Abstract
Sheer enormity of lignocellulosics makes them potential feedstock for biofuel production but, their conversion into fermentable sugars is a major hurdle. They have to be pretreated physically, chemically, or biologically to be used by fermenting organisms for production of ethanol. Each lignocellulosic substrate is a complex mix of cellulose, hemicellulose and lignin, bound in a matrix. While cellulose and hemicellulose yield fermentable sugars, lignin is the most recalcitrant polymer, consisting of phenyl-propanoid units. Many microorganisms in nature are able to attack and degrade lignin, thus making access to cellulose easy. Such organisms are abundantly found in forest leaf litter/composts and especially include the wood rotting fungi, actinomycetes and bacteria. These microorganisms possess enzyme systems to attack, depolymerize and degrade the polymers in lignocellulosic substrates. Current pretreatment research is targeted towards developing processes which are mild, economical and environment friendly facilitating subsequent saccharification of cellulose and its fermentation to ethanol. Besides being the critical step, pretreatment is also cost intensive. Biological treatments with white rot fungi and Streptomyces have been studied for delignification of pulp, increasing digestibility of lignocellulosics for animal feed and for bioremediation of paper mill effluents. Such lignocellulolytic organisms can prove extremely useful in production of bioethanol when used for removal of lignin from lignocellulosic substrate and also for cellulase production. Our studies on treatment of hardwood and softwood residues with Streptomyces griseus isolated from leaf litter showed that it enhanced the mild alkaline solubilisation of lignins and also produced high levels of the cellulase complex when growing on wood substrates. Lignin loss (Klason lignin) observed was 10.5 and 23.5% in case of soft wood and hard wood, respectively. Thus, biological pretreatment process for lignocellulosic substrate using lignolytic organisms such as actinomycetes and white rot fungi can be developed for facilitating efficient enzymatic digestibility of cellulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The important drivers for the increased interest, globally, in non-petroleum based sources of energy, the so-called alternative fuels, are increasing demand for energy, fast depleting resources and increasing problem of CO2 emissions. A first-generation of fuels and chemicals is being produced from high-value sugars and oils. Meanwhile, a second-generation, based on cheaper and more abundant lignocellulosic biomass is being developed.
By comparison to feedstock for first-generation biofuels, lignocellulosic biomass is generally (a) not edible and therefore does not compete directly with food production; (b) can be bred specifically for energy purposes, thereby enabling higher production per unit land area; and (c) represents more of the above-ground plant material, thereby further increasing land-use efficiency [1]. Lignocellulosic biomass is, therefore, considered as the only foreseeable, feasible and sustainable resource for renewable fuel. Agricultural wastes such as crop residues, food processing wastes and forestry residues are potential sources of sugars. Annual production of biomass is estimated to be 1 × 1010 MT worldwide [2]. However, an accurate estimation of biomass availability in India is non-existent and the only statistics that are available are on agricultural production. According to a recent survey on the generation and availability of various biomass residues employed by the NIIST (National Institute for Interdisciplinary Science and Technology), the major agro-residues in terms of volumes generated (in million metric tons—MMT) were found to be rice straw (112), rice husk, (22.4) wheat straw (109.9), sugarcane tops (97.8) and bagasse (101.3) (Fig. 1) [3].
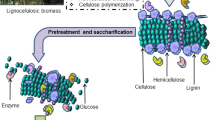
Biomass contains about 40–50% cellulose, a glucose polymer; 25–35% hemicellulose, a sugar heteropolymer; 15–20% lignin, a non-fermentable phenyl-propane unit; plus lesser amounts of minerals, oils, soluble sugars, and other components [4]. The relative proportions of these three components in lignocellulosic feedstock vary, depending on the species involved. The technological outline developed for the production of fuel from lignocellulose involves pretreatment of biomass, enzymatic hydrolysis of polysaccharides into monomeric sugars, and their fermentation to alcohol. Cellulose and hemicellulose, being polysaccharides, can be hydrolysed into sugars. Lignin is not a polysaccharide and is not fermented to produce liquid biofuels, but instead can be recovered and used as a fuel for heat and electricity at an ethanol production facility [1].
The main technological hurdles in the conversion of biomass to ethanol are:
-
i.
Separation of lignin from cellulose and hemicellulose to make them susceptible to hydrolysis
-
ii.
Optimization of hydrolysis of cellulose and hemicellulose that takes place at different rates and temperatures
-
iii.
Fermentation of the complex variety of sugars, some of which cannot be fermented by standard yeasts that are used in grain ethanol industry; most of the pentoses from hemicellulose are particularly difficult to ferment to ethanol by industrial strains
-
iv.
Improvement of fermenting yeasts or bacterial strains in terms of their ethanol tolerance, pH and temperature tolerance and expansion of their substrate range
A pretreatment step is essential to effectively prepare cellulose for enzymatic hydrolysis with high yields. A wide range of thermal, mechanical and chemical pretreatment methods and combinations thereof have been reported. Recently, the environment friendly approach of microbial pretreatment has received renewed attention for enhancing enzymatic saccharificaton of lignocellulosic biomass. This method employs micro-organisms, mainly white and soft-rot fungi, actinomycetes, and bacteria which degrade lignin, the most recalcitrant polymer in biomass.
Biological delignification, when integrated with solid-state culture technology to produce and use a low-cost cellulase on specific lignocellulosic materials, would result in a low-cost biological technology which integrates lignin removal, cellulose hydrolysis, and fermentation to convert lignocellulose to alcohol [5].
Major Challenges in Conversion of Biomass to Ethanol
Lignocellulosic biomass as substrate for bioethanol presents a different set of challenges as compared to the molasses or starch based alcohol production [3]. First, the cellulose and hemicellulose portions of the biomass must be broken down into sugars and a variety of pretreatments are required to carry out this saccharification step in an efficient and low-cost manner. Second, these sugars, which are a complex mixture of 5-carbon and 6-carbon sugars, must be fermented to make bioethanol. Low efficiency due to the natural recalcitrance of lignocellulose to deconstruction and high cost of enzymatic conversion, therefore, form the major bottlenecks in this technology.
The Natural Recalcitrance of Lignocellulose
It is one of the key factors preventing enzymatic conversion of lignocellulosic substrates to fermentable sugars. The factors that contribute to the recalcitrance of lignocellulose to enzymatic conversion include the following [6]:
Lignin and Hemicellulose Contents
The presence of lignin and hemicellulose makes the access of cellulase enzymes to cellulose difficult [7], thus reducing the efficiency of hydrolysis. Lignin acts as a physical barrier, preventing the digestible parts of the substrate from being hydrolysed, and binds non-productively to the cellulolytic enzymes [8]. The presence of hemicellulose reduces the mean pore size of the substrate and therefore reduces the accessibility of cellulose to hydrolytic enzymes.
Cellulose Crystallinity
The degree of polymerization and cellulose crystallinity have been considered as important factors in determining the hydrolysis rates of relatively refined cellulosic substrate [9]. Crystallinity of cellulose, along with the glucan chain length, makes the hydrolysis process difficult.
Accessible Surface Area
The effect of this area may correlate with crystallinity or lignin protection or hemicellulose presentation or all of them [10].
Porosity
The pore size of the substrate in relation to the size of the enzymes is another limiting factor in the enzymatic hydrolysis of lignocellulosic biomass. Cellulases can get trapped in the pores if the internal area is much larger than the external area which is the case for many lignocellulosic materials [11].
Thus, it is necessary to remove the lignin and hemicellulose, decrease the crystallinity of cellulose, increase the accessible surface area and porosity by pretreatment technology for achieving acceptable enzymatic digestibility.
Enzyme Cost
Higher amounts and different types of enzymes are required to achieve high sugar yields from both cellulose and hemicellulose fractions, thus the enzymatic saccharification of cellulose incur high cost. In this context, development of cellulases and other accessory enzymes needed for complete degradation of lignocellulose components is an important issue. New balanced enzymatic complexes containing optimal combinations to effectively modify the complex structure of lignocellulosic materials are to be developed [6].
Pretreatment Technologies for Lignocellulosic Biomass
Pre-treatment of biomass promotes disruption of the lignocellulosic matrix. An effective and economical pretreatment should meet the following requirements according to Taherzadeh and Karimi [10].
-
(a)
Avoiding destruction of hemicelluloses and cellulose
-
(b)
Avoiding formation of possible inhibitors for hydrolytic enzymes and fermenting microorganisms and production of less residues
-
(c)
Minimizing the energy demand
-
(d)
Reducing the cost of size reduction for feedstocks and cost of material for construction of pretreatment reactors
-
(e)
Consumption of little or no chemical and using a cheap chemical
A multitude of different pretreatment technologies have been developed during the last decades. They can be classified into physical, chemical and physico-chemical, according to the different forces of energy applied in the pretreatment process. Alvira et al. [6] describes these processes in detail, a list of which is given below.
-
A.
Physical Pretreatments
-
(1)
Mechanical Comminution The objective is a reduction in particle size and crystallinity of lignocellulose in order to increase the surface area and reduce the degree of polymerization. Methods of chipping, grinding and milling [12] can be used to improve the enzymatic hydrolysis. However, taking into account the high energy requirements, this process is not economically feasible.
-
(2)
Extrusion It disrupts the lignocellulose structure and increases the accessibility of carbohydrates to enzyme attack. The materials are subjected to heating, mixing and shearing resulting in physical and chemical modifications [13]. However, the process is novel and not widely applied.
-
B.
Chemical Pretreatments
-
(1)
Alkali Pretreatment Alkali pretreatments increase cellulose digestibility by enhancing lignin solubilisation. Sodium, calcium and ammonium hydroxides are suitable for the process. NaOH causes swelling, increasing the internal surface of cellulose and decreasing the degree of polymerization and crystallinity, which provokes lignin disruption. Lime pretreatment [14] removes amorphous substances. Lignin removal increases enzyme effectiveness by reducing non-productive adsorption sites for enzymes and by increasing cellulose accessibility. This method is more effective on agricultural residues than on wood materials [15].
-
(2)
Acid Pretreatment The main objective is to solubilise the hemicellulose fraction of the biomass and to make the cellulose more accessible to enzymes. This can be performed with concentrated or diluted acid [16] but utilization of concentrated acid is less attractive due to the formation of inhibiting compounds, equipment corrosion, and high operational and maintenance costs [17]. Dilute acid pretreatment is probably the most commonly applied method among the chemical pretreatment methods. It can be used either as a pretreatment of lignocellulose for enzymatic hydrolysis, or as the actual method of hydrolysing to fermentable sugars [10]. High rate of hydrolysis is obtained with dilute acids, but they generate toxic degradation products.
-
(3)
Ozonolysis Ozone is a powerful oxidant that shows high delignification efficiency. The pretreatment is done at room temperature and normal pressure and does not lead to the formation of inhibitory compounds. An important drawback is the large amounts of ozone needed, which can make the process economically unviable [18].
-
(4)
Organosolv Process Organosolv process [19] achieves high lignin removal and minimum cellulose loss. Numerous organic or aqueous solvent mixtures can be utilized, including methanol, ethanol, acetone, ethylene glycol and tetrahydrofurfuryl alcohol, in order to solubilise lignin and enable the recovery of relatively pure lignin [20]. But, the high commercial prices of solvents hinder their industrial applications.
-
C.
Physico-Chemical Pretreatments
-
(1)
Steam Explosion It is a hydrothermal pretreatment in which the biomass is subjected to pressurised steam [21] for a period ranging from seconds to several minutes, and then suddenly depressurized. In combination with the partial hemicellulose hydrolysis and solubilisation, the lignin is redistributed and to some extent removed from the material [22]. Though the process is cost effective, it generates toxic compounds and the hemicellulose degradation is partial [23].
-
(2)
Ultrasound Pretreatment The effect of ultrasound on lignocellulosic biomass has been employed for extracting hemicelluloses, cellulose and lignin but less research has been addressed to study the susceptibility of lignocellulosic materials to hydrolysis [24].
-
(3)
CO 2 Explosion The method is based on the utilization of CO2 as a supercritical fluid [25] so that lignin is removed effectively thereby increasing substrate digestibility. The disadvantage of the method is the very high pressure requirements.
-
(4)
Liquid Hot Water (LHW) Pretreatment It utilizes pressurized hot water [26] at pressure less than 5 Mpa and temperature range of 170–230°C for several minutes followed by decompression up to atmospheric pressure. The solubilised hemicellulose and lignin are present in low concentrations, however, the water and energy demand are high.
-
(5)
Ammonia Fibre Explosion (AFEX) Biomass is treated with liquid anhydrous ammonia at 60–100°C and high pressure for varying periods of time [27, 28]. The pressure is then released causing a rapid expansion of the gas that result in swelling and physical disruption of biomass fibres and partial decrystallization of cellulose. However, the process does not work for raw materials with high lignin content [29].
-
D.
Thermo-Chemical Processes
The thermo-chemical processes generally use much higher temperatures and pressures, like gasification (where the biomass is converted into synthesis gas, also called syngas, which is a mixture of hydrogen and carbon monoxide), or pyrolysis (heating of organic material in the absence of oxygen). These methods are used to produce a wider variety of fuels than biochemical conversion processes [1].
Biological Pretreatment
All the above mentioned pretreatments employ methods which are harsh and cost/energy intensive. On the contrary, biological pretreatment processes are mild and environment friendly. They employ micro-organisms, mainly white and soft-rot fungi, actinomycetes, and bacteria which degrade lignin, the most recalcitrant polymer in biomass, through the action of lignin-degrading enzymes such as peroxidases and laccases.
Phanerochaete chrysosporium has been the model organism for studies of lignin degradation by white rot fungi [30]. Fungi breakdown lignin anaerobically through the use of a family of extracellular enzymes collectively termed “lignases” [31]. Two families of lignolytic enzymes are widely considered to play a key role in the enzymatic degradation: phenol oxidase (laccase) and peroxidases (lignin peroxidase, LiP and manganese peroxidase, MnP) [32, 33]. Other enzymes whose roles have not been fully elucidated include glyoxal oxidase, glucose oxidase, oxido-reductase and methanol oxidase [34].
Laccases, belonging to the blue copper oxidase enzyme family, are similar to other phenol-oxidizing enzymes which preferably polymerize lignin by coupling of the phenoxy radicals produced from oxidation of lignin phenolic groups [35]. The importance of laccase in lignin degradation is attributed to its ability to oxidize non-phenolic lignin model compounds via certain redox mediators. Laccase was demonstrated to be present in fungi for the first time by both Bertrand and Laborde in 1896 [35]. Recently some bacterial laccases have also been characterized from Azospirillum lipoferum, Bacillus subtilis etc. [35]. LiP catalyzes the cleavage of β-o-4 ether bonds and of Cα–Cβ bonds in lignin, thereby causing depolymerization of lignin [34]. It also catalyzes hydroxylation, quinine formation, and aromatic ring cleavage. MnP is a Mn(II) and H2O2 dependent oxidase which oxidizes lignin, phenols, phenolic lignin model compounds, and high molecular weight chlorolignins [34]. The combination in white rot fungi, of laccase with either LiP and/or MnP seems to be a more common combination of enzymes than the LiP/MnP pattern found in Phanerochaete chrysosporium [34].
Trichoderma reesei was one of the first cellulolytic organisms isolated in 1950s. By 1976, an impressive collection of more than 14,000 fungi which were active against cellulose and other insoluble fibres were collected [36]. Trichoderma reesei, though a good producer of hemi-and cellulolytic enzymes, is unable to degrade lignin.
Some actinomycetes were studied for their role in lignin biodegradation [37]. These degraded lignin into low molecular weight fragments. Fungal peroxidases, ligninase and manganese peroxidase which have been implicated in the biodegradation of lignin were discovered in Phanerochaete chrysosporium [38, 39]. Based on this, biological delignification of wood and paddy straw for ethanol production using Phanerochaete chrysosporium was taken up [5]. But, the extent of delignification was insufficient to expose a significant fraction of cellulose for enzymatic hydrolysis.
Microbial pretreatment has been previously explored to upgrade lignocellulosic materials for feed and paper applications. In bio-pulping where lignocellulolytic enzymes were used, tensile, tear and burst indices of the resultant paper improved, brightness of pulp was increased with an improved energy saving of 30–38% [40].
The industrial scale implementation of lignocellulose-based biotechnologies utilizing the ability of an appropriate microorganism to selectively degrade lignin was realized when Malherbe and Cloete [33] reiterated that the primary objective of lignocellulose treatment by the various industries is to access the potential of the cellulose encrusted by lignin within the lignocellulose matrix. Studies have shown that Lentinus edodes [41], Pleurotes spp. [42], Penicillium camemberti [43] grown at 25–35°C for 3–22 days resulted in 45–75% and 65–80% holocellulose and lignin degradation, respectively.
Recent studies by Kuhar et al. [44] have shown that fungal pretreatment of wheat straw for 10 days with a high lignin-degrading and low cellulose producing fungal isolate, RCK-1, resulted in a reduction in acid loading for hydrolysis, an increase in the release of fermentable sugars and a reduction in the concentration of fermentation inhibitors. Ethanol yield and volumetric productivity from RCK-1 treated wheat straw (0.48 g/g and 0.54 g/lh, respectively) were higher than the untreated wheat straw (0.36 g/g and 0.30 g/lh, respectively).
An evaluation of biological pretreatment of sugarcane trash using eight different bacteria and fungi was performed on the basis of quantitative changes in the components of the sugarcane trash, production of the cellulase enzyme complex, total protein and release of reducing sugars by different bioagents as well as the interaction among different chemical parameters affecting the pretreatment. In this case, the microbial pretreatment of trash increased accessibility of sugars for enzymatic hydrolysis [45].
Jian et al. [46] investigated the potential of microbial pretreatment of cotton stalks by Phanerochaete chrysosporium to degrade lignin and facilitate fuel ethanol production under two culture conditions: submerged cultivation and solid-state cultivation. In this study, the fungal pretreatment of cotton stalks by Phanerochaete chrysosporium showed significant lignin and hemicellulose degradation when compared with untreated stalks. However, the main challenge of fungal pretreatment was found to be the improvement in selectivity for preferential lignin degradation by applying cellulase-deficient or non-cellulose utilizing white rot fungi, thus preserving more cellulose.
Xu et al. [47] reported that the total sugar yield of rice hull after the combination pretreatment of hydrogen peroxide treatment and fungal treatment was higher than that after the sole pretreatment. The effect of a 15 day bio-treatment with the white rot fungus, Irpex lacteus CD2 on sodium hydroxide pretreatment of cornstalks under mild reaction condition was also investigated [47]. It was found that the biotreatment did not have a large effect on components of cornstalk, but it enhanced significantly delignification and xylan loss during the mild alkaline pretreatment. This further suggested that the synergistic effects of the pretreatments resulted in significant reduction in the recalcitrance of cornstalks to enzymatic deconstruction in comparison with the sole alkaline pretreatment, thus improving the enzymatic digestibility of glucan.
An evaluation of biological pretreatment methods, using various micro-organisms on different lignocellulosic substrates and the resultant delignification and enhancement in digestibility has been summarized in the Table 1.
Studies conducted on solid-state fermentation of hardwood and softwood substrates at 37°C by Streptomyces griseus B1, isolated from leaf litter, caused much higher loss of Klason lignin content than uninoculated substrate which showed hardly any loss when treated with 0.1 N alkali for 2 h. Hardwood substrates showed higher lignin loss (23.4%) after inoculation with S. griseus as compared to softwood substrates which showed 10.5% loss. The organism also chemically modified the lignins but it did not use simple lignin monomers as carbon/energy source [56].
Thus, from the reports available, it is evident that white rot fungi and actinomycete can be used to remove lignin from lignocellulosic substrates, and further studies are required to shorten the incubation time and to optimise the delignification process. The importance of enzymatic hydrolysis has increased the focus on search for high cellulase-producing organisms; the production of hypercellulolytic mutants of organisms suitable for cellulase production; genetic modification to develop high cellulase-producing organisms with high specific activity; and theoretical studies on the mechanism of action of a multi-enzyme system on a complex polymer [57].
Economic Evaluation
Although, huge information about the different pretreatment methods has been reported, few references exist on the economic aspects of these methods. Eggeman and Elander performed process and economic analysis of pretreatment technologies and concluded that there is little differentiation between the projected economic performances of various physical and chemical pretreatment options, as the low-cost pretreatment reactors in some processes counterbalanced with pretreatment catalyst recovery or higher costs of ethanol product recovery [58].
Biological delignification processes are being developed for their integration in biomass to ethanol process. Solid-state fermentation is the method of choice for biological delignification. Capital and operating costs for solid-state fermentation can be kept low, and the lignocellulosic substrate is likely to be the major component of the cost of the delignified product. Experience in the operation of biological delignification processes at pilot plant or larger scale is needed to establish realistic process costs [59].
Conclusion
Although, the properties of the cellulase enzyme complex have a significant effect on how effectively a lignocellulosic material will be hydrolysed, it is the biomass pretreatment and the intrinsic structure/composition of the substrate itself that are primarily responsible for its subsequent hydrolysis by cellulases. The conditions employed in the chosen pretreatment will affect various substrate characteristics, which in turn govern the susceptibility of the substrate to hydrolysis by cellulases and the subsequent fermentation of the released sugars. Choosing the appropriate pretreatment is frequently a compromise between minimizing the degradation of the hemicellulose and cellulose components while maximizing the ease of hydrolysis of the cellulosic substrate. Therefore, treatment of lignocellulosic substrate with lignin-degrading and low cellulase producing organisms can effectively render cellulose in lignocellulosic substrate vulnerable to hydrolysis with cellulases. The effectiveness of pretreatment affects both the up-stream selection of biomass, the efficiency of recovery of the overall cellulose, hemicellulose and lignin components and the chemical and morphological characteristics of the resulting cellulosic component, which governs downstream hydrolysis and fermentation.
Microbial pretreatment offers advantages such as low capital cost, low energy, little dependence of chemicals, and mild environmental conditions. However, the main drawback is the low hydrolysis rate obtained in most biological processes as compared to other technologies. To move forward, a cost-competitive biological pretreatment of lignocellulose and improved hydrolysis leading eventually to improved fuel yields, there is a need to keep on studying and testing more micro-organisms for their ability to delignify the plant material quickly and efficiently.
Although, some pilot plants for production of biofuels exist currently, second-generation biofuels still remain a product of the future. Larson [1] predicts that substantial commercial production using biochemical processing will only begin in the next decade.
References
Larson ED (2008) Biofuel production technologies: status, prospects and implications for trade and development. Paper presented at the United Nations conference on trade and development (UNCTAD) 2008
Sánchez OJ, Cardona CA (2008) Trends in biological production of fuel ethanol from different feedstocks. Bioresour Technol 99:5270–5295
Sukumaran RK, Surender VJ, Sindhu R, Binod P, Janu KU, Sajna KV, Rajasree KP, Pandey A (2010) Lignocellulosic ethanol in India: prospects, challenges and feedstock availability. Bioresour Technol 101:4826–4833
Holtzapple MT (1993) Cellulose, hemicelluloses, and lignin. In: Macrae R, Robinson RK, Sadler MJ (eds) Encyclopedia of food science, food technology, and nutrition. Academic Press, London
Bradley C, Wood P, Kearns R, Black B (1989) Biological delignification of wood and straw for ethanol production via solid state culture. Final Report, Montana Department of Natural Resources and Conservation, Montana, 1989
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Mansfield SD, Mooney C, Saddler JN (1999) Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol Prog 15:804–816
Esteghlalian AR, Srivastava V, Gilkes N, Gregg DJ, Saddler JN (2001) An overview of factors influencing the enzymatic hydrolysis of lignocellulosic feedstocks. In: Himmel ME, Baker W, Saddler JN (eds) Glycosyl hydrolases for biomass conversion. ACS, Washington, DC, pp 100–111
Chang VS, Holtzapple M (2000) Fundamental factors affecting biomass reactivity. Appl Biochem Biotechnol 84–86:5–37
Taherzadeh JM, Karimi K (2008) Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci 9:1621–1651
Chandra RP, Bura R, Mabee WE, Berlin A, Pan X, Saddler JN (2007) Substrate pretreatment: the key to effective enzymatic hydrolysis of lignocellulosics? Adv Biochem Eng Biotechnol 108:67–93
Tassinari T, Macy C, Spano L, Ryu DDY (1980) Energy requirements and process design considerations in compression-milling pretreatment of cellulosic wastes for enzymatic hydrolysis. Biotechnol Bioeng 22:1689–1705
Karunanithy C, Muthukumarappan K and Julson JL (2008) Influence of high shear bioreactor parameters on carbohydrate release from different biomasses. American Society of Agricultural and Biological Engineers Annual International Meeting 2008. ASABE 084114. ASABE, St. Joseph
Playne MJ (1984) Increased digestibility of bagasse by pretreatment with alkalis and steam explosion. Biotechnol Bioeng 26:426–433
Kumar R, Wyman CE (2009) Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol Prog 25:302–314
Knappert H, Grethlein H, Converse A (1981) Partial acid hydrolysis of poplar wood as a pretreatment for enzymatic hydrolysis. Biotechnol Bioengineering Symp 11:67–77
Wyman CE (1996) Handbook on bioethanol: production and utilization. Taylor Francis, Washington, p 417
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Wood TM, Saddler JN (1988) Increasing the availability of cellulose in biomass materials. Methods Enzymol 160:3–11
Zhao H, Cheng K, Liu D (2009) Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl Microbiol Biotechnol 82:815–827
Delong EA (1981) Methods for rendering lignin separable from cellulose and hemicelluloses and the product so formed. Canadian Patent 1096374
Pan X, Xie D, Gilkes N, Gregg DJ, Saddler JN (2005) Strategies to enhance the enzymatic hydrolysis of pretreated softwood with high residual lignin content. Appl Biochem Biotechnol A 124:1069–1079
Oliva JM, Sáez F, Ballesteros I, Gónzalez A, Negro MJ, Manzanares P, Ballesteros M (2003) Effects of lignocellulosic degradation compounds from steam explosion pretreatment on ethanol fermentation by thermotolerant yeast Kluyveromyces marxianus. Appl Microbiol Biotechnol 105:141–154
Sun RC, Tomkinson RC (2002) Characterization of hemicelluloses obtained by classical and ultrasonically assisted extractions from wheat straw. Carbohydr Polym 50:263–271
Zheng Y, Lin HM, Tsao GT (1998) Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol Prog 14:890–896
Bobleter O, Cocin R (1979) Degradation of poplar lignin by hydrothermal treatment. Cellul Chem Technol 13:583–593
Dale BE (1986) Method for increasing the reactivity and digestibility of cellulose with ammonia. US patent no. 4600590
Holtzapple MT, Jun J-H, Ashok G, Patibandla SL, Dale BE (1991) The ammonia freeze explosion process: a practical lignocellulose pretreatment. Appl Biochem Biotechnol 28–29:59–74
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY (2005) Coordinated development of leading biomass pretreatment technologies. Bioresour Technol 96:1959–1966
Kirk TM, Farrell R (1987) Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol 41:465–505
Howard RL, Abotsi E, Jansen van Rensberg EL, Howard S (2003) Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr J Biotechnol 2:602–619
Krause DO, Denman SE, Mackie RI (2003) Opportunities to improve fibre degradation in the rumen: microbiology, ecology, and genomics. FEMS Microbiol Rev 797:1–31
Malherbe S, Cloete TE (2003) Lignocellulosic biodegradation: fundamentals and applications: a review. Environ Sci Biotechnol 1:105–114
Eriksson K-EL (2000) Lignocellulose, lignin, ligninases. In: Schaechter M (ed) Encyclopedia of microbiology, vol 3. Academic press, San Diego, pp 39–48
Kunamneni A, Ballesteros A, Plou FJ, Alcalde M (2007) Fungal laccase—a versatile enzyme for biotechnological applications. In: Mendez-Vilas A (ed) Communicating current research and educational topics and trends in applied microbiology. Formatex Research Center, Badajoz, pp 223–245
Mandels M, Sternberg D (1976) Recent advances in cellulase technology. Fermentation technology 54:267–286
Crawford DL, Barder MJ, Pometto AL III, Crawford RL (1982) Chemistry of softwood lignin degradation by Streptomyces viridosporus. Arch Microbiol 131:140–145
Glenn KJ, Gold HM (1983) Decolorization of several polymeric dyes by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol 45:1741–1747
Kirk TK, Tien M, Johnsrud SC, Eriksson KE (1986) Lignin degrading activity of Phanerochaete chrysosporium Burds: comparison of cellulase-negative and other strains. Enzym Microbial Technol 8:75–80
Scott GM, Aktar M, Lentz MJ (1998) New technology for paper making: commercalising biopulping. Tappi J 81:220–225
Songulashvili G, Elisashvili V, Penninckx M, Metreveli E, Hadar Y, Aladashvili N, Asatiani M (2005) Bioconversion of plant raw materials in value-added products by Lentinus edodes (Berk.) Singer and Pleurotus spp. Int J Med Mushrooms 7(3):467–468
Ragunathan R, Swaminathan K (2004) Bioconversion of agro-wastes by fungus, Pleurotus spp. Biol Mem 30:1–6
Taseli BK (2008) Fungal treatment of hemp-based pulp and paper mill wastes. Afr J Biotechnol 7:286–289
Kuhar S, Nair LM, Kuhad RC (2008) Pretreatment of lignocellulosic material with fungi capable of higher lignin degradation and lower carbohydrate degradation improves substrate acid hydrolysis and eventual conversion to ethanol. Can J Microbiol 54:305–313
Singh P, Suman A, Tiwari P, Arya N, Gaur A, Shrivastava AK (2008) Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J Microbiol Biotechnol 24:667–673
Jian S, Ratna R, Sharma-Shivappa, Chinn M, Howell N (2008) Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioenergy 33:88–96
Xu C, Ma F, Zhang X (2009) Lignocellulose degradation and enzyme production by Irpex lacteus CD2 during solid-state fermentation of corn stover. J Biosci Bioeng 108:372–375
Reid ID (1985) Biological delignification of aspen wood by solid-state fermentation with the white-rot fungus, Merulius tremellosus. Appl Env Microbiol 50:133–139
Regalado V, Rodriguez A, Perestelo F, Carnicero A, Fuente G, Falcon MA (1997) Lignin degradation and modification by the soil inhabiting fungus Fusarium proliferatum. Appl Environ Microbiol 63:3716–3718
Zhang X, Yu H, Huang H, Liu Y (2007) Evaluation of biological pretreatment with white rot fungi for the enzymatic hydrolysis of bamboo culms. Int Biodeterior Biodegrad 60:159–164
Zhang X, Xu C, Wang H (2007) Pretreatment of bamboo residues with Coriolus versicolor for enzymatic hydrolysis. J Biosci Bioeng 104:149–151
Hamisan AF, Abd-Aziz S, Kamaruddin K, Md. Shah UK, Shahab N, Hassan MA (2009) Delignification of oil palm empty fruit bunch using chemical and microbial pretreatment methods. Int J Agric Res 4:250–256
Yu H, Du W, Zhang J, Ma F, Zhang X, Zhong W (2010) Fungal treatment of cornstalks enhances the delignification and xylan loss during mild alkaline pretreatment and enzymatic digestibility of glucan. Bioresour Technol 101:6728–6734
Wan C, Li Y (2010) Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour Technol 101:6398–6403
Wan C, Li Y (2010) Microbial delignification of corn stover by Ceriporiopsis subvermispora for improving cellulose digestibility. Enzyme Microbial Technol 47:31–36
Arora A, Nain L, Gupta JK (2005) Solid-state fermentation of wood residues by Streptomyces griseus B1, a soil isolate, and solubilisation of lignins. World J Microbiol Biotechnol 21:303–308
Bon EPS and Ferrara MA (2007) Bioethanol production via enzymatic hydrolysis of cellulosic biomass. In: The Role of Agricultural Biotechnologies for Production of Bioenergy in Developing Countries. FAO. Available via http://www.fao.org/biotech/seminaroct2007.htm. Cited 12 October 2007
Eggeman T, Elander RT (2005) Process and economic analysis of pretreatment technologies. Bioresour Technol 96:2019–2025
Reid ID (1989) Solid-state fermentations for biological delignification. Enzyme Microb Technol 11:786–803
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saritha, M., Arora, A. & Lata Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J Microbiol 52, 122–130 (2012). https://doi.org/10.1007/s12088-011-0199-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-011-0199-x