Abstract
Species with relatively small, membraneous, black ascomata, with or without long necks, unitunicate, cylindrical asci with apical rings and fusiform, hyaline ascospores with or without mucilaginous sheaths are common in freshwater habitats in tropical and temperate regions. Many of these taxa have originally been recorded as Annulatascaceae-like taxa. Twenty genera have been included in the family Annulatascaceae, mostly based on morphological characters, while molecular work and phylogenetic analyses are lacking for many genera. In this study, nine new Annulatascaceae-like taxa collected from Thailand were morphologically examined. Pure cultures obtained from single ascospores were used in molecular studies. The nine new strains and several other strains of Annulatascaceae-like Sordariomycetes species were used to establish phylogenetic and evolution relationships among the taxa, based on combined LSU, SSU, ITS and RPB2 sequence data. Phylogenetic analyses provide evidence to introduce one new order and six new families, to accommodate taxa excluded from Annulatascaceae sensu stricto. A new order Atractosporales is established based on the molecular study, including three new introduced families Conlariaceae, Pseudoproboscisporaceae and Atractosporaceae. Conlariaceae is introduced for the genus Conlarium which comprises two species, Conlarium duplumascosporun and a new Hyphomycetous asexual morph taxon Conlarium aquaticum which has subglobose or irregular, brown, clathrate, muriform conidia. Pseudoproboscisporaceae includes Pseudoproboscispora and Diluviicola, while Atractosporaceae includes the genera Rubellisphaeria and Atractospora. Barbatosphaeria, Xylomelasma and Ceratostomella form a distinct stable lineage which is introduced as a new family Barbatosphaeriaceae in Diaporthomycetidae families incertae sedis. A new family Lentomitellaceae is introduced in Diaporthomycetidae families incertae sedis, to accommodate the genus Lentomitella. Woswasiaceae is introduced to accommodate Woswasia, Xylochrysis and Cyanoannulus in Diaporthomycetidae families incertae sedis. Three new species of Fluminicola viz. F. saprophytica, F. thailandensis and F. aquatica are introduced. A new sexual morph, Dictyosporella thailandensis, is reported and Dictyosporella is excluded from Annulatascaceae and placed in Diaporthomycetidae genera incertae sedis. The first sexual morph of Sporidesmium, S. thailandense is also described. The new species Atractospora thailandensis, Diluviicola aquatica and Pseudoproboscispora thailandensis are also introduced. Platytrachelon is added to Papulosaceae based on phylogenetic analysis and morphological characters. Aquaticola, Fusoidispora and Pseudoannulatascus are excluded from Annulatascaceae and placed in Diaporthomycetidae genera incertae sedis. Mirannulata is accommodated in Sordariomycetes, genera incertae sedis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of species from the family Annulatascaceae and Annulatascaceae-like taxa are recorded from freshwater habitats in tropical and temperate areas (Goh and Hyde 1996; Hyde and Goh 1998; Tsui et al. 2000; Ho et al. 2001; Cai et al. 2002a, 2003; Shearer and Raja 2010). Annulatasceae and Annulatascaceae-like species appear to be predominantly saprobic on submerged wood, but individual species are also known on dead petioles of palms, submerged stems of grasses and decaying bamboo (Fröhlich and Hyde 2000; Cai et al. 2002b; Abdel-Wahab et al. 2011; Boonyuen et al. 2012).
The genus Annulatascus was introduced based on two tropical freshwater taxa, A. velatispora K.D. Hyde as the type species, and A. bipolaris K.D. Hyde, both of which occurred on submerged wood in northern Queensland, Australia (Hyde 1992; Dayarathne et al. 2016). The genus was originally described as representing taxa having black ascomata with long necks, unitunicate, cylindrical asci with massive, refractive, apical rings and fusiform, hyaline, aseptate ascospores with or without mucilaginous sheaths (Hyde 1992). Later, A. bipolaris was transferred to Cataractispora as C. bipolaris (K.D. Hyde) K.D. Hyde (Hyde et al. 1999), because ascospores have a polar pad which unfurl to form long threads in water, a unique and contrasting feature from those in Annulatascus. However, this transfer has not yet been supported by molecular data.
A key to species and a comparative table of morphological features of Annulatascus was provided by Boonyuen et al. (2012) and Luo et al. (2015). Placement of species in this genus based solely on the relatively massive apical ascal ring is not very reliable and molecular data has demonstrated that Annulatascus is polyphyletic (Raja et al. 2003; Campbell and Shearer 2004; Abdel-Wahab et al. 2011; Luo et al. 2015). Phylogenetic analyses in Campbell and Shearer (2004) demonstrated that A. triseptatus S.W. Wong, K.D. Hyde & E.B.G. Jones clustered in a distinct clade which is distantly related to A. velatispora. Morphologically, the ascomata of A. triseptatus are oblate, roughened, and with short setose-like hyphae, a doughnut-shaped ascus apical ring with a subapical flange that is not visible in glycerin, unlike the globose or subglobose, glabrous ascomata and sphaerical ascus apical ring without a subapical flange in A. velatispora. A new genus Annulusmagnus J. Campb. & Shearer was established for Annulatascus triseptatus based on morphological characters and molecular data. Luo et al. (2015) introduced Pseudoannulatascus Z.L. Luo, Maharachch. & K.D. Hyde for Annulatascus biatriisporus K.D. Hyde. Pseudoannulatascus biatriisporus (K.D. Hyde) Z.L. Luo, Maharachch. & K.D. Hyde differs from Annulatascus species in the size of asci and ascospores, the latter which have swollen ends (Hyde 1995; Boonyuen et al. 2012; Luo et al. 2015).
Annulatascus was first placed in Lasiosphaeriaceae Nannf. (Hyde 1992), then transferred to Annulatascaceae S.W. Wong, K.D. Hyde & E.B.G. Jones with the help of ultrastructural data (sensu Barr 1990; Wong et al. 1998). One of the most distinct morphological characters of Annulatascaceae is asci with a relatively massive, refractive, wedge-shaped, J-, apical ring (Wong et al. 1998). Ultrastructural studies on Annulatascaceae-like taxa using Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) have been published (Hyde et al. 1998, 1999; Wong et al. 1999a, b; Wong and Hyde 1999). Diluviicola K.D. Hyde, S.W. Wong and E.B.G. Jones is similar to Annulatascus, Pseudoproboscispora Punith. and Rivulicola K.D. Hyde in having a relatively massive, refractive, apical rings, however, the general structure of the apical rings is different at the TEM level (Hyde et al. 1997, 1998; Wong et al. 1999a; Wong and Hyde 1999; Boonyuen et al. 2012). Hyde et al. (1998) provided a comparative table of three Annulatascaceae-like taxa, including Annulatascus bipolaris, Diluviicola capensis K.D. Hyde, S.W. Wong & E.B.G. Jones and Proboscispora aquatica S.W. Wong & K.D. Hyde, which have unfurling polar appendages at the light and ultrastructural microscope levels. The unique appendage unfurling mechanism and ontogeny were used to separate these three Annulatascaceae-like taxa. Fluminicola S.W. Wong, K.D. Hyde & E.B.G. Jones was introduced to accommodate Annulatascaceae-like taxa with ascospores having a unique appendage form which are adpressed to the ascospore tip and then curled backwards to form an irregular bifurcate or cup-like structure after release in water (Wong et al. 1999b). This unfurling mechanism and ontogeny of appendages can also be used to distinguish Fluminicola from other similar genera. Although ultrastructural studies can separate the above genera, they all have relatively massive apical rings and are saprobic on submerged wood in freshwater. Ho and Hyde (2000) concluded that ultrastructural studies supported the description of new genera after reexamining several species in Annulatascaceae at the ultrastructural level, combining the molecular study of Ranghoo et al. (1999).
Ranghoo et al. (1999) initially reported that Annulatascaceae was a distinct family, using LSU rDNA molecular data. Ho and Hyde (2000) concluded that more sequences of Annulatascaceae-like taxa are needed in phylogenetic analyses to strengthen the data. Based on analyses of sequence data, the placements of some Annulatascaceae-like taxa have been changed. Ceriospora caudae-suis Ingold was introduced by Ingold (1951) who commented that the length and coiling of the ascospore appendages made it different from all other species in the genus. Fallah and Shearer (2001) further described this species, C. caudae-suis, where the ellipsoidal ascospores were overlappingly uni-seriate in the cylindrical asci, which had a J-, apical ring, whereas C. dubyi Niessl, the type species of this genus, has fusiform ascospores arranged overlappingly bi- and triseriate in cylindric-clavate asci, and ascus with a J + , and plug-like apical ring. A phylogenetic analyses carried out by Campbell et al. (2003) showed that C. caudae-suis formed a sister clade with Pseudoproboscispora sp. with strong support and shared similar morphological characters. Thus, Campbell et al. (2003) transferred C. caudae-suis to Pseudoproboscispora caudae-suis (Ingold) J. Campb., Shearer, J.L. Crane & Fallah. Aquaticola ellipsoidea W.H. Ho, K.M. Tsui, Hodgkiss & K.D. Hyde was transferred to Atractospora ellipsoidea (W.H. Ho, K.M. Tsui, Hodgkiss & K.D. Hyde.) Réblová & J. Fourn. based on its ascomata lying horizontally to the host surface, long-pedicellate asci and fusiform ascospores. These characters are typical of the genus Atractospora Réblová & J. Fourn., and Aquaticola ellipsoidea nested in the Atractospora clade with strong support (Réblová et al. 2016). Based on differences in morphology between Ascolacicola austriaca Réblová, Winka & Jaklitsch and A. aquatica Ranghoo & K.D. Hyde, the type species of this genus, the former formed a sister clade with the genus Annulusmagnus J. Campb. & Shearer, and a new genus Ascitendus J. Campb. & Shearer was established (Campbell and Shearer 2004).
It has also been demonstrated using molecular data that several Annulatascaceae-like genera do not belong in the family Annulatascaceae. Brunneosporella Ranghoo & K.D. Hyde and Fluminicola S.W. Wong, K.D. Hyde & E.B.G. Jones were placed in Annulatascaceae due to their cylindrical asci with a large apical ring (Wong et al. 1999b; Ranghoo et al. 2001). However, they clustered with Papulosa Kohlm. & Volkm.-Kohlm. (Abdel-Wahab et al. 2011) in the molecular analysis and were placed in Papulosaceae Winka & O.E. Erikss. by Maharachchikumbura et al. (2015).
A latest morphology based key of genera which belong to or are referred to the family Annulatascaceae was provided by Maharachchikumbura et al. (2016). A comprehensive description of Annulatascaceae was also provided, as “ascomata pale brown to black, or reddish-brown, immersed, semi-immersed, erumpent or superficial, with black or hyaline necks which rarely with setae, hyphae or hairs; peridium coriaceous or membranous, composed of variable cells; asci cylindrical, usually with a massive, refractive, discoid or wedge-shaped, J-, apical ring; ascospores hyaline to brown, aseptate to septate, with or without appendages, sheaths and germ pores” and with a Taeniolella-like asexual morph and mostly saprobic on submerged wood, rarely on bamboo or other substrates in terrestrial habitats (Maharachchikumbura et al. 2016).
A Taeniolella-like asexual morph, was reported in Annulatascaceae from a culture of Chaetorostrum quincemilense Zelski, Raja, A.N. Mill. & Shearer (Zelski et al. 2011). With use of molecular technique, asexual genera can be confidently linked to Annulatascaceae and their sexual morphs (Shenoy et al. 2007, 2010). Ariyawansa et al. (2015) introduced Dictyosporella Abdel-Aziz as an asexual genus in the family Annulatascaceae, but this taxon clustered distantly with other members of Annulatascaceae. Its morphological characters are similar to those of Conlarium F. Liu & L. Cai, however, the two genera are distantly separated and Dictyosporella formed a weakly-supported clade with the sexual genus Cyanoannulus Raja, J. Campb. & Shearer (Ariyawansa et al. 2015).
Although most Annulatascaceae-like taxa clustered together as a distinct clade and separated from other families in earlier studies (Réblová 2006, 2013; Réblová et al. 2014), as more and more taxa were included in phylogenetic analyses, Annulatascaceae was revealed to be polyphyletic (Vijaykrishna et al. 2005, 2006). In a phylogenetic tree in Luo et al. (2015), five genera were placed in Annulatascaceae sensu stricto and ten genera were placed in Annulatascaceae sensu lato. Maharachchikumbura et al. (2016) concluded that 20 genera belong to or should be referred to the family Annulatascaceae. Annulatascaceae remains a heterogeneous assemblage of many genera representing different morphotypes and their phylogenetic positions are not well-studied and natural placements being doubtful due to dearth of DNA sequence data. Thus, new morphological and molecular data are required to clarify their affinities and solve the polyphyly of this family (Maharachchikumbura et al. 2016).
We are studying freshwater fungi along a north/south latitudinal gradient in the Asian region (Hyde et al. 2016). During an ongoing study on these microfungi in Thailand, we encountered several morphologically similar specimens of freshwater Sordariomycetes which are mostly saprobic on submerged bamboo, and were superficially identified as Annulatascaceae-like species. In this paper, we use nine new strains of Annulatascaceae-like taxa and other strains in Sordariomycetes to establish phylogenetic and evolutionary trees. One new order, six new families and nine new species are introduced to accommodate taxa excluded from Annulatascaceae sensu stricto.
Materials and methods
Specimen examination
Decaying wood was collected from Prachuap Khiri Khan, a small river in Thailand in July 2015, following the procedures described in Kurniawati et al. (2010). Samples were placed in Ziploc plastic bags with sterile moist tissue paper and taken to the laboratory. The samples were processed and examined following the methods of Taylor and Hyde (2003). Specimens were examined under a Nikon SMZ-171 dissecting microscope to locate fruiting bodies. Photomicrographs were made under a Nikon ECLIPSE Ni compound microscope with a Cannon EOS 600D camera and an OLYMPUS BX53 compound microscope with a MicroPublisher 5.0 RTV camera. India ink was used for revealing gelatinous sheaths of the ascospores. The fungal structures were measured using Tarosoft (R) Image Frame Work program and images were processed with Adobe Photoshop CS5 Extended version 12.0 × 32 software (Adobe Systems, USA). Isolations were made from single ascospores or conidia as detailed in Chomnunti et al. (2014), on 2% water agar (WA) and then incubated overnight at room temperature or in an incubator (25 °C). Colonies were examined and recorded every seven days. Herbarium specimens are deposited in the herbarium of Mae Fah Luang University (MFLU), Chiang Rai, Thailand and Herbarium of Cryptogams, Kunming Institute of Botany Academia Sinica (HKAS), Kunming, China. Living cultures are deposited in the Mae Fah Luang University Culture Collection (MFLUCC) and Kunming Institute of Botany Culture Collection (KUMCC). Facesoffungi and Index Fungorum numbers are registered as in Jayasiri et al. (2015) and Index Fungorum (2017).
DNA extraction, PCR amplification and sequencing
All cultures were grown on PDA at 25 °C until enough mycelium was obtained and a Biospin Fungus Genomic DNA Extraction Kit (Bioer Technology Co., Ltd., Hangzhou, P.R. China) was used to extract total genomic DNA from the fresh mycelium in terms of instructions of manufacturer. DNA amplification was performed by polymerase chain reaction (PCR). For nucleotide sequence comparisons, fragments of four loci were analysed: LSU, SSU, ITS and RNA polymerase II subunit 2 (RPB2). Primer pairs LROR/LR5 for LSU, NS1/NS4 for SSU, ITS5/ITS4 for ITS and fRPB2-5f/fRPB2-7cR for RPB2 were utilized to amplify. The amplifications were carried out in a 25 μL reaction volume containing 9.5 μL ddH2O, 12.5 μL 2 × PCR Master Mix, 1 μL of DNA template, 1 μL of each primer (10 μM). The PCR thermal cycles for the amplification of the gene regions were as described in Réblová et al. (2011). The PCR products were viewed on 1% agarose electrophoresis gels stained with ethidium bromide. Purification and sequencing of PCR products of four loci were carried out by Shanghai Sangon Biological Engineering Technology & Services Co. Shanghai, P.R. China. DNASTAR Lasergene SeqMan Pro v. 8.1.3 was used to obtain consensus sequences from sequences generated from forward and reverse primers.
Phylogenetic analyses
Sequences generated in this study (Table 1) were supplemented with additional sequences obtained from GenBank, based on blast searches and the literature. Multiple sequence alignments were generated with MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html); the alignments were visually improved with Mesquite v. 2.75 (Maddison and Maddison 2011) and MEGA v. 5.2.2 (Kumar et al. 2012). Phylogenetic analyses of the combined aligned dataset consisted of maximum likelihood (ML), Bayesian Inference (BI) and maximum parsimony (ML) analyses. Ambiguously aligned regions were excluded from all analyses and gaps were treated as “missing data” in the parsimony analyses. A ML analysis was performed using raxmlGUI v. 1.3 (Silvestro and Michalak 2011). The optimal ML tree search was conducted with 1000 separate runs, using the default algorithm of the program from a random starting tree for each run. The final tree was selected among suboptimal trees from each run by comparing likelihood scores under the GTR + GAMMA substitution model. Suitable models for the Bayesian analyses were first selected using models of nucleotide substitution for each gene, as determined using MrModeltest v. 2.2 (Nylander 2004), and included for each gene partition. The Bayesian analyses (MrBayes v. 3.2.1; Ronquist et al. 2012) of four simultaneous Markov Chain Monte Carlo (MCMC) chains were run from random trees for 10,000,000 generations and sampled every 1000 generations. The temperature value was lowered to 0.15, burn-in was set to 0.25, and the run was automatically stopped as soon as the average standard deviation of split frequencies reached less than 0.01. The MP analysis was performed with PAUP v.4.0b10 (Swofford 2003). Trees were inferred by using the heuristic search option with TBR branch swapping and 1000 random sequence additions. The maximum number of retained trees was limited to 5000, branches of zero length were collapsed and all multiple equally most parsimonious trees were saved. Tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), homoplasy index (HI), and log likelihood (−ln L) (HKY model) values were calculated. The robustness of the equally most parsimonious trees was evaluated by 1000 bootstrap replications (Felsenstein 1985) resulting from a maximum parsimony analysis, each with 10 replicates of random stepwise addition of taxa. The Kishino–Hasegawa tests (Kishino and Hasegawa 1989) were performed to determine whether the trees were significantly different. The resulting trees were printed with FigTree v. 1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/) and the layout was completed with Adobe Illustrator CS v. 6.
The aligned datasets from phylogenetic analyses was used in molecular dating analysis. The analysis was performed using BEAST package 1.8.0 (Drummond et al. 2012). The aligned data was partitioned for each LSU, SSU, ITS and RPB2 dataset and these were loaded to BEAUti 1.8.0. for setting up prior parameters and preparing the XML file. Unlinked substitution and clock models were selcected to independently estimate for each gene partition. Taxa sets were developed for each calibration of the common ancestor nodes, associated with the most recent common ancestor, TMRCA. Since different researches have used different prior parameters and calibrations points which can affect the analysis (Gueidan et al. 2011; Prieto and Wedin 2013; Beimforde et al. 2014; Hongsanan et al. 2016; Pérez-Ortega et al. 2016; Hyde et al. 2017), thus we used the divergence time estimates from the recent paper (Hyde et al. 2017) as the reference to decide calibration points used in this study. The most recent common ancestor of Sordariomycetes and Leotiomycetes was set follow Hyde et al. (2017) using normal distribution (mean = 295, SD = 45, with 97.5% CI of 383MYA), and the Sordariomycetes crown with a normal distribution (mean = 250, SD = 45, with 97.5% of CI = 338 MYA). Paleoophiocordyceps coccophagus Sung et al. was used as fossil calibration of Hypocreales, because it is morphologically similar to Ophiocordyceps (Sung et al. 2008; Samarakoon et al. 2016; Hyde et al. 2017). Thus, this fossil data was used for calibration of the Ophiocordyceps crown in Samarakoon et al. (2016) and Hyde et al. (2017), with an exponential distribution (offset = 100, mean = 27.5, with 97.5% CI of 200 Mya). jModeltest 2.1.1 were used to find the best model for each gene partition; GTR + GAMMA for LSU, ITS and RPB2, TrNef + I+G for SSU. We used TN93 with setting ‘‘All Equal’’ for the base frequencies to replace TrNef + I+G. The Lognormal distribution of rates with uncorrelated relaxed clockmodel (ucld) was selected for the analyses. We used the Yule process tree prior to model the speciation of nodes in the topology with a randomly generated starting tree. The number of generations was set with 100 million generations, sampling parameters every 1000 generations. After the XML file was generated from BEAUti 1.8.0, the file was loaded and run in BEAST 1.8.0. The effective sample sizes were checked by loaded.log file into Tracer v.1.6, the acceptable values are higher than 200. The first 20,000 trees representing the burn-in phase suggested by Tracer v.1.6 were discarded, thus 80,000 trees were used in LogCombiner 1.8.0. The maximum clade credibility (MCC) tree was generated by abalysing the file from LogCombiner in TreeAnnotator 1.8.0. The molecular dating tree was viewed in FigTree (Rambaut 2006).
Results
Phylogenetic study
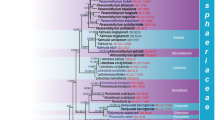
Recent familial classifications of Sordariomycetes provided by Maharachchikumbura et al. (2016) and updated in Hongsanan et al. (2017) are followed here. The placement of taxa at the family level were analyzed using LSU, SSU, ITS rDNA and protein coding RPB 2 sequence data, with Leotia lubrica (Scop.) Pers. as the outgroup taxon (Fig. 1). The alignment comprised 124 strains (including the outgroup taxon Leotia lubrica) with an alignment length of 4511 characters. With parsimony analysis, 1871 characters were constant and 1857 parsimony informative. The parsimony analysis of the data matrix resulted in seven equally parsimonious trees and the first tree (TL = 15,393, CI = 0.297, RI = 0.470, HI = 0.703, RC = 0.140) is shown here (Fig. 1). The GTR + G model with gamma-distributed rate for LSU and the GTR + I+G model with inverse gamma-distributed rate for SSU, RPB2 and ITS were selected and included for each gene partition in Bayesian analyses. Both ML, MP and BI analyses generated trees with similar topologies.
Maximum likelihood (ML) majority rule consensus tree for the combined LSU, SSU, ITS and RPB2 sequence alignment of Diaporthomycetidae and related taxa belonging to Sordariomycetes. RAxML bootstrap support values (ML), maximum parsimony bootstrap support values (MP) are given at the nodes (ML/MP) and Bayesian posterior probabilities (PP) marked with circles. The orders Myrmecridiales, Phomatosporales, Diaporthales, Togniniales, Calosphaeriales, Magnaporthales, Sordariales, Boliniales, Chaetosphaeriales, Xylariales, Amplistromatales, Trichosphaeriales and Ophiostomatales are compressed. Types are in bold and newly introduced sequence data are in violet. The tree is rooted to Leotia lubrica
The Maximum clade credibility tree of Annulatascaceae obtained from BEAST software has a topology which differs little from the topology of the phylogenetic tree. The differences could be found in the genus Dictyosporella which is presented in the clade of Atractosporales, while the genus is close to Distoseptisporaceae in phylogenetic tree. Aquaticola hyalomura strain R038 is closely related to Bullimyces communis strain AF281-3 and Ceratolenta caudata strain CBS 125234 with high PP support in the MCC tree; however, these have low support in phylogenetic tree. The members of Atractosporaceae, Barbatosphaeriaceae and Woswasiaceae formed as distinct clades with high PP support in the MCC tree. Hongsanan et al. (2017) and Hyde et al. (2017) recommended the ranking for families in Sordariomycetes at ca 50–150 MYA. Atractosporaceae has a stem age at ca 98 MYA and Barbatosphaeriaceae has stem age at ca 110 MYA, and Woswasiaceae has stem age at ca 115 MYA which support their family status. Atractosporales has low PP support in the MCC tree. Hyde et al. (2017) recommend the ranking for ordinal status as 130–250 MYA. Although, the stem age of Atractosporales is ca 114 MYA which does not support its ordinal status, the introduction is based on morphological characters.
One new order and six new families are introduced. Nine new strains of Annulatascaceae-like taxa were added, including eight sexual morphs and one asexual morph, which are introduced as new species.Table 2 denotes the main changes.
Species of Sporidesmiaceae form a weak clade (Figs. 1, 2) as in Su et al. (2016), and its sister group is not stable. It is sister to the clade comprising of Mirannulata, Dictyosporella species and Distoseptisporaceae in the phylogenetic tree (Fig. 1) and Papulosaceae in the evolution tree (Fig. 2). Therefore, we maintain Sporidesmiaceae in Diaportheomycetidae, families incertae sedis as in Maharachchikumbura et al. (2016). The sexual morph has never been linked by molecular analysis. In this study, we report the first sexual morph for Sporidesmium sensu stricto.
Mirannulata samuelsii has a sister relationship to different groups in Diaporthomycetidae Distoseptisporaceae in the phylogenetic tree (Fig. 1) and clade comprising of Calosphaeriales Togniniales and Diaporthales in the evolution tree (Fig. 2). However, it was shown sister to Lautospora (Jones et al. 2015) which was placed in Lautosporaceae, Sordariomycetes, families incertae sedis in later study (Maharachchikumbura et al. 2016). Thus, we suggest place Mirannulata in Sordariomycetes, genera incertae sedis.
Dictyosporella is sister to different groups in Diaportheomycetidae: Distoseptisporaceae in the phylogenetic tree (Fig. 1) and the new family Pseudoproboscisporaceae in the evolution tree (Fig. 2). Dictyosporella is characterized by (1) immersed ascomata with subhyaline erumpentt necks, (2) unitunicate, long cylindrical asci with sharply tapered pedicels and a small, unrefractive, wedge-shaped, J-apical ring, (3) uni-seriate, hyaline, fusiform, 3-septate ascospores with sheaths and bipolar appendages, and helicoid, brown to black, globose or subglobose conidia with holoblastic conidiogenous cells. According to phylogenetic result, a new sexual morph is introduced and Dictyosporella is moved from Annulatascaceae to Diaportheomycetidae, genera incertae sedis.
A new family Lentomitellaceae is introduced in Diaportheomycetidae, families incertae sedis, to accommodate the genus Lentomitella which has dark ascomata with long or short necks, cylindrical, short-pedicellate or sessile asci with a distinct, refractive, apical ring and mostly hyaline, 1–2-seriate mostly aseptate ascospores. In this study, Ascocollumdensa clusters with the family Lentomitellaceae in the phylogenetic analysis (Fig. 1), but the species clusters at base of the evolution tree (Fig. 2). Thus, it is removed from Lentomitellaceae. Although the genera Pseudoannulatascus and Fusoidispora form a sister clade with Lentomitellaceae in the phylogenetic tree (Fig. 1), we suggest to place them in Diaporthomycetidae genera incertae sedis.
A new order Atractosporales is established based on the molecular study, for three new families Conlariaceae, Pseudoproboscisporaceae and Atractosporaceae, which forms a distinct group of aquatic Sordariomycetes. Dark brown ascomata with dark brown, erect or lateral long or short necks, clavate but mostly cylindrical asci with a refractive, J-, apical ring, and uni-seriate to biseriate, hyaline, aseptate to multi-septate ascospores with or without sheaths or appendages are prominent characters of Atractosporales. Conlariaceae is introduced for the genus Conlarium comprising two species, C. duplumascospora and C. aquaticum sp. nov. Pseudoproboscisporaceae includes Pseudoproboscispora and Diluviicola, while Atractosporaceae includes the genera Rubellisphaeria and Atractospora. The ascomata of Pseudoproboscisporaceae and Atractosporaceae often lie parallel to the host surface. The ascospores of Atractosporaceae lack appendages, unlike in Conlariaceae and Pseudoproboscisporaceae which have appendages at one or both ends.
The family Papulosaceae forms a clade as in previous studies with each genus having clearly resolved relationships. The genus Platytrachelon affiliated to Papulosaceae in both the phylogenetic (Fig. 1) and evolution trees (Fig. 2). This is similar to the results of Réblová (2013), Réblová et al. (2014) and Jaklitsch et al. (2013). Thus, Platytrachelon is added to Papulosaceae based on the phylogenetic analysis and morphological characters. Three new species of Fluminicola are introduced.
Woswasiaceae is introduced to accommodate Woswasia, Xylochrysis and Cyanoannulus in Diaporthomycetidae families incertae sedis. Woswasiaceae is distinctive by colourful, globose to subglobose ascomata with a cylindrical neck, ascomata nesting together in stromatic or astromatic structures. Asci are unitunicate, cylindrical to fusoid, with a J-, wedge-shaped, apical ring. Eight uniseriate, or rarely 2–4 ascospores are found in each ascus and are globose, subglobose or ellipsoidal, hyaline, unicellular or septate, verruculose or smooth-walled, thin-walled or thick-walled.
Three genera Barbatosphaeria, Xylomelasma and Ceratostomella form a stable clade which is introduced as a new family Barbatosphaeriaceae in Diaporthomycetidae families incertae sedis. Natantiella is close to Ophiostomatales, but with weak support. This result is inconsistent with Senanayake et al. (2016). Therefore, we place Natantiella in Sordariomycetes, genera incertae sedis as reported in Maharachchikumbura et al. (2016).
Four strains Vertexicola confusa, Rhodoveronaea varioseptata, Rhamphoria pyriformis and Rhamphoria delicatula have only LSU sequence data and form a stable clade as in previous analyses (Réblová and Štěpánek 2009; Jaklitsch et al. 2013; Jones et al. 2015) and are sister to Trichosphaeriales. However, the relationships among taxa in this clade and sister clade are unstable. Vertexicola confusa is distant from the other three species (Réblová et al. 2016) and the whole clade separated from Trichosphaeriales in Senanayake et al. (2016). Vertexicola confusa was mentioned in Ranghoo et al. (1999) with only sequence data. Characters include black perithecia with long necks, cylindrical asci with a relative large, refractive, apical ring and ascospores with appendages. It has never been validly published in any mycological journal. The asexual morph Rhodoveronaea varioseptata, type species of Rhodoveronaea, was established by Arzanlou et al. (2007) for ramichloridium-like fungi having septate conidia with a basal and marginal conidial frill. The genus Rhamphoria is an archaic species introduced in 1876, with R. delicatula as the type (Niessl 1876). Fifteen records are listed under this genus in Index Fungorum (2017) and all were introduced before 1930, except R. bevanii in 1977 and R. separata in 1948. The type species needs to be recollected in order to obtain the pivotal characters of this genus. In the present, we place Vertexicola, Rhodoveronaea and Rhamphoria in Diaporthomycetidae genera incertae sedis instead of Annulatascaceae and Sordariomycetidae, genera incertae sedis as in Maharachchikumbura et al. (2016). With exploration of further populations, the clade Rhodoveronaea and Rhamphoria may need to be raised to family rank.
Although most of the new taxa introduced in this study are single collections with a single isolate we prefer to move forward by introducing them as the taxa are relatively rare and difficult to find. The results however, advance the phylogenetic understanding of these freshwater fungi, which have been poorly understood for a long period (Vijaykrishna et al. 2006). We expect further studies to reveal a better undertsanding of the phylogeny and evolution of this poorly represented group of fungi (Vijaykrishna et al. 2006). Our ideas towards introducing new species follow Jeewon and Hyde (2016) and those for higher taxa of Liu et al. (2016).
Taxonomy
Annulatascales D’souza, Maharachch. & K.D. Hyde, Fungal Divers. 72: 212
An order comprising a single family Annulatascaceae was introduced by Maharachchikumbura et al. (2015). Species typically occur on wood in freshwater habitats and have cylindrical, thin-walled asci, with a massive, J-, refractive, apical ring and ascospores usually having appendages or sheaths (Maharachchikumbura et al. 2015). Twenty genera are included in Annulatascaceae, but the inclusion of many are doubtful, due to lack of molecular data (Maharachchikumbura et al. 2015). Annulatascaceae has been reported as closely related to Cordanales and Papulosaceae (Maharachchikumbura et al. 2015), Thyridiaceae and Ophiostomataceae (Maharachchikumbura et al. 2016) or Rhamphoria pyriformis (Pers.) Höhn., Vertexicola confusa and Bullimyces communis A. Ferrer, A.N. Mill., Sarmiento & Shearer (Senanayake et al. 2016). In our phylogenetic analyses, Annulatascaceae appears to have a distant relationship with Bullimyces communis and Ceratolenta caudata Réblová, as well as Papulosaceae.
In Annulatascaceae, Submersisphaeria aquatica K.D. Hyde and Pseudoproboscispora caudae-suis (Ingold) J. Campb., Shearer, J.L. Crane & Fallah (Group 1) cluster together with Ascitendus J. Campb. & Shearer and Annulusmagnus J. Campb. & Shearer (Group 2, Fig. 1), as shown in previous studies (Jaklitsch et al. 2013; Jones et al. 2015; Maharachchikumbura et al. 2015, 2016; Senanayake et al. 2015, 2016). The two groups may need to be raised to family rank when the tree is better populated.
Atractosporales H. Zhang, K.D. Hyde & Maharachch., ord. nov.
Index Fungorum: IF553756; Facesoffungi number: FoF 03333
Sexual morph: Ascomata immersed or semi-immersed, astromatic, subglobose to conical, dark brown, with a lateral neck, often lying horizontally to the substrate surface. Ostiole periphysate. Peridium leathery to fragile, two-layered. Paraphyses abundant. Asci 8-spored, unitunicate, cylindrical, pedicellate, apically rounded, with a distinct, refractive, relatively large, wedge-shaped, J-, apical ring, Ascospores uni-seriate or obliquely uni-seriate, hyaline, fusiform, aseptate or transversely septate. Asexual morph: Hyphomycetous. Colonies dark brown to black, planar, smooth. Conidiophores micronematous or semi-macronematous, mononematous, septate, branched or unbranched, straight or flexuous, hyaline, becoming brown at maturity. Conidiogenous cells determinate, holoblastic, doliiform, cylindrical. Conidia brown, muriform, irregularly globose or subglobose. Conidia produced in culture of Conlarium duplumascospora.
Type family: Atractosporaceae H. Zhang, K.D. Hyde & Maharachch.
Notes: Analyses of combined LSU, SSU, ITS and RPB2 sequence data in the phylogenetic tree (Fig. 1) reveals that Atractosporaceae, Conlariaceae and Pseudoproboscisporaceae group together, forming a group of aquatic Sordariomycetes and this lineage is introduced here as Atractosporales as an order of Diaporthomycetidae. Dark brown ascomata with dark brown, erect or lateral long or short necks, clavate but mostly cylindrical asci with a refractive, J-, apical ring, uni-seriate to biseriate, hyaline, aseptate to multi-septa ascospores with or without sheaths or appendages are prominent characters of Atractosporales. Conlariaceae is introduced for the genus Conlarium comprising two species, Conlarium duplumascospora and Conlarium aquaticum sp. nov. Pseudoproboscisporaceae includes Pseudoproboscispora and Diluviicola, while Atractosporaceae includes the genera Rubellisphaeria and Atractospora. The ascomata of Pseudoproboscisporaceae and Atractosporaceae often lie parallel to the host surface. The ascospores of Atractosporaceae lack appendages, unlike in Conlariaceae and Pseudoproboscisporaceae which have appendages at one or both ends. The phylogenetic position of Aquaticola hyalomura is not stable in our two trees, thus it is not included in Atractosporales.
Atractosporaceae H. Zhang, K.D. Hyde & Maharachch., fam. nov.
Index Fungorum: IF553757; Facesoffungi number: FoF 03334
Saprobic on submerged deciduous wood. Sexual morph: Ascomata immersed or semi-immersed, astromatic, subglobose to conical, dark brown, with a lateral neck, often lying horizontally on the substrate surface. Ostiole periphysate. Peridium leathery to fragile, two-layered. Paraphyses abundant, persistent, cylindrical, flexuous. Asci 8-spored, unitunicate, pedicellate, apically rounded or obtuse, with a J-, relatively small, apical ring, range from 3–4.5 μm diam., 1.5–3.5 μm high. Ascospores uni-seriate, hyaline, fusiform, aseptate or transversely septate. Asexual morph: Undetermined.
Type genus: Atractospora Réblová & J. Fourn., Mycol. Progr. 15(no. 21): 8 (2016)
Notes: This group is grossly under sampled resulting with very few taxa having molecular data. This is partly because the habitats in which they are found are poorly investigated, rather inconspicuous in size and few are known in cultures. Many of these taxa may have originally been recorded as Annulatascaceae-like taxa. The family forms a distinct clade in the phylogenetic tree (Fig. 1), which was also shown in the study of Réblová et al. (2016). Atractospora and Rubellisphaeria are morphologically quite similar and represent a distinct group of aquatic Sordariomycetes. The genera included in this family share freshwater habitats and similar characters in having immersed to semi-immersed, usually light brown to dark brown ascomata, with a relatively thin peridium, comprising a few layers of cells of textura angularis. The asci are mostly cylindrical with apical rings, while ascospores are hyaline and uni-seriate in the asci and most lack sheaths.
Atractospora comprises four species with the type, Atractospora decumbens Réblová & J. Fourn. having dark brown ascomata, long cylindrical asci with a slender pedicel and hyaline, 3-septate, uni-seriate ascospores (Réblová et al. 2016). All species except, A. ellipsoidea, have fusiform, 3-septate, relatively thick-walled ascospores (Ho et al. 1999; Réblová et al. 2016). The monotypic genus Rubellisphaeria has oblique, globose to subglobose ascomata with subhyaline to reddish-brown walls and ellipsoidal ascospores with a delayed formation of the middle septum (Réblová et al. 2016). Aquaticola hongkongensis also nests in this clade, but it has never been validly published (Ranghoo et al. 1999).
We collected a new species in this study which is typical of Atractospora, however the ascospores lack septa and the colonies on PDA are dark brown to grey. The phylogeny places the taxa in Atractospora.
Atractospora Réblová & J. Fourn., Mycol. Progr. 15(no. 21): 8 (2016)
For description refer to (Réblová et al. 2016).
Notes: Atractospora was established by Réblová et al. (2016), and typified by A. reticulata Réblová & J. Fourn. Réblová et al. (2016) reported the genus as characterized by dark, immersed to semi-immersed ascomata lying horizontally on the host, with necks, pedicellate asci with a wedge-shaped, refractive, J-, apical ring and uni-seriate, hyaline, fusiform, aseptate or transversely septate, thick-walled ascospores with smooth or ornamented walls. However, both Atractospora ellipsoidea and our new species A. thailandensis have thin-walled ascospores. Therefore, we extend the generic description to include thin-walled ascospores.
Asexual structures were produced from vegetative mycelium of Atractospora decumbens and described as brown, aseptate, ellipsoidal to subglobose, thick-walled cells often forming short chains of three to five cells (Réblová et al. 2016). The authors did not regard these as asexual morph of A. decumbens.
Atractospora thailandensis W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553212; Facesoffungi number: FoF 03335; Fig. 3
Atractospora thailandensis (holotype) a, b Appearance of dark brown to black ascomata semi-immersed on host. c Ostiole with long neck. d Vertical section of ascoma. e Structure of peridium. f Paraphyses. g–i Unitunicate asci. j Immature ascospore in ascus. k–l Mature ascospores. m Colony on PDA (from front). n Colony on PDA (from reverse). Scale bars a–c = 100 μm; d, g = 20 μm; e–f, i–l = 10 μm; h = 5 μm
Etymology: In reference to the host location, Thailand, where the holotype was collected.
Holotype: HKAS 96226
Saprobic on decaying wood submerged in freshwater. Sexual morph: Ascomata 150–200 μm high, 120–160 μm diam., dark brown to black, gregarious or solitary, semi-immersed, globose to subglobose, unilocular, with long, subcylindrical neck, thick-walled, ostiolate, lying horizontal to the substrate surface. Ostiole up to 170 μm long, subcylindrical, with long neck, straight or slight curved, filled with hyaline, septate periphysis, centrally located. Peridium 19–23 μm thick, comprising outer layers of brown, thick-walled, compressed cells of textura angularis, which are inwardly hyaline. Paraphyses ca 6.5 μm diam. at the base, up to 1 μm at the apex, tapering or suddenly tapering at the apex, numerous, cylindrical, unbranched, hyaline, septate, slightly constricted at the septa. Asci 175–215 × 9–12 μm (\({\bar{x}}=195.7 \times 10.5\,\upmu\text{m}, \;\text{n}=10\)), 8-spored, unitunicate, cylindrical with a slender tapering pedicel, up to 74 μm long, apically rounded or slightly obtuse, with a indistinct, not refractive, wedge-shaped, J-, apical ring, 2.8–3.3 μm high × 3–4 μm wide. Ascospores 20–26 × 7–9 μm (\( \bar{x} =23.2 \times 8.3\,\upmu\text{m}, \; n=20\)), uni-seriate, hyaline, aseptate, fusiform to long citriform, ends sharply tapered, with 2–4 prominent guttules, thin-walled. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged wood in a small river, 30 July 2015, W. Dong 56B (HKAS 96226, holotype); ex-type culture KUMCC 16-0067
Culture characteristics: On PDA, colony circular, reaching 5 mm in 30 days at 25 °C, dark brown to grey from above, black from below, surface hairy with raised elevation, dry, edge entire.
Notes: Atractospora thailandensis forms characteristic dark ascomata, lying horizontally on the host, with upright necks, cylindrical asci with a cylindrical, refractive, J-, apical ring and slender tapering pedicel and fusiform, hyaline, aseptate ascospores, with 2–4 prominent guttules. This is the only species of Atractospora lacking septa. A. thailandensis is most similar to A. ellipsoidea, but the former has larger ascospores (20–26 × 7–9 μm vs. 12–14 × 5–7 μm). Molecular data also separates these species (Fig. 1).
Conlariaceae H. Zhang, K.D. Hyde & Maharachch., fam. nov.
Index Fungorum: IF553758; Facesoffungi number: FoF 03336
Saprobic on submerged wood. Sexual morph: Ascomata perithecioid, gregarious, coriaceous, superficial or partially immersed, dark brown to black, globose to subglobose, smooth. Ostiole elongate, cylindrical, straight or slightly flexuous. Peridium composed of several layers of cells of textura angularis, which are usually darker in the outer layer and pale in the inner layer. Paraphyses cylindrical, hyaline, septate, cells sometimes slightly inflated between the septa, branched, tapering or not, not embedded in a matrix. Asci 8-spored, unitunicate, cylindrical, short pedicellate, apically rounded, with a distinct, refractive, massive, barrel-shaped, apical ring, 4 µm high × 5 µm wide. Ascospores biseriate, hyaline, fusiform, straight or slightly curved, aseptate to multi-septate, guttulate, without appendages or appendages at one or both ends. Asexual morph: Hyphomycetous. Colonies dark brown to black. Mycelium mostly immersed, consisting of branched, septate, thin-walled, smooth, pale brown to brown hyphae. Conidiophores micronematous or semi-macronematous, mononematous, septate or aseptate, unbranched or irregularly branched, straight or flexuous, hyaline, becoming brown when old. Conidiogenous cells holoblastic, determinate, doliiform, cylindrical. Conidia brown, muriform, irregularly globose or subglobose, septate, constricted at the septa (asexual morph description from Liu et al. 2012).
Type genus: Conlarium F. Liu & L. Cai
Notes: This group was introduced to accommodate a single genus Conlarium with both sexual and asexual morphs. Species of Conlarium formed a sister clade to members of Atractosporaceae and Pseudoproboscisporaceae in our phylogenetic analyses. Conlariaceae is characterized by superficial, dark, globose to subglobose ascomata with cylindrical, mostly straight necks, cylindrical asci with a distinct, refractive, massive, barrel-shaped, apical ring and short pedicel and biseriate, fusiform, aseptate to multi-septate, guttulate ascospores with or without appendages. The distinct morphological characters and phylogeny warrant a new family Conlariaceae. The prime features distinguishing Conlariaceae from Atractosporaceae are superficial, ascomata with mostly straight necks, biseriate ascospores with globose or papillary appendages at one or both ends. In Atractosporaceae, ascomata are immersed to semi-immersed, with a lateral neck, often lying horizontally on the substrate surface. The ascospores are mostly uni-seriate in the asci and most lack sheaths.
Conlarium F. Liu & L. Cai, Mycologia 104(5): 1180 (2012)
For description refer to Liu et al. (2012).
Conlarium aquaticum W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553759; Facesoffungi number: FoF 03337; Fig. 4
Etymology: In reference to the aquatic habitat.
Holotype: MFLU 15-2703
Saprobic on decaying, submerged wood in freshwater. Sexual morph: Undetermined. Asexual morph: Colonies sporodochial, broadly punctiform, gregarious or scattered, raised, dark brown to black, velvety. Mycelium mostly immersed on natural substratum, consisting of branched, septate, thin-walled, smooth, pale brown to brown hyphae. Conidiophores absent or reduced to conidiogenous cells. Conidiogenous cells up to 18 µm long, monoblastic, holoblastic, integrated, determinate, cylindrical, hyaline to pale brown, smooth. Conidia 45–70 × 20–57 µm (\( \bar{x}=54.9 \times 39.3 \) µm, n = 30), acrogenous, solitary, dry, subglobose, ellipsoidal, oblong or irregular, brown, clathrate, muriform, 6–12-transversely septate, 4–10-longitudinal septate, slightly constricted at septa, smooth, thin-walled. Conidial secession schizolytic.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged wood in a small river, 30 July 2015, W. Dong 136A (MFLU 15-2703, holotype); ex-type culture MFLUCC 15-0992.
Culture characteristics: On PDA, colony circular, reaching 10 mm in 30 days at 25 °C, grey to dark brown from above, black from below, umbonate, rough, wrinkled, dry, edge undulate.
Notes: The monotypic genus Conlarium was established to accommodate the holomorphic species C. duplumascospora (Liu et al. 2012). Phylogenetic analyses showed that our isolate separate with C. duplumascospora with high support (100% ML, 100% MP, 100% PP) (Fig. 1). Morphologically, C. aquaticum is similar to the asexual morph of Conlarium in muriform conidia. However, they can be distinguished by the number of septa and conidial size (2–4 transversely septa, 1–3 longitudinal septa, 15.5–35 × 11–26.5 µm in C. duplumascospora vs. 6–12 transverse septa, 4–10 longitudinal septa, 45–70 × 20–57 µm in C. aquaticum).
Pseudoproboscisporaceae H. Zhang, K.D. Hyde & Maharachch., fam. nov.
Index Fungorum: IF553760; Facesoffungi number: FoF 03338
Saprobic on decaying bamboo submerged in freshwater. Sexual morph: Ascomata scattered or solitary, immersed with neck erumpent through host surface, dark brown to black, ostiolate. Ostiole central, erect, dark brown, apical with mass of releasing spores yellowish. Peridium comprising several layers of flattened cells. Paraphyses wide, hypha-like, septate, tapering. Asci 8-spored, cylindrical, unitunicate, pedicellate, with a relatively large, J-, apical ring. Ascospores uniseriate or overlappingly uniseriate, ellipsoidal or fusiform, aseptate or 1–3-septate, hyaline, with bipolar filamentous appendages. Appendages initially coiled or proboscis-like then unfurling to form long threads on release. Asexual morph: Undetermined.
Type genus: Pseudoproboscispora Punith.
Notes: This family includes the genera Cateractispora, Pseudoproboscispora and Diluviicola based on molecular study. The prominent morphological characters are appendages which are at first coiled and then unfurl in water to form long threads. The ascomata are light brown to dark brown, with central or lateral dark brown necks. Asci are clavate to cylindrical, with a refractive, wedge-shaped, J-, apical ring. Ascospores are uni-seriate to biseriate, fusiform, hyaline, 0–1- or 3-septa, with appendages.
Cateractispora Ranghoo, K.D. Hyde & E.C.Y. Liew has never been validly published. We observed a photo-plate of this species in the PhD thesis of Ranghoo (1998) without any description. The plate shows that ascospores appendages of C. recepticuli are at first coiled and proboscispora-like, and then unfurl in water to form long polar, thread-like appendages, as in Pseudoproboscispora aquatica (S.W. Wong & K.D. Hyde) Punith. (= Proboscispora aquatica S.W. Wong & K.D. Hyde), the type species of Pseudoproboscispora. It has also been observed by Cai et al. (2006), in which they named the same plate as P. aquatica. According to morphological characters, Cateractispora is likely to be an invalidly published synonym of Pseudoproboscispora. However, Pseudoproboscispora caudae-suis (Ingold) J. Campb., Shearer, J.L. Crane & Fallah clusters in Annulusmagnaceae distant from C. recepticuli. Pseudoproboscispora should be compared with Diluviicola as both have unfurling appendages. These taxa can be separated as the appendages are cap-like in Diluviicola and stretch to form filaments and are proboscis-like in Pseudoproboscispora and unwind.
Aquaticola hyalomura W.H. Ho, K.M. Tsui, Hodgkiss & K.D. Hyde also clusters as a sister group to Pseudoproboscisporaceae in the phylogenetic tree (Fig. 1). However, it clustered in a different clade in the MCC tree (Fig. 2). Réblová et al. (2016) showed that Aquaticola is polyphyletic. This is confirmed in our study as A. hongkongensis and A. hyalomura (type species) clustered in two distinct clades. Due to this unstable phylogenetic relationship, Aquaticola hyalomura is not placed in Pseudoproboscisporaceae.
Our new collection MFLU 15-2705 has immersed, dark brown ascomata with central, erect, dark brown necks and hyaline ascospores with proboscis-like appendages, which unfurl to form thread-like appendages. The species is typical of Pseudoproboscispora. Another new collection MFLU 15-2701 is typical of Diluviicola.
Diluviicola K.D. Hyde, S.W. Wong & E.B.G. Jones, Fungal Diversity Res. Ser. 1: 141 (1998)
For description refer to Hyde et al. (1998).
Diluviicola aquatica W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553762; Facesoffungi number: FoF 03340; Fig. 5
Diluviicola aquatica (holotype) a–b Ascomata semi-immersed or superficial on host. c Vertical section of ascoma. d Structure of peridium. e Paraphyses. f–h, j–k Unitunicate asci. i Ascospore with long appendages. l Ascospore mounted in India ink. m–o Ascospores mounted in water. p Germinating ascospore. q Colony on PDA (from front). r Colony on PDA (from reverse). Scale bars b = 100 μm; c, g = 20 μm; d–f, h–i, l–p = 10 μm; j = 2 μm; k = 5 μm
Etymology: In reference to aquatic habitat.
Holotype: MFLU 15-2701
Saprobic on decaying bamboo submerged in freshwater. Sexual morph: Ascomata 70–100 μm high, 115–145 μm diam., scattered or solitary, semi-immersed or superficial, subglobose or ellipsoidal, dark brown, ostiolate. Ostiole 35–45 μm long, 18–22 μm wide, dark brown, growing laterally to the host surface, curving upwards. Peridium 15–20 μm thick, two-layered, outer layer comprising 4–5 layers of dark brown, large, compressed cells of textura angularis, inner layer comprising 3–4 layers of hyaline, compressed, elongate cells. Paraphyses numerous, cylindrical, unbranched, hyaline, septate, constricted at the septa, tapering towards the apex, ca 6.2 μm diam. at the base, up to 1.5 μm at the apex, not embedded in a gelatinous matrix. Asci 120–180 × 14–20 μm (\(\bar{x} = 143.1 \times 16.3\,\upmu\text{m},\; \text{n}=10\)), 8-spored, unitunicate, clavate to cylindrical, pedicellate, with a distinct, refractive, wedge-shaped, J-, apical ring, 3.2–4 μm high × 4.2–4.5 μm wide. Ascospores 23–28 × 7–10 μm (\(\bar{x} = 25.3 \times 8.3 \upmu\text{m}, \;\text{n}=20\)), obliquely uni-seriate or partially biseriate or sometimes triserial, fusiform, straight or slightly curved, smooth or rugose, aseptate when young, 0–1-septate when mature, slightly constricted at the septa, hyaline, thin-walled, guttulate, with bipolar filamentous appendages. Appendages initially coiled or cap-like then unfurl to form long threads after releasing, up to 98 μm long. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged bamboo in a small river, 30 July 2015, W. Dong 55A (MFLU 15-2701, holotype); ex-type culture MFLUCC 15-0986.
Culture characteristics: On PDA, colony circular, reaching 10 mm in 40 days at 25 °C, dark brown to black from above, black from below, raised, rough, dry, edge entire or undulate.
Notes: Diluviicola capensis, the type species of Diluviicola, is characterized by semi-immersed or superficial, dark brown ascomata with short, dark brown necks growing laterally to the host surface and curving upwards, clavate to cylindrical asci with a distinct, refractive, wedge-shaped, J-, apical ring and obliquely uni-seriate or partially biseriate or sometimes triseriate, fusiform, 0–1-septate, hyaline, guttulate ascospores with cap-like appendages, which form long threads after releasing (Hyde et al. 1998). Diluviicola aquatica resembles D. capensis in having black ascomata with short necks growing laterally to the ascomata, fusiform, hyaline ascospores with cap-like appendages and the same unfurling mechanism (Hyde et al. 1998). However, D. aquatica was considered to differ from D. capensis as the latter has obpyriform ascomata with hyaline necks and uni-seriate ascospores as compared with subglobose or ellipsoidal ascomata with dark brown necks and mostly biseriate ascospores in D. aquatica.
Pseudoproboscispora Punith., Kew Bull. 54(1): 234 (1999)
For description refer to Wong and Hyde (1999).
Pseudoproboscispora thailandensis W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553761; Facesoffungi number: FoF 03339; Fig. 6
Etymology: In reference to the freshwater habitat.
Holotype: MFLU 15-2705
Saprobic on decaying bamboo submerged in freshwater. Sexual morph: Ascomata scattered or solitary, immersed with neck erumpent through host surface, dark brown to black, ostiolate. Ostiole 200–300 μm long, 30–70 μm wide, central, erect, dark brown, apical with mass of releasing spores appear yellowish. Peridium comprising several layers of compressed brown-walled cells. Paraphyses wide, hypha-like, septate, tapering distally. Asci deliquesced. Ascospores 23–27 × 7–9 μm ({\(\bar{x} = 24.5 \times 8.2\,upmu\text{m}, \;\text{n}=15\)), fusiform, less straight or mostly curved, smooth, aseptate, hyaline, minutely guttulate, with bipolar filamentous appendages. Appendages initially coiled or proboscis-like then unfurl to form long threads after releasing. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged bamboo in a small river, 30 July 2015, W. Dong 41C (MFLU 15-2705, holotype); ex-type culture MFLUCC 15-0989.
Culture characteristics: On PDA, colony circular, reaching 5 mm in 30 days at 25 °C, dark brown to black from above, dark brown from below, raised, rough, dry, edge entire.
Notes: Pseudoproboscispora thailandensis has the typical appendages and unfurling mechanism found in Pseudoproboscispora. P. aquatica, the type species, has a hyaline neck, while P. thailandensis has a dark brown neck. In addition, it differs in having aseptate rather than three-septate ascospores. The asci were not seen in this collection because they had deliquesced. Like many aquatic ascomycetes, the asci can only be seen at an early stage.
Diaporthomycetidae families incertae sedis
Barbatosphaeriaceae H. Zhang, K.D. Hyde & Maharachch., fam. nov.
Index Fungorum: IF553763; Facesoffungi number: FoF 03341
Saprobic on decaying wood or other plant materials. Sexual morph: Ascomata astromatic, leathery to fragile, dark brown to black, solitary or usually aggregated in circular to oval nests or in short rows, globose to subglobose, slightly roughened, with long necks, both venter and neck are sparsely covered with a pubescence that disappears with age, necks remaining covered with septate hairs, ostiolate. Ostiole cylindrical, long, straight to slightly flexuous, periphysate, when in circular groups decumbent to perpendicular, covering, piercing the periderm in a group but never united in a disk. Peridium two layered, outer layer composed of brown cells of textura prismatica, with wide lumina tending to be longer toward the interior and becoming smaller outwards, inner layer composed of elongate, hyaline cells of textura prismatica. Paraphyses abundant, persistent, cylindrical, unbranched, hyaline, septate, constricted at the septa, tapering to the apex, longer than the asci. Asci unitunicate, clavate, tapering toward the stipe, with a shallow, refractive, J-, apical ring, floating freely in centrum at maturity. Ascospores 1- or 2-seriate, oblong to ellipsoidal, septate, non- or slightly constricted at the median septum, hyaline, smooth-walled. Asexual morph: Hyphomycetous. Ramichloridium- and Sporothrix-like. Conidiophores of the sporothrix-type micronematous to semi-micronematous, unbranched or branched, cylindrical to flask- or irregular-shaped, hyaline, denticulate. Conidiogenous cells polyblastic, integrated, and terminal with several denticles producing conidia holoblastically. Conidia ellipsoidal to suballantoid, curved, unicellular, hyaline. Conidiophores of the Ramichloridium-type macronematous, simple, erect, septate, brown, paler and thin-walled toward the apex. Conidiogenous cells polyblastic, integrated, terminal, cylindrical, tapering with rachis, proliferating sympodially. Conidia ellipsoidal, straight or curved, unicellular, hyaline (Réblová 2007). Conidia produced in culture of Barbatosphaeria barbirostris.
Type genus: Barbatosphaeria Réblová
Notes: This family presently comprises three genera, Barbatosphaeria, Ceratostomella and Xylomelasma, which was also apparent in Réblová et al. (2016). The genera are morphologically quite similar and represent a distinct group. They share similar characters in having dark, long-necked, astromatic ascomata, mostly surrounded by sparse mycelium or pubescence, clavate or cylindrical-clavate asci, arising from ascogenous hyphae and mostly ellipsoidal ascospores. In our study, Barbatosphaeriaceae forms a sister clade to Natantiella ligneola and the order Ophiostomatales Nannf. However, it formed a sister clade comprising of Ophiostomatales, Phomatosporales and Amplistromatales. Therefore, Barbatosphaeriaceae can not be phylogenetically assigned to any orders and we place it in Diaporthomycetidae families incertae sedis.
Barbatosphaeria is phenotypically similar to Lentomitella in ascospore morphology and the long-necked, dark ascomata surrounded by sparse mycelium or pubescence. However, Barbatosphaeria can be distinguished as ascomata form circular formations with the pubescence disappearing with age; clavate asci arising from croziers on proliferating ascogenous hyphae without a high-level branching system and with a shallow apical ring and basal pedicel and smooth ascospores (Réblová 2007). Both genera are clearly separated in our phylogenetic tree (Fig. 1) and other studies (Réblová 2007).
Ceratostomella sensu lato comprises 110 species in Index Fungorum (2017), with the type, Ceratostomella vestita Sacc. Lentomitella was introduced by Höhnel (1905) based on C. vestita to accommodate taxa distinct from Ceratostomella in having ornamented (longitudinally striate) ascospore walls. Ceratostomella was discussed and redescribed by Réblová (2006), who emended the generic concept based on lectotype species C. rostrata (Tode: Fr.) Sacc. Ceratostomella can be characterized by dark, astromatic, elongate ascomata surrounded by sparse mycelium; clavate to cylindrical-clavate asci arising from branched, discrete ascogenous hyphae, with short pedicels and a shallow, indistinct, J- apical ring and pale brown aseptate, ascospores ranging from suballantoid to irregularly ellipsoid, to globose, to reniform,, sometimes with terminal pores (from emended generic concept in Réblová 2006).
Xylomelasma is similar to Ceratostomella in having pigmented ascospores before discharge, similar ascogenous hyphae and asci floating within the centrum at maturity, but differs in having cylindrical, septate paraphyses, generally larger cylindrical asci with a distinct, refractive, apical ring and ellipsoidal to oblong, obliquely uni-seriate ascospores (Réblová 2006).
The genus Natantiella was shown to cluster with Barbatosphaeriaceae in Senanayake et al. (2016). In our study, however, it is close to Ophiostomatales, but with weak support. This is similar to Jaklitsch et al. (2013) and Réblová et al. (2014). Therefore, we place Natantiella in Sordariomycetes, genera incertae sedis as in Maharachchikumbura et al. (2016).
Lentomitellaceae H. Zhang, K.D. Hyde & Maharachch., fam. nov.
Index Fungorum: IF553764; Facesoffungi number: FoF 03342
Saprobic on branch or decorticated wood. Sexual morph: Ascomata solitary or gregarious, immersed to superficial, globose, subglobose or conical, glabrous or slightly roughened, dark brown. Necks dark brown, central, elongate, periphysate. Peridium coriaceous, two-layered, outer layer composed of brown, thick-walled cells of textura prismatica to textura angularis, externally with a row of heavily melanized cells, inner layer composed of subhyaline to hyaline, thinner-walled, elongated and compressed cells. Paraphyses numerous, cylindrical, tapering. Asci 8-spored, cylindrical-clavate, sessile, with a refractive, J-, apical ring. Ascospores obliquely 1–2-seriate, hyaline, ellipsoidal, aseptate to several septate. Asexual morph: Hyphomycetous. Conidiophores macronematous, mononematous, hyaline, short. Conidiogenous cells hyaline, cylindrical, tapering toward the apex, terminal or intercalary, bearing several hyaline denticles. Conidia ellipsoidal to globose, apiculate at the base, aseptate, (Conidia produced in culture of Lentomitella crinigera, description from Réblová 2006).
Type genus: Lentomitella Höhn.
Notes: Lentomitellaceae is introduced to accommodate the presently monotypic genus Lentomitella in Diaportheomycetidae, families incertae sedis. The genus is characterized by dark ascomata, with long or short necks, cylindrical, short-pedicellate or sessile asci, with a distinct, refractive, apical ring and mostly hyaline, 1–2-seriate mostly aseptate ascospores. Lentomitella was segregated from Ceratostomella by Höhnel (1905), based on the type species C. vestita Sacc. Lentomitella accommodates taxa that have dark brown ascomata with long necks, cylindrical-clavate, sessile asci with a distinct, J-, apical ring, relatively wide, distinctly tapering paraphyses, hyaline, ellipsoidal, aseptate or several-septate ascospores, often with longitudinal ridges and a hyphomycetous asexual morph (Réblová 2006). Sequence data and morphological characters also warrant the distinction between Lentomitella and Ceratostomella (Réblová 2006). Lentomitella comprises seven species in Index Fungorum (2017), with the type L. vestita (Sacc.) Höhn, with the current name L. cirrhosa (Réblová 2006). All species, except L. pallibrunnea (pale brown ascospores) and L. tropica (pale brown ascospores), have hyaline ascospores. A mucilaginous sheath was observed in some spores of L. unipretoriae (Marincowitz et al. 2008).
The clade comprising species of Pseudoannulatascus, Fusoidispora and Ascocollumdensa forms a sister clade with Lentomitellaceae in phylogenetic tree with weak support (Fig. 1). The monotypic genus Pseudoannulatascus was segregated from Annulatascus to accommodate Annulatascus biatriisporus K.D. Hyde based on molecular study and morphological differences that are large asci, and larger, long-fusiform ascospores with swollen ends (Hyde 1995; Luo et al. 2015). The ascomata are immersed, ellipsoidal with long, black and cylindrical necks, asci unitunicate, cylindrical, short-pedicellate with a large, refractive, spherical, apical ring; and ascospores overlapping uni-seriate, aseptate, hyaline, long fusiform with a thin mucilaginous sheath (Luo et al. 2015). Fusoidispora is characterized by dark brown ascomata lying horizontal to the host surface with very short necks, long cylindrical, pedicellate asci with a refractive, discoid, J-, apical ring and hyaline, fusoid to sickle-shaped, guttulate, aseptate to rarely 5-septate ascospores with globose mucilaginous pads at the ends (Vijaykrishna et al. 2005). Pseudoannulatascus and Fusoidispora were accepted in Annulatascaceae in the study of Maharachchikumbura et al. (2016). But they are distant from Annulatascaceae in our phylogenetic tree. Therefore, we suggest placement of Pseudoannulatascus and Fusoidispora in Diaporthomycetidae genera incertae sedis rather than Annulatascaceae until more evidence is available. The monotypic genus Ascocollumdensa was introduced by Ranghoo et al. (1999) with A. aquatica Ranghoo, K.D. Hyde & E.C.Y. Liew as the type species. It has never been validly published. It was briefly described as having “black perithecia with long necks, cylindrical asci with a relative large refractive apical ring and ascospores with appendages”. It needs recollecting, examining and sequencing because it has been placed in Xylariomycetidae, Diaporthomycetidae and Lulworthomycetidae in different papers. We do not include Ascocollumdensa in Lentomitellaceae until more evidence is available.
Papulosaceae Winka & O.E. Erikss., Mycoscience 41 (2): 102 (2000)
Notes: The family Papulosaceae was introduced by Winka and Eriksson (2000) to accommodate the monotypic marine genus Papulosa. Maharachchikumbura et al. (2015) placed two freshwater genera, Brunneosporella (as Ascobrunneispora aquatica Ranghoo, K.D. Hyde & E.C.Y. Liew) and Fluminicola in this family. It is characterized by immersed or semi-immersed, black to dark brown, globose to ellipsoidal ascomata, with long periphysate necks, cylindrical asci with a short pedicel and a refractive, discoid, J-, apical ring, and uniseriate to biseriate, hyaline or brown, ellipsoidal or fusiform, unicellular to 3-septate ascospores, with or without cup-like, bipolar appendages (Kohlmeyer and Volkmann-Kohlmeyer 1993; Maharachchikumbura et al. 2016). Maharachchikumbura et al. (2016) placed the genus in Diaporthomycetidae families incertae sedis and provided a discussion concerning the family. Su et al. (2016) showed that Papulosaceae formed a sister clade to Sporidesmiaceae and Trichosphaeriaceae. In our study, it clusters with Annulatascales (Fig. 1) and Sporidesmiaceae (Fig. 2).
The monotypic genus Platytrachelon, typified by P. abietis (Réblová) Réblová also nests in Papulosaceae with strong support in our study, as shown in previous studies (Jaklitsch et al. 2013; Réblová 2013; Réblová et al. 2014). The genus was placed in Diaporthomycetidae genera incertae sedis in Maharachchikumbura et al. (2016) due to its simple and inconspicuous morphology. Platytrachelon abietis is characterized by immersed, dark brown to black, globose to subglobose ascomata with long, central necks, cylindrical-clavate asci with a long pedicel and a refringent wedge-shaped apical ring, and uniseriate, hyaline, fusiform, 3(–5)-septate ascospores (Réblová 2013). The genus produces a hyphomycetous asexual morph which has ellipsoid, pale brown, 1-septate conidia. Platytrachelon abietis fits the characters of Papulosaceae well except for the freshwater habitat. We therefore place the genus in Papulosaceae.
Fluminicola S.W. Wong, K.D. Hyde & E.B.G. Jones, Fungal Diversity Res. Ser. 2: 190 (1999)
Notes: The genus Fluminicola was established for the freshwater species F. bipolaris (Wong et al. 1999b). It is characterized by cylindrical asci with a relatively massive, refractive apical ring and fusiform, hyaline ascospores with irregular bifurcate or cup-like bipolar appendages (Wong et al. 1999b). Fluminicola was placed in Annulatascaceae by Wong et al. (1999b), and transferred to Papulosaceae by Réblová (2013) based on phylogenetic analyses. This was also shown by Maharachchikumbura et al. (2015) and our study (Fig. 1). The asexual morph of Fluminicola remains to be discovered.
Fluminicola aquatica W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553765; Facesoffungi number: FoF 03344; Fig. 7
Fluminicola aquatica (holotype) a, b Appearance of necks on host. c Vertical section of ascoma. d Structure of peridium. e Paraphyses. f–i Unitunicate asci. j Young ascospore with bipolar appendages (arrowed). k–l Mature ascospore with bipolar appendages (arrowed). m Germinating ascospore. n Colony on PDA (from front). o Colony on PDA (from reverse). Scale bars a = 200 μm; b = 100 μm; c = 50 μm; d–g, m = 10 μm; h–i = 20 μm; j–l = 5 μm
Etymology: In reference to the freshwater habitat.
Holotype: MFLU 15-2710
Saprobic on decaying wood submerged in freshwater. Sexual morph: Ascomata 140–180 μm high, 100–140 μm in diam., scattered or solitary, immersed with neck erumpent through host surface, uniloculate, ellipsoidal, glabrous, brown, coriaceous. Ostiole 155–175 μm long, 55–65 μm wide, cylindrical, central, brown, periphysate. Peridium 15–25 μm wide, two-layered, outer layer comprising 5–6 layers of dark brown, thick-walled, large cells of textura angularis, inner layer comprising 4–5 layers of pale brown to hyaline, thin-walled, compressed cells of textura angularis. Paraphyses ca 6.6 μm diam. at the base, up to 1.9 μm at the apex, tapering, cylindrical, unbranched, hyaline, septate, constricted at the septa. Asci 180–200 × 9–14 μm (\(\bar{x} = 185.7 \times 10.2\,\upmu\text{m},\; \text{n}=10\)), 8-spored, unitunicate, cylindrical, subcylindrical or lanceolate, with a short, narrow pedicel, ca 20 μm long, with an indistinct, refractive, relatively small, discoid, J-, apical ring, 1.9–2.1 μm high × 2.7–3 μm wide. Ascospores 19–22 × 6–8 μm (\(\bar{x} = 20.4 \times 6.7\,\upmu\text{m}, \text{n}=20 \)), obliquely uni-seriate, hyaline, fusiform, straight or slightly curved, with a single prominent guttule, aseptate when young, becoming 1–2 guttulate and 3-septate when mature, slightly constricted at the septa, smooth and thin-walled, with bifurcate bipolar appendages. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged wood in a small river, 30 July 2015, W. Dong 42B (MFLU 15-2710, holotype); ex-type culture MFLUCC 15-0962.
Culture characteristics: On PDA, colony circular, 10 mm in 15 days at 25 °C, dark brown to black from above, black from below, umbonate, rough, dry, edge entire.
Notes: Morphologically, our collection should be placed in Fluminicola as it has characteristic features of cylindrical asci with an apical ring and fusiform, hyaline ascospores with bifurcate bipolar appendages. Fluminicola aquatica is similar to F. bipolaris. It differs from F. bipolaris in the colour of the ascomata (brown vs. black) and longer asci (180–200 × 9–14 μm vs. 107–192 × 9–12 μm). Additionally, the arrangement of ascospores in the asci can distinguish the taxa. Fluminicola bipolaris has a relatively massive, refractive, apical ring (3–6 μm high × 1.5–3 μm wide), while F. aquatica has a relatively small, apical ring (1.9–2.1 μm high × 2.7–3 μm wide). Fluminicola coronata Ranghoo, K.D. Hyde & E.C.Y. Liew has never been validly published. It was described as having “black perithecia with long necks, cylindrical asci with a relative large refractive apical ring and ascospores with appendages” (Ranghoo et al. 1999). Molecular data confirmed that F. aquatica can be distinguished from F. coronata and the other two taxa within this genus defined in this study.
Fluminicola saprophytica W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553766; Facesoffungi number: FoF 03345; Fig. 8
Fluminicola saprophytica (holotype) a, b Appearance of necks on host. c Vertical section of ascoma. d Structure of peridium. e Paraphyses. f–h Unitunicate asci. i Germinating ascospore. j–m Ascospores mounted in water. n Ascospores mounted in India ink. o Colony on PDA (from front and reverse). Scale bars a = 100 μm; b = 200 μm; c = 50 μm; d–g, i = 10 μm; h, j–n = 5 μm
Etymology: In reference to saprobic life mode of the fungus.
Holotype: MFLU 15-2694
Saprobic on decaying bamboo submerged in freshwater. Sexual morph: Ascomata 200–230 μm high, 145–175 μm diam., scattered or solitary, immersed with neck erumpent through host surface, globose, subglobose or ellipsoidal, black. Ostiole central, brown, with straight necks. Peridium 12–23 μm thick, two-layered, outer wall comprising 3–4 layers of brown, thick-walled, large cells of textura angularis to textura prismatica, inner wall comprising 2–3 layers of pale brown to hyaline, thin-walled, compressed cells of textura angularis. Paraphyses ca 6.5 μm diam. at the base, up to 2.5 μm at the apex, tapering, sparse, unbranched, hyaline, septate, constricted at the septa, often with minute guttules. Asci 120–140 × 8–12 μm (\(\bar{x} = 116.2 \times 9.4\,\upmu\text{m}, \;\text{n}=10 \)), 8-spored, unitunicate, cylindrical or subcylindrical, pedicellate, tapering to a point, apically rounded or slightly obtuse, with an indistinct, refractive, small, discoid, J-, apical ring, 2.4–2.7 μm high × 2.4–2.9 μm wide. Ascospores 15–19 × 6–7 μm (\(\bar{x} = 17.0 \times 6.5\,\upmu\text{m},\; \text{n}=10 \)), obliquely uni-seriate and partially overlapping, 3-septate, fusiform, straight or slightly curved, constricted at the septa, with 4 prominent guttules, hyaline, smooth and thick-walled, with an irregular sheath. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged bamboo in a small river, 30 July 2015, W. Dong 04A (MFLU 15-2694, holotype); ex-type culture MFLUCC 15-0976.
Culture characteristics: On PDA, colony irregular, reaching 15 mm in 40 days at 25 °C, dark brown to grey from above, black from below, umbonate, veined or rough, dry, edge curled.
Notes: The ascospores of Fluminicola saprophytica are of similar length and shape as those of the type species, F. bipolaris, but lack bifurcate, bipolar appendages in contrast to the latter species. It also differs from F. bipolaris in its smaller apical rings, 2.4–2.7 μm high × 2.4–2.9 μm wide vs. 3–6 μm high × 1.5–3 μm wide. Fluminicola saprophytica is similar to F. aquatica in having immersed ascomata with neck erumpent through host surface, and 3-septate, fusiform, guttulate, hyaline ascospores. However, F. saprophytica has smaller ascospores with an irregular sheath and without bifurcate bipolar appendages contrasting with those of F. aquatica. Fluminicola saprophytica clustered together with F. aquatica in our phylogenetic tree (Fig. 1).
Fluminicola thailandensis W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553767; Facesoffungi number: FoF 03346; Fig. 9
Fluminicola thailandensis (holotype) a appearance of necks on host. b Vertical section of ascoma. c Structure of peridium. d Paraphyses. e–h Unitunicate asci. i–k Ascospores mounted in water. l Ascospore mounted in Melzer’s Reagent. m Ascospores mounted in India ink clearly showing mucilaginous sheath. n Germinating ascospore. o Colony on PDA (from front). p Colony on PDA (from reverse). Scale bars a = 100 μm; b = 30 μm; c–f, h, k, n = 10 μm; g, i–j, l–m = 5 μm
Etymology: In reference to Thailand, where the holotype was collected.
Holotype: MFLU 15-2704
Saprobic on decaying bamboo submerged in freshwater. Sexual morph: Ascomata 60–100 μm high, 130–180 μm diam., scattered or solitary, deeply immersed with neck erumpent through host surface, ellipsoidal with the base tortuous toward the interior, black. Ostiole central or lateral, brown, with straight upright, black necks, ca 230 μm long. Peridium 14–18 μm thick, two-layered, outer layer comprising 3–4 layers of dark brown, thick-walled, large cells of textura angularis, inner layer comprising 2–3 layers of pale brown, thin-walled, compressed cells of textura angularis. Paraphyses ca 2.7 μm diam. at the base, up to 0.5 μm at the apex, tapering, sparse, unbranched, thick-walled, brown, septate, not constricted at the septa. Asci 186–193 × 8–12 μm (\(\bar{x} = 189.5 \times 10.2\,\upmu\text{m},\; \text{n}=10\)), 8-spored, unitunicate, cylindrical, pedicellate, up to 52 μm long, tapering to a point, apically rounded or slightly obtuse, with an indistinct, refractive, small, discoid, J-, apical ring, 1.5–3 μm high × 2.7–3.9 μm wide. Ascospores 14–20 × 7–9 μm (\(\bar{x} = 19.2 \times 7.6\,\upmu\text{m},\; \text{n}=20\)), uni-seriate or overlapping uni-seriate, 3-septate, fusiform, constricted at the septa, with 2 prominent guttules, hyaline, smooth and thick-walled, with a thin mucilaginous sheath. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged wood in a small river, 30 July 2015, W. Dong 22A (MFLU 15-2704, holotype); ex-type culture MFLUCC 15-0984.
Culture characteristics: On PDA, colony irregular, reaching 15 mm in 25 days at 25 °C, dark brown to black from above, black from below, raised, rough, dry, edge undulate.
Notes: Fluminicola thailandensis is most similar to F. saprophytica in having fusiform, 3-septate, guttulate ascospores. However, it can be distinguished from F. saprophytica by its longer asci (186–193 × 8–12 μm vs. 120–140 × 8–12 μm) and wider ascospores (7–9 μm vs. 6–7 μm). In our phylogenetic tree, two taxa formed a sister clade with strong support (100% ML, 100% MP) (Fig. 1).
Sporidesmiaceae Fr. [as ‘Sporidemsiacei’], Summa veg. Scand., Sectio Post. (Stockholm): 504 (1849)
Saprobic on woody plants in terrestrial and aquatic habitats. Sexual morph: Ascomata scattered or solitary, immersed, uniloculate, subglobose or ellipsoidal, coriaceous. Ostiole cylindrical, periphysate. Peridium three-layered. Paraphyses numerous, tapering, hypha-like. Asci 8-spored, unitunicate, long cylindrical, with a tapering pedicel, with a distinct, J-, apical ring. Ascospores obliquely uni-seriate, fusiform, hyaline. Asexual morph: Hyphomycetous. Colonies hairy or velvety, effuse, brown, grey or black. Conidiophores macronematous, mononematous, solitary or sometimes aggregated, unbranched, brown. Conidiogenous cells monoblastic, holoblastic, terminal, cylindrical, brown, proliferating once percurrently at apex. Conidia acrogenous, solitary, brown, obclavate.
Type genus: Sporidesmium Link
Notes: The family Sporidesmiaceae was introduced by Fries (1849), but the taxon has not been widely used in modern polymorphic classification. The family is typified by Sporidesmium with S. ehrenbergii as the lectotype species (see below) and Shenoy et al. (2006) showed the genus to be polyphyletic. Su et al. (2016) treated Ellisembia as a synonym of Sporidesmium based on molecular data and placed it in the monotypic Sporidesmiaceae and this is followed here. In our study, Sporidesmiaceae shows a sister relationship to Distoseptisporaceae and Lentomitellaceae in the phylogenetic tree (Fig. 1), while it clusters with Papulosaceae in the evolution tree (Fig. 2). We report the first molecular-based sexual morph for Sporidesmiaceae in this study.
Sporidesmium Link, Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 41 (1809)
Synonymy:
=Podoconis Boedijn, Bull. Jard. bot. Buitenz, 3 Sér. 13(1): 133 (1933)
=Ellisembia Subram., Proc. Indian natn Sci. Acad., Part B. Biol. Sci. 58(4): 183 (1992)
Facesoffungi number: FoF 01750
Saprobic on woody plants in terrestrial and aquatic habitats. Sexual morph: Ascomata scattered or solitary, immersed with neck erumpent through host surface, uniloculate, subglobose or ellipsoidal, glabrous, brown, coriaceous. Ostiole cylindrical, periphysate. Peridium three-layered, outer layer comprising several layers of dark brown to black, oblong and rounded cells, middle layer comprising 3–4 layers of pale brown to hyaline, compressed cells of textura prismatica, inner layer comprising 4–5 layers of hyaline, large cells of textura angularis or irregular cells. Paraphyses numerous, tapering, hypha-like, hyaline, septate, unbranched, persistent, embedded in a gelatinous matrix. Asci 8-spored, unitunicate, long cylindrical, with a tapering pedicel, apically rounded, with a distinct, relatively small, refractive, wedge-shaped, J-, apical ring. Ascospores obliquely uni-seriate, fusiform, hyaline, 3-septate. Asexual morph: Hyphomycetous. Colonies hairy or velvety, effuse, brown, grey or black. Mycelium superficial or immersed, composed of branched, septate, thin-walled, smooth, hyaline to pale brown hyphae. Conidiophores macronematous, mononematous, solitary, or sometimes aggregated in groups, cylindrical, straight or curved, erect or somewhat repent, unbranched, brown, septate, smooth-walled. Conidiogenous cells monoblastic, integrated, terminal, cylindrical, brown, prolife rating percurrently at apex. Conidia acrogenous, solitary, pale brown to brown, obclavate, or cylindrical with tapering ends, with hyaline apex and truncate base, euseptate or distoseptate, length determinate, truncate at the base, some with basal scar, smooth-walled (description of asexual morph from Su et al. 2016).
Lectotype: Sporidesmium ehrenbergii M.B. Ellis
Notes: The generic concept of Sporidesmium has been defined and emended several times in its history (Link 1809; Ellis 1958, 1971; Wu and Zhuang 2005; Shenoy et al. 2006; Su et al. 2016). Su et al. (2016) provided a detailed discussion on this genus and provided a description based on the lectotype S. ehrenbergii M.B. Ellis. The type species of this genus needs to be epitypified (sensu Ariyawansa et al. 2014), in order to fix the generic concept (Su et al. 2016). The sexual morph of Sporidesmium has never been confirmed. In this study, we collected a sexual morph taxon which clustered with Sporidesmium species with high support (Fig. 1). This is the first sexual morph of Sporidesmium confirmed by molecular study.
Sporidesmium thailandense W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index Fungorum: IF553768; Facesoffungi number: FoF 03347; Fig. 10
Sporidesmium thailandense (holotype) a, b Appearance of ascomata on host. c Vertical section of ascoma. d Peridium. e Paraphyses. f–g Unitunicate asci. h Ascus with a wedge-shaped apical ring. i–n Ascospores. i is mounted in India ink showing the sheath. o Ascospore with germ tube on substratum. p Germinating ascospore. q Colony on PDA (from front). r Colony on PDA (from reverse). Scale bars a, b = 100 μm; c = 50 μm; d, f, g, p = 20 μm; e = 5 μm; h–o = 10 μm
Holotype: MFLU 15-2709
Etymology: In reference to Thailand, where the holotype was collected.
Saprobic on decaying wood submerged in freshwater. Sexual morph: Ascomata 150–190 μm high, 190–230 μm in diam., scattered or solitary, immersed with neck erumpent through host surface, uniloculate, subglobose or ellipsoidal, glabrous, brown, coriaceous. Ostiole 290–320 μm long, 80–90 μm wide, cylindrical, central or lateral, brown at the base, becoming hyaline towards the apex, periphysate. Peridium 25–35 μm thick, three-layered, outer layer comprising several layers of dark brown to black, oblong and rounded cells, middle layer comprising 3–4 layers of pale brown to hyaline, compressed cells of textura prismatica, inner layer comprising 4–5 layers of hyaline, large cells of textura angularis or irregular cells. Paraphyses ca 3.5 μm diam. at the base, up to 1 μm at the apex, tapering, numerous, hypha-like, hyaline, septate, unbranched, persistent, embedded in a gelatinous matrix. Asci 160–220 × 11–14 μm (\(\bar{x} = 189.1 \times 12.5\,\upmu\text{m},\; \text{n}=10 \)), 8-spored, unitunicate, long cylindrical, with a tapering pedicel up to 22 μm long, apically rounded, with a distinct, relatively small, refractive, wedge-shaped, J-, apical ring, 2–2.2 μm high × 4.5–4.8 μm wide. Ascospores 23–28 × 8–10 μm (\(\bar{x} = 25.2 \times 8.9\,\upmu\text{m},\; \text{n}=20\)), not overlapping, obliquely uni-seriate, fusiform, straight or slightly curved, hyaline, 3-septate, slightly constricted at the septa, thin-walled, guttulate when young, with 2–5 prominent guttules when mature, with a thin sheath. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged wood in a small river, 30 July 2015, W. Dong 11A (MFLU 15-2709, holotype); ex-type culture MFLUCC 15-0964.
Culture characteristics: On PDA, colony irregular, reaching 10 mm in 40 days at 25 °C, white to yellow from above, yellow from below, umbonate, rough or wrinkled, dry, edge undulate.
Notes: The sexual morphs of Sporidesmium have been associated with the dothideomycetous genera, Akaropeltella, Eupelte and Placosoma, as well as the sordariomycetous genera Miyoshiella and Umbrinosphaeria (Casagrande 1969; Sivanesan 1984; Hyde et al. 2011; Wijayawardene et al. 2012). Miyoshiella fusispora Kawamura (type), M. larvata Réblová and M. triseptata (Shoemaker & White) Réblová were considered to be associated with Sporidesmium in Réblová (1999). They all have cylindrical asci with an apical ring and fusiform, 3-septate ascospores (Shoemaker 1985; Réblová 1999), which are similar to the new collection. However, Miyoshiella can be separated by the characters of ascomata (superficial, black, pyriform ascomata often associated with asexual morph in Miyoshiella vs. immersed, brown, subglobose or ellipsoidal ascomata with neck erumpent through host surface and without asexual morph surrounded in the new collection) and peridium (two-layered peridium with outer layer comprising dark cells of textura angularis, inner layer comprising hyaline, compressed cells in Miyoshiella vs. three-layered peridium with outer layer comprising dark, oblong and rounded cells, middle layer comprising brown, compressed cells of textura prismatica, inner layer comprising hyaline, large cells of textura angularis or irregular cells in the new collection) (Shoemaker 1985; Réblová 1999). Umbrinosphaeria caesariata (Clinton & Peck) Réblová resembles the new collection in having cylindrical, pedicellate asci and fusiform, septate, guttulate ascospores, but it differs by its larger ascomata often sparsely covered with conidiophores and sterile, and 7(–12)-septate, 2–3-seriate ascospores (middle cells becoming brown when old) (Réblová 1999). None of these taxa can be linked by molecular data and as this character form is common amongst freshwater fungi, we treat the new collection as a distinct species until the above taxa are sequenced. Therefore, this sexual morph is introduced as a new species in Sporidesmium and it is the first sexual morph species proven by molecular studies.
Sporidesmium thailandense is similar to Fluminicola saprophytica in having immersed ascomata with necks erumpent through host surface, cylindrical asci and obliquely uni-seriate, fusiform, 3-septate, guttulate ascospores. However, they can be distinguished by the characters of neck (black in F. saprophytica vs. brown at the base, becoming hyaline towards the apex in S. thailandense), size and shape of apical ring (2.4–2.7 μm high × 2.4–2.9 μm wide, indistinct, discoid apical ring in F. saprophytica vs. 2–2.2 μm high × 4.5–4.8 μm wide, distinct, wedge-shaped apical ring in S. thailandense) and spore size (15–19 × 6–7 μm in F. saprophytica vs. 23–28 × 8–10 μm in S. thailandense). Additionally, ascospores lack of sheaths in F. saprophytica.
Woswasiaceae H. Zhang, K.D. Hyde & Maharachch., fam. nov.
Index Fungorum: IF553769; Facesoffungi number: FoF 03348
Saprobic, hyperparasitic or hypersaprotrophic on stromata of Diaporthe. Sexual morph: Ascomata aggregated or in groups, stromatic, immersed to erumpent, globose, subglobose or ellipsoid, coloured, seated in a stroma or basal stroma. Ostiole centrally located, cylindrical, upright or slightly horizontal to the substrate surface, pale brown, dark brown to black. Paraphyses abundant, persistent, hyaline, septate. Asci 8-spored, unitunicate, cylindrical to fusoid, with a distinctive, J-, apical ring. Ascospores uni-seriate or overlapping uni-seriate, globose, subglobose, ellipsoidal or fusiform, hyaline, unicellular or septate, thin-walled or thick-walled, verruculose or smooth-walled, with or without gelatinous sheath. Asexual morph: Produced in culture with or without sporodochial conidiomata. Conidiophores aggregated, hyaline to subhyaline, with a clavate or penicillate head or conidiogenous cells developing directly on hyphae. Conidiogenous cells monoblastic or polyblastic, terminal, sympodially proliferating, cylindrical, hyaline. Conidia ellipsoid to obovoid, tapering towards the base, hyaline, aseptate, smooth-walled (sexual morph and asexual morph description from Jaklitsch et al. 2013 and Réblová et al. 2014).
Type genus: Woswasia Jaklitsch, Réblová & Voglmayr
Notes: Woswasiaceae is distinctive among lignicolous Diaporthomycetidae in forming coloured, globose to subglobose ascomata with a cylindrical neck and ascomata nested together in stromatic or nonstromatic tissues. Asci are unitunicate, cylindrical to fusoid, with a J-, apical ring. Ascospores are globose, subglobose or ellipsoidal, hyaline, unicellular or septate, verruculose or smooth-walled and thin-walled or thick-walled (Jaklitsch et al. 2013 and Réblová et al. 2014). Three genera, Woswasia, Xylochrysis and Cyanoannulus are included according to our phylogenetic analysis. A close relationship of Woswasia and Xylochrysis is supported in both the phylogenetic (Fig. 1) and evolution trees (Fig. 2) as well as in recent studies (Jaklitsch et al. 2013; Réblová et al. 2014; Senanayake et al. 2016). Xylochrysis is phenotypically more similar to Woswasia as it often has aggregated astromatic ascomata or with bases slightly immersed and seated on a small, basal stroma. The venter is surrounded by a golden yellow layer of cells. Asci are cylindrical, with a J-, wedge-shaped, apical ring (Réblová et al. 2014). The asexual morphs produced from culture have similar holoblastic conidiogenesis and branched conidiophores with clavate heads (Réblová et al. 2014). Réblová et al. (2014) compared the two genera and mentioned the main differences as phenotypical characters of ascomata (ascomata with stromata with colour changing with the addition of water and 3% KOH in Woswasia, whereas astromatic or base seated on an inconspicuous stroma which colour not changing in Xylochrysis) and morphology of ascospores (globose to subglobose, verruculose and thick-walled in Woswasia, whereas ellipsoidal, smooth and thin-walled in Xylochrysis). In addition, Woswasia is apparently hyperparasitic or hypersaprotrophic on stromata of Diaporthe oncostoma (Duby) Fuckel (Jaklitsch et al. 2013), while Xylochrysis is saprobic and terrestrial (Réblová et al. 2014).
Cyanoannulus, monotypic with C. petersenii Raja. is stable and close to Woswasia and Xylochrysis, but has weak support (Fig. 1, Réblová et al. 2014, Senanayake et al. 2016). It was saprobic on submerged wood in freshwater streams and previously placed in Annulatascaceae based on LSU sequence data (Raja et al. 2003). Cyanoannulus is characterized by immersed pale reddish-brown, globose ascomata, slightly horizontal to the substrate surface, central, long, cylindrical necks becoming tapering and lighter towards the apex, fusoid asci with a large, bipartite, J-, but cotton blue positive, apical ring and thick-walled ascospores with a narrow channel at the apices and with a mucilaginous sheath (Raja et al. 2003). The characters, such as coloured ascomata, morphology of asci fits Woswasiaceae well. Therefore, no other placment is presently more suitable than Woswasiaceae for Cyanoannulus. Woswasia resembles Cyanoannulus in having pale brown to dark ascomata with long necks, a two layered peridium, J-, but cotton blue positive asci and hyaline ascospores. Woswasia is easily distinguished from Cyanoannulus by its characteristic colour changing stromata that is dark pink, purple or brown on surface when dry, purple or nearly violet when rehydrated, turning immediately green to black after the addition of 3% KOH; the stromata surface comprises a layer of intricate, hyaline, pink to brownish hyphae; ascomata separate towards the margins, are then superficial, the venter entirely enclosed in a dense pink hyphal subiculum with a glabrous neck (Jaklitsch et al. 2013). Cyanoannulus has no stromata. Because Woswasiaceae has been shown sister to Phomatosporales (Fig. 1), Magnaporthales (Senanayake et al. 2016) or Ceratolenta caudata (Jaklitsch et al. 2013; Réblová et al. 2014), we place the genus in Diaportheomycetidae, families incertae sedis until more taxa are discovered.
Diaporthomycetidae genera incertae sedis
Aquaticola W.H. Ho, K.M. Tsui, Hodgkiss & K.D. Hyde, Fungal Diversity Res. Ser. 3: 88 (1999)
Notes: The genus Aquaticola is polyphyletic (Réblová et al. 2016) and therefore its characters must be taken from the type species, Aquaticola hyalomura W.H. Ho, K.M. Tsui, Hodgkiss & K.D. Hyde (Ho et al. 1999) whose placement is not stable. It clusters close to the family Pseudoproboscisporaceae in our phylogenetic tree (Fig. 1), while it is close to Bullimyces communis and Ceratolenta caudata in the evolution tree (Fig. 2). This species which occurs on submerged wood in freshwater, has white to pale brown ascomata, small, broadly oblong asci with a relatively wide, ring-like, apical ring and tapering base, and small, overlapping uni-seriate to biseriate ascospores with distinct guttules. Aquaticola ellipsoidea W.H. Ho, K.M. Tsui, Hodgkiss & K.D. Hyde is somewhat similar, but has dark brown to black ascomata, cylindrical asci and 1-celled, inequilaterally fusoid to rhomboid ascospores (Ho et al. 1999). In the phylogenetic tree (Fig. 1), A. ellipsoidea clusters with Atractospora species and was synonymized as Atractospora ellipsoidea by Réblová et al. (2016). Aquaticola hongkongensis Ranghoo, K.D. Hyde & E.C.Y. Liew which has never been validly published and groups with Atractospora species. The type species of Aquaticola needs to be recollected and epitypified (sensu Ariyawansa et al. 2014) to establish if Atractospora is a synonym, however, they appear to be morphologically distinct. We tentatively place Aquaticola in Diaporthomycetidae genera incertae sedis instead of in Annulatascaceae (Maharachchikumbura et al. 2016).
Dictyosporella Abdel-Aziz, in Ariyawansa et al., Fungal Diversity 75: 143 (2015)
Saprobic on grass stem or decaying bamboo submerged in freshwater. Sexual morph: Ascomata scattered or solitary, immersed with neck erumpent through host surface, uniloculate, globose, subglobose or ellipsoidal, glabrous, brown, coriaceous. Ostiole long, cylindrical, periphysate. Peridium comprising of several layers of brown, thick-walled, compressed cells of textura porrecta. Paraphyses numerous, cylindrical, hyaline, septate, unbranched, persistent. Asci 8-spored, unitunicate, long cylindrical, with a tapering pedicel, apically rounded, with an indistinct, J-, apical ring. Ascospores uni-seriate or overlapping uni-seriate, fusiform, hyaline, 3-septate, with filamentous bipolar appendages. Asexual morph: Mycelium superficial and immersed in the substrate, composed of branched, septate, smooth, yellow–brown to brown hyphae. Conidiophores reduced. Conidiogenous cells holoblastic. Conidia terminal and lateral, unicellular, determinate, helicoid when young and quickly becoming a mass of cells, brown to black. Conidial cells are generally similar in shape, colour and size that are globose, subglobose, rounded or with truncate base, brown to dark-brown (from Ariyawansa et al. 2015).
Notes: The genus Dictyosporella was introduced for an asexual morph species D. aquatica, which is characterized by helicoid, brown to black, globose or subglobose conidia (Ariyawansa et al. 2015). Dictyosporella was placed in the family Annulatascaceae (Ariyawansa et al. 2015; Maharachchikumbura et al. 2016). In our phylogeny (Fig. 1), Dictyosporella grouped in Distoseptisporaceae, while in the evolution tree Dictyosporella clustered with Pseudoproboscisporaceae. Given this, we suggest that Dictyosporella is temporarily assigned to Diaportheomycetidae, genera, incertae sedis.
Phylogenetic analyses showed that our collection grouped together with Dictyosporella aquatica with high support, but is a different species (Fig. 1). Thus, a new species, Dictyosporella thailandensis is introduced in Dictyosporella. This is the first account of a sexual morph for this genus.
Dictyosporella thailandensis W. Dong, H. Zhang & K.D. Hyde, sp. nov.
Index fungorum: IF553770; Facesoffungi number: FoF 03349; Fig. 11
Dictyosporella thailandensis (holotype) a Appearance of necks on host. b Vertical section of ascoma. c Structure of peridium. d Paraphyses. e–h Unitunicate asci. i–l Ascospores mounted in water. m Ascospore mounted in India ink showing the appendages. n Colony on PDA (from front). o Colony on PDA (from reverse). Scale bars b = 30 μm; c, d, h–m = 10 μm; e, f = 20 μm; g = 5 μm
Etymology: In reference to Thailand, where the holotype was collected.
Holotype: MFLU 15-2706
Saprobic on decaying bamboo submerged in freshwater. Sexual morph: Ascomata 50–100 μm high, 40–90 μm in diam., scattered or solitary, immersed with neck erumpent through host surface, uniloculate, globose, subglobose or ellipsoidal, glabrous, brown, coriaceous. Necks 70–100 μm long, 40–60 μm wide, cylindrical, central or slightly lateral, hyaline to pale yellow, thin-walled, periphysate. Peridium 14–19 μm thick, comprising of several layers of brown, thick-walled, compressed cells of textura porrecta. Paraphyses ca 3.5 μm diam. at the base, up to 1 μm at the apex, tapering, numerous, cylindrical, hyaline, septate, unbranched, persistent. Asci 130–155 × 9–12 μm (\(\bar{x} = 138.6 \times 10.5\,\upmu\text{m},\; \text{n}=10\)), 8-spored, unitunicate, long cylindrical, pedicellate, up to 35 μm long, suddenly tapered at one-third, apically rounded, with an indistinct, small, refractive, wedge-shaped, J-, apical ring, 1.3–1.6 μm high × 2.1–2.3 μm wide. Ascospores 18–22 × 6–8 μm (\(\bar{x} = 20 \times 7.1\,\upmu\text{m},\; \text{n}=20\)), uni-seriate or overlapping uni-seriate, fusiform, straight, hyaline, 3-septate, slightly constricted at the septa, thin-walled, with large prominent guttules when old, with filamentous bipolar appendages. Asexual morph: Undetermined.
Material examined: THAILAND, Prachuap Khiri Khan, on submerged bamboo in a small river, 30 July 2015, W. Dong 22B (MFLU 15-2706, holotype); ex-type culture MFLUCC 15-0985.
Culture characteristics: On PDA, colony circular, researching 30 mm in 35 days at 25 °C, brown to grey from above, dark brown from below, flat, surface rough, dry, edge entire.
Notes: Dictyosporella thailandensis is characterized by immersed ascomata with subhyaline, erumpent necks, long, unitunicate cylindrical asci with tapering pedicels, and small, refractive, wedge-shaped, J-, apical rings, and uni-seriate, hyaline, fusiform, 3-septate ascospores with bipolar appendages. Its ascospores are quite similar to the phylogenetically unrelated taxa, Fluminicola aquatica and Sporidesmium thailandense. However, in Fluminicola aquatica, ascomata have central, relatively long, dark brown necks and ascospores have bifurcate bipolar appendages, while in Dictyosporella thailandensis, ascomata have lateral, short, hyaline to pale yellow necks and ascospores have filamentous bipolar appendages. Sporidesmium thailandense differs in its necks (brown at the base, becoming hyaline towards the apex) and larger ascospores (23–28 × 8–10 μm vs. 18–22 × 6–8 μm), as well as ascospores that lack apppendages. Line drawing of the peridia of D. thailandensis as well as other Annulatascaceae-like new species are shown in Fig. 12.
Peridium of new species. a Atractospora thailandensis (HKAS 96226, holotypus). b Diluviicola aquatica (MFLU 15-2701, holotypus). c Fluminicola aquatica (MFLU 15-2710, holotypus). d Fluminicola saprophytica (MFLU 15-2694, holotypus). e Fluminicola thailandensis (MFLU 15-2704, holotypus). f Sporidesmium thailandense (MFLU 15-2709, holotypus). g Dictyosporella thailandensis (MFLU 15-2706, holotypus). Scale bars 50 μm
References
Abdel-Wahab MA, Abdel-Aziz FA, Mohamed SS, Abdel-Aziz AE (2011) Annulatascus nilensis sp. nov., a new freshwater ascomycete from the River Nile. Egypt. IMA Fungus 2:1–6
Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EBG et al (2014) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers 69:57–91
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B et al (2015) Fungal diversity notes 111–252-taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–274
Arzanlou M, Groenewald JZ, Gams W, Braun U et al (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud Mycol 58:57–93
Barr ME (1990) Prodromus to nonlichenized, pyrenomycetous members of class Hymenoascomycetes. Mycotaxon 39:43–184
Beimforde C, Feldberg K, Nylinder S, Rikkinen J et al (2014) Estimating the phanerozoic history of the Ascomycota lineages: combining fossil and molecular data. Mol Phylogenet Evol 78:386–398
Boonyuen N, Sri-Indrasutdhi V, Suetrong S, Sivichai S, Jones EBG (2012) Annulatascus aquatorba sp. nov., a lignicolous freshwater ascomycete from Sirindhorn Peat Swamp Forest, Narathiwat. Thailand. Mycologia 104:746–757
Cai L, Lumyong P, Zhang KQ, Hyde KD (2002a) New species of Annulatascus and Saccardoella from the Philippines. Mycotaxon 84:255–263
Cai L, Tsui CKM, Zhang KQ, Hyde KD (2002b) Aquatic fungi from Lake Fuxian, Yunnan, China. Fungal Divers 9:57–70
Cai L, Zhang KQ, McKenzie EHC, Hyde KD (2003) Freshwater fungi from bamboo and wood submerged in the Liput River in the Philippines. Fungal Divers 13:1–12
Cai L, Hyde KD, Tsui CKM (2006) Genera of freshwater fungi. Fungal Divers Res Ser 18:1–261
Campbell J, Shearer CA (2004) Annulusmagnus and Ascitendus, two new genera in the Annulatascaceae. Mycologia 96:822–833
Campbell J, Shearer CA, Crane JL, Fallah PM (2003) A reassessment of two freshwater ascomycetes, Ceriospora caudae-suis and Submersisphaeria aquatica. Mycologia 95:41–53
Casagrande F (1969) Ricerche biologiche e sistematiche su particolari ascomiceti pseudosferiali. J Phytopathol 66:97–136
Chomnunti P, Hongsanan S, Aguirre-Hudson B, Tian Q et al (2014) The sooty moulds. Fungal Divers 66:1–36
Dayarathne MC, Maharachchikumbura SSN, Phookamsak R, Fryar S et al (2016) Morpho-molecular characterization and epitypification of Annulatascus velatisporus. Mycosphere 7:1389–1398
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973
Ellis MB (1958) Clasterosporium and some allied Dematiaceae-Phragmosporae. I Mycol Pap 7:1–89
Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew
Fallah PM, Shearer CA (2001) Freshwater ascomycetes: new or noteworthy species from north temperate lakes in Wisconsin. Mycologia 93:566–602
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fries EM (1849) Summa vegetabilium scandinaviae. Part 2. Bonnier, Leipzig
Fröhlich J, Hyde KD (2000) Palm microfungi. Fungal Divers Res Ser 3:1–393
Goh TK, Hyde KD (1996) Biodiversity of freshwater fungi. J Ind Microbiol Biotech 17:328–345
Gueidan C, Ruibal C, de Hoog GS, Schneider H (2011) Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biol. 115:987–996
Ho WH, Hyde KD (2000) A new family of freshwater ascomycetes. Fungal Divers 4:21–36
Ho WH, Tsui CKM, Hodgkiss IJ, Hyde KD (1999) Aquaticola, a new genus of Annulatascaceae from freshwater habitats. Fungal Divers 3:87–97
Ho WH, Hyde KD, Hodgkiss JI, Yanna (2001) Fungal communities on submerged wood from streams in Brunei, Hong Kong, and Malaysia. Mycol Res 105:1492–1501
Höhnel FV (1905) Mykologishe fragmente CVI–CXVII. Ann Mycol 3:548–560
Hongsanan S, Sánchez-Ramírez S, Crous PW, Ariyawansa HA et al (2016) The evolution of fungal epiphytes. Mycosphere 7:1690–1712
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Diversity 84:25–41
Hyde KD (1992) Tropical Australian freshwater fungi. II. Annulatascus velatispora gen. et sp. nov., A. bipolaris sp. nov. and Nais aquatica sp. nov. (Ascomycetes). Aust Systematic Botany 5:117–124
Hyde KD (1995) Tropical Australian freshwater fungi. VII. New genera and species of Ascomycetes. Nova Hedwigia 61:119–140
Hyde KD, Goh TK (1998) Fungi on submerged wood in Lake Barrine, north Queensland, Australia. Mycol Res 102:739–749
Hyde KD, Read SJ, Jones EBG, Moss ST (1997) Tropical Australian freshwater fungi. XII. Rivulicola incrustata gen. et sp. nov. and notes on Ceratosphaeria lampadophorai. Nova Hedwigia 64:185–196
Hyde KD, Wong SW, Jones EBG (1998) Diluviocola capensis gen. and sp. nov., a freshwater ascomycete with unique polar caps on the ascospores. Fungal Divers 1:133–146
Hyde KD, Wong SW, Jones EBG (1999) Cataractispora gen. nov. with three new freshwater lignicolous species. Mycol Res 103:1019–1031
Hyde KD, McKenzie EHC, KoKo TW (2011) Towards incorporating anamorphic fungi in a natural classification–checklist and notes for 2010. Mycosphere 2:1–88
Hyde KD, Fryar S, Tian Q, Bahkali AH, Xu JC (2016) Lignicolous freshwater fungi along a north–south latitudinal gradient in the Asian/Australian region; can we predict the impact of global warming on biodiversity and function? Fungal Ecol 19:190–200
Hyde KD, Maharachchikumbura SSN, Hongsanan S, Samarakoon MC et al (2017) The ranking of fungi: a tribute to David L. Hawksworth on his 70th birthday. Fungal Diversity 84:1–23
Ingold CT (1951) Aquatic ascomycetes: Ceriospora caudae-suis nov. sp. and Ophiobolus tyhae. Trans Br Mycol Soc 34:210–215
Jaklitsch WM, Réblová M, Voglmayr H (2013) Molecular systematics of Woswasia atropurpurea gen. et sp. nov. (Sordariomycetidae), a fungicolous ascomycete with globose ascospores and holoblastic conidiogenesis. Mycologia 105:476–485
Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat DJ (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers 74:3–18
Jeewon R, Hyde KD (2016) Establishing species boundaries and new taxa among fungi: recommendations to resolve taxonomic ambiguities. Mycosphere 7:1669–1677
Jones EBG, Suetrong S, Sakayaroj J, Bahkali AH et al (2015) Classification of marine Ascomycota, Basidiomycota, Blastocladiomycota and Chytridiomycota. Fungal Divers 73:1–72
Kishino H, Hasegawa M (1989) Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data. J Mol Evol 29:170–179
Kohlmeyer J, Volkmann-Kohlmeyer B (1993) Two new genera of Ascomycotina from saltmarsh Juncus. Syst Ascomycetum 11:95–103
Kumar S, Stecher G, Peterson D, Tamura K (2012) MEGACC: computing core of molecular evolutionary genetics analysis program for automated anditerative data analysis. Bioinformatics 28:2685–2686
Kurniawati E, Zhang H, Chukeatirote E, Sulistyowati L et al (2010) Diversity of freshwater ascomycetes in freshwater bodies at Amphoe Mae Chan, Chiang Rai. Cryptogr Mycol 31:323–331
Link HF (1809) Observationes in ordines plantarumnaturales. Dissertatio I. Mag. Ges. Naturf. Freunde Berlin 3:3–42
Liu F, Hu DM, Cai L (2012) Canlarium duplumascospora gen. et. sp nov and Jobellisia guangdongensis sp nov from freshwater habitats in China. Mycologia 104:1178–1186
Liu NG, Ariyawansa HA, Hyde KD, Maharachchikumbura SSN et al (2016) Perspectives into the value of genera, families and orders in classification. Mycosphere 7:1649–1668
Luo ZL, Maharachchikumbur SSN, Liu XY, Li SH et al (2015) Annulatascus saprophyticus sp. nov. and Pseudoannulatascus gen. nov. to accommodate Annulatascus biatriisporus (Annulatascales, Sordariomycetes) from Thailand. Phytotaxa 239:174–182
Maddison WP, Maddison DRV (2011) Mesquite: a modular system for evolutionary analysis, version 2.75. http://mesquiteproject.org
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC et al (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC et al (2016) Families of Sordariomycetes. Fungal Divers 79:1–317
Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ (2008) Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series 7:1–166
Niessl G (1876) Notizen über neue und kritische Pyrenomyceten. Verh Naturforsch Ver Brünn 14:165–218
Nylander JAA (2004) MrModeltest 2.0. Program distributed Author. Evolutionary Biology Centre, Uppsala University, Uppsala
Pérez-Ortega S, Garrido-Benavent I, Grube M, Olmo R, de los Ríos A (2016) Hidden diversity of marine borderline lichens and a new order of fungi: Collemopsidiales (Dothideomyceta). Fungal Divers 80:285–300
Prieto M, Wedin M (2013) Dating the diversification of the major lineages of Ascomycota (Fungi). PLoS ONE 8(6):e65576
Raja HA, Campbell J, Shearer CA (2003) Freshwater ascomycetes: Cyanoannulus petersenii, a new genus and species from submerged wood. Mycotaxon 88:1–17
Rambaut A (2006) FigTree v1.0. http://beast.bio.ed.ac.uk/FigTree
Ranghoo VM (1998) Phylogeny of freshwater ascomycetes. Dissertation, University of Hong Kong
Ranghoo VM, Hyde KD, Liew ECY, Spatafora JW (1999) Family placement of Ascotaiwania and Ascolacicola based on DNA sequences from the large subunit rRNA gene. Fungal Divers 2:159–168
Ranghoo VM, Tsui CK, Hyde KD (2001) Brunneosporella aquatica gen. et sp. nov., Aqualignicolahyalina gen. et sp. nov., Jobellisiaviridifusca sp. nov. and Porosphaerellopsis bipolaris sp. nov. (ascomycetes) from submerged wood in freshwater habitats. Mycol Res 105:625–633
Réblová M (1999) Studies in Chaetosphaeria sensu lato III. Umbrinosphaeria gen. nov. and Miyoshiella with Sporidesmium anamorphs. Mycotaxon 71:13–43
Réblová M (2006) Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia 98:68–93
Réblová M (2007) Barbatosphaeria gen. et comb. nov., a new genus for Calosphaeria barbirostris. Mycologia 99:723–732
Réblová M (2013) Two taxonomic novelties in the Sordariomycetidae: Ceratolenta caudata gen. et sp. nov. and Platytrachelon abietis gen. et comb. nov. for Ceratosphaeria abietis. Mycologia 105:462–475
Réblová M, Štěpánek V (2009) New fungal genera, Tectonidula gen. nov. for Calosphaeria-like fungi with holoblastic-denticulate conidiogenesis and Natantiella gen. nov. for three species segregated from Ceratostomella. Mycol Res 113:991–1002
Réblová M, Gams W, Seifert KA (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud Mycol 68:163–191
Réblová M, Štěpánek V, Schumacher RK (2014) Xylochrysis lucida gen. et sp. nov., a new lignicolous ascomycete (Sordariomycetidae) with holoblastic conidiogenesis. Mycologia 106:564–572
Réblová M, Fournier J, Štěpánek V (2016) Two new lineages of aquatic ascomycetes: Atractospora gen. nov. and Rubellisphaeria gen. et sp. nov., and a sexual morph of Myrmecridium montsegurinum sp. nov. Mycol Prog 15:1–18
Ronquist F, Teslenko M, van der Mark P, Ayres DL et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large modelspace. Syst Biol 61:539–542
Samarakoon MC, Hyde KD, Promputtha I, Hongsanan S et al (2016) Evolution of Xylariomycetidae (Ascomycota: Sordariomycetes). Mycosphere 7:1746–1761
Senanayake IC, Maharachchikumbura SSN, Hyde KD, Bhat DJ et al (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73:73–144
Senanayake IC, Al-Sadi AM, Bhat JD, Camporesi E et al (2016) Phomatosporales ord. nov. and Phomatosporaceae fam. nov., to accommodate Lanspora, Phomatospora and Tenuimurus, gen. nov. Mycosphere 7:628–641
Shearer CA, Raja HA (2010) Freshwater ascomycetes database. http://fungi.life.illinois.edu/. Accessed April 2017
Shenoy BD, Jeewon R, Wu WP, Bhat DJ et al (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Fungal Divers 56:95–129
Shenoy BD, Jeewon R, Hyde KD (2007) Impact of DNA sequence-data on the taxonomy of anamorphic fungi. Fungal Diversity 26:1–54
Shenoy BD, Jeewon R, Wang H, Amandeep K et al (2010) Sequence data reveals phylogenetic affinities of fungal anamorphs Bahusutrabeeja, Diplococcium, Natarajania, Paliphora, Polyschema, Rattania and Spadicoides. Fungal Divers 44:161–169
Shoemaker RA (1985) Lasiosphaeria caesariata with Sporidesmium hormiscioides and L. triseptata with S. adscendens. Sydowia. Ann Mycol Ser 2:278–283
Silvestro D, Michalak I (2011) RaxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Sivanesan A (1984) The bitunicate ascomycetes and their anamorphs. J. Cramer, Vaduz
Su HY, Hyde KD, Maharachchikumbura SSN, Ariyawansa HA et al (2016) The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province. China. Fungal Divers 80:1–35
Sung GH, Poinar GO Jr, Spatafora JW (2008) The oldest fossil evidence of animal parasitism by fungi supports a Cretaceous diversification of fungal e arthropod symbioses. Mol Phylogenet Evol 49:495–502
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland
Taylor JE, Hyde KD (2003) Microfungi of tropical and temperate palms. Fungal Divers Res Ser 12:1–459
Tsui KM, Hyde KD, Hodgkiss IJ (2000) Biodiversity of fungi on submerged wood in Hong Kong streams. Aquat Microb Ecol 21:289–298
Vijaykrishna D, Jeewon R, Hyde KD (2005) Fusoidispora aquatica: a new ascomycete from Hong Kong based on morphology and phylogeny inferred from rDNA gene sequences. Sydowia 57:267–280
Vijaykrishna D, Jeewon R, Hyde KD (2006) Molecular taxonomy, origins and evolution of freshwater ascomycetes. Fungal Divers 23:351–390
Wijayawardene NN, McKenzie EHC, Hyde KD (2012) Towards incorporating anamorphic fungi in a natural classification–checklist and notes for 2011. Mycosphere 3:157–228
Winka K, Eriksson OE (2000) Papulosa amerospora accommodated in a new family (Papulosaceae, Sordariomycetes, Ascomycota) inferred from morphological and molecular data. Mycoscience 41:97–103
Wong SW, Hyde KD (1999) Proboscispora aquatica gen. et sp. nov., from wood submerged in freshwater. Mycol Res 103:81–87
Wong SW, Hyde KD, Jones EBG (1998) Annulatascaceae, a new ascomycete family from the tropics. Syst Ascomycetum 16:17–25
Wong SW, Hyde KD, Jones EBG, Moss ST (1999a) Ultrastructural studies on the aquatic ascomycetes Annulatascus velatisporus and A. triseptatus sp. nov. Mycol Res 103:561–571
Wong SW, Hyde KD, Jones EBG (1999b) Ultrastructural studies on freshwater ascomycetes, Fluminicola bipolaris gen. et sp. nov. Fungal Divers 2:189–197
Wu WP, Zhuang WY (2005) Sporidesmium, Endophragmiella and related genera from China. Fungal Divers Res Ser 15:1–351
Zelski SE, Raja HA, Miller AN, Shearer CA (2011) Chaetorostrum quincemilensis, gen. et sp. nov., a new freshwater ascomycete and its Taeniolella-like anamorph from Peru. Mycosphere 2:593–600
Acknowledgements
This work was mainly supported by National Natural Science Foundation of China (Project ID: NSF 31500017 to Huang Zhang) and Scientific Foundation of Kunming University of Science and Technology (Project ID: 14118899 to Huang Zhang). Thanks are extended to Saranyaphat Boonmee for their assistance in microscope use.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Dong, W., Hyde, K.D. et al. Towards a natural classification of Annulatascaceae-like taxa: introducing Atractosporales ord. nov. and six new families. Fungal Diversity 85, 75–110 (2017). https://doi.org/10.1007/s13225-017-0387-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-017-0387-z