Abstract
Objective
Testicular tissues and sperms are typically vulnerable to oxidative stress and inflammation. Despite functioning as a signaling molecule in different physiological and pathological processes, hydrogen sulfide (H2S) role in the reproductive system is not fully recognized. This study aimed to assess whether H2S could counteract the testicular damage that acrylamide (AC) causes in male rats.
Methods
Forty male rats were equally divided randomly into four groups: normal control, AC, H2S, and AC + H2S who received normal saline, 40 mg/Kg of AC, 200 µg/Kg of sodium hydrosulfide NaHS (H2S donor), and 40 mg/Kg of AC + 200 µg/Kg of NaHS by intraperitoneal injection for 14 consecutive days, respectively. Body and testes weights, sperm count and motility, lactate dehydrogenase isoenzyme-x (LDH-X), serum testosterone level, oxidative parameters, the expression level of inducible nitric oxide synthase (iNOS), inflammatory cytokines, and histopathological alterations were evaluated.
Results
The reduction in relative testicular weights, sperm count, and motility served as evidence of the harmful effects of AC. However, these values were reversed when H2S and AC were combined. Additionally, AC significantly decreased serum testosterone level, testicular LDH-X activity, superoxide dismutase, catalase, and reduced glutathione. While malondialdehyde, expression levels of iNOS protein and inflammatory cytokines (TNF-α, IL-1β, and IL-6) levels were elevated. Interestingly, the co-administration of H2S with AC reversed these values, demonstrating an opposing effect in the previous parameters.
Conclusion
H2S exhibited a protective effect in the rat model of testicular toxicity induced by AC, which may be associated with the suppression of iNOS expression, proinflammatory cytokines, and the inhibition of oxidative stress injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acrylamide (AC) is a water-soluble carcinogenic substance implicated in the production of polyacrylamide and acrylamide copolymers that are applied in the manufacture of papers and plastics [1]. AC has been detected in many sources that are usually in contact with humans like cosmetic products, creams, and lotions [2]. Besides, AC is generated naturally in different starchy meals during frying or baking at a temperature exceeding 120 °C and low humidity [3]. Reducing sugars and amino acids (particularly asparagine), which are naturally found in many meals, are primarily responsible for AC formation [4].
Acrylamide has several detrimental effects on humans and animals. Short-time exposure of humans to AC leads to somnolence and hallucinations [5].While on chronic exposure, AC has been recognized to cause nerve damage, numbness, and negative impacts on male reproduction, [6]. Furthermore, reddish rashes and peeling are commonly observed with skin exposure [5]. Regarding animal studies, long-term exposure to AC has been reported to cause testicular toxicity [7], neurotoxicity [6], nephrotoxicity [8], and hepatotoxicity [9] in rats.
The toxicity of AC is attributed to the generation of some inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β, and IL-6 [10] and the induction of oxidative stress via depletion of reduced glutathione (GSH) [11]. AC is absorbed from the intestine and binds with GSH, resulting in a decrease in cellular GSH content, consequently the rise of reactive oxygen species (ROS) levels, and eventually the emergence of clinical diseases. Moreover, AC is transformed by cytochrome P450 2E1 to generate additional toxic metabolites that can conjugate to GSH, resulting in further depletion [5].
Hydrogen sulfide (H2S) is a gasotransmitter substance that originated naturally in humans by three enzymes comprising cystathionine γ lyase, cystathionine β synthase, and 3-mercaptopyruvate sulfurtransferase [12]. Growing studies reveal the beneficial role of sodium hydrosulfide (NaHS) supplements as H2S donors in different pathological conditions where H2S has been demonstrated to have multiorgan protective effects on neurotoxicity [13], nephrotoxicity [14], hepatotoxicity [15], cardiovascular diseases [16] and several types of wounds [17].
To the best of our information, the influence of H2S on AC-induced testicular toxicity is not evaluated yet. Therefore, the present in vivo study was designed to investigate the protective activity of H2S towards AC-induced testicular toxicity and clarify some potential underlying mechanisms that may be implicated.
Results
Effects of H2S administration on body and testes weights in rats exposed to AC
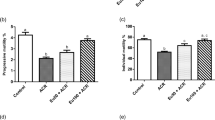
Treatment of male rats with AC revealed a significant decrease in body weight (Fig. 1a) as compared to control animals (p < 0.0001). In addition, the testes’ relative weight was reduced significantly in the AC-treated group (p < 0.05 vs control) (Fig. 1b). However, concurrent administration of NaHS with AC significantly increased body weight as compared to the AC-treated group (p < 0.01) and increased relative testes weight compared to AC group (p < 0.05).
Effect of H2S and /or acrylamide (AC) on rat body weight (a) and relative testes weight (b), epididymal sperm count (c) and percent of sperm motility (d). All data are expressed as the mean ± SD (n = 10). The AC group compared with control, AC + H2S group compared with AC. Differences were considered statistical significance at *p < 0.05 vs control, *** p < 0.001 vs control, **** p < 0.0001 vs control, # p < 0.05 vs AC, ## p < 0.01 vs AC and #### p < 0.0001 vs AC
Effects of H2S treatment on sperm parameters in rats exposed to AC
Sperm count and percent of sperm motility were significantly decreased in response to treatment with AC compared to the control group (p < 0.001 and p < 0.0001, respectively). In contrast, concurrent administration of NaHS with AC significantly restored sperm count to normalcy (P < 0.05) and increased percent of sperm motility (p < 0.0001) as compared to AC-treated group (Fig. 1c, d).
Impact of H2S administration on serum testosterone and testicular lactate dehydrogenase isoenzyme-X (LDH-X) in rats exposed to AC
Animals treated with AC showed a significant decrease in serum testosterone level (p < 0.0001) (Fig. 2a) as compared to the related control. Nonetheless, animals concurrently treated with NaHS showed a marked increase in serum testosterone levels with respect to the AC group (p < 0.001). Treatment with AC at a dose of 40 mg/kg significantly decreased LDH-X activity (p < 0.0001 vs control) (Fig. 2b). Concomitant supplementation of NaHS ameliorated LDH-X activity toward normalcy (p < 0.0001 vs AC) but did not reach a normal level.
Effects of H2S administration on the serum testosterone (a) and testicular LDH-X (b) in rats treated with acrylamide (AC). All data are expressed as the mean percentage relative to control ± SD (n = 10). The AC group compared with control, AC + H2S group compared with AC. Differences were considered statistical significance at **** p < 0.0001 vs control, ### p < 0.001 vs AC and #### p < 0.0001 vs AC
Effects of H2S administration on testicular oxidative stress markers in rats exposed to AC
Animals treated with AC showed a reduction in the activities of catalase (CAT) and superoxide dismutase (SOD) (p < 0.0001) (Fig. 3a, b) as compared to the corresponding control. Treatment with NaHS reverted the reduction in CAT and SOD activities induced by AC (p < 0.001 vs AC). GSH level was significantly decreased in response to AC treatment (p < 0.0001) (Fig. 3c) as compared to the related control. However, co-treatment with NaHS and AC partially normalized GSH levels (p < 0.01). Moreover, the malondialdehyde (MDA) level was significantly increased in response to AC treatment (Fig. 3d) as compared to the related control (P < 0.0001). Notably, treatment with NaHS significantly decreases MDA level to some extent and co-treatment of NaHS with AC resulted in a significant decrease in MDA level with respect to the AC group (p < 0.0001).
Effects of H2S treatment on the oxidative stress markers; CAT (a), SOD (b), GSH (c) and MDA (d) levels in testicular tissues of rats treated with acrylamide (AC). Data are presented as the mean percentage relative to control ± SD (n = 10). The AC group compared with control, the AC + H2S group compared with AC. Differences were considered statistical significance at **** p < 0.0001 vs control, ## p < 0.01 vs AC, ### p < 0.001 vs AC and #### p < 0.0001 vs AC
Induction of testicular inducible nitric oxide synthase (iNOS) expression by AC and the inhibitory effect of H2S
As shown in Fig. 4, the protein expression of iNOS was detected in the testicular tissues of control rats at a low level (Fig. 4a). At the same time, AC treatment markedly increased iNOS protein expression (Fig. 4b). On the other hand, the administration of NaHS did not change the expression pattern of iNOS compared to the control group (Fig. 4c). Interestingly, rats subjected to AC/NaHS co-treatment revealed a marked reduction in testicular expression of iNOS (Fig. 4d). Quantitative image analysis (Fig. 4e) indicated that AC treatment increased iNOS protein contents in testicular tissue as compared to the control group (p < 0.01). While AC/NaHS co-administration induced a marked decrease in iNOS protein contents compared to the AC-treated group (p < 0.05).
Effects of H2S treatment on iNOS expression in the testes of rats treated with acrylamide (AC). Representative fluorescence photomicrographs of testis obtained from control (a), AC (b), H2S (c), and AC + H2S (d) treated rats, probed for iNOS (green fluorescence) (arrows) and DAPI as the counterstain (blue fluorescence). Fluorescence intensity of iNOS measured from testes e was obtained from five fields of each section (minimally two rats in each group), using ImageJ software. Values are presented as the mean ± SD (n = 10). Differences were considered statistical significance at ** p < 0.01 vs control and # p < 0.05 vs AC
Acrylamide induced testicular inflammatory biomarkers (TNF-α, IL-1β, and IL-6) and the inhibitory effect of H2S.
The impact of H2S on pro-inflammatory mediators in the testes of rats treated with AC is presented in Fig. 5. Animals treated with AC alone caused a significant increase in the levels of tissue TNF-α, IL-1β and IL-6 (p < 0.0001) (Fig. 5a–c, respectively) as compared to the corresponding control. Conversely, co-treatment with NaHS and AC was markedly exhibited reduced pro-inflammatory TNF-α, IL-1β, and IL-6 biomarkers levels compared to AC only treated rats (p < 0.0001).
Effects of H2S treatment on proinflammatory cytokine biomarkers; TNF- α (a), IL-1β (b) and IL-6 (c) levels in testicular tissues of rats treated with acrylamide (AC). Results expressed as the mean ± SD (n = 10). The AC group compared with control, the AC + H2S group compared with AC. Differences were considered statistical significance at **** p < 0.0001 vs control, and #### p < 0.0001 vs AC
Effects of H2S on histopathological alterations
Histopathological examination of control and NaHS groups revealed normal testicular histology in the seminiferous tubules and interstitial fields (Fig. 6a, c). Treatment of animals with AC significantly disrupted the normal architecture of the testis and resulted in the appearance of multinucleated giant cells lying close to the seminiferous tubules, while the others were located nearby spermatogenetic cells (Fig. 6b). In the AC/NaHS-treated group, there were few congested blood vessels in the interstitial tissue and multinucleated giant cells disappeared. Moreover, other testicular structures showed nearly normal histology, as shown in the control and NaHS groups (Fig. 6d). Johnsen’s scores (Fig. 6e) and seminiferous tubule diameters (Fig. 6f) in AC-treated rats were significantly lower than the control group, while in AC/NaHS-treated group were higher than the AC-treated group.
Effect of H2S treatment on the histopathological features of the testes of rats treated with acrylamide (AC) stained with H&E. a Normal structure of the testis is seen in the control group. b The appearance of multinucleated giant cells lying close to seminiferous tubules in the AC group (Arrows). c Normal structure is seen in the H2S group. d Testis structure in the AC + H2S shows improvement of testicular tissue toward normal appearance except for some congested blood vessels in interstitial tissues (arrow heads) (H&E staining, scale bar = 50 µm). e Johnsen’s score of groups presented as mean ± SD. f Average diameter of seminiferous tubules for control and treated groups expressed as mean ± SD. The AC group compared with control, the AC + H2S group compared with AC. Differences were considered statistical significance at **** p < 0.0001 vs control, and ## p < 0.01 vs AC
Discussion
In this study, AC administration resulted in a significant reduction in total body and relative testicular weights. The reduction in body weight may be due to the remarkable effect of AC on appetite and hence the reduction in food intake [18]. While the reduction in relative testicular weight may be due to AC-induced testicular atrophy [19]. Based on our findings, the administration of NaHS enhanced total body and testicular weights in AC-treated rats. The positive impacts of H2S are due to the fact that H2S has an orexigenic effect [20], and exerts anti-apoptotic activity on testicular cells [21].
We revealed that AC treatment significantly decreased sperm count, sperm motility, and serum testosterone and this may be due to oxidative stress and apoptosis of Sertoli cells [22] or due to the decrease in testosterone level induced by AC in which the production and maturation of sperm are under the control of testosterone [23]. However, concurrent administration of H2S significantly restored sperm count to normalcy and increased sperm motility. The effects of H2S in the alleviation of spermatogenic failure may be due to the restoration of normal testosterone levels, and the anti-inflammatory/anti-oxidant properties of H2S [24].
The inhibitory effect of AC on serum testosterone may be due to the oxidative stress and inflammation induced by AC in Leydig cells [22]. Induction of iNOS and subsequent release of NO may also account for the reduction in serum testosterone [25]. Moreover, AC disrupts the rate-limiting steps in the steroidogenic pathway in Leydig cells [26]. Concurrent administration of NaHS inverts the reduction in testosterone. Possible mechanisms by which H2S can protect from androgenic reduction include its anti-oxidant/anti-inflammatory functions and its effect on the secretion of luteinizing hormone and hence the production of testosterone [27].
Lactate dehydrogenase-X plays a vital role in the process of spermatogenesis and has been shown to play a vital role in sperm survival and motility. It is involved in the regulation of glycolysis in the sperm flagellum and serves as an indicator of normal spermatozoal metabolism [28]. The relation between the activity of LDH-X and sperm motility and sperm count [29] has previously been investigated. In the present study, the treatment with AC significantly decreased LDH-X activity which is indicative of testicular toxicity, and this finding supports the explanation of the reduction of sperm count and motility found in this study. Concurrent administration of NaHS with AC significantly increased LDH-X activity toward normalcy when compared to the AC group suggesting the cytoprotective action of H2S against AC-induced testicular damage.
Interestingly, the male reproductive system is particularly susceptible to the harmful effects of ROS, lipid peroxidation, and inflammatory mediators. Antioxidants play a vital role in the maintenance of the vitality of spermatozoa through the inactivation of ROS to keep only a small amount necessary to maintain normal cell function [30]. In the current study, treatment with AC created a state of oxidative stress by lowering GSH, CAT, and SOD with a subsequent increase in lipid peroxidation represented by a high MDA level. Acrylamide is predominantly metabolized and excreted as a metabolite of GSH-conjugates in the urine which results in a reduction in cellular GSH [5]. Co-treatment with NaHS significantly increased SOD and CAT activities and GSH with a subsequent decrease in MDA testicular content as compared to the AC group, indicating the antioxidant power of H2S. This power is mediated through different mechanisms including direct suppression of ROS, enhancing the expression of antioxidant enzymes, upregulating the expression of genes involved in GSH biosynthesis, and modulation of cellular GSH levels [31].
Inflammation is closely linked to testicular dysfunction. Nuclear factor kappa B (NF-κB) has been known to be the core regulator of the inflammatory process and supports the transcription of many pro-inflammatory mediators, such as IL-1β, IL-6, TNF-α, and iNOS. Acrylamide is well known to induce NF-κB expression [7]. In line with Pan et al. AC significantly increased the expression of TNF- α, IL-1β, IL-6, and iNOS as compared to the control group [10]. Increased expression of iNOS and subsequent release of nitric oxide (NO) in the presence of ROS may drive the formation of peroxynitrite [32]. This may explain a possible mechanism by which AC induces testicular damage through the induction of a state of nitrosative stress. O’Bryan et al. [33] reported that the upregulation of iNOS may contribute to the damage of seminiferous epithelium. It has been noted that H2S has the potential role as an inhibitor of peroxynitrite-mediated cytotoxicity and intracellular protein nitration [34]. Our results fit well with a study executed by Yang et al. who reported that H2S exhibits a protective effect on the heart and kidney by suppressing iNOS activity and expression with a concurrent decrease in the NO and the inhibition of oxidative stress injury [35]. Results of the present study revealed that co-administration of NaHS with AC significantly counteracts the effect of AC on the level of the iNOS protein.
Elevation of pro-inflammatory cytokines due to AC toxicity is linked to testicular failure on both spermatogenesis and steroidogenesis levels. On the level of spermatogenesis, infiltration of leucocytes into semen occurs in conditions of inflammation and induces the production of anti-sperm antibodies. Inflammation also increases sperm flagellar membrane rigidity by decreasing the lipid content of the membrane leading to decreases sperm motility and causing sperm agglutination and asthenozoospermia [36]. Sanocka et al. [36] confirmed that pro-inflammatory cytokines induce ROS production and oxidative stress through activation of the xanthine oxidase system. On the level of steroidogenesis, TNF-α suppresses the gene expression of steroidogenic enzymes in Leydig cells via activation of NF-κB. This repression of the steroidogenic enzymes leads to a decrease in the production of testosterone [37]. IL-6 may also have potential adverse effects on male reproductive function via persistent testicular resistance to luteinizing hormone action and/or suppression of Leydig cell steroidogenesis [38]. The modulatory effect of H2S pro-inflammatory cytokines production might be due to inhibition of leukocyte infiltration to sites of inflammation, suppression of leukocyte adherence to the vascular endothelium, and reduction of the expression of several pro-inflammatory cytokines [39] through suppression of histone acetylation and hence reduction in the gene transcription of the pro-inflammatory cytokines [40].
Acrylamide is a classical inducer of multinucleated giant cell formation in the testis [41]. The histopathological examinations further confirm AC-induced testicular toxicity, in which a significant disruption of the normal testicular architecture and appearance of multinucleated giant cells located nearby the seminiferous tubules and spermatogenetic cells have been reported. Co-administration of NaHS with AC showed disappearance of multinucleated giant cells and restoration of normal testicular structures as in the control group. Reduction in Johnsen’s testicular score and diameter of seminiferous tubules was detected in the testicular tissues of AC-treated rats. On the other hand, NaHS prevented the decrease in Johnsen’s score and seminiferous tubules diameter when administered concurrently with AC, These results are in accordance with the study of Azarbarz et al. [42]. This highlights the cytoprotective role of H2S against AC-induced testicular toxicity.
Materials and methods
Materials
Acrylamide and the NaHS (H2S donor) were purchased from Sigma-Aldrich (St Louis, MO, USA).
Animals
Forty male Sprague–Dawley rats, 13–14 weeks old, weighing 130–140 g, were obtained from El-Nile Company, Cairo, Egypt. Rats were housed in well-ventilated cages under constant conditions of 22 ± 2 °C room temperature, 50–70% humidity, and 12 h day/night cycle. Animals received a standard diet and water ad libitum and were allowed to acclimate to the new surroundings for 1 week before the beginning of the experiment.
Experimental design
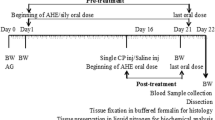
Rats were weighed at the beginning of the experiment and divided randomly into four groups (10 rats for each): group received normal saline (control group), group received 40 mg/Kg of AC [43] (AC group), group received 200 µg/Kg of NaHS [42] (H2S group) and the group received 40 mg/Kg of AC and 200 µg/Kg of NaHS (AC with H2S group). Saline, AC, and NaHS were administrated every day intraperitoneally for 14 consecutive days. Twenty-four hours after the last dose, animals were weighed and anesthetized by ether, and blood samples were collected from the retro-orbital sinus and centrifuged to obtain the serum which was stored at − 80 °C until use. Rats were killed, and the testes and caudal epididymis were excised, weighed, and washed with ice-cold saline. the right testis of each animal was processed for histological evaluation, whereas the left one was homogenized in ice-cold 0.15 M KC1 (10% w/v), then centrifuged at 9000 g for 10 min at 4 °C and the supernatant was immediately collected and processed for biochemical studies and enzyme activity assays.
Determination of sperm parameters
The caudal epididymis was minced with anatomical scissors in pre-warmed (37 °C) normal saline. One drop of sperm suspension was placed on a glass slide to analyze 200 motile sperm in 4 different fields. The motility of the epididymal sperm was evaluated using a phase-contrast microscope at a magnification of 400 × within 2–4 min of their isolation from the epididymis. Motility was defined as a sperm that showed any motion of the flagellum during an observation period of 30s and the motility of the sperm was expressed as a percentage of motile sperm in total sperm. The total sperm count was determined by using a Neubauer hemocytometer as described by Yokoi et al. [44].
Determination of serum testosterone
Serum testosterone was determined using the rat testosterone competitive ELISA kit (LifeSpan Bioscience, Inc, USA) of catalog NO. LS-F28083 according to the manufacturer’s protocol.
Determination of LDH-X
Lactate dehydrogenase isoenzyme-x was determined in the supernatant of tissue homogenate using rat LDH-X ELISA kit (MyBioSource, CA, USA) of catalog NO. MBS3808917 according to the manufacturer’s protocol.
Determination of testicular oxidative stress
Lipid peroxidation was investigated by measuring the levels of MDA using thiobarbituric acid (TBA) test [45]. The MDA level was expressed as nmol/g of tissue. Reduced glutathione was assessed by the method of Beutler and Mary [46] using a colorimetric assay kit (Bio-Diagnostic, Egypt) according to the manufacturer’s protocol, GSH concentrations were expressed as mg/g of tissue. The testicular activities of antioxidant enzymes CAT and SOD were determined by the methods of Aebi [47] and Nishikimi et al. [48] respectively, using a colorimetric assay kit (Bio-Diagnostic, Egypt) according to the manufacturer’s protocol, the activities were expressed as U/g of tissue.
Immunofluorescence staining for determination of iNOS
Immunofluorescence staining procedures were performed according to the method of Abdel-Bakky et al. [49]. Briefly, paraffinized tissue sections were dissolved in an oven for 20 min at 60 °C, then de-paraffinized for 15 min in 100% xylene. The sections were rehydrated by immersion in graded ethanol concentrations: 100%, 90%, 70%, 50%, and finally 30% for 5 min in each concentration. Following rehydration, tissue sections were washed for 15 min with distilled water, and incubated in a microwave oven with Dako solution (0.01 M sodium citrate buffer, pH 6) for 20 min. Then tissue sections were cooled, washed for 15 min with phosphate-buffered saline with 0.05% TWEEN 20 (PBST) at pH 7.4, fixed using methanol for 10 min, and then blocked with blocking buffer for 1 h at room temperature. Hereafter, the tissue sections were incubated overnight with the primary antibodies (mouse polyclonal iNOS) in a concentration of 1:500 at 4 °C in a cool dark place. The stained sections were washed for 5 min three times with PBST, and the bound antibodies were detected by secondary antibodies (goat anti-rabbit Cy3) for 30 min. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) fluorescent stain and washed for 30 min by PBST. Finally, tissue sections were mounted using anti-fade mounting medium Fluoromount® (Sigma-Aldrich, St. Louis, MO) and visualized by fluorescence microscopy Leica DM5000 B, (Leica, Germany). The average fluorescence intensity of 3–5 microscopic fields was measured using Image-J/ NIH software for each tissue section and normalized to DAPI intensity.
Determination of testicular proinflammatory cytokines (TNF-α, IL-1β and IL-6)
Estimation of TNF-α, IL-1β, and IL-6 levels in testicular tissue homogenate was conducted by a rat-specific ELISA kit (Cloud-Clone Corp., Houston, USA) of catalog NO. SEA133Ra, SEA563Ra, and SEA079Ra, respectively. The procedures of the test were followed as specified by the manufacturer.
Histopathological assessment
Testes were fixed in Bouin's solution, and the tissue was processed and embedded in paraffin blocks for the preparation of 5-µm thick sections to study the histological changes using hematoxylin and eosin stain according to the method of Bancroft and Gamble [50]. After that, the stained sections were examined and photographed using a light microscope (Olympus BX 41, Japan).
Johnsen's testicular score [51, 52] was used to estimate the spermatogenesis process in the seminiferous tubules on a scale of 1–10 according to the following criteria: 10 = complete spermatogenesis and perfect tubules; 9 = many spermatozoa present and disorganized spermatogenesis; 8 = only a few spermatozoa present; 7 = many spermatids but no spermatozoa present; 6 = only a few spermatids present; 5 = many spermatocytes but no spermatozoa or spermatids present; 4 = only a few spermatocytes present; 3 = only spermatogonia present; 2 = only sertoli cells present and no germ cells; 1 = no germ cells or sertoli cells present.
Histomorphometric assay
For quantitative examination, the average diameter of seminiferous tubules [53] in ten tubules per testicular section and ten sections per group were quantified at × 40 magnifications by using calibrated OLYSIA Soft Imaging System GmbH, version 3.2 (Japan).
Statistical analysis
All statistical analysis was performed using GraphPad Prism software, version 6. Data were expressed as mean ± SD, where SD stands for standard deviation (SD). Differences between obtained values were compared by one-way analysis of variance (ANOVA) followed by Tukey’s test as a post hoc for multiple comparisons. Differences were considered statistical significance at *p < 0.05 vs control ** p < 0.01 vs control, *** p < 0.001 vs control, **** p < 0.0001 vs control, # p < 0.05 vs AC, ## p < 0.01 vs AC, ### p < 0.001 vs AC and #### p < 0.0001 vs AC.
Conclusion
The current study revealed that H2S alleviated the testicular toxicity induced by AC, and its mechanism might be associated with the suppression of proinflammatory cytokines and iNOS expression, reduction in the production of lipid peroxides, and thereby inhibition of the oxidative stress injury to protect the testicular tissues.
Data availability
Data available on request from corresponding author.The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
References
Braun O, Coquery C, Kieffer J, Blondel F, Favero C, Besset C, Mesnager J, Voelker F, Delorme C, Matioszek D (2021) Spotlight on the life cycle of acrylamide-based polymers supporting reductions in environmental footprint: review and recent advances. Molecules 27:42. https://doi.org/10.3390/molecules27010042
Lee B-M, Kwon S, Cho YM, Kim K-B, Seo K, Min CS, Kim K (2019) Perspectives on trace chemical safety and chemophobia: risk communication and risk management. J Toxicol Environ Health A 82:186–199. https://doi.org/10.1080/15287394.2019.1618680
Paul V, Ezekiel R, Pandey R (2016) Acrylamide in processed potato products: progress made and present status. Acta Physiol Plant 38:1–23. https://doi.org/10.1007/s11738-016-2290-8
Ciesarová Z, Kukurová K, Torbica A, Belović M, Horváthová J, Daško Ľ, Jelemenská V (2021) Acrylamide and 5-hydroxymethylfurfural in thermally treated non-wheat flours and respective breads. Food Chem 365:130491. https://doi.org/10.1016/j.foodchem.2021.130491
Kunnel SG, Subramanya S, Satapathy P, Sahoo I, Zameer F (2019) Acrylamide induced toxicity and the propensity of phytochemicals in amelioration: a review. Cent Nervous Syst Agents Med Chem Former Curr Med Chem-Cent Nervous Syst Agents 19:100–113. https://doi.org/10.2174/1871524919666190207160236
Pennisi M, Malaguarnera G, Puglisi V, Vinciguerra L, Vacante M, Malaguarnera M (2013) Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health 10:3843–3854. https://doi.org/10.3390/ijerph10093843
Kucukler S, Caglayan C, Darendelioğlu E, Kandemir FMJLS (2020) Morin attenuates acrylamide-induced testicular toxicity in rats by regulating the NF-κB, Bax/Bcl-2 and PI3K/Akt/mTOR signaling pathways. Life Sci 261:118301. https://doi.org/10.1016/j.lfs.2020.118301
Kandemir FM, Yıldırım S, Kucukler S, Caglayan C, Darendelioğlu E, Dortbudak MB (2020) Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: a multi-biomarker approach. Food Chem Toxicol 138:111190. https://doi.org/10.1016/j.fct.2020.111190
Al-Qahtani F, Arafah M, Sharma B, Siddiqi N (2017) Effects of alpha lipoic acid on acrylamide-induced hepatotoxicity in rats. Cell Mol Biol 63:1–6. https://doi.org/10.14715/cmb/2017.63.6.1
Pan X, Wu X, Yan D, Peng C, Rao C, Yan H (2018) Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol Lett 288:55–64. https://doi.org/10.1016/j.toxlet.2018.02.002
Dasari S, Ganjayi M, Gonuguntla S, Kothapalli S, Konda P, Basha S, Kutagolla P, Meriga B (2018) Evaluation of biomarkers distress in acryla-mide-induced hepatic and nephrotoxicity of albino wistar rat. Adv Anim Vet Sci 6:427–435. https://doi.org/10.17582/journal.aavs/2018/6.10.427.435
Giuffrè A, Vicente JBJOM, Longevity C (2018) Hydrogen sulfide biochemistry and interplay with other gaseous mediators in mammalian physiology. Oxid Med Cell Longev. https://doi.org/10.1155/2018/6290931
Calabrese V, Scuto M, Salinaro AT, Dionisio G, Modafferi S, Ontario ML, Greco V, Sciuto S, Schmitt CP, Calabrese EJ, Peters V (2020) Hydrogen sulfide and carnosine: modulation of oxidative stress and inflammation in kidney and brain axis. Antioxidants (Basel) 9:1303. https://doi.org/10.3390/antiox9121303
Cao X, Xiong S, Zhou Y, Wu Z, Ding L, Zhu Y, Wood ME, Whiteman M, Moore PK, Bian J-s (2018) Renal protective effect of hydrogen sulfide in cisplatin-induced nephrotoxicity. Antioxid Redox Signal 29:455–470. https://doi.org/10.1089/ars.2017.7157
Fan H-N, Wang H-J, Yang-Dan C-R, Ren L, Wang C, Li Y-F, Deng Y (2013) Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep 7:247–253. https://doi.org/10.3892/mmr.2012.1153
Li Z, Polhemus DJ, Lefer DJ (2018) Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ Res 123:590–600. https://doi.org/10.1161/CIRCRESAHA.118.311134
Goren I, Köhler Y, Aglan A, Pfeilschifter J, Beck K-F, Frank S (2019) Increase of cystathionine-γ-lyase (CSE) during late wound repair: hydrogen sulfide triggers cytokeratin 10 expression in keratinocytes. Nitric Oxide 87:31–42. https://doi.org/10.1016/j.niox.2019.03.004
Chu P, Lin L, Chen P, Su T, Lin C (2017) Negative association between acrylamide exposure and body composition in adults: NHANES, 2003–2004. Nutr Diabet 7:e246–e246. https://doi.org/10.1038/nutd.2016.48
Burek J, Albee R, Beyer J, Bell T, Carreon R, Morden D, Wade C, Hermann E, Gorzinski S (1980) Subchronic toxicity of acrylamide administered to rats in the drinking water followed by up to 144 days of recovery. J Environ Pathol Toxicol 4:157–182
Slade E, Williams L, Gagnon JJPR (2018) Hydrogen sulfide suppresses ghrelin secretion in vitro and delays postprandial ghrelin secretion while reducing appetite in mice. Physiol Rep 6:e13870. https://doi.org/10.14814/phy2.13870
Ning JZ, Li W, Cheng F, Rao T, Yu WM, Ruan Y, Yuan R, Zhang XB, Du Y, Xiao CC (2018) The protective effects of GYY4137 on ipsilateral testicular injury in experimentally varicocele-induced rats. Exp Ther Med 15:433–439. https://doi.org/10.3892/etm.2017.5417
Yilmaz BO, Yildizbayrak N, Aydin Y, Erkan M (2017) Evidence of acrylamide-and glycidamide-induced oxidative stress and apoptosis in Leydig and Sertoli cells. Hum Exp Toxicol 36:1225–1235. https://doi.org/10.1177/0960327116686818
Sharpe R, Maddocks S, Millar M, Kerr J, Saunders P, McKinnell C (1992) Testosterone and spermatogenesis identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J Androl 13:172–184
Wang J, Wang W, Li S, Han Y, Zhang P, Meng G, Xiao Y, Xie L, Wang X, Sha J (2018) Hydrogen sulfide as a potential target in preventing spermatogenic failure and testicular dysfunction. Antioxid Redox Signal 28:1447–1462. https://doi.org/10.1089/ars.2016.6968
Kostic T, Andric S, Maric D, Stojilkovic S, Kovacevic RJJE (1999) Involvement of inducible nitric oxide synthase in stress-impaired testicular steroidogenesis. J Endocrinol 163:409–416. https://doi.org/10.1677/joe.0.1630409
Yildizbayrak N, Erkan M (2018) Acrylamide disrupts the steroidogenic pathway in Leydig cells: possible mechanism of action. Toxicol Environ Chem 100:235–246. https://doi.org/10.1080/02772248.2018.1458231
Chen H-J, Ngowi EE, Qian L, Li T, Qin Y-Z, Zhou J-J, Li K, Ji X-Y, Wu D-D (2021) Role of hydrogen sulfide in the endocrine system. Front Endocrinol. https://doi.org/10.3389/fendo.2021.704620
Khanna S, Lakhera PC, Khandelwal S (2011) Interplay of early biochemical manifestations by cadmium insult in sertoli-germ coculture: an in vitro study. Toxicology 287:46–53. https://doi.org/10.1016/j.tox.2011.05.013
Abd-Ellah M, Aly H, Mokhlis H, Abdel-Aziz A (2016) Quercetin attenuates di-(2-ethylhexyl) phthalate-induced testicular toxicity in adult rats. Hum Exp Toxicol 35:232–243. https://doi.org/10.1177/0960327115580602
Oyagbemi AA, Adedara IA, Saba AB, Farombi EO (2010) Role of oxidative stress in reproductive toxicity induced by co-administration of chloramphenicol and multivitamin-haematinics complex in rats. Basic Clin Pharmacol Toxicol 107:703–708. https://doi.org/10.1111/j.1742-7843.2010.00561.x
Corsello T, Komaravelli N, Casola A (2018) Role of hydrogen sulfide in NRF2-and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 7:129. https://doi.org/10.3390/antiox7100129
Beck K-F, Euler J, Eisel F, Beck M, Köhler Y, Sha LK, von Knethen A, Longen S, Pfeilschifter JJBP (2015) Cytokines induce protein kinase A-mediated signalling by a redox-dependent mechanism in rat renal mesangial cells. Biochem Pharmacol 93:362–369. https://doi.org/10.1016/j.bcp.2014.11.009
O’Bryan MK, Schlatt S, Gerdprasert O, Phillips DJ, de Kretser DM, Hedger MP (2000) Inducible nitric oxide synthase in the rat testis: evidence for potential roles in both normal function and inflammation-mediated infertility. Biol Reprod 63:1285–1293. https://doi.org/10.1095/biolreprod63.5.1285
Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK (2004) The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’? J Neurochem 90:765–768. https://doi.org/10.1111/j.1471-4159.2004.02617.x
Yang R, Jia Q, Ma S, Cui S, Liu X, Wang Y, Gao Q (2017) Effect of hydrogen sulfide on inducible nitric oxide synthase in kidneys of Type 1 diabetic rats. J Cent South Univ Med Sci 42:389–394. https://doi.org/10.11817/j.issn.1672-7347.2017.04.004
Sanocka D, Jędrzejczak P, Szumała-Kaękol A, Frączek M, Kurpisz M (2003) Male genital tract inflammation: the role of selected interleukins in regulation of pro-oxidant and antioxidant enzymatic substances in seminal plasma. J Androl 24:448–455. https://doi.org/10.1002/j.1939-4640.2003.tb02693.x
Hong CY, Park JH, Ahn RS, Im SY, Choi H-S, Soh J, Mellon SH, Lee KJM, Biology C (2004) Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol 24:2593–2604. https://doi.org/10.1128/MCB.24.7.2593-2604.2004
Tsigos C, Papanicolaou DA, Kyrou I, Raptis SA, Chrousos GP (1999) Dose-dependent effects of recombinant human interleukin-6 on the pituitary-testicular axis. J Interferon Cytokine Res 19:1271–1276. https://doi.org/10.1089/107999099312948
Wallace JL (2007) Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci 28:501–505. https://doi.org/10.1016/j.tips.2007.09.003
Rios E, Szczesny B, Soriano FG, Olah G, Szabo CJ (2015) Hydrogen sulfide attenuates cytokine production through the modulation of chromatin remodeling. Int J Mol Med 35:1741–1746. https://doi.org/10.3892/ijmm.2015.2176
Yassa HA, George SM, Refaiy AERM, Moneim EM (2014) Camellia sinensis (green tea) extract attenuate acrylamide induced testicular damage in albino rats. Environ Toxicol 29:1155–1161. https://doi.org/10.1002/tox.21846
Azarbarz N, Shafiei Seifabadi Z, Moaiedi MZ, Mansouri E (2020) Assessment of the effect of sodium hydrogen sulfide (hydrogen sulfide donor) on cisplatin-induced testicular toxicity in rats. Environ Sci Pollut Res 27:8119–8128. https://doi.org/10.1007/s11356-019-07266-5
Alturfan AA, Tozan-Beceren A, Sehirli AO, Demiralp E, Sener G, Omurtag GZ (2012) Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol Biol Rep 39:4589–4596. https://doi.org/10.1007/s11033-011-1249-5
Yokoi K, Uthus EO, Nielsen FH (2003) Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 93:141–154. https://doi.org/10.1385/BTER:93:1-3:141
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278. https://doi.org/10.1016/0003-2697(78)90342-1
Beutler E, Yeh MK (1963) Erythrocyte glutathione reductase. Blood 21:573–585. https://doi.org/10.1182/blood.V21.5.573.573
Aebi H (1984) Catalase in vitro. In: Oxygen radicals in biological systems, vol 105. Methods in enzymology. Academic Press, pp 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–854. https://doi.org/10.1016/s0006-291x(72)80218-3
Abdel-Bakky MS, Hammad MA, Walker LA, Ashfaq MK (2011) Silencing of tissue factor by antisense deoxyoligonucleotide prevents monocrotaline/LPS renal injury in mice. Arch Toxicol 85:1245–1256. https://doi.org/10.1007/s00204-011-0663-8
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Sciences. https://doi.org/10.1016/C2015-0-00143-5
Hozyen HF, Khalil HMA, Ghandour RA, Al-Mokaddem AK, Amer MS, Azouz RA (2020) Nano selenium protects against deltamethrin-induced reproductive toxicity in male rats. Toxicol Appl Pharmacol 408:115274. https://doi.org/10.1016/j.taap.2020.115274
Johnsen SG (1970) Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1:2–25. https://doi.org/10.1159/000178170
Mirhoseini M, Talebpour Amiri F, Karimpour Malekshah AA, Rezanejad Gatabi Z, Ghaffari E (2017) Protective effects of melatonin on testis histology following acute torsion-detorsion in rats. Int J Reprod Biomed 15:141–146. https://doi.org/10.29252/ijrm.15.3.141
Acknowledgements
The authors acknowledge with thanks Faculty of Pharmacy, Al-Azhar University, for technical and financial support. This study did not receive any specific grant from funding agencies.
Author information
Authors and Affiliations
Contributions
HAM, AA conceived and designed the study. HAM, MHR, IGS, MGE, AAE, AA performed the practical experiments. EGK, MHG collected and analyzed data. HAM, MGE, AAE, AA, HSG wrote and revised the manuscript: All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Hamada Ahmed Mokhlis, Mohammed Helmy Rashed, Ibrahim Ghalib Saleh, Mahmoud Gomaa Eldeib, Ahmed A. El-Husseiny, Emad Gamil Khidr, Maher H. Gomaa, Hesham S. Gad, and Ahmed Aglan declare that they have no conflicts of interest.
Ethical approval
All experimental procedures for animals in this study were conducted in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources for the National Research Council. Approval by the Institutional Animal Care and Use Committee of the Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt was obtained before the animal experiments were performed (Azhar-Pharmacy-2022–005).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mokhlis, H.A., Rashed, M.H., Saleh, I.G. et al. Hydrogen sulfide alleviates acrylamide-induced testicular toxicity in male rats. Toxicol. Environ. Health Sci. 15, 41–51 (2023). https://doi.org/10.1007/s13530-022-00156-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13530-022-00156-3