Abstract
Cisplatin (CIS) is an antineoplastic drug able to produce free radicals that are capable to induce various side effects in different tissues. Hydrogen sulfide (H2S) has notable antioxidant, anti-apoptotic, and anti-inflammatory effects in different systems but its role in male reproductive system is not fully understood. In the present research, the effect of sodium hydrosulfide (NaHS) on cisplatin-induced testicular toxicity in male rats was studied. Thirty-two Sprague–Dawley rats were equally divided into 4 groups. The control group was treated with normal saline by intraperitoneal injection. The NaHS group received NaHS (200 μg/kg/day) intraperitoneally for 15 days. The CIS group received single dose of cisplatin (5 mg/kg) intraperitoneally, while the combination of CIS and NaHS was given to the CIS+ NaHS group. At the end of the study, body and testicular weights, plasma testosterone level, histological and morphometrical alterations, inflammation via IL-1β protein, lipid peroxidation, and activity of antioxidant enzymes (including glutathione peroxidase, superoxide dismutase, and catalase) of testicular tissue were evaluated. CIS injection revealed a significant decrease (p < 0.01) in body and testis weights, plasma testosterone concentration, diameter of seminiferous tubules, germinal epithelium thickness, the number of Sertoli cells, spermatogonia and spermatocyte, Johnsen’s testicular score, and testicular antioxidant enzymes, whereas it caused a significant increase (p < 0.01) in lumen diameter of the seminiferous tubules, level of lipid peroxidation, and IL-1β protein expression when compared with the control group. NaHS administration to CIS-treated rats provided marked improvement (p < 0.05) in all biochemical, histological, and morphometrical changes induced by CIS. The beneficial effects of NaHS were mediated, at least partly, by its antioxidant and anti-inflammatory properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cisplatin (CIS), cis-diamminedichloroplatinum (II), is widely used as a standard anticancer drug for treating different cancers such as head, neck, lung, bladder, and testicular cancers (Abdellatief et al. 2017; Sherif et al. 2014; Abdel-Daim et al. 2019a, b). CIS, a molecule of DNA-alkylating, runs its antitumor effects by creating cross-links and double-strand breaks of DNA; these functions inhibit transcription and replication of DNA, leading to apoptosis (Reddy et al. 2016). Moreover, CIS generates oxidative stress by generating free radicals which progress cellular injury and necrosis via the tissues lipid peroxidation, DNA damage, and denaturation of protein (Fallahzadeh et al. 2017; Abdel-Daim et al. 2019a, b). Even though CIS is a potent anticancer drug, but its application has been restricted for two reasons: development of resistance to CIS and intense toxicity in some organs, like kidney, peripheral nerves, and testis (Abdel-Moneim et al. 2014). The mechanisms involved in testicular injuries created by CIS include physiological and histopathological disorders caused by oxidative damage and DNA lesion (Almeer and Abdel Moneim 2018). Biological compounds with antioxidant effects can supply to the conservation of tissues and organs against harmful effects of reactive oxygen species (ROS) and other free radicals generated by CIS (Shokrzadeh et al. 2014).
Hydrogen sulfide (H2S) is now believed to be the third human endogenous gaseous transmitter (Mansouri 2018). It has been reported which H2S creates notable antioxidant, anti-apoptotic, and anti-inflammatory effects in different organs (Lobb et al. 2014; Li et al. 2006). Endogenous H2S are principally produced by cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3MST). CBS and 3MST are mainly expressed in the brain and nervous system, but CSE is suggested to be predominantly in the cardiovascular system (Kimura 2013). Also, it is determined which both CSE and CBS are expressed in rat testes, while there is little knowledge about 3MST expression, and its function in the reproductive system so far (Sugiura et al. 2005; Li et al. 2015). NaHS has been largely applied in clinical experiments to estimate the biological effects of H2S (Li et al. 2015; Kimura 2014). However, NaHS liberates huge content of H2S in a short time and cannot efficiently follow the biological track of H2S which is naturally produced (Liu et al. 2013).
In light of all this information, in this study, we aimed to investigate the possible testicular protective potential of NaHS, a hydrogen sulfide (H2S) donor, against the oxidative injuries induced by cisplatin in testes of rat.
Materials and methods
Chemicals
Cisplatin, NaHS, and other chemicals and reagents were obtained from Sigma Aldrich chemical Co. (St. Louis, USA). Antibody against IL-1β was purchased from Zellbio GmbH (Germany). Rat testosterone ELISA kit was obtained from MyBioSource Inc. (USA). Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) kits were purchased from Randox Lab (Crumlin, UK).
Animals
In this study, 32 adult male Sprague–Dawley rats (9–10 weeks old, 180–200 g) were procured from animal house of Ahvaz Jundishapur University of Medical Science (Ahvaz, Iran). Throughout the experiment, rats had free access to food and water in a 12-h light/dark cycle and a temperature (22 ± 2 °C) and relative humidity (40–70%). All experimental procedures were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR. AJUMS. ABHC. REC.1397.011) and followed the National Institutes of Health guidelines.
Experimental protocol
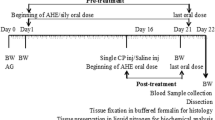
After 5-day acclimatization to the home cage, the rats were randomly divided into four groups (all n = 8). The control group received normal saline via intraperitoneal injection. The NaHS group received NaHS dissolved in saline, intraperitoneally at the dose of 200 μg/kg/day for 15 days; the dose and time of administration were selected based on previous study (Karimi et al. 2017). The CIS group received a single injection of cisplatin (5 mg/kg) intraperitoneally. CIS+ NaHS group received a single injection of cisplatin (5 mg/kg) intraperitoneally (Mercantepe et al. 2018) and then received NaHS (200 μg/kg/day) for a period of 15 days.
Sample collection and homogenate preparation
At the end of the experimental period (16th day), animals of all groups were weighed and killed under anesthesia with ketamine (75 mg/kg) and xylazine (10 mg/kg). Blood samples were drawn from left ventricle, transferred into heparinized tubes, and centrifuged at 3000×g for 10 min to obtain plasma. Then, testes were removed, released from the epididymis and adjacent tissues, and weighed. One of the testes was fixed in 10% formalin for histological and immunohistochemical evaluations. The other testes and plasma samples were stored at − 20 °C for biochemical analyses. For biochemical analyses, testicular tissues were minced in a glass and homogenized in potassium phosphate buffer (10 mM, pH 7.4) at a concentration of 5% (w/v) using a homogenizer (Model silent crusher-M; Heidolph Instruments, Donau, Germany). The homogenates were centrifuged at 16000×g at 4 °C for 20 min. Protein concentration of the samples was determined using the method of Bradford (Bradford 1976).
Relative organ weight
The relative organ weight of each animal was calculated as follows:
Epididymal sperm count
Epididymal sperm count was determined by the method described earlier (Yokoi and Mayi 2004). Briefly, the epididymis was cut into small pieces in 5 mL normal saline, located in a roller for 10 min, and permitted to incubate at room temperature for 2 min. After incubation, the supernatant was diluted 1:100 with 1 mL formalin (35%) and 5 g sodium bicarbonate. The sperms were counted by using hemocytometer. Almost 10 μL of the specimen was transferred to the counting chamber and was allowed to stand for 5 min. The sperms were then counted by light microscope. The total epididymal sperm numbers obtained from the counting were expressed as the number of sperms × 106/mL.
Testosterone assay
The level of plasma testosterone was determined by ELISA according to the instructions of the kit and expressed as ng/ml.
Morphometrical and histological examination
The fixed testes, embedded in paraffin, sectioned at 5 μm, and were stained with hematoxylin and eosin. Light microscope was used to evaluate the testicular tissue injury and Johnsen’s testicular score was performed for all groups. All cross-sectioned seminiferous tubules were assessed, and a score between 1 and 10 was given to each tubule based on Johnsen’s criteria. The histologic score criteria were as follows: (1) no seminiferous epithelium; (2) no germinal cells, only Sertoli cells; (3) only spermatogonia; (4) no spermatozoa or spermatids, few spermatocytes; (5) no spermatozoa or spermatids, many spermatocytes; (6) no spermatozoa, no late spermatids, few early spermatids; (7) no spermatozoa, no late spermatids, many early spermatids; (8) less than five spermatozoa per tubule, few late spermatids; (9) slightly impaired spermatogenesis, many late spermatids, disorganized epithelium; and (10) full spermatogenesis (Yoshida et al. 1997). Motic software (Micro-Optic Industrial Group CO., LTD., UK) was used to measure diameter of seminiferous tubules, germinal epithelium thickness, and diameter of seminiferous lumen as described previously (Farsani et al. 2018). Furthermore, the average number of Sertoli cells, spermatogonia, and spermatocytes were calculated in seminiferous tubules in stages VII or VIII in each animal using a light microscopy with an objective lens × 100. For each animal, 25 tubules were assessed.
Immunohistochemistry examination
Immunohistochemistry analysis was performed as explained in our previous study (Karimi et al. 2017). The tissue sections (5 μm) were deparaffinized with xylene and dehydrated with different degrees of alcohol. The sections were incubated in citrate buffer (10 mM, pH 6.0) in 98 °C for 20 min for unmasking of antigen. After washing with phosphate buffer solution (PBS), endogenous peroxidase was inactivated by 1% H2O2 for 15 min. To block non-specific reactions, they were incubated with 1.5% blocking serum for 1 h. Then, sections were incubated with primary rabbit polyclonal antibody against IL-1β (dilution 1:500) overnight at 4 °C. Sections were washed again in PBS and were incubated with secondary conjugated antibody (goat anti-rabbit IgG/HRP conjugate, dilution 1:1000) for 1 h, after that, sections were washed with PBS. Diaminobenzidin was used as color determining substrate. At the last stages, sections were washed with tap water for 10 min then were stained with hematoxylin. IL-1β positive in germinal epithelium was evaluated by light microscope and scored as follows (Çeribaşı et al. 2012). Score 0: negative stained epithelial cells; score 1: < 25% positive stained epithelial cells; score 2: 26–50% positive stained epithelial cells; score 3: 51–75% positive stained epithelial cells; score 4: > 75% positive stained epithelial cells. In this method, appendix tissue was used as negative control. Negative control was done by deleting the primary antibody; consequently, immunoreactions did not occur.
Oxidative stress evaluation
To determine the content of lipid peroxidation, malondialdehyde (MDA) as an index of lipid peroxidation was measured as previously described (Mehrzadi et al. 2018). The MDA level was expressed as nmol/mg protein. The glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) activity were spectrophotometrically determined by Ransel and Ransod kits respectively. As described in our previous study (Khorsandi et al. 2017), the activity of catalase (CAT) was spectrophotometrically determined by measuring the decomposition of hydrogen peroxide (H2O2) at 240 nm and was expressed as U/mg protein.
Statistical analysis
The SPSS software for windows version 16.0 was used for the statistical analyses (Spss Inc. Chicago, 2007). All data were presented as mean ± SD. One-way analysis of variance (ANOVA) and post hoc Tukey test were used to determine differences between the groups. p < 0.05 indicated that the differences were statistically significant.
Results
Body weight, testicular weight, and testes/body weight ratio
In the CIS group, body weight gain and testicular weights were significantly (p < 0.001) lower than the control and NaHS weights (Table 1). However, relative testis weight that was obtained at the end of the experiment was lower (nonsignificant) than control and NaHS groups. The rats treated with CIS+ NaHS showed significantly higher body weight gain and testicular weights (p < 0.001, p < 0.01) than CIS-treated rats. The NaHS treatment alone did not affect on the final body and testicular weights compared with control and CIS+ NaHS groups.
Sperm count
Epididymal sperm concentration is presented in Fig. 1. Administration of CIS alone caused a significant decrease (p < 0.001) in sperm concentration when compared with the values of the control and NaHS groups. While sperm count was significantly increased (p < 0.001) in CIS+ NaHS compared with CIS. Sperm count between groups control, NaHS and CIS+ NaHS was not statistically significant.
Plasma testosterone
Figure 2 indicates that plasma testosterone concentration was significantly lower (p < 0.01) in the CIS-treated rats (3.78 ng/mL) than in the control (8.42 ng/mL) and NaHS (9.08 ng/mL) groups. However, decreased testosterone levels significantly increased (p < 0.05) with administration of NaHS in CIS+ NaHS group (6.48 ng/mL). Testosterone levels showed no significant difference between NaHS, control, and CIS+ NaHS groups.
Histopathological findings
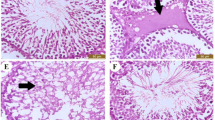
Histological appearances of testicular tissues showed a normal arrangement of germinal epithelium in the control and NaHS groups, whereas CIS injection caused structural changes in testicular tissue such as disorganization, degeneration, desquamation, necrosis and reduction of germinal cells, seminiferous tubules atrophy, thickening in basement membrane of tubules, and interstitial edema. NaHS administration to CIS-treated group caused an amelioration in testicular tissue when compared with the CIS group; however, a little amount of interstitial edema and mild degeneration can be seen in the seminiferous tubules. (Fig. 3a). Also, a significant (p < 0.001) reduction in Johnsen’s testicular score was indicated in the CIS group when compared with the control and NaHS groups. However, NaHS injection to CIS-treated rats significantly (p < 0.001) prevented the decrease induced by CIS in this parameter. There was no difference between the groups of control, NaHS, and CIS+ NaHS (Fig. 3b).
a Microscopic slides of testicular tissues of in different groups. Photomicrograph of the testicular tissue of the control and NaHS groups showing normal seminiferous tubules at all stages of spermatogenic cells and the interstitial tissue filling the space between the seminiferous tubules. Photomicrograph of the testicular tissue of rats treated with CIS indicating abnormal seminiferous tubules (black arrow) degenerative alterations (white arrow) in spermatogenic cells and the detachment of the spermatogenic epithelium. Photomicrograph of the testicular tissue of rats treated with CIS and NaHS indicating a restoration of spermatogenic epithelium in most seminiferous tubules; however, a little amount of interstitial edema and mild degeneration can be seen in the seminiferous tubules, hematoxylin and eosin (× 150). b Johnsen’s testicular score in different groups. All data are expressed as the mean ± SD (n = 8). The CIS group compared with control, the CIS+ NaHS group compared with CIS. ***p < 0.001
Morphometrical analysis
As shown in Table 2, seminiferous tubule diameter, germinal epithelium height, and the mean number of Sertoli cells, spermatogonia, and spermatocytes decreased (p < 0.01), whereas the lumen diameter of the seminiferous tubules increased (p < 0.01) after the CIS treatment compared with those in the control and NaHS groups. But the injection of NaHS along with CIS significantly (p < 0.05) restored the morphometric parameters toward the control value. The morphometric parameters in control, NaHS, and CIS+ NaHS groups were almost identical.
Immunohistochemistry findings
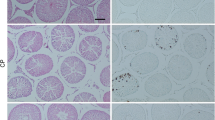
Immunohistochemical analysis revealed a higher expression of IL-1β protein in the CIS group than in the control and NaHS groups. However, expression of IL-1β protein showed a significant decrease in the CIS+ NaHS group when compared with the CIS group (Fig. 4a). Figure 4 b showed that the number of IL-1β-positive cells in the germinal epithelium of CIS-treated group was markedly increased (p < 0.001) when compared with the control and NaHS groups. However, administration of NaHS to animals treated with CIS showed a significant decrease (p < 0.001) in increased IL-1β-positive cell counts due to CIS treatment. There were no significant difference in IL-1β expression between the control, NaHS, and CIS+ NaHS groups.
Effects of NaHS treatment on IL-1β expression in the testes of rats treated with cisplatin (CIS). a Representative microscopic slides of staining from the different groups (× 300). In the control and NaHS groups, very little brown staining was observed. In contrast, in the CIS group, high brown staining within germinal epithelium was seen. The intensity of brown staining was reduced in the CIS+ NaHS group. b immunohistochemical scores were evaluated. All data are expressed as the mean ± SD (n = 8). The CIS group compared with control, the CIS+ NaHS group compared with CIS. ***p < 0.001
Oxidative stress markers
Table 3 shows level of MDA and activity of antioxidant enzymes in testicular tissue. CIS injection induced a significant elevation (p < 0.001) in levels of testicular MDA when compared with the control and NaHS groups but NaHS injection to CIS-treated group significantly decreased (p < 0.001) the level of increased MDA compared with the CIS group. Activity of GSH-Px, SOD, and CAT was significantly (p < 0.01) lower in the CIS-treated group than in the control and NaHS groups. However, this activity in the CIS+ NaHS group was significantly higher (p < 0. 01) than in the CIS-treated group. NaHS administration alone had no significant effect on MDA levels and antioxidant enzyme activities when compared with the control and CIS+ NaHS groups.
Discussion
The results of this study indicate that NaHS provides testicular protection, via reversing CIS-induced testicular oxidative stress and inflammation, and by increasing the activity of antioxidant enzymes.
Many chemotherapy drugs utilized in cancer can cause toxic side effects in different organs. Testicular damage in the male reproductive system is one of the side effects of using these drugs. Spermatogenic cells in testes are targeted by cytotoxic drugs due to their abundant mitotic activity. Spermatogonia injuries cause prolongated sterility or oligozoospermia (Howell and Shalet 2001; Endo et al. 2003). The chance of amelioration of spermatogenesis after cytotoxic injury, and also the degree and rate of amelioration, is associated with the drug and the dose used. CIS is one of the extensively utilized cytotoxic drugs, which disturbs testicular functions and spermatogenesis (Amin et al. 2012; Ateşşahin et al. 2006). The testicular weight and histopathological findings in testes are the most important indexes to detect CIS-induced male reproductive toxicity. According to earlier studies, exposure to CIS reduces body and testes weight (Soni et al. 2016). In current research, the CIS administration alone significantly reduces weight of body and testes comparing to the control group. The body weight loss of CIS-treated rats maybe due to the toxic effects of CIS on the gastrointestinal tract such as, decrease in appetite, ingestion, and assimilation of food (Karimi et al. 2017). Moreover, the testes weight loss may be due to severe testicular parenchymal atrophy and spermatogenic injuries. A decrease in weight of testes of CIS-treated rats shows decreased spermatogenesis and steroidogenesis (Amin et al. 2012). In this study, CIS administration remarkably decreased testosterone hormone level. This can be because of dysfunction of Leydig cells, which produce gonadotropin and decrease activity of mitochondrial side-chain cleavage and cytochrome P-450 (García et al. 2012). Moreover, CIS impacts on the function of Sertoli cells and reduces the androgen-binding proteins expression. Actually, hormonal disorders induced by CIS are mediated by its impacts on the hypothalamic-pituitary gonadal axis (Almeer and Abdel Moneim 2018). Based on the achieved findings, NaHS treatment considerably enhanced body weight, testes weight, and testosterone hormone level by comparing with CIS administration. These effects are probably due to the fact that H2S increases appetite, decreases apoptosis in testicular cells, and increases blood flow to organs (Ahmad et al. 2016; Ning et al. 2018; Zhou et al. 2018).
In present study, significant reduction in sperm count was recorded in the CIS-treated group as compared with control but NaHS treatment in combination with CIS improved sperm count. These outcomes display which treatment with NaHS can protect decrement in sperm production within the seminiferous tubules by reducing the side effects of CIS. As previously reported, cisplatin disrupts spermatogenesis and reduces number and motility of spermatozoa. These effects of CIS on sperm are related to its ability to induce oxidative stress. CIS induces ROS production in the testes and sperms, and caused apoptosis in the germinal epithelium (Jahan et al. 2018). H2S can mitigate the spermatogenic failure via the combination of anti-oxidative and anti-inflammatory properties (Wang et al. 2018).
Histologically, CIS reduces the size and number of the seminiferous tubules and seminiferous epithelial layers. Also, CIS causes degeneration and vacuolation in spermatogonia, spermatocytes, and in a small number of germ cells, and inhibits cellular maturation (Fallahzadeh et al. 2017; Mohammadnejad et al. 2012; Almeer and Abdel Moneim 2018;). An earlier investigation showed that spermatogenic disorder occurred trough interaction with damaged DNA synthesis in stem cells and Sertoli cell dysfunction that were directly generated by anticancer drugs (Nambu and Kumamoto 1995). In current study degeneration, desquamation, disorganization, decrease in the number of Sertoli cells, spermatogonia and spermatocytes, reduce in the thickness of the seminiferous epithelium, diameter of seminiferous tubules, and Johnsen’s testicular score were observed in testicular tissues of CIS-treated rats. These results are in accordance with the previous studies. The injuries found in the structure of testicular tissues in current study have been caused by the direct or indirect impact of CIS; the latter induces lipid peroxidation which is a chemical mechanism that can impair testicular structure and function. However, histological and morphometrical evaluation of the testes in NaHS receiving group indicated a reduction in structural variations caused by CIS. Formation of ROS has a main role in CIS-induced damage but different antioxidants and thiol compounds have been indicated to have a protective role against toxicity induced by CIS. There is evidence to suggest that H2S has potent antioxidant effects in various organs (Karimi et al. 2017). This antioxidant activity of NaHS probably reduced tissue damage.
The current research presented that CIS increased IL-1β expression. Inflammation plays role in CIS-induced tissue toxicity (Zhu et al. 2017). CIS initiates the NF-κB pathway, thereby stimulating the expression of a group of inflammatory cytokines, comprising IL-1β (Eid et al. 2016). NaHS treatment inhibited inflammatory cytokine formation in CIS-induced reproductive toxicity. The results of this work are in accordance with the study of Guo et al. (2013), in which NaHS controlled cardiotoxicity via decreasing the formation of IL-1β, and other pro-inflammatory cytokines.
CIS disturbs the oxidant/antioxidant balance of the testicular tissue (Anand et al. 2015). In this research, CIS considerably elevated MDA and depleted SOD, CAT, and GSH-Px activities in the testes tissue, representing that the molecules of enzymatic antioxidant were insufficient for scavenging free radicals generated by CIS. MDA acts as an indicator for oxidative stress which is produced due to the cellular polyunsaturated fatty acids peroxidation (Chirino and Pedraza-Chaverri 2009). SOD acts as the first preventive antioxidant enzyme, which neutralizes singlet oxygen (1O2) and spontaneously dismutates superoxide radicals (O−2) to H2O2. H2O2 is effectively decomposed by CAT, so suppressing lipid peroxidation. GSH-Px along with GSH catalyzes depletion of H2O2 and lipid peroxides (Zhao et al. 2014). Reduction of these antioxidant enzymes and molecules may be related with an uncontrollable accumulation of H2O2, which inhibits antioxidant defense systems of testicular. Though treating with NaHS weakened oxidative stress of testes and improved the antioxidant defense system in the testicular tissue, representing that NaHS suppresses oxidative stress induced by CIS. As stated, the antioxidant properties of H2S have been well showed earlier. H2S has been presented to exert antioxidant activities via multiple mechanisms comprising direct suppressing of ROS, modulation of GSH cellular levels, and enhancing of antioxidant enzymes expression (Corsello et al. 2018). However, due to some limitations, we were not able to perform any further tests. Therefore, we suggest that further inflammatory factors, apoptotic pathways, and some markers associated with fertility can be done in other studies.
In conclusion, CIS injection in rats induces inflammation and structural alterations in testicular tissues via induction of oxidative stress as determined by increased generation of MDA and reduced activity of antioxidant enzymes. However, NaHS treatment protected the testes against CIS-induced toxicity owing to its anti-inflammatory and antioxidant properties.
References
Abdel-Daim MM, Abushouk AI, Donia T, Alarifi S, Alkahtani S, Aleya L, Bungau SG (2019a) The nephroprotective effects of allicin and ascorbic acid against cisplatin-induced toxicity in rats. Environ Sci Pollut Res Int 26(13):13502–13509. https://doi.org/10.1007/s11356-019-04780-4
Abdel-Daim MM, Aleya L, El-Bialy BE, Abushouk AI, Alkahtani S, Alarifi S, Alkahtane AA, Albasher G, Ali D, Almeer RS, Al-Sultan NK, Alghamdi J, Alahmari A, Bungau SG (2019b) The ameliorative effects of ceftriaxone and vitamin E against cisplatin-induced nephrotoxicity. Environ Sci Pollut Res Int 26(15):15248–15254. https://doi.org/10.1007/s11356-019-04801-2
Abdellatief SA, Galal AA, Farouk SM, Abdel-Daim MM (2017) Ameliorative effect of parsley oil on cisplatin-induced hepato-cardiotoxicity: a biochemical, histopathological, and immunohistochemical study. Biomed Pharmacother 86:482–491. https://doi.org/10.1016/j.biopha.2016.12.038
Abdel-Moneim AE, Othman MS, Ahmed M, Aref AM (2014) Azadirachta indica attenuates cisplatin-induced nephrotoxicity and oxidative stress. Biomed Res Int: Article ID 647131, 11 pages. https://doi.org/10.1155/2014/647131
Ahmad A, Druzhyna N, Szabo C (2016) Delayed treatment with sodium hydrosulfide improves regional blood flow and alleviates cecal ligation and puncture (clp)-induced septic shock. Shock 46(2):183–193. https://doi.org/10.1097/SHK.0000000000000589
Almeer RS, Abdel Moneim AE (2018) Evaluation of the protective effect of olive leaf extract on cisplatin-induced testicular damage in rats. Oxidative Med Cell Longev: Article ID 8487248. 11 pages. https://doi.org/10.1155/2018/8487248
Amin A, Abraham C, Hamza AA, Abdalla ZA, Al-Shamsi SB, Harethi SS, Daoud S (2012) A standardized extract of Ginkgo biloba neutralizes cisplatin-mediated reproductive toxicity in rats. J Biomed Biotechnol: 11 pages, article 362049. https://doi.org/10.1155/2012/362049
Anand H, Misro MM, Sharma SB, Prakash S (2015) Protective effects of Eugenia jambolana extract versus N-acetyl cysteine against cisplatin-induced damage in rat testis. Andrologia 47(2):194–208. https://doi.org/10.1111/and.12247
Ateşşahin A, Sahna E, Türk G, Ceribaşi AO, Yilmaz S, Yüce A, Bulmuş O (2006) Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res 41(1):21–27. https://doi.org/10.1111/j.1600-079X.2006.00327.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Çeribaşı AO, Sakin F, Türk G, Sönmez M, Ateşşahin A (2012) Impact of ellagic acid on adriamycin-induced testicular histopathological lesions, apoptosis, lipid peroxidation and sperm damages. Exp Toxicol Pathol 64(7–8):717–724. https://doi.org/10.1016/j.etp.2011.01.006
Chirino YI, Pedraza-Chaverri J (2009) Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol 61(3):223–242. https://doi.org/10.1016/j.etp.2008.09.003
Corsello T, Komaravelli N, Casola A (2018) Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 7(10):E129. https://doi.org/10.3390/antiox7100129
Eid AH, Abdelkader NF, Abd El-Raouf OM, Fawzy HM, El-Denshary ES (2016) Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Arch Pharm Res 39(12):1693–1702. https://doi.org/10.1007/s12272-016-0833-6
Endo F, Manabe F, Takeshima H, Akaza H (2003) Protecting spermatogonia from apoptosis induced by doxorubicine using the luteinizing hormone-releasing hormone analog leuprorelin. Int J Urol 10:72–77. https://doi.org/10.1046/j.1442-2042.2003.00572.x
Fallahzadeh AR, Rezaei Z, Rahimi HR, Barmak MJ, Sadeghi H, Mehrabi S, Rabani SM, Kashani IR, Barati V, Mahmoudi R (2017) Evaluation of the effect of pentoxifylline on cisplatin-induced testicular toxicity in rats. Toxicol Res 33(3):255–263. https://doi.org/10.5487/TR.2017.33.3.255
Farsani BE, Karimi S, Mansouri E (2018) Pravastatin attenuates testicular damage induced by doxorubicin - a stereological and histopathological study. J Basic Clin Physiol Pharmacol 30(1):103–109. https://doi.org/10.1515/jbcpp-2018-0073
García MM, Acquier A, Suarez G, Gomez NV, Gorostizaga A, Mendez CF, Paz C (2012) Cisplatin inhibits testosterone synthesis by a mechanism that includes the action of reactive oxygen species (ROS) at the level of P450scc. Chem Biol Interact 199(3):185–191. https://doi.org/10.1016/j.cbi.2012.08.012
Guo R, Wu K, Chen J, Mo L, Hua X, Zheng D, Chen P, Chen G, Xu W, Feng J (2013) Exogenous hydrogen sulfide protects against doxorubicin-induced inflammation and cytotoxicity by inhibiting p38MAPK/NFκB pathway in H9c2 cardiac cells. Cell Physiol Biochem 32(6):1668–1680. https://doi.org/10.1159/000356602
Howell SJ, Shalet SM (2001) Testicular function following chemotherapy. Hum Reprod Update 7:363–369. https://doi.org/10.1093/humupd/7.4.363
Jahan S, Munawar A, Razak S, Anam S, Ul Ain Q, Ullah H, Afsar T, Abulmeaty M, Almajwal A (2018) Ameliorative effects of rutin against cisplatin-induced reproductive toxicity in male rats. BMC Urol 18:107. https://doi.org/10.1186/s12894-018-0421-9
Karimi A, Absalan F, Khorsandi L, Valizadeh A, Mansouri E (2017) Sodium hydrogen sulfide (NaHS) ameliorates alterations caused by cisplatin in filtration slit diaphragm and podocyte cytoskeletal in rat kidney. J Nephropathol 6(3):150–156. https://doi.org/10.15171/jnp.2017.26
Khorsandi L, Orazizadeh M, Moradi-Gharibvand N, Hemadi M, Mansouri E (2017) Beneficial effects of quercetin on titanium dioxide nanoparticles induced spermatogenesis defects in mice. Environ Sci Pollut Res Int 24(6):5595–5606. https://doi.org/10.1007/s11356-016-8325-2
Kimura H (2013) Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem Int 63:492–497. https://doi.org/10.1016/j.neuint.2013.09.003
Kimura H (2014) The physiological role of hydrogen sulfide and beyond. Nitric Oxide 41:4–10. https://doi.org/10.1016/j.niox.2014.01.002
Li L, Bhatia M, Moore PK (2006) Hydrogen sulphide-a novel mediator of inflammation? Curr Opin Pharmacol 6:125–129. https://doi.org/10.1016/j.coph.2005.10.007
Li G, Xie ZZ, Chua JM, Wong PC, Bian J (2015) Hydrogen sulfide protects testicular germ cells against heat-induced injury. Nitric Oxide 46:165–171. https://doi.org/10.1016/j.niox.2014.10.005
Liu Z, Han Y, Li L, Lu H, Meng G, Li X, Shirhan M, Peh MT, Xie L, Zhou S, Wang X, Chen Q, Dai W, Tan CH, Pan S, Moore PK, Ji Y (2013) The hydrogen sulfide donor, GYY4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E(−/-) mice. Br J Pharmacol 169:1795–1809. https://doi.org/10.1111/bph.12246
Lobb I, Zhu J, Liu W, Haig A, Lan Z, Sener A (2014) Hydrogen sulfide treatment ameliorates long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Can Urol Assoc J 8:E413–E418. https://doi.org/10.5489/cuaj.1694
Mansouri E (2018) Effects of sodium hydrogen sulfide (a H2S donor) on acute kidney injury. J Nephropathol 7(1):1–3. https://doi.org/10.15171/jnp.2018.01
Mehrzadi S, Fatemi I, Malayeri AR, Khodadadi A, Mohammadi F, Mansouri E, Rashno M, Goudarzi M (2018) Ellagic acid mitigates sodium arsenite-induced renal and hepatic toxicity in male wistar rats. Pharmacol Rep 70(4):712–719. https://doi.org/10.1016/j.pharep.2018.02.007
Mercantepe T, Unal D, Tümkaya L, Yazici ZA (2018) Protective effects of amifostine, curcumin and caffeic acid phenethyl ester against cisplatin-induced testis tissue damage in rats. Exp Ther Med 15(4):3404–3412. https://doi.org/10.3892/etm.2018.5819
Mohammadnejad D, Abedelahi A, Soleimani-rad J, Mohammadi-roshandeh A, Rashtbar M, Azami A (2012) Degenerative effect of cisplatin on testicular germinal epithelium. Adv Pharm Bull 2(2):173–177. https://doi.org/10.5681/apb.2012.026
Nambu A, Kumamoto Y (1995) Effect of follicle-stimulating hormone (FSH) on protection or acceleration to recovery from spermatogenic damage induced by anti-cancer agents. (In Japanese). Nihon Hinyokika Gakkai Zasshi 86(7):1231–1239
Ning JZ, Li W, Cheng F, Rao T, Yu WM, Ruan Y, Yuan R, Zhang XB, Du Y, Xiao CC (2018) The protective effects of GYY4137 on ipsilateral testicular injury in experimentally varicocele-induced rats. Exp Ther Med 15(1):433–439. https://doi.org/10.3892/etm.2017.5417
Reddy KP, Madhu P, Reddy PS (2016) Protective effects of resveratrol against cisplatin-induced testicular and epididymal toxicity in rats. Food Chem Toxicol 91:65–72. https://doi.org/10.1016/j.fct.2016.02.017
Sherif IO, Abdel-Aziz A, Sarhan OM (2014) Cisplatin-induced testicular toxicity in rats: the protective effect of arjunolic acid. J Biochem Mol Toxicol 28(11):515–521. https://doi.org/10.1002/jbt.21593
Shokrzadeh M, Ahmadi A, Chabra A, Naghshvar F, Salehi F, Habibi E, Haghi-Aminjan H (2014) An ethanol extract of Origanum vulgare attenuates cyclophosphamide-induced pulmonary injury and oxidative lung damage in mice. Pharm Biol 52(10):1229–1236. https://doi.org/10.3109/13880209.2013.879908
Soni KK, Kim HK, Choi BR, Karna KK, You JH, Cha JS, Shin YS, Lee SW, Kim CY, Park JK (2016) Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: reactive oxygen species and endoplasmic reticulum stress. Drug Des Devel Ther 10:3959–3968. https://doi.org/10.2147/DDDT.S120014
Sugiura Y, Kashiba M, Maruyama K, Hoshikawa K, Sasaki R, Saito K, Kimura H, Goda N, Suematsu M (2005) Cadmium exposure alters metabolomics of sulfur-containing amino acids in rat testes. Antioxid Redox Signal 7:781–787. https://doi.org/10.1089/ars.2005.7.781
Wang J, Wang W, Li S, Han Y, Zhang P, Meng G, Xiao Y, Xie L, Wang X, Sha J, Chen Q, Moore PK, Wang R, Xiang W, Ji Y (2018) Hydrogen sulfide as a potential target in preventing spermatogenic failure and testicular dysfunction. Antioxid Redox Signal 28(16):1447–1462. https://doi.org/10.1089/ars.2016.6968
Yokoi K, Mayi ZK (2004) Organ apoptosis with cytotoxic drugs. Toxicology 290:78–85
Yoshida A, Miura K, Shirai M (1997) Evaluation of seminiferous tubule scores obtained through testicular biopsy examinations of nonobstructive azoospermic men. Fertil Steril 68(3):514–518. https://doi.org/10.1016/s0015-0282(97)00239-2
Zhao YM, Gao LP, Zhang HL, Guo JX, Guo PP (2014) Grape seed proanthocyanidin extract prevents DDP-induced testicular toxicity in rats. Food Funct 5(3):605–611. https://doi.org/10.1039/c3fo60486a
Zhou J, Lv XH, Fan JJ, Dang LY, Dong K, Gao B, Song AQ, Wu WN (2018) GYY4137 promotes mice feeding behavior via arcuate nucleus sulfur-sulfhydrylation and ampk activation. Front Pharmacol 9:966. https://doi.org/10.3389/fphar.2018.00966
Zhu X, Jiang X, Li A, Zhao Z, Li S (2017) S-Allylmercaptocysteine attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation. Nutrients 9(2):E166. https://doi.org/10.3390/nu9020166
Funding
This research project was supported by a grant from the Deputy of Research and Technology Development of the Ahvaz Jundishapur University of Medical Sciences (Grant No. 96s62).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were done in accordance with the ethics committee of Ahvaz Jundishapur University of Medical Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Mohamed Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azarbarz, N., Shafiei Seifabadi, Z., Moaiedi, M.Z. et al. Assessment of the effect of sodium hydrogen sulfide (hydrogen sulfide donor) on cisplatin-induced testicular toxicity in rats. Environ Sci Pollut Res 27, 8119–8128 (2020). https://doi.org/10.1007/s11356-019-07266-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07266-5