Abstract
Intramuscular fat (IMF) content is an important trait closely related to meat quality, which is highly variable among pig breeds from diverse genetic backgrounds. High-throughput sequencing has become a powerful technique for analyzing the whole transcription profiles of organisms. In order to elucidate the molecular mechanism underlying porcine meat quality, we adopted RNA sequencing to detect transcriptome in the longissimus dorsi muscle of Wei pigs (a Chinese indigenous breed) and Yorkshire pigs (a Western lean-type breed) with different IMF content. For the Wei and Yorkshire pig libraries, over 57 and 64 million clean reads were generated by transcriptome sequencing, respectively. A total of 717 differentially expressed genes (DEGs) were identified in our study (false discovery rate < 0.05 and fold change > 2), with 323 up-regulated and 394 down-regulated genes in Wei pigs compared with Yorkshire pigs. Gene Ontology analysis showed that DEGs significantly related to skeletal muscle cell differentiation, phospholipid catabolic process, and extracellular matrix structural constituent. Pathway analysis revealed that DEGs were involved in fatty acid metabolism, steroid biosynthesis, glycerophospholipid metabolism, and protein digestion and absorption. Quantitative real time PCR confirmed the differential expression of 11 selected DEGs in both pig breeds. The results provide useful information to investigate the transcriptional profiling in skeletal muscle of different pig breeds with divergent phenotypes, and several DEGs can be taken as functional candidate genes related to lipid metabolism (ACSL1, FABP3, UCP3 and PDK4) and skeletal muscle development (ASB2, MSTN, ANKRD1 and ANKRD2).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owing to the improvement of people’s life standard, high quality meat is increasingly popular with consumers. However, to pursue more pork production during the past decades, Western commercial pig breeds have been received persistent artificial selection by animal breeders. This process not only leads to swine carrying lower backfat thickness and higher lean meat percentage, but also is accompanied by the deterioration of pork quality. For example, intramuscular fat (IMF) content is a key meat quality trait correlated with tenderness, flavor and juiciness (Fortin et al. 2005), while the significant decrease of IMF content is an important factor causing poor meat quality of modern lean pigs (Pietruszka et al. 2015). Accordingly, pork quality requires improving to sufficiently meet the current market demand, and one possible approach is to enhance the IMF content of pigs (Schwab et al. 2009). It is well known that there are significant differences in lipid deposition between obese and lean pig breeds, resulting in swine present distinct carcass and meat characteristics, such as lean meat percentage, backfat thickness, and IMF content. However, little is known about the genomic regulation of different meat quality in these pig breeds. Therefore, it is necessary to clarify the molecular mechanism of meat quality traits, especially the intramuscular fat content, in fat-type and lean-type swine, which is conducive to the genetic improvement of pork quality.
Some candidate genes have been reported to be significantly associated with porcine IMF content (Li et al. 2010; Xue et al. 2015), which can improve the muscle quality through molecular marker assisted breeding. Nevertheless, quantitative traits are generally regulated by multiple genes, so that the candidate gene method is incomplete to study meat quality traits. Furthermore, the genetic factors controlling porcine meat quality are still not quite clear. Consequently, detection of transcription profiles in muscle from the whole genome level, which is helpful to better know the regulatory mechanism of pork quality. Due to the rapid development of biotechnology, the traditional microarray analysis has disadvantage to detect genome-wide profiling of organisms. At present, RNA sequencing (RNA-Seq) has become a powerful high-throughput technology for analyzing complex traits in animals (Wickramasinghe et al. 2014). As the main site of meat production, skeletal muscles (e.g. longissimus dorsi and semimembranosus muscles) are extensively used as experimental materials for pork quality research. Previous studies have highlighted the advantages of transcriptome sequencing for comprehensively investigating molecular features of porcine skeletal muscle. For example, RNA-Seq technology has been used to detect the transcription profiling in the longissimus dorsi muscle of a single pig breed with extreme meat quality traits, in order to identify differentially expressed genes (DEGs) associated with intramuscular fat content (Lim et al. 2017) and lipid traits (Cardoso et al. 2017). Furthermore, transcriptome sequencing has also been adopted to examine the skeletal muscle in multiple pig breeds (Ropka-Molik et al. 2015; Li et al. 2016; Chen et al. 2017), which could identify the genes regulating pork quality traits with breed specific. These studies directly gain insight into global gene expression in porcine muscle so as to reveal the causal genes and pathways that determine meat quality. However, transcriptome sequencing is applied to analyzing the genetic regulation of pork quality remains inadequate, and the functional genes controlling meat quality in different pig breeds is not fully elucidated. Consequently, investigating muscle transcriptome of divergent phenotype pigs is essential to deciphering the regulatory mechanism of pork quality differences. Yorkshire pig is a famous Western lean-type breed widely raised in the world, whereas its muscle quality is relatively poor. Compared with the foreign lean pig breeds, Chinese indigenous breeds have drawn much attention for their excellent pork quality. Wei pig is a famous native black breed with superior meat quality, high reproductive performance and slow growth rate, mainly distributed in the southern region of Anhui province, China. As a typical fat-type breed, Wei pig is characterized by strong fat deposition capacity and low lean meat percentage. Accordingly, the two pig breeds can be taken as ideal animal models to comparatively study the clear distinction of meat quality. Previous studies on fine muscle properties of Wei pigs were only based on conventional determination of meat quality traits; however, the transcriptome analysis of skeletal muscle in Wei pigs has not been carried out so far.
Considering the extreme phenotypes between Chinese and Western pig breeds from diverse genetic backgrounds, comparing their muscle transcription features can explore the difference of pork quality. In this study, RNA-Seq was utilized to analyze transcriptome profiling in the longissimus dorsi muscle of Wei and Yorkshire pigs with divergent IMF content, in order to identify differentially expressed genes and metabolic pathways regulating meat quality. The results can gain further insight into the molecular mechanism of skeletal muscle in different pig breeds, and provide some useful information for genetically improving pork quality.
Materials and methods
Animals and IMF content measurement
Six female pigs (Wei pigs, n = 3; Yorkshire pigs, n = 3) were reared under similar conditions in Antai Pig Breeding Co., Ltd (Anhui, China), and these healthy pigs were slaughtered at similar weight of 90 kg. Then, the longissimus dorsi muscle at the 3rd/4th last rib was collected and divided into two parts, one part was rapidly frozen in liquid nitrogen for transcriptome sequencing, and another part was stored in 4 °C for measuring the IMF content.
Intramuscular fat content was determined by measuring the crude fat of muscle using Soxhlet Extraction with petroleum ether (Tyra and ŻAk 2012), and each sample was repeated three times.
RNA isolation, library construction and sequencing
Total RNA was extracted from porcine muscle using Trizol reagent (Invitrogen), and RNA integrity was checked by a Bioanalyzer 2100 (Agilent Technologies). Then, qualified total RNA (RNA integrity number > 7 and 28S/18S > 0.7) was purified by RNAClean XP Kit and RNase-Free DNase Set.
Six sequencing libraries were generated using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA) following the manufacturer’s instructions. Briefly, mRNA was isolated from total RNA using oligo (dT) magnetic beads (Invitrogen) and fragmented by an RNA fragmentation kit (Ambion). Then, mRNA fragments were used to synthesize the first-strand cDNA with random primers and reverse transcriptase, and further synthesize the second-strand cDNA with RNase H and DNA polymerase I. After end repair, A-tailing addition and adaptor ligation, the cDNA fragments were purified and enriched to construct the library. The six libraries were sequenced at the Shanghai Biotechnology Corporation, and 150 bp paired-end reads were generated using an Illumina HiSeq platform. The RNA-Seq data have been submitted to the Gene Expression Omnibus (GEO) database (Accession no. GSE99092).
RNA-Seq data analysis
Clean reads were obtained by removing adaptor sequences, low quality reads and rRNA reads from raw reads, and then mapped to the pig genome (Sus scrofa 10.2) using TopHat software with a maximum of 2 bp mismatches (Trapnell et al. 2009). The gene expression level was calculated using the fragments per kilobase per of exon model per million mapped reads (FPKM) method (Mortazavi et al. 2008). Differential expression analysis was performed by cuffdiff software (Trapnell et al. 2010), and P value was adjusted using the false discovery rate (FDR) method. The screening criteria of differentially expressed genes (DEGs) were designated as FDR < 0.05 and fold change (FC) > 2.
Enrichment analysis of differentially expressed genes was carried out using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Significance levels were calculated using modified Fisher’s exact test and P < 0.05 was considered the level of significance.
Quantitative real time PCR validation
To validate the transcriptome sequencing data of skeletal muscle, 11 genes (six up-regulated and five down-regulated genes) related to meat quality were selected to quantify the expression levels using quantitative real time PCR (Q-PCR), and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. Primers used for Q-PCR are listed in Supplementary material 1.
Total RNA (0.5 μg) was reverse-transcribed into 20 μL cDNA using a ReverTra Ace qPCR RT Kit (TOYOBO, Japan). Q-PCR was performed using the Power SYBR Green PCR Master Mix (ABI, USA) on ABI Prism 7900HT Sequence Detection System. The reaction system consisted of 10 μL 2× SYBR Green Buffer, 1 μL each forward and reverse primer (10 μM), 7 μL ddH2O, and 1 μL cDNA. The thermal cycling conditions were 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 60 s. Each sample was performed with three replications, and cycle threshold (Ct) values were calculated using the 2− ΔCt method. Relative gene expression levels of two pig breeds were compared by t-test using SAS software (version 9.0), and P < 0.05 was considered the significant difference.
Results
Measurement of intramuscular fat content
The measured results of intramuscular fat content between Wei and Yorkshire pigs are shown in Fig. 1. The longissimus dorsi muscle of Wei pigs containing significantly higher IMF content than Yorkshire pigs (P < 0.05), indicating that the two swine breeds were fit for identifying DEGs associated with meat quality traits.
Summary of RNA-Seq data
The transcriptome in porcine skeletal muscle was detected by RNA-Seq, and yielded over 65 million raw reads in each library (Supplementary material 2). After filtering the unqualified raw reads, 57–94 million clean reads were obtained from six RNA-Seq libraries. About 72.87% of clean reads were mapped to the reference genome in Wei pigs, which was lower than 75.59% of Yorkshire pigs. Of the mapped reads, 82.22, 3.82 and 13.95% of reads were respectively located in exon, intron and intergenic regions in Wei pigs, while 82.78, 3.30 and 13.92% of reads were respectively located in exon, intron and intergenic regions in Yorkshire pigs. The results showed that sequencing reads were high quality and sufficient to cover the pig genome, which ensure that analyze the muscle transcriptome profiling between the two swine breeds.
Identification of differentially expressed genes
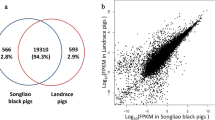
The distribution range of gene expressed abundance was similar between Wei and Yorkshire pigs. About 97% of genes were expressed at low levels (0–100 FPKM), and no more than 3% of genes were expressed at medium levels (100–1000 FPKM), and less than 1% of genes were expressed at high levels (> 1000 FPKM) (Supplementary material 3). A total of 17,246 and 17,517 genes were expressed in muscle of Wei and Yorkshire pigs, respectively. Of these, 199 genes were only expressed in Wei pigs, 470 genes were only expressed in Yorkshire pigs, and 17,047 genes were co-expressed in both breeds (Supplementary material 4). In this study, 717 differentially expressed genes were identified (FDR < 0.05 and FC > 2), with 323 up-regulated and 394 down-regulated genes in Wei pigs compared to Yorkshire pigs (Supplementary material 5).
Functional analysis of differentially expressed genes
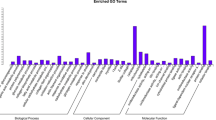
To annotate biological functions of the 717 DEGs, GO and KEGG enrichment analyses were carried out in our study. The results of GO analysis showed that the DEGs mainly involved in skeletal muscle cell differentiation, glycerophospholipid catabolic process, triglyceride biosynthetic process, phospholipid catabolic process, regulation of lipid storage, extracellular matrix structural constituent, collagen trimer, and collagen fibril organization (Fig. 2). In addition, some DEGs were enriched the GO terms that may be correlated with the different muscle quality between Wei and Yorkshire pigs, such as muscle structure development, muscle organ development, lipid storage, cellular lipid catabolic process, cellular response to lipid, and extracellular matrix (Supplementary material 6).
KEGG analysis showed that the DEGs were enriched in pathways related to meat quality, including fatty acid metabolism, steroid biosynthesis, glycerophospholipid metabolism, and protein digestion and absorption (Fig. 3). Besides, the PPAR signaling pathway, adipocytokine signaling pathway, and glycerolipid metabolism were enriched, but the three pathways did not reach at the significant level (Supplementary material 7). Particularly, several DEGs involved in fatty acid metabolism and the PPAR signaling pathway are highlighted, which can be taken as functional candidate genes associated with the difference in IMF content.
Validation of DEGs by quantitative real time PCR
To validate the outcomes of transcriptome sequencing, the mRNA levels of 11 selected DEGs were detected by Q-PCR using the same muscle samples. The expression levels of ACSL1, FABP3, ANKRD2, TXNIP, PDK4 and UCP3 genes were up-regulated, while MSTN, ANKRD1, COL3A1, SPARC and COL1A2 genes were down-regulated in Wei pigs compared to Yorkshire pigs (Fig. 4). The fold change of gene expression in Q-PCR had a same tendency with RNA-Seq data, and the Pearson correlation coefficient (r = 0.995, P < 0.01) was highly significant based on log2 FC, demonstrating that the results of high-throughput sequencing were reliable.
Discussion
In recent years, increasing attention has been paid to the genetic improvement of muscle quality. Previous reports have demonstrated that there are obvious differences in meat quality traits of different genetic background pig breeds (Dai et al. 2009; Yu et al. 2013). Chinese local pig breeds have universal characteristics of higher lipid deposition capacity and lower lean meat percentage than Western commercial pig breeds, indicating that these extreme phenotype breeds could fit for underlying the variation of pork quality. In the present study, to explore the hereditary elements of meat quality differences, transcriptome profiles of the longissimus dorsi muscle were compared between Wei and Yorkshire pigs with divergent IMF content. A total of 717 DEGs were identified in the two pig breeds, enabling to reveal the genes and biological processes that control muscle development and fat storage. Previous studies showed that a number of DEGs were identified in longissimus dorsi muscle of lean and obese type pigs using transcriptome sequencing (Zhao et al. 2011; Wang et al. 2015; Li et al. 2016), but only a few DEGs of these reports overlap with our results mainly due to the effect of pig breed. However, the different transcriptome profiling may explain phenotypic differences in these pig breeds, and identification of DEGs is beneficial to understanding the regulatory mechanism of pork quality.
The difference of lipid deposition in muscle can lead to the change of IMF content, which is an important reason affecting meat quality in pigs. Since divergent IMF content in the longissimus dorsi muscle of Wei and Yorkshire pigs, we mainly focus on the metabolic processes involved in lipid absorption, synthesis and degradation. According to the annotating results of DEGs, some GO terms and pathways are directly relevant to lipid metabolism, including phospholipid catabolic process, triglyceride biosynthetic process, fatty acid metabolism, and steroid biosynthesis. Of these, several DEGs participating in lipid metabolic processes were identified, which may account for the higher IMF content in Wei pigs compared with Yorkshire pigs. Low density lipoprotein receptor (LDLR) is a cell-surface protein involved in cholesterol homeostasis and lipid metabolism. Zeng et al. (2014) found that LDLR gene variants associated with serum lipid parameters in pigs, and Serão et al. (2011) reported that the LDLR mRNA level had a correlation with porcine IMF content. Therefore, LDLR can be considered as a candidate gene for lipid deposition in porcine skeletal muscle. Scavenger receptor class B member 1 (SCARB1) plays a role in cholesterol homeostasis, and its genetic variations were significantly associated with high-density lipoprotein cholesterol levels in humans (Niemsiri et al. 2015), suggesting that the biological roles of SCARB1 relate to lipid metabolism. In this study, LDLR and SCARB1 genes were enriched in the phospholipid catabolic process, so down-regulated levels of them may reduce lipolysis and promote lipid storage in skeletal muscle of Wei pigs. Owing to the mRNA abundances of LDLR and SCARB1 were low in muscle of Wei and Yorkshire pigs, they probably not the critical DEGs determining IMF content in the two pig breeds. Moreover, three DEGs, acyl-CoA synthetase long-chain family member 1 (ACSL1), fatty acid desaturase 1 (FADS1) and acetyl-CoA acetyltransferase 2 (ACAT2), were closely associated with fatty acid metabolism in our study. The ACSL1 gene variant was significantly associated with polyunsaturated fatty acids in skeletal muscle of beef cattle (Widmann et al. 2011), and overexpressed ACSL1 could enhance fatty acid uptake in mouse 3T3-L1 adipocytes (Zhan et al. 2012). These results indicate that ACSL1 can become a critical candidate gene regulating fatty acid metabolism in pig muscle. A previous study reported that ACSL1 mRNA levels in longissimus dorsi muscle of two Chinese native pig breeds (Diannan small ear and Tibet pigs) were significantly higher than Yorkshire pigs (Li et al. 2012). Moreover, RNA-Seq also revealed that ACSL1 was up-regulated in muscle of Chinese local Wannanhua pigs and Laiwu pigs compared to Yorkshire pigs, respectively (Li et al. 2016; Chen et al. 2017). These findings were similar to our results that muscular ACSL1 was highly expressed in Wei pigs compared with Yorkshire pigs. Consequently, the increased expression of ACSL1 may be associated with higher intramuscular fat content in Chinese indigenous breeds compared to Western commercial breeds. FADS1 is an enzyme involved in the synthesis of polyunsaturated fatty acids. Han et al. (2013) reported that FADS1 gene polymorphisms related to fatty acid composition in the brisket adipose tissue of beef steers, suggesting that the FADS1 gene may play a crucial role in fatty acid metabolism in skeletal muscle of pigs. Whether the down-regulation of FADS1 gene could cause different polyunsaturated fatty acid content in muscle between Wei and Yorkshire pigs need further studies. The protein encoded by ACAT2 gene catalyzes the cholesterol and fatty acids to generate cholesterol esters (Zhang et al. 2010); hence, it decreasing expression in our study may modulate lipid synthesis in porcine skeletal muscle. However, the expression levels of FADS1 and ACAT2 were very low (FPKM < 5) in both pig breeds, so they probably not the key genes regulating lipid storage in skeletal muscle.
In addition, other DEGs involved in lipid metabolism were also screened by RNA-Seq, such as heart fatty acid binding protein (FABP3, or H-FABP), uncoupling protein 3 (UCP3) and pyruvate dehydrogenase kinase 4 (PDK4). The known lipid-related gene of FABP3 was enriched in the PPAR signaling pathway in our study. Previous studies showed that FABP3 gene polymorphisms were significantly associated with porcine IMF content (Li et al. 2010; Cho et al. 2011). Moreover, the FABP3 mRNA level in skeletal muscle of Chinese local Wujin pigs was higher than Western Landrace pigs (Zhao et al. 2009), which was similar to our results. Overexpression of FABP3 significantly promoted the differentiation of 3T3-L1 preadipocytes and increased triacylglycerol levels (Yi et al. 2014); therefore, the increased expression level of FABP3 gene may contribute to intramuscular lipid deposition in Wei pigs. UCP3 is a mitochondrial membrane protein involved in lipid metabolism, and overexpressing UCP3 in mouse skeletal muscle could increase fatty acid oxidation (Wang et al. 2003). It was reported that UCP3 gene polymorphisms were significantly associated with IMF content in a Pietran × Jinhua F2 pig population (Chen et al. 2011), indicating it may be a candidate gene affecting meat quality. Additionally, PDK4 is an enzyme involved in lipid homeostasis (Distel et al. 2017). The PDK4 gene was highly expressed in muscle of Meishan pigs (a Chinese native breed) compared to Yorkshire pigs, and a mutation in its intron 9 was significantly correlated with IMF content (Lan et al. 2009), suggesting PDK4 may relate to intramuscular lipid deposition in pigs. Particularly, Chen et al. (2017) found that UCP3 and PDK4 were more highly expressed in skeletal muscle of Laiwu pigs than Yorkshire pigs, and Li et al. (2016) reported that FABP3, UCP3 and PDK4 were markedly up-regulated in the longissimus dorsi muscle of Wannanhua pigs compared with Yorkshire pigs. In our findings, both RNA-Seq and Q-PCR methods revealed that FABP3, UCP3 and PDK4 gene expression levels were up-regulated, which may be one reason for the higher IMF content in Wei pigs than Yorkshire pigs. These observations indicate that high expression levels of FABP3, UCP3 and PDK4 probably have positive effects on lipid deposition in porcine skeletal muscle. Consequently, FABP3, UCP3 and PDK4 can be taken as functional genes defining the differences of IMF content in divergent pig breeds, and polymorphisms of them may become important molecular markers for genetic improvement of pork quality.
In the present study, function annotation showed that several DEGs were involved in the GO terms of skeletal muscle cell differentiation. As a typical obese breed, Wei pigs carrying lower lean meat production than introduced lean-type pigs. Ankyrin repeat and SOCS box containing protein 2 (ASB2) is a negative regulator of muscle mass (Davey et al. 2016), implying that the high expression level of ASB2 was not conducive to muscle growth of Wei pigs. On the contrary, myostatin (MSTN) is a famous negative regulator of muscle growth (Thomas et al. 2000), and the results from our study showed that muscular MSTN was decreased expression in Wei pigs, indicating that MSTN less inhibit muscle growth of Wei pigs than Yorkshire pigs at adult stage. Given that ASB2 and MSTN directly take part in muscle development, they can be important genes modulating muscle growth and meat mass in pigs. In our study, ankyrin repeat domain 1 (ANKRD1) and 2 (ANKRD2) belonging to the muscle ankyrin repeat protein family were highlighted based on their regulatory role in skeletal muscle cell differentiation (Wang et al. 2011). In swine, the ANKRD1 gene was reported to affect pathways related to meat quality traits (Ponsuksili et al. 2009), and the expression level of ANKRD1 was up-regulated in Large White pigs with lower IMF content compared with Basque pigs (Damon et al. 2012). Additionally, the ANKRD2 gene polymorphism was remarkably associated with loin depth and firmness traits (Sun et al. 2011). The expression levels of ANKRD2 and ANKRD1 were respectively up-regulated in the Wei and Yorkshire pigs, suggesting that the two genes have different roles in skeletal muscle growth. Due to ANKRD1 and ANKRD2 participate in the skeletal muscle development, differential expression of them may correspond to the muscle physiology differences between Wei and Yorkshire pigs.
Collagen is the elementary constituent of the extracellular matrix, which is a key element affecting muscular tenderness of pigs (Wheeler et al. 2000). In this study, some DEGs were involved in biological processes related to collagen protein metabolism, including GO terms (extracellular matrix structural constituent, collagen trimer, and collagen fibril organization) and pathway (protein digestion and absorption). In comparison to Yorkshire pigs, the collagen genes (COL1A2, COL3A1, COL4A2, COL5A1, COL5A2, COL11A1 and COL14A1) were down-regulated in skeletal muscle of Wei pigs with high IMF content, indicating that expression patterns of collagen genes are associated with the differences in pork quality in the two pig breeds. Óvilo et al. (2014) reported that six collagen genes (e.g. COL5A1 and COL14A1) were down-regulated in Iberian pigs with higher IMF content compared with Duroc × Iberian pigs, and Li et al. (2016) showed that the muscular expression levels of COL1A2 and COL3A1 in Wannanhua pigs were lower than Yorkshire pigs, which were consistent with the results in our study. Lim et al. (2017) also found that collagen genes were differentially expressed in muscle of Berkshire pigs with different IMF content, but they (e.g. COL1A2, COL5A1 and COL14A1) were significantly up-regulated in the pigs with higher IMF content. These observations showed that collagen proteins play roles in the skeletal muscle formation of pigs, however, the inconsistent results of collagen gene expression patterns probably due to the breed-specific during muscle development. Though the genetic effects of these collagen genes on porcine meat quality are rarely reported, they might potentially affect pork quality by regulating muscular components of Wei and Yorkshire pigs.
Conclusion
In this study, transcriptome sequencing was performed in the longissimus dorsi muscle of Wei and Yorkshire pigs. A total of 717 differentially expressed genes were identified, and functional analysis showed that they were involved in skeletal muscle cell differentiation, phospholipid catabolic process, and extracellular matrix structural constituent. Several DEGs relate to lipid metabolism (ACSL1, FABP3, UCP3 and PDK4) and skeletal muscle development (ASB2, MSTN, ANKRD1 and ANKRD2), and can be taken as important candidate genes for improving the pork quality by marker assisted selection. The results contribute to better exploring transcriptional profiling in muscle of different pig breeds, and provide a foundation for further elucidating the genetic mechanism of meat quality.
References
Cardoso TF, Cánovas A, Canela-Xandri O, González-Prendes R, Amills M, Quintanilla R (2017) RNA-Seq based detection of differentially expressed genes in the skeletal muscle of Duroc pigs with distinct lipid profiles. Sci Rep 7:40005
Chen Z, Zhao XF, Hao Z, Guo XL, Jiang XL, Xu NY (2011) Association of porcine UCP3 gene polymorphisms with fatness traits in a Pietrain × Jinhua F2 population. Afr J Biotechnol 10:3296–3300
Chen W, Fang GF, Wang SD, Wang H, Zeng YQ (2017) Longissimus lumborum muscle transcriptome analysis of Laiwu and Yorkshire pigs differing in intramuscular fat content. Genes Genom 39:759–766
Cho KH, Kim MJ, Jeon GJ, Chung HY (2011) Association of genetic variants for FABP3 gene with back fat thickness and intramuscular fat content in pig. Mol Biol Rep 38:2161–2166
Dai FW, Feng DY, Cao QY, Ye H, Zhang CM, Xia WG, Zuo JJ (2009) Developmental differences in carcass, meat quality and muscle fibre characteristics between the Landrace and a Chinese native pig. S Afr J Anim Sci 39:267–273
Damon M, Wyszynska-Koko J, Vincent A, Hérault F, Lebret B (2012) Comparison of muscle transcriptome between pigs with divergent meat quality phenotypes identifies genes related to muscle metabolism and structure. PLoS ONE 7:e33763
Davey JR, Watt KI, Parker BL, Chaudhuri R, Ryall JG, Cunningham L, Qian HW, Sartorelli V, Sandri M, Chamberlain J, James DE, Gregorevic P (2016) Integrated expression analysis of muscle hypertrophy identifies Asb2 as a negative regulator of muscle mass. JCI Insight 1:e85477
Distel E, Cadoudal T, Collinet M, Park EA, Benelli C, Bortoli S (2017) Early induction of pyruvate dehydrogenase kinase 4 by retinoic acids in adipocytes. Mol Nutr Food Res 61:1600920
Fortin A, Robertson WM, Tong AKW (2005) The eating quality of Canadian pork and its relationship with intramuscular fat. Meat Sci 69:297–305
Han C, Vinsky M, Aldai N, Dugan MER, Mcallister TA, Li C (2013) Association analyses of DNA polymorphisms in bovine SREBP-1, LXRα, FADS1 genes with fatty acid composition in Canadian commercial crossbred beef steers. Meat Sci 93:429–436
Lan J, Lei MG, Zhang YB, Wang JH, Feng XT, Xu DQ, Gui JF, Xiong YZ (2009) Characterization of the porcine differentially expressed PDK4 gene and association with meat quality. Mol Biol Rep 36:2003–2010
Li XP, Kim SW, Choi JS, Lee YM, Lee CK, Choi BH, Kim TH, Choi YII, Kim JJ, Kim KS (2010) Investigation of porcine FABP3 and LEPR gene polymorphisms and mRNA expression for variation in intramuscular fat content. Mol Biol Rep 37:3931–3939
Li QG, Tao Z, Shi LH, Ban DM, Zhang B, Yang YZ, Zhang H, Wu CX (2012) Expression and genome polymorphism of ACSL1 gene in different pig breeds. Mol Biol Rep 39:8787–8792
Li XJ, Zhou J, Liu LQ, Qian K, Wang CL (2016) Identification of genes in longissimus dorsi muscle differentially expressed between Wannanhua and Yorkshire pigs using RNA-sequencing. Anim Genet 47:324–333
Lim KS, Lee KT, Park JE, Chung WH, Jang GW, Choi BH, Hong KC, Kim TH (2017) Identification of differentially expressed genes in longissimus muscle of pigs with high and low intramuscular fat content using RNA sequencing. Anim Genet 48:166–174
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628
Niemsiri V, Wang XB, Pirim D, Radwan ZH, Bunker CH, Barmada MM, Kamboh MI, Demirci FY (2015) Genetic contribution of SCARB1 variants to lipid traits in African Blacks: a candidate gene association study. BMC Med Genet 16:106
Óvilo C, Benítez R, Fernández A, Núñez Y, Ayuso M, Fernández AI, Rodríguez C, Isabel B, Rey AI, López-Bote C, Silió L (2014) Longissimus dorsi transcriptome analysis of purebred and crossbred Iberian pigs differing in muscle characteristics. BMC Genom 15:413
Pietruszka A, Jacyno E, Kawecka M, Biel W (2015) The relation between intramuscular fat level in the longissimus muscle and the quality of pig carcasses and meat. Ann Anim Sci 15:1031–1041
Ponsuksili S, Murani E, Phatsara C, Schwerin M, Schellander K, Wimmers K (2009) Porcine muscle sensory attributes associate with major changes in gene networks involving CAPZB, ANKRD1, and CTBP2. Funct Integr Genom 9:455–471
Ropka-Molik K, Żukowski K, Eckert R, Piórkowska K, Oczkowicz M, Gurgul A, Szmatoła T (2015) Whole transcriptome analysis of the porcine muscle tissue of breeds differing in muscularity and meat quality traits. Livest Sci 182:93–100
Schwab CR, Baas TJ, Stalder KJ, Nettleton D (2009) Results from six generations of selection for intramuscular fat in Duroc swine using real-time ultrasound. I. Direct and correlated phenotypic responses to selection. J Anim Sci 87:2774–2780
Serão NVL, Veroneze R, Ribeiro AMF, Verardo LL, Braccini Neto J, Gasparino E, Campos CF, Lopes PS, Guimarães SEF (2011) Candidate gene expression and intramuscular fat content in pigs. J Anim Breed Genet 128:28–34
Sun L, Dong XJ, Fan B, Liu B (2011) The association of ANKRD2 with loin depth and muscle firmness in pigs. J Anim Vet Adv 10:1462–1468
Thomas M, Langly B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515
Tyra M, ŻAk G (2012) Analysis of relationships between fattening and slaughter performance of pigs and the level of intramuscular fat (IMF) in longissimus dorsi muscle. Ann Anim Sci 12:169–178
Wang S, Subramaniam A, Cawthorne MA, Clapham JC (2003) Increased fatty acid oxidation in transgenic mice overexpressing UCP3 in skeletal muscle. Diabetes Obes Metab 5:295–301
Wang LJ, Lei MG, Xiong YZ (2011) Molecular characterization and different expression patterns of the muscle ankyrin repeat protein (MARP) family during porcine skeletal muscle development in vitro and in vivo. Anim Biotechnol 22:87–99
Wang ZX, Li QG, Chamba YZ, Zhang B, Shang P, Zhang H, Wu CX (2015) Identification of genes related to growth and lipid deposition from transcriptome profiles of pig muscle tissue. PLoS ONE 10:e0141138
Wheeler TL, Shackelford SD, Koohmaraie M (2000) Variation in proteolysis, sarcomere length, collagen content, and tenderness among major pork muscles. J Anim Sci 78:958–965
Wickramasinghe S, Cánovas A, Rincón G, Medrano JF (2014) RNA-sequencing: a tool to explore new frontiers in animal genetics. Livest Sci 166:206–216
Widmann P, Nuernberg K, Kuehn C, Weikard R (2011) Association of an ACSL1 gene variant with polyunsaturated fatty acids in bovine skeletal muscle. BMC Genet 12:96
Xue W, Wang W, Jin B, Zhang X, Xu X (2015) Association of the ADRB3, FABP3, LIPE, and LPL gene polymorphisms with pig intramuscular fat content and fatty acid composition. Czech J Anim Sci 60:60–66
Yi B, Wang JG, Wang S, Yuan DL, Sun JZ, Li ZL, Mao YP, Hou Q, Liu WQ (2014) Overexpression of Banna mini-pig inbred line fatty acid binding protein 3 promotes adipogenesis in 3T3-L1 preadipocytes. Cell Biol Int 38:918–923
Yu KF, Shu G, Yuan FF, Zhu XT, Gao P, Wang SB, Wang LN, Xi QY, Zhang SQ, Zhang YL, Li Y, Wu TS, Yuan L, Jiang QY (2013) Fatty acid and transcriptome profiling of longissimus dorsi muscles between pig breeds differing in meat quality. Int J Biol Sci 9:108–118
Zeng Z, Chen R, Liu C, Yang H, Chen C, Huang L (2014) Evaluation of the causality of the low-density lipoprotein receptor gene (LDLR) for serum lipids in pigs. Anim Genet 45:665–673
Zhan TZ, Poppelreuther M, Ehehalt R, Füllekrug J (2012) Overexpressed FATP1, ACSVL4/FATP4 and ACSL1 increase the cellular fatty acid uptake of 3T3-L1 adipocytes but are localized on intracellular membranes. PLoS ONE 7:e45087
Zhang ZQ, Chen HZ, Yang RF, Zhang R, Jia YY, Xi Y, Liu DP, Liang CC (2010) Regulation of acyl-coenzyme A: cholesterol acyltransferase 2 expression by saturated fatty acids. Chin Med Sci J 25:222–227
Zhao SM, Ren LJ, Chen L, Zhang X, Cheng ML, Li WZ, Zhang YY, Gao SZ (2009) Differential expression of lipid metabolism related genes in porcine muscle tissue leading to different intramuscular fat deposition. Lipids 44:1029–1037
Zhao X, Mo DL, Li AN, Gong W, Xiao SQ, Zhang Y, Qin LM, Niu YN, Guo YX, Liu XH, Cong PQ, He ZY, Wang C, Li JQ, Chen YS (2011) Comparative analyses by sequencing of transcriptomes during skeletal muscle development between pig breeds differing in muscle growth rate and fatness. PLoS ONE 6:e19774
Acknowledgements
This work was supported by the Provincial Natural Science Research Project of Anhui Colleges (KJ2016A180 and KJ2017ZD43), the Anhui Provincial Science and Technology Major Special Project (16030701066), the Anhui Provincial Science and Technology Plan Project (1704a07020093), and Open Fund of Anhui Province Key Laboratory of Local Livestock and Poultry Genetical Resource Conservation and Breeding (AKLGRCB2017012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Jingen Xu, Chonglong Wang, Erhui Jin, Youfang Gu, Shenghe Li and Qinggang Li declare that they have no conflict of interest.
Ethical approval
This work was approved by the Animal Welfare and Use Committee of Anhui Science and Technology University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, J., Wang, C., Jin, E. et al. Identification of differentially expressed genes in longissimus dorsi muscle between Wei and Yorkshire pigs using RNA sequencing. Genes Genom 40, 413–421 (2018). https://doi.org/10.1007/s13258-017-0643-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-017-0643-3