95 % for all blends and varies irregularly depending on the amount of hemp in the composite. The crosslinking density (ν) of samples increases as the amount of hemp in blends increases, because hemp acts as a filler in natural rubber blends and leads to the reinforcement of blends. The water uptake and swelling parameters increases also with increasing the amount of fiber content due to the hydrophilic characteristics of hemp. The water absorption becomes constant after 1,176 h. In these composites, the water is absorbed mainly by the hemp. Obtaining polymeric composites based on hemp and natural rubber is an efficient method of exploiting hemp waste from the textile industry. The use of natural fibers in composites has increased due to their relative cheapness, their ability to be recycled and for the fact that they can compete well in terms of strength per weight of material.
References
Hussain AI, Abdel-Kader AH, Ibrahim AA (2010) Effect of modified linen fiber waste on physico-mechanical properties of polar and non-polar rubber. Nat Sci 8(7):82–93
Karus M, Vogt D (2004) European hemp industry: cultivation, processing and product lines. Euphytica 140:7–12
Osabohien E, Egboh SHO (2008) Utilization of bowstring hemp fiber as a filler in natural rubber compounds. J Appl Polym Sci 107(1):210–214
Van Duin M (2002) Chemistry of EPDM cross-linking. Kautsch Gummi Kunstst 55(4):150–156
Hertz DL Jr (1984) Elastomerics. Theory and practice of vulcanization. A Publication of Communication Channels Inc, USA
Stelescu MD, Georgescu M, Manaila E (2010) Aspects regarding crosslinking of a natural rubber blend. In: Proceedings of the 3rd international conference advanced materials and systems ICAMS 2010, 16–18 September, Bucharest, Romania, 313–318
Manaila E, Stelescu MD, Craciun G (2011) Characteristics of natural rubber blends vulcanized with electron beam and microwave. Leather Footwear J 11(1):43–52
Mathew NM (2001) Natural rubber. In: White JR, De SK (eds) Rubber technologist’s handbook. Rapra Technology Limited, Shropshire, UK, p 38
Dluzneski PR (2001) Peroxide vulcanization of elastomers. Rubber Chem Technol 74:451–492
Stelescu MD, Manaila E, Zuga N (2011) The use of polyfunctional monomers in the radical cure of chlorinated polyethylene. Polym J 43(9):792–800
Stelescu MD, Manaila E, Craciun G, Zuga N (2012) Crosslinking and grafting ethylene vinyl acetate copolymer with accelerated electrons in the presence of polyfunctional monomers. Polym Bull 68(1):263–285
Quirk RP (1988) Overview of curing and crosslinking of elastomers. Prog Rubber Plast Technol 4(1):31–45
Ogunniyi DS (1999) Peroxide vulcanisation of rubber. Prog Rubber Plast Technol 15(2):95–112
Van Duin M, Dees M, Dikland H (2008) Advantages of EPDM rubber products with a third monomer Part I—improved peroxide curing efficiency in window gasket applications. Kautsch Gummi Kunstst 49:233–243
Kalia S, Kumar A, Kaith BS (2011) Sunn hemp cellulose graft copolymers polyhydroxybutyrate composites: morphological and mechanical studies. Adv Mater Lett 2(1):17–25
Stelescu MD, Manaila E, Craciun G, Dumitrascu M (2014) New green polymeric composites based on hemp and natural rubber processed by electron beam irradiation. Sci World J 2014:684047-1–684047-13
Manaila E, Craciun G, Stelescu MD, Ighigeanu D, Ficai M (2014) Radiation vulcanization of natural rubber with polyfunctional monomers. Polym Bull 71(1):57–82
Paul A, Joseph K, Thomas S (1997) Effect of surface treatments on the electrical properties of low-density polyethylene composites reinforced with short sisal fibers. Compos Sci Technol 57(1):67–79
Joseph K, Thomas S, Pavithran C (1996) Effect of chemical treatment on the tensile properties of short sisal fibre-reinforced polyethylene composites. Polymer 37(23):5139–5149
Kumar R, Obrai S, Sharma A (2011) Chemical modifications of natural fiber for composite material. Pelagia Research Library, Der Chemica Sinica 2(4):219–228
Bledzki AK, Gassan J (1999) J Composites reinforced with cellulose based fibres. Prog Polym Sci 24(2):221–274
Espert A (2003) Natural fibres/polypropylene composites from residual and recycled materials: surface modification of cellulose fibers, properties and environmental degradation. Thesis KTH Fiber—och polymerteknologi, pp 1–5
Lopez-Manchado MA, Herrero B, Arroyo M (2003) Preparation and characterization of organoclay nanocomposites based on natural rubber. Polym Int 52(7):1070–1077
Chenal JM, Chazeau L, Guy L, Bomal Y, Gauthier C (2007) Molecular weight between physical entanglements in natural rubber: a critical parameter during strain-induced crystallization. Polymer 48(4):1042–1046
Kraus G (1963) Swelling of filler-reinforced vulcanizates. J Appl Polym Sci 7(3):861–871
Mathew L, Ulahannan J, Joseph R (2006) Effect of curing temperature, fibre loading and bonding agent on the equilibrium swelling of isora-natural rubber composites. Compos Interface 13(4–6):391–401
Jacob M, Thomas S, Varughese KT (2004) Mechanical properties of sisal/oil palm hybrid fiber reinforced natural rubber composites. Compos Sci Technol 64(7–8):955–965
Geethamma VG, Thomas S (2004) Transport of organic solvents through coir-fiber-reinforced natural rubber composites: a method for evaluating interfacial interaction. J Adhes Sci Technol 18(8):951–966
Dong Z, Liu M, Jia D, Zhou Y (2013) Synthesis of natural rubber-g-maleic anhydride and its use as a compatibilizer in natural rubber/short nylon fiber composites. Chin J Polym Sci 31(8):1127–1138
Mathew L, Joseph KU, Joseph R (2006) Swelling behaviour of isora/natural rubber composites in oils used in Automobiles. Bull Mater Sci 29(1):91–99
Ismail H, Edyham MR, Wirjosentono B (2002) Bamboo fibre filled natural rubber composites: the effects of filler loading and bonding agent. Polym Test 21(2):139–144
Yang HS, Kim HJ, Son J, Park HJ, Lee BJ, Hwang TS (2004) Rice-husk flour filled polypropylene composites; mechanical and morphological study. Compos Struct 63:305–312
Arroyo M, Lopez-Manchado MA, Herrero B (2003) Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 44(8):2447–2453
Thomas S, Chan CH, Pothen LA, Joy J, Maria JH (2014) Natural rubber materials: composites and nanocomposites, Chapt. 5. In: Samsuri A (ed) Strength and durability of natural rubber and chemically modified natural rubber. The Royal Society of Chemistry, Cambridge, pp 172–173
Dhakal HN, Zhang ZY, Richardson MOW (2007) Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos Sci Technol 67(7–8):1674–1683
Ismail H, Edyham MR, Wirjosentono B (2001) Dynamic properties and swelling behaviour of bamboo filled natural rubber composites : the effect of bonding agent. Iran Polym J 10(6):377–383
Thomas S, Pothan LA (2009) Natural fibre reinforced polymer composites: from macro to nanoscale, Chapt. 10. In: John MJ, Anandjiwala RD, Thomas S (eds) Lignocellulosic fiber reinforced rubber composites. Old City Publishing, Inc, Philadelphia, pp 255–258
Ciolacu D, Ciolacu F, Popa VI (2011) Amorphous cellulose—structure and characterization. Cellulose Chem Technol 45(1–2):13–21
Bodirlau R, Teaca CA (2009) Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom J Phys 54(1–2):93–104
Pang AL, Ismail H (2013) Tensile properties, water uptake and thermal properties of polypropylene/waste pulverized tire/kenaf (PP/WPT/KNF) composites. Bio Resour 8(1):806–817
Litvinov VM, De PP (2002) Spectroscopy of rubbers and rubbery materials, Chapt. 5. In: Infrared spectroscopy of rubbery materials. Rapra Technology Limited, Shropshire, pp 57, 84, 226
Ali AMM, Subban RHY, Bahron H, Winie T, Latif F, Yahya MZA (2008) Grafted natural rubber based polymer electrolytes: ATR-FTIR and conductivity studies. Ionics 14(6):491–500
Liang CY, Marchessault RH (1959) Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native celluloses. J Polym Sci 37(132):385–395
Eng AH, Tanaka Y, Gan SN (1992) FTIR studies on amino groups in purified Hevea rubber. J Nat Rubber Res 7:152–155
Chaikumpollert O, Yamamoto Y, Suchiva K, Kawahara S (2012) Protein-free natural rubber. Colloid Polym Sci 290:331–338
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manaila, E., Stelescu, M.D., Craciun, G. et al. Effects of benzoyl peroxide on some properties of composites based on hemp and natural rubber. Polym. Bull. 71, 2001–2022 (2014). https://doi.org/10.1007/s00289-014-1168-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1168-4