Abstract
Tissue factor (TF) is involved in monocrotaline (MCT)/lipopolysaccharide (LPS) hepatotoxicity. It is not known whether MCT/LPS can cause renal toxicity and whether TF is involved in this toxicity. Thus, the present study was undertaken to investigate the potential renal toxicity after MCT/LPS co-treatment and the involvement of TF in this toxicity. MCT was delivered to ND4 male mice (200 mg/kg) per os followed 4 h later by treatment with LPS ip (6 mg/kg) to investigate its effect on kidney. We injected TF antisense oligonucleotide (TF-AS) intravenously (i.v) in mice prior to LPS treatment, to block TF, and measured their blood urea nitrogen (BUN), creatinine (CRE), alkaline phosphatase (ALP), and potassium. In MCT/LPS co-treated group, fibrin was detected on the glomerular capillary lumina, distal tubules of renal cortex, and the necrotic tubules of renal medulla. An elevation of BUN, creatinine, and the BUN/creatinine ratio was seen in mice with MCT/LPS co-treatment, compared to animals receiving LPS or MCT alone. Simultaneously, an aggressive tubular necrosis was seen in the medullary tubules in the same group which may account for the oliguria observed in these animals. Fourfold inductions in the plasma TF level was detected at 10 h after MCT/LPS co-treatment which increased to 18-fold at 24 h. Increased blood level of leptin, interleukin-6 (IL-6) and downregulation of tubular chemokine (C-X-C motif) ligand 16 (CXCL16) are characteristic features in MCT/LPS co-treated animal. On the other hand, mice injected with TF-AS in the presence of MCT/LPS co-treatment showed no elevation of the blood BUN, creatinine, potassium, and normal levels of the proinflammatory molecules. TF-AS injection significantly prevented glomerular and tubular fibrin deposition, tubular necrosis, and improvement of the animal survivability. Renal toxicity involving TF can be prevented successfully by the use of TF-AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation, elaboration of cytokines, and activation of coagulation system are important mechanisms of injury in different organs (Goodman et al. 1996; Idell 1994; Rinaldo and Rogers 1982). Coagulation system activation is a part of the inflammatory response mediated through tissue factor (TF), a transmembrane glycoprotein that is expressed on the extracellular membrane of various cells, particularly around blood vessels, to reduce bleeding after injury (Mackman 2005). It functions as a receptor for the coagulation factor VII/VIIa that expressed on vessel walls or on the cells around the microvascular system (Nemerson 1988). Though normally absent on unstimulated monocytes or endothelial cells, it can be induced on these cells by various physiological activation factors in vitro. Expression of TF on cell surface and its appearance as a soluble molecule are characteristic features of acute and chronic inflammation in conditions such as sepsis, atherosclerosis, Crohn’s disease, systemic lupus erythematosus, and allograft rejection (More et al. 1993; Taylor et al. 1991; Tremoli Jr. et al. 1999; Wakita et al. 1999; Weinberg et al. 1993). Beside its role in the initiation of homeostasis, TF has also been identified as a mediator of arteriosclerosis, tumor metastasis, and angiogenesis (Belting et al. 2004; Bromberg et al. 1999; Mach et al. 1997).
The renal expression of TF is restricted to the glomerular podocytes, Bowman’s epithelium and, to some extent, the tubular loop of Henle (Osterholm et al. 2005). TF plays an important role in the development of renal injury after ischemia and reperfusion in rats (Ushigome et al. 2002). Deposited fibrin (in the form of fibrinoid necrosis) is the end-product of TF activation and is characteristic of severe acute rejection of renal allografts (Osterholm et al. 2005). In the healthy body, the natural anticoagulants predominate and strictly control the activities of the pro-coagulants, thus maintaining a non-thrombogenic environment. However, the presence of inflammatory mediators, such as the cytokines TNF-α, IL-1β, and IL-6, has the tendency to shift the hemostatic balance in favor of coagulation and thrombosis (Esmon 2003). Lipopolysaccharide (LPS) and certain cytokines increase TF expression in inflammatory and endothelial cells, which could activate intravascular coagulation (Bierhaus et al. 1995; Cunningham et al. 1999). TF is the main initiator of protease coagulation cascade in vivo (Bazan 1990). Several studies have also described the proinflammatory and the profibrotic effects of TF (Erlich et al. 2000; Taylor et al. 1998). The proinflammatory role of TF has been demonstrated in a number of animal models, including sepsis (Welty-Wolf et al. 2001), anti-GBM nephritis (Erlich et al. 1997), and delayed-type hypersensitivity (Imamura et al. 1993).
It is previously known that ip injection of both MCT and LPS induces synergistic hepatotoxicity that involves the activation of the coagulation system (Yee et al. 2000). We developed a hepatotoxicity model in mice by the administration of a sub-toxic oral MCT and a sub-toxic ip LPS and observed oliguria and increased plasma creatinine in these animals (Abdel-Bakky et al. 2010). To our knowledge, no investigations have been reported on the effect of MCT/LPS on the kidney. Therefore, a compelling question arises whether the co-treatment with po MCT/ip LPS would produce renal toxicity and the possible contribution of TF in this type of toxicity.
Materials and methods
Antibodies and chemicals
Polyclonal rabbit anti-human fibrin antibody was obtained from Dako (Carpinteria, CA). Monoclonal anti-mouse CXCL16 was purchased from R&D (Minneapolis, MN). Goat anti-mouse Alexa fluor 488 was obtained from Invitrogen (Carlsbad, TX). Cy3-conjugated goat anti-rabbit antibody was purchased from Jackson Immunoresearch (West Grove, PA). 4′,6′-Diamidino-2-phenylindole (DAPI), lipopolysaccharide (LPS) and monocrotaline (MCT) were purchased from Sigma–Aldrich (St. Louis, MO). Rabbit polyclonal IgG for tissue factor and mouse monoclonal IgG1 for Wilms’ tumor (WT1) were purchased from Santa Cruz biotechnology, inc. (Santa Cruz, CA).
Tissue factor oligonucleotides
The oligonucleotides (ODNs) used in these studies were purchased from integrated DNA technologies (San Diego, CA). The following sequences of the mouse TF antisense, sense, and scrambled nucleotides were used:
Antisense TF (TF-AS), 5′-CATGGGGATAGCCAT-3′; Sense TF (TF-SE), 5′-ATGGCTATCCCCATG-3′; Scrambled TF (TF-SC), 5′-TGACGCAGAGTCGTA-3′. Both TF-SC and TF-SE were used as controls.
Animal model
Male ND-4 mice were obtained from Harlan Lab (Indianapolis, IN) at 5 weeks of age and 21–24 g of weight upon receipt. Prior to the experiment, mice were fasted for 12 h and randomly assigned into seven groups. Vehicle/vehicle: saline (po) followed 4 h by saline (ip); MCT/vehicle: MCT (po) followed 4 h by saline (ip); vehicle/LPS: saline (po) followed 4 h by LPS (ip); MCT/LPS: MCT (po) followed 4 h by LPS (ip); The other three groups received ODNs (TF-SC, TF-SE, and TF-AS, 5.6 mg/kg) in MCT/LPS-treated mice right after MCT administration and 3.5 h before LPS administration. Following the above-mentioned treatments, food was made available ad libitum. MCT (200 mg/kg) and LPS (6 mg/kg) doses were selected for this study based on our previous work (Abdel-Bakky et al. 2010). TF ODNs (5.6 mg/kg) i.v. were selected during our preliminary work.
All animal study protocols were approved by the Institutional Animal Care and Use Committee (IACUC), University of Mississippi.
Sample collection
After 10, 16, and 24-h posttreatments, blood samples were collected, and then, the mice were euthanized by CO2 asphyxiation. Kidney samples were fixed in 10% formalin and processed by standard histological techniques.
Immunofluorescence analysis on tissue sections
Paraffin tissue sections were deparaffinized in xylene, rehydrated through a graded ethanol series, and washed in 10 mM phosphate-buffered 150 mM saline, pH 7.4. Antigen retrieval was performed by incubating the tissue sections for 20 min in 0.01 M sodium citrate buffer, pH 6.0, in a microwave oven (500 W). To block endogenous peroxidase, slides were incubated with absolute methanol for 30 min. After incubation with blocking buffer (0.1% Triton X-100/PBS containing 1% BSA and 10% horse serum) for 1 h, tissue sections were incubated with the first antibodies (diluted in 1% BSA/10% horse serum/PBS/0.1% Triton X-100) as required. Following washing, bound antibodies were detected by goat anti-rabbit Cy3 (Molecular Probes) or goat anti-mouse Alexa 488 (Invitrogen) secondary antibodies. Nuclei were stained with DAPI, and slides were mounted in Fluoromount G (Biozol, Southern Biotech, Birmingham, AL). Evaluation was performed by fluorescence microscopy (Carl Zeiss, CA).
Histopathological evaluation and morphometry
Kidney sections were processed routinely and embedded in paraffin blocks. Slides were prepared (4 μm) and stained with hematoxylin (RICCA Chemical Co., Arlington, TX) and eosin (EMD Chemicals, Gibbstown, NJ). The slides were observed blinded and analyzed by light microscopy for renal injury.
Fluorometric analyses
Fluorometric intensity of at least 5–8 low-power microscopic fields (100×) was measured for each tissue section using ImageJ/NIH software.
Blood chemistry
Blood samples (100 μL), 10, 16, and 24 h after different treatments, obtained by venipuncture were analyzed using the VetScan® Comprehensive Diagnostic Profile reagent rotor used with the VetScan Chemistry Analyzer (Abaxis Inc., Union City, CA). The blood samples were collected in heparinized micropipette and transferred into the reagent rotor and run within 30 min of collection. A quantitative determinations of albumin (ALB), alkaline phosphatase (ALP), amylase (AMY) total calcium (Ca++), creatinine (CRE), globulin (GLOB), glucose (GLU), phosphorus (PHOS), potassium (K+), sodium (Na+), total bilirubin (TBIL), total protein (TP), and blood urea nitrogen (BUN) were obtained.
Plasma cytokine measurement
Plasma was analyzed with the Quantibody® Mouse Cytokine Custom Array (Raybiotech, Norcross, GA) for the following cytokines: Interleukin-1β (IL-1β), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-13 (IL-13), interleukin-12p70 (IL-12p70), keratinocyte-derived chemokine (KC), leptin, tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF). Slides were incubated with the plasma samples and analyzed in accordance with the manufacturer’s instructions.
Tissue factor ELISA
Plasma TF antigen was measured using a monoclonal antibody capture system ELISA (IMUBIND tissue factor ELISA kit; American Diagnostica Inc., Greenwich, CT), following the procedure according to the vendor’s instructions.
Statistical analysis
Data were analyzed by one-way ANOVA followed by Tukey’s test for multiple comparisons using Graph Pad prism 5.0 software (La Jolla, CA). A P value of less than 0.05 was considered to show a significant difference among the groups.
Results
Renal toxicity developed in mice by (po) MCT/(ip) LPS co-treatment
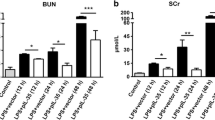
Oral administration of 200 mg/kg MCT followed 4 h by ip 6 mg/kg of LPS produced renal dysfunction manifested by progressive oliguria, rising serum CRE (2.0-fold), BUN (5.8-fold), BUN/CRE ratio (3.0-fold), PHOS (1.5-fold), and reduction in the blood ALP (0.45-fold) compared to the vehicle-treated animals. As seen in Table 1, 24 h after MCT/LPS administration, blood level of TP, ALB, K+, and GLU was significantly reduced compared to the vehicle-treated mice. Animals administered MCT or LPS showed no change in most of the above parameters (Table 1).
Effect of MCT/LPS co-treatment or TF-ODNs on the plasma and tissue TF
Plasma TF level in the vehicle, MCT, LPS, and MCT/LPS co-treated mice at 10 and 24 h after treatment was measured using TF-specific ELISA. Elevation of TF was only observed at 24 h after MCT/LPS co-treatment; at this point, plasma TF was highly elevated (18-fold) compared to vehicle group. However, in the MCT/LPS group, treatment with TF-AS, 30 min after MCT administration and 3.5 h before LPS injection, caused a 16-fold reduction in the plasma TF at 24 h compared to MCT/LPS co-treatment. No significant change in plasma TF was observed in MCT or LPS-treated groups at 24 h compared to vehicle (Fig. 1a, b). In addition, tissue TF was highly accumulated in the tubules and in the glomerular cells of MCT/LPS co-treated mice compared to vehicle as shown with specific TF immunofluorescence in renal tissues. On the other hand, injection of TF-AS in MCT/LPS co-treated mice showed considerably less accumulation of TF in the tubules and in the glomeruli compared to MCT/LPS co-treated mice (Fig. 1c). No changes appeared in plasma or tissue TF in MCT/LPS-TF-SC-, or MCT/LPS-TF-SE-treated mice compared to MCT/LPS co-treatment alone.
Effect of different treatments on tissue and plasma TF. a Plasma TF level of vehicle-, MCT-, or LPS-treated mice was significantly unchanged. TF levels 24 h after induction of renal toxicity by MCT/LPS co-treatment were strongly induced (≈18-fold) compared to the vehicle-treated mice. Data present as mean ± SEM. a significantly different from vehicle-treated animals, b significantly different from MCT-treated animals, c significantly different from LPS-treated animals. b MCT/LPS co-treatment elevating plasma TF level that was lowered significantly by TF-AS injection as measured by TF-specific ELISA. Data represent mean ± SEM. a significantly different from vehicle-treated animals, b significantly different from MCT/LPS co-treated animals, c significantly different from MCT/LPS-TF-SC-treated animals, d significantly different from MCT/LPS-TF-SE-treated animals. c Immunofluorescence staining of TF in kidney sections of animals co-treated with MCT/LPS, showing increased deposition in the tubular and glomerular cells compared to vehicle-treated mice 24 h after co-treatment. Similar deposition pattern of renal TF is seen in MCT/LPS-TF-SC and MCT/LPS-TF-SE compared to MCT/LPS co-treated mice. Slight deposition of TF is seen in the renal tubules or glomeruli in TF-AS-injected mice
Effect of TF ODN treatment on the animal survivability
Animals treated with MCT/LPS, MCT/LPS-TF-SC, MCT/LPS-TF-SE, or MCT/LPS-TF-AS were observed for the survivability (n = 10). In MCT/LPS-, and MCT/LPS-TF-SC-treated groups, 60% of mice survived beyond day 4. Thirty percent of the mice survived in MCT/LPS-TF-SE-treated group until day 4. However, in the group treated with TF-AS, 100% survived beyond day 4 (Table 2).
Effect of TF ODNs on the blood chemistry
Blood levels of BUN, creatinine, ALP, TP, ALB, GLOB, K+, PHOS, Na+, GLU, and Ca++ were measured after MCT/LPS and/or TF ODNs treatment. Twenty-four h after MCT/LPS, MCT/LPS-TF-SC, or MCT/LPS-TF-SE administration, the levels of BUN, CRE, GLOB, and PHOS were significantly increased. On the other hand, blood ALP, TP, ALB, K+, and GLU levels were significantly reduced compared to the vehicle-treated group. Injecting the animals with TF-AS 30 min after MCT administration and 3.5 h before LPS clearly prevented the deleterious effect of MCT/LPS co-treatment. The BUN/CRE ratio was significantly increased after MCT/LPS, MCT/LPS-TF-SC, or MCT/LPS-TF-SE administration compared to the vehicle-treated mice. Apparently, TF-AS injection prevented the elevation of BUN, CRE, and GLOB and also prevented the reduction in blood ALP and GLU compared to that observed in mice injected with MCT/LPS-TF-SE (Table 3).
Microscopic findings of the kidney
Normal mice kidneys showed a low level of fibrin deposition in the glomerular cells (Fig. 2a), the cortical (Fig. 2b), and the medullary tubules (Fig. 2c). On the other hand, a significant increase in fibrin deposition in MCT/LPS-, MCT/LPS-TF-SC-, and MCT/LPS-TF-SE-treated mice was observed relative to the vehicle-treated group. This inducible deposition was seen mainly in the cortex on the apical side of the distal tubules and collecting ducts (Fig. 2b). In contrast, both the proximal tubules and the loop of Henle had no fibrin deposition. Figure 2c showed the deposition of fibrin protein that was restricted only on the necrotic medullary tubules especially in basolateral side and interstitially between the tubules (the kind of tubules was difficult to define because of the structural loss). The glomerular podocytes and the glomerular basement membrane also showed fibrin deposition after MCT/LPS, MCT/LPS-TF-SC, and MCT/LPS-TF-SE treatment (Fig. 2a). In contrast, TF-AS injection markedly reduced fibrin deposition in the glomeruli and tubules both in the cortex and medulla (Fig. 2a–c). To identify fibrin-expressing cells in glomeruli of mice, we performed double immunofluorescence analysis in renal sections of MCT/LPS co-treated mice. As shown in Fig. 2d, fibrin (red) was co-expressed with WT1 (green), which has been described to be only expressed in the nuclei of glomerular podocytes (Mundlos et al. 1993). Marked tubular collapse, hemorrhage, and the necrotic medullary tubules were seen in MCT/LPS-treated mice analyzed by H&E staining. MCT/LPS-TF-SC and MCT/LPS-TF-SE showed the same histopathological features as MCT/LPS co-treated mice. However, the kidney sections of MCT/LPS-TF-AS-injected mice showed normal structure similar to those of vehicle-treated mice (Fig. 2e).
Effect of ODNs treatment on fibrin deposition at 24 h in the kidneys after MCT/LPS co-treatment. Mice kidney sections were analyzed for fibrin deposition with immunofluorescence staining using antibody against fibrin followed by Cy3-coupled secondary antibody. a Fibrin deposition was absent in the renal glomeruli of the vehicle-treated mice. Very low deposition of fibrin was seen in the renal glomeruli of MCT-, or LPS-treated mice. Treating the animals with MCT/LPS, MCT/LPS-TF-SC or MCT/LPS-TF-SE strongly induced fibrin deposition in the glomerular capillary lumen. TF-AS injection prevent fibrin upregulation in the glomeruli. b The cortical tubules were negative for fibrin in vehicle-, MCT-, and LPS-treated mice. MCT/LPS, MCT/LPS-TF-SC, and MCT/LPS-TF-SE showed strong induction in fibrin deposition, mostly in the apical side of the distal tubules as well as in the collecting ducts (yellow arrow). No expression was seen in the proximal. In addition, MCT/LPS-TF-AS-injected mice showed an absence of tubular fibrin deposition. c Low deposition of fibrin was seen in the medullary tubules in vehicle, MCT, and LPS. MCT/LPS, MCT/LPS-TF-SC, and MCT/LPS-TF-SE were strongly induced fibrin especially in the necrotic medullary tubules (yellow arrows), whereas TF-AS prevents fibrin deposition. d Paraffin sections of MCT/LPS-treated mice renal tissue were stained with a polyclonal antibody against fibrin (red, upper left plate) and monoclonal IgG1 for WT-1 (green, upper right plate). Double immunofluorescence staining for merged picture (lower right plate) and surface blot (lower left plate) showing yellow fluorescence resulting from the co-localization of red fibrin and green WT1 fluorescence. e Histopathological evaluation of kidney sections of mice after vehicle, MCT/LPS with or without TF ODNs injection treatment. Remarkable lesions were seen in the MCT/LPS-, MCT/LPS-TF-SC-, and MCT/LPS-TF-SE-treated groups in the form of pronounced hemorrhage collapsing and necrotic medullary tubules (yellow arrows) that are absent in MCT/LPS-TF-AS-injected mice. Bar: a 10 μm; b and d 25 μm; c and e 100 μm (color figure online)
TF-AS attenuated renal inflammation and selectively decreased leptin and interleukin-6 (IL-6) production
In conjunction with modulated blood chemistry, serum TF, and renal fibrin deposition in mice, TF-AS injection also significantly diminished blood IL-6 and leptin (inflammatory cytokines) production induced by MCT/LPS in mice. Ten plasma cytokines were analyzed to determine the extent of the systemic inflammatory response including IL-6, interleukin-1β (IL-1β), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF), leptin, interleukin-4 (IL-4), interleukin-13 (IL13), interleukin-12p70 (IL-12p70), and keratinocyte-derived chemokine (KC) at 10, 16, and 24 h after MCT/LPS co-treatment (data not shown). MCT/LPS co-treatment strongly induced IL-6, IL-1β, IL-10, TNF-α, and VEGF at 10 h; however, by 24 h, the levels declined, while leptin, IL-12p70, and KC started to increase at 10 h and the peak level was at 24 h. As seen in Fig. 3a and c, in MCT/LPS co-treated mice, the plasma leptin and IL-6 were significantly increased at 10 h compared to vehicle, MCT, or LPS-treated mice and the levels remained elevated at 24 h as well. On the other hand, the blocking effect of TF-AS was obviously seen in leptin and IL-6 levels but not on the rest of the previously mentioned cytokines. In other words, leptin (Fig. 3b) and IL-6 (Fig. 3d) were the only cytokines that were reduced to their basal levels after TF-AS injection.
Serum level of leptin, IL-6 and tissue expression of CXCL16 in mice after various treatments. a Serum leptin levels started to increase 10 h and sustained for 24 h after MCT/LPS co-treatment. MCT or LPS by 24 h showed low leptin level compared to vehicle-treated mice. b Plasma leptin 24 h after MCT/LPS-, MCT/LPS-TF-SC-, and MCT/LPS-TF-SE-treated mice showed significant leptin elevation that returned to the initial value in MCT/LPS co-treated animals injected with TF-AS. c Similarly, IL-6 level increased 10 h after MCT/LPS co-treatment and remained elevated at 16 and 24 h. No significant change was seen in IL-6 in MCT or LPS alone except in LPS at 10 h. d Plasma IL-6 remained elevated 24 h after treatment with MCT/LPS, MCT/LPS-TF-SC, and MCT/LPS-TF-SE but retained toward initial values in MCT/LPS animals treated injected with TF-AS. e CXCL16 expression in vehicle-treated kidney section was in the basolateral side in distal and collecting ducts in cortical tubules (yellow arrows). Treating the animals with MCT/LPS, MCT/LPS-TF-SC, or MCT/LPS-TF-SE reduced the tubular CXCL16 expression, and its presence (positive green fluorescence) in the tubular interstitial space (yellow arrows) correlated with the presence of migrating immune cells (blue DAPI fluorescence). Data represent mean ± SEM and were analyzed by one-way ANOVA with Tukey’s test for multiple comparisons. a and c: a significantly different from vehicle-treated animals, b significantly different from MCT-treated animals, c significantly different from LPS-treated animals. For b and d: a significantly different from vehicle-treated animals, b significantly different from MCT/LPS-treated animals, c significantly different from MCT/LPS-TF-SC-treated animals, d significantly different from MCT/LPS-TF-SE-treated animals (color figure online)
TF-AS attenuated renal inflammation and selectively prevented downregulation of tubular chemokine (C-X-C motif) ligand 16 (CXCL16) induced by MCT/LPS
In glomerular podocytes and in the basolateral side of the distal tubules in vehicle-treated mice, we detected CXCL16 expression by immunofluorescence (green). Treating the animals with MCT/LPS, MCT/LPS-TF-SC, or MCT/LPS-TF-SE led to the absence of CXCL16 in the tubules and its presence in the interstitial space; the interstitial CXCL16 correlated with the presence of migrating leukocyte cells (blue DAPI fluorescence). The absence of the interstitial CXCL16 and its presence in the tubular cells were observed in TF-AS-injected mice (Fig. 3e).
Discussion
TF is not detectable in the human blood stream, but expressed on the surface of monocytes and endothelial cells in a number of pathological conditions. Unstable angina pectoris, endotoxemia with or without disseminated intravenous coagulation (DIC), some malignancies, and certain immunological diseases have been suggested to be associated with increased monocytic TF levels (Edgington et al. 1992; Rothberger et al. 1984). In the extrinsic pathway, when factor VIIa is bound to its cofactor, TF can activate factor X directly. The activation of factor X leads to thrombin generation, fibrin formation, and platelets activation. Local fibrin deposition is frequently observed in many diseases (Dorfleutner et al. 2004). In the kidney, TF expression is restricted to the glomerular podocytes, bowman epithelium, and the tubular loop of Henle (Belting et al. 2004; Bromberg et al. 1999; Mach et al. 1997). The present report showed that co-treatment with sub-toxic doses of oral MCT and ip LPS did induce renal injury, whereas the individual treatments did not. The toxicity was confirmed with elevation of the renal toxicity markers including blood BUN, creatinine, BUN/creatinine ratio, K+, and ALP. Since oliguria was a characteristic feature in the animals after the co-treatment with MCT/LPS, it was difficult to collect urine for urinalysis. We have documented upregulation of the serum TF level at 10 h and a progressive increase in its level up to 24 h after MCT/LPS co-treatment. Similarly, renal TF protein was upregulated at 24 h after MCT/LPS co-treatment. These data are similar to those shown earlier (Frank et al. 2005) where upregulation of TF mRNA was reported in renal ischemia reperfusion (I/R) murine model. After prolonged renal ischemia and contralateral nephrectomy in a rat model, TF was upregulated in the kidney with increased TF staining in glomeruli, blood vessels, and stimulated monocytes (Ushigome et al. 2002). The same study, using a less severe model, has subsequently shown protection from tubular necrosis by the use of TF antisense oligonucleotides (Matsuyama et al. 2003). The mechanism underlying TF-mediated injury was not explored, although it was postulated that necrosis was caused by microcirculatory incompetence and microthrombus formation. An important therapeutic concept in support of anti-TF strategies to target many toxicity types involves the known cross talk between coagulation and inflammation through TF-VIIa complex, factor Xa, thrombin, and fibrin (Cirino et al. 1997; Cunningham et al. 1999; Johnson et al. 1998; Shin et al. 1999). TF-VIIa has intracellular calcium-dependent signaling functions (Rottingen et al. 1995) and perhaps other intracellular signaling effects via phosphorylation of mitogen activated protein kinases (Poulsen et al. 1998; Sorensen et al. 1999). These effects enhance cytokine production (Camerer et al. 2000) and inflammation, and may alter endothelial permeability. Factor Xa and thrombin also have potent proinflammatory effects, mediated in part by nuclear factor-β activation (Shapiro et al. 1996; Shin et al. 1999; Stankova et al. 1995). In the present study, the glomerular capillary lumens, glomerular podocytes, distal tubular epithelial cells in the cortical region, and the necrotic tubules in the medullary area showed extensive deposition of fibrin in MCT/LPS co-treated mice kidney. Blocking of TF by TF-AS ODNs led to decreased renal injury, fibrin deposition, plasma leptin level, and loss of tubular CXCL16 induced by MCT/LPS. These results provided direct evidence that TF is the principal initiator of fibrin deposition which means that the extrinsic pathway of coagulation cascade is the main contributor to the current model of renal toxicity. Also, TF blockade would inhibit downstream activation of factor X and generation of thrombin and fibrin, all of which have potential proinflammatory actions that could be important in producing tissue injury. This may offer an advantage over other therapeutic strategies targeting steps in the coagulation cascade downstream to TF that show limited ability to hamper subsequent TF-dependent coagulation. Synthetic TF ODN is thought to prolong organ allograft survival by blocking the expression of specific targeted proteins or mRNA, arresting translation, inhibiting processing, or degrading the targeted RNA (Akhtar et al. 1991; Crooke 1992; Hoke et al. 1991; Milligan et al. 1993). Nakamura et al. (2002) demonstrated that use of AS ODNs method could be effective in protecting the liver from I/R injury. In preclinical studies, it could be effective in protecting the kidney and prolong the animal survivability from I/R injury (Matsuyama et al. 2003). In our study, we found also that TF-AS did prolong the animal survival rate of MCT/LPS co-treated mice, indicating the effectiveness of AS method in the prevention of renal toxicity in our model.
Leptin, a peptide hormone of the long-chain helical cytokine family, is predominantly produced by white adipose cells (Zhang et al. 1994). Leptin has been reported to have direct pathological effects on renal resident cells. It was also reported that leptin could stimulate cellular proliferation in vitro, TGF-β1 synthesis, and type IV collagen production (Wolf et al. 1999). Moreover, an increase in glomerular TGF-β activation after leptin infusion was seen signifying a close regulatory connection of leptin and the TGF-β axis (Gunduz et al. 2005). Kumpers et al. (2007), demonstrated that leptin deficiency leads to significantly less inflammation, tissue damage, fibrotic changes, and matrix accumulation in ob/ob mice after unilateral ureteral obstruction (UUO) compared to db/db mice and respective controls. Similarly, Guagnano et al. (2003) showed a significant positive correlation between leptin and FVIIa in obese women. In the present report, leptin plasma level was increased after MCT/LPS co-treatment but was decreased almost to the basal level after AS-TF ODN injection indicating that leptin could be a downstream event to the coagulation system activated by MCT/LPS co-treatment. To our knowledge, this is the first report to exhibit a relation between plasma leptin level and TF activation in the renal injury induced by MCT/LPS.
Interleukin (IL)-6 is a pleiotropic cytokine that has many biological properties. It is primarily involved in the regulation of immune and inflammatory responses. IL-6 can induce a prothrombotic state by increasing expression of fibrinogen, tissue factor, factor VIII, and von Willebrand factor, as well as by activating endothelial cells and increasing platelet production (Kerr et al. 2001). In the intestine, increased expression of IL-6 contributes to gut I/R injury (Cuzzocrea et al. 1999). Similarly in renal I/R injury, a significant and sustained increase in the expression of the IL-6 gene was reported (Takada et al. 1997). In our study, the increase in IL-6 level caused by MCT/LPS was prevented by injection of TF-AS, whereas injection of TF-SC or TF-SE did not prevent its increase. It appears that elevation of leptin and IL-6 in renal injury induced by MCT/LPS is a TF-dependent process.
The role of CXCL16, a transmembrane chemokine, in the progression of kidney diseases has been recently described. So far, in humans, an important role of CXCL16 has only been suggested in lupus patients and in kidney transplanted patients with the diagnosis of acute interstitial rejection (Schramme et al. 2008). In the present report, we found that reduced expression of CXCL16 in the tubular cells after MCT/LPS co-treatment correlated with the presence of migrated leukocytes. We hypothesize that this finding may be due to increased proteinase(s) responsible for the CXCL16 shedding, consequently converting CXCL16 from the membrane into the soluble chemoattractant form, which may function to recruit leukocytes into the site of renal injury. Further work is needed to confirm this hypothesis. Finally, it is possible that MCT/LPS induces the expression of TF through a loop involving proinflammatory molecules such as leptin, IL-6, and CXCL16, initiating blood coagulation on glomerular endothelial or distal tubular cells that are efficiently blocked by TF-AS injection. These investigations support the feasibility of using TF-AS ODNs as a new approach for some inflammation-induced xenobiotic toxicity in kidney.
In summary, this is the first report that shows induction of renal injury by sub-toxic doses of oral MCT and ip LPS in a mouse model and that this injury is mediated by TF. In addition, TF-AS ODNs ameliorated renal damage by inhibiting TF overexpression, suggesting that renal toxicity mediated by TF can be prevented successfully by the use of TF-AS.
References
Abdel-Bakky MS, Hammad MA, Walker LA, Ashfaq MK (2010) Developing and characterizing a mouse model of hepatotoxicity using oral pyrrolizidine alkaloid (monocrotaline) administration, with potentiation of the liver injury by co-administration of LPS. Nat Prod Commun 5:1457–1462
Akhtar S, Kole R, Juliano RL (1991) Stability of antisense DNA oligodeoxynucleotide analogs in cellular extracts and sera. Life Sci 49:1793–1801
Bazan JF (1990) Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA 87:6934–6938
Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, Carmeliet P, Mueller BM, Friedlander M, Ruf W (2004) Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med 10:502–509
Bierhaus A, Zhang Y, Deng Y, Mackman N, Quehenberger P, Haase M, Luther T, Muller M, Bohrer H, Greten J (1995) Mechanism of the tumor necrosis factor alpha-mediated induction of endothelial tissue factor. J Biol Chem 270:26419–26432
Bromberg ME, Sundaram R, Homer RJ, Garen A, Konigsberg WH (1999) Role of tissue factor in metastasis: functions of the cytoplasmic and extracellular domains of the molecule. Thromb Haemost 82:88–92
Camerer E, Gjernes E, Wiiger M, Pringle S, Prydz H (2000) Binding of factor VIIa to tissue factor on keratinocytes induces gene expression. J Biol Chem 275:6580–6585
Cirino G, Cicala C, Bucci M, Sorrentino L, Ambrosini G, DeDominicis G, Altieri DC (1997) Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Invest 99:2446–2451
Crooke ST (1992) Therapeutic applications of oligonucleotides. Annu Rev Pharmacol Toxicol 32:329–376
Cunningham MA, Romas P, Hutchinson P, Holdsworth SR, Tipping PG (1999) Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood 94:3413–3420
Cuzzocrea S, De SG, Costantino G, Ciliberto G, Mazzon E, De SA, Caputi AP (1999) IL-6 knock-out mice exhibit resistance to splanchnic artery occlusion shock. J Leukoc Biol 66:471–480
Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W (2004) Cross-talk of integrin alpha3beta1 and tissue factor in cell migration. Mol Biol Cell 15:4416–4425
Edgington TS, Mackman N, Fan ST, Ruf W (1992) Cellular immune and cytokine pathways resulting in tissue factor expression and relevance to septic shock. Nouv Rev Fr Hematol 34(Suppl):S15–S27
Erlich JH, Holdsworth SR, Tipping PG (1997) Tissue factor initiates glomerular fibrin deposition and promotes major histocompatibility complex class II expression in crescentic glomerulonephritis. Am J Pathol 150:873–880
Erlich JH, Boyle EM, Labriola J, Kovacich JC, Santucci RA, Fearns C, Morgan EN, Yun W, Luther T, Kojikawa O, Martin TR, Pohlman TH, Verrier ED, Mackman N (2000) Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia-reperfusion injury by reducing inflammation. Am J Pathol 157:1849–1862
Esmon CT (2003) Inflammation and thrombosis. J Thromb Haemost 1:1343–1348
Frank RD, Schabbauer G, Holscher T, Sato Y, Tencati M, Pawlinski R, Mackman N (2005) The synthetic pentasaccharide fondaparinux reduces coagulation, inflammation and neutrophil accumulation in kidney ischemia-reperfusion injury. J Thromb Haemost 3:531–540
Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR (1996) Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med 154:602–611
Guagnano MT, Romano M, Falco A, Nutini M, Marinopiccoli M, Manigrasso MR, Basili S, Davi G (2003) Leptin increase is associated with markers of the hemostatic system in obese healthy women. J Thromb Haemost 1:2330–2334
Gunduz Z, Dursun N, Akgun H, Ozturk F, Okur H, Koc N (2005) Renal effects of long-term leptin infusion and preventive role of losartan treatment in rats. Regul Pept 132:59–66
Hoke GD, Draper K, Freier SM, Gonzalez C, Driver VB, Zounes MC, Ecker DJ (1991) Effects of phosphorothioate capping on antisense oligonucleotide stability, hybridization and antiviral efficacy versus herpes simplex virus infection. Nucleic Acids Res 19:5743–5748
Idell S (1994) Extravascular coagulation and fibrin deposition in acute lung injury. New Horiz 2:566–574
Imamura T, Iyama K, Takeya M, Kambara T, Nakamura S (1993) Role of macrophage tissue factor in the development of the delayed hypersensitivity reaction in monkey skin. Cell Immunol 152:614–622
Johnson K, Choi Y, DeGroot E, Samuels I, Creasey A, Aarden L (1998) Potential mechanisms for a proinflammatory vascular cytokine response to coagulation activation. J Immunol 160:5130–5135
Kerr R, Stirling D, Ludlam CA (2001) Interleukin 6 and haemostasis. Br J Haematol 115:3–12
Kumpers P, Gueler F, Rong S, Mengel M, Tossidou I, Peters I, Haller H, Schiffer M (2007) Leptin is a coactivator of TGF-beta in unilateral ureteral obstructive kidney disease. Am J Physiol Renal Physiol 293:F1355–F1362
Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P (1997) Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation 96:396–399
Mackman N (2005) Tissue-specific hemostasis in mice. Arterioscler Thromb Vasc Biol 25:2273–2281
Matsuyama M, Yoshimura R, Akioka K, Okamoto M, Ushigome H, Kadotani Y, Nakatani T, Yoshimura N (2003) Tissue factor antisense oligonucleotides prevent renal ischemia-reperfusion injury. Transplantation 76:786–791
Milligan JF, Matteucci MD, Martin JC (1993) Current concepts in antisense drug design. J Med Chem 36:1923–1937
More L, Sim R, Hudson M, Dhillon AP, Pounder R, Wakefield AJ (1993) Immunohistochemical study of tissue factor expression in normal intestine and idiopathic inflammatory bowel disease. J Clin Pathol 46:703–708
Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B (1993) Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development 119:1329–1341
Nakamura K, Kadotani Y, Ushigome H, Akioka K, Okamoto M, Ohmori Y, Yaoi T, Fushiki S, Yoshimura R, Yoshimura N (2002) Antisense oligonucleotide for tissue factor inhibits hepatic ischemic reperfusion injury. Biochem Biophys Res Commun 297:433–441
Nemerson Y (1988) Tissue factor and hemostasis. Blood 71:1–8
Osterholm C, Veress B, Simanaitis M, Hedner U, Ekberg H (2005) Differential expression of tissue factor (TF) in calcineurin inhibitor-induced nephrotoxicity and rejection—implications for development of a possible diagnostic marker. Transpl Immunol 15:165–172
Poulsen LK, Jacobsen N, Sorensen BB, Bergenhem NC, Kelly JD, Foster DC, Thastrup O, Ezban M, Petersen LC (1998) Signal transduction via the mitogen-activated protein kinase pathway induced by binding of coagulation factor VIIa to tissue factor. J Biol Chem 273:6228–6232
Rinaldo JE, Rogers RM (1982) Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med 306:900–909
Rothberger H, Barringer M, Meredith J (1984) Increased tissue factor activity of monocytes/macrophages isolated from canine renal allografts. Blood 63:623–628
Rottingen JA, Enden T, Camerer E, Iversen JG, Prydz H (1995) Binding of human factor VIIa to tissue factor induces cytosolic Ca2 + signals in J82 cells, transfected COS-1 cells, Madin–Darby canine kidney cells and in human endothelial cells induced to synthesize tissue factor. J Biol Chem 270:4650–4660
Schramme A, Abdel-Bakky MS, Gutwein P, Obermuller N, Baer PC, Hauser IA, Ludwig A, Gauer S, Schafer L, Sobkowiak E, Altevogt P, Koziolek M, Kiss E, Grone HJ, Tikkanen R, Goren I, Radeke H, Pfeilschifter J (2008) Characterization of CXCL16 and ADAM10 in the normal and transplanted kidney. Kidney Int 74:328–338
Shapiro PS, Evans JN, Davis RJ, Posada JA (1996) The seven-transmembrane-spanning receptors for endothelin and thrombin cause proliferation of airway smooth muscle cells and activation of the extracellular regulated kinase and c-Jun NH2-terminal kinase groups of mitogen-activated protein kinases. J Biol Chem 271:5750–5754
Shin H, Kitajima I, Nakajima T, Shao Q, Tokioka T, Takasaki I, Hanyu N, Kubo T, Maruyama I (1999) Thrombin receptor mediated signals induce expressions of interleukin 6 and granulocyte colony stimulating factor via NF-kappa B activation in synovial fibroblasts. Ann Rheum Dis 58:55–60
Sorensen BB, Freskgard PO, Nielsen LS, Rao LV, Ezban M, Petersen LC (1999) Factor VIIa-induced p44/42 mitogen-activated protein kinase activation requires the proteolytic activity of factor VIIa and is independent of the tissue factor cytoplasmic domain. J Biol Chem 274:21349–21354
Stankova J, Rola-Pleszczynski M, D’Orleans-Juste P (1995) Endothelin 1 and thrombin synergistically stimulate IL-6 mRNA expression and protein production in human umbilical vein endothelial cells. J Cardiovasc Pharmacol 26(Suppl 3):S505–S507
Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL (1997) The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest 99:2682–2690
Taylor FB Jr, Chang AC, Esmon CT, Hinshaw LB (1991) Baboon model of Escherichia coli sepsis: description of its four stages and the role of tumor necrosis factor, tissue factors, and the protein C system in septic shock. Curr Stud Hematol Blood Transfus 58:8–14
Taylor FB, Chang AC, Peer G, Li A, Ezban M, Hedner U (1998) Active site inhibited factor VIIa (DEGR VIIa) attenuates the coagulant and interleukin-6 and -8, but not tumor necrosis factor, responses of the baboon to LD100 Escherichia coli. Blood 91:1609–1615
Tremoli E, Camera M, Toschi V, Colli S (1999) Tissue factor in atherosclerosis. Atherosclerosis 144:273–283
Ushigome H, Sano H, Okamoto M, Kadotani Y, Nakamura K, Akioka K, Yoshimura R, Ohmori Y, Yoshimura N (2002) The role of tissue factor in renal ischemic reperfusion injury of the rat. J Surg Res 102:102–109
Wakita Y, Wada H, Nakase T, Nakasaki T, Shimura M, Hiyoyama K, Mori Y, Gabazza EC, Nishikawa M, Deguchi K, Shiku H (1999) Aberrations of the tissue factor pathway in patients positive for lupus anticoagulant. Clin Appl Thromb Hemost 5:10–15
Weinberg JB, Wortham TS, Misukonis MA, Patton KL, Chitneni SR (1993) Synovial mononuclear phagocytes in rheumatoid arthritis and osteoarthritis: quantitative and functional aspects. Immunol Invest 22:365–374
Welty-Wolf KE, Carraway MS, Miller DL, Ortel TL, Ezban M, Ghio AJ, Idell S, Piantadosi CA (2001) Coagulation blockade prevents sepsis-induced respiratory and renal failure in baboons. Am J Respir Crit Care Med 164:1988–1996
Wolf G, Hamann A, Han DC, Helmchen U, Thaiss F, Ziyadeh FN, Stahl RA (1999) Leptin stimulates proliferation and TGF-beta expression in renal glomerular endothelial cells: potential role in glomerulosclerosis [see comments]. Kidney Int 56:860–872
Yee SB, Kinser S, Hill DA, Barton CC, Hotchkiss JA, Harkema JR, Ganey PE, Roth RA (2000) Synergistic hepatotoxicity from coexposure to bacterial endotoxin and the pyrrolizidine alkaloid monocrotaline. Toxicol Appl Pharmacol 166:173–185
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Acknowledgments
The authors wish to thank Mr. Rajnish Sahu (NCNPR, University of Mississippi) for his help in the use of fluorescent microscope. This work was supported in part by the United States Department of Agriculture, Agricultural Research Service, Specific Cooperative agreement [58-6408-2-0009] and US Food and Drug Administration [5U01FD002071-09 and 1U01FD003871-011].
Author information
Authors and Affiliations
Corresponding author
Additional information
Mohamed Sadek Abdel-Bakky and Mohamed A. Hammad have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Abdel-Bakky, M.S., Hammad, M.A., Walker, L.A. et al. Silencing of tissue factor by antisense deoxyoligonucleotide prevents monocrotaline/LPS renal injury in mice. Arch Toxicol 85, 1245–1256 (2011). https://doi.org/10.1007/s00204-011-0663-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0663-8