Abstract
Strains of a coelomycete isolated from grapevine cankers in southeastern Australia and identified as Dothiorella iberica in previous studies are redescribed in this study as a novel species based on morphological characters and phylogenetic analyses of DNA sequences of the internal transcribed spacer region (ITS1-5.8S-ITS2), and partial sequences of the translation elongation factor 1-α and β-tubulin genes. Dothiorella vidmadera sp. nov. is most closely related to D. iberica, D. americana and D. sarmentorum, but differs in morphological characters and DNA sequences. All four species are known to be associated with Botryosphaeria dieback of grapevines. Additionally, an unidentified ascomycete isolated from dead wood of grapevines in Western Australia is described. Phylogenetically, strains were most closely related to Spencermartinsia viticola, and bore conidia with morphological characters and dimensions consistent with published descriptions. However, ascospores were shorter and narrower than previously reported and lacked the terminal apiculi that typify the genus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Botryosphaeriaceae is a species-rich family that comprises taxa that are frequently associated with the canker disease of grapevines (Vitis vinifera L.), known as ‘Botryosphaeria dieback’ (Úrbez-Torres 2011; Liu et al. 2012). Symptoms of this disease include wood cankers, shoot and branch dieback, bleached, necrotic or discoloured canes, bud necrosis, graft failure, fruit rot and a characteristic wedge-shaped lesion in the trunks and cordons of infected vines (van Niekerk et al. 2006).
While Botryosphaeria, Diplodia, Neofusicoccum and Lasiodiplodia are genera most frequently associated with Botryosphaeria dieback (Úrbez-Torres et al. 2006; Pitt et al. 2010), Dothiorella and Spencermartinsia are also routinely found on grapevines, and to date four species have been isolated from V. vinifera including Dothiorella americana J.R. Úrbez-Torres et al. (Úrbez-Torres et al. 2012), D. iberica A.J.L. Phillips et al. (Phillips et al. 2005; Úrbez-Torres et al. 2007), D. sarmentorum (Fr.) A.J.L. Phillips et al. (Martin and Cobos 2007; Gramaje et al. 2009), and D. viticola (≡Spencermartinsia viticola (A.J.L. Phillips & J. Luque) A.J.L. Phillips et al.) (Luque et al. 2005; Phillips et al. 2008).
In a major survey of 91 vineyards throughout southeastern Australia, eight species in the Botryosphaeriaceae, including two species of Dothiorella were routinely isolated from grapevine cankers (Pitt et al. 2010). Based on morphology and phylogenetic analyses of DNA sequences derived from the ribosomal DNA internal transcribed spacer (ITS) region (ITS1-5.8S-ITS2) and partial sequences of the translation elongation factor 1-α (TEF) gene, taxa were identified as D. viticola and D. iberica, respectively. However, phylograms from that study clearly show that the strains previously identified as D. iberica occupy a discrete, strongly supported branch separate from ex-type specimens of this species, and hence represent a novel species, that we describe here as Dothiorella vidmadera W.M. Pitt et al.
The aim of this study was to use morphological techniques and more comprehensive phylogenetic analyses that combined in addition to DNA sequences of the ITS and TEF regions, partial sequences of the β-tubulin (BT) gene to legitimately redescribe putative Dothiorella iberica strains misidentified in previous works. Additionally, in November 2009 during surveys of wine-growing regions in Western Australia (WA), an ascomycete with brown, 1-septate ascospores closely resembling Spencermartinsia viticola was isolated from dead wood of grapevines. The phylogenetic relationships and morphological characters of this species are documented.
Materials and methods
Collection, isolation, culturing and morphological characterization of isolates
Between November 2006 and April 2008, 91 vineyards throughout southeastern Australia were surveyed. Wood samples were taken from 2239 grapevines displaying symptoms of Botryosphaeria dieback, and fungal isolations from wood samples were carried out as described previously by Pitt et al. (2010). Based on conidial morphology and phylogenetic analyses of DNA sequences derived from the ITS and TEF genes, eight species in the Botryosphaeriaceae including putative strains of Dothiorella iberica were identified.
In November 2009, fruiting bodies were collected on dead wood of grapevines in WA. Isolations of fungi from these samples were made directly from ascospores on potato dextrose agar supplemented with 50 μg/mL of streptomycin sulfate (PDA-Strep), with isolates subsequently pure cultured by transfer of hyphal tips. Fungal strains derived from ascospores were tentatively identified to “genus” based on colony characteristics after 3 to 4 weeks incubation at 25 °C. Pure cultures were then transferred to 1.5 % water agar containing triple autoclaved Pinus radiata needles, and pycnidia induced by 4 to 6 weeks incubation under near ultraviolet light (UVB, 315–280 nm) at room temperature (~25 °C). Conidial morphology was then examined and by comparison to published descriptions (Luque et al. 2005), isolates further identified to “species” based on anamorphic and teleomorphic features such as propagule size, shape, colour and septation.
Microscopic examinations were carried out on an Olympus Provis AX70TRF microscope (Olympus Optical Co. Ltd., Japan) fitted with a ColorView IIIu digital camera (Soft Imaging Systems (SIS) GmbH, Munster, Germany). Pycnidia and perithecial contents from fruiting bodies were mounted in water and observed by bright field microscopy. Digital images of representative fungal strains as well as the lengths and widths of their respective conidia and/or ascospores were recorded using analySIS LS Research 2.41 software (SIS). Mean, standard deviation and 95 % confidence limits of propagule dimensions were recorded for at least 50 conidia/ascospores per specimen. To study colony morphology, cultures were maintained in incubators under controlled conditions of intermittent fluorescent light (12 h) at 25 °C for 8 weeks. Colony colours were recorded according to Rayner (1970).
Cardinal temperatures for growth were also determined. Five-mm-diameter mycelial plugs taken from the margins of actively growing 4-day-old cultures of each strain were transferred to PDA-strep and three plates per strain incubated in the dark at 5, 10, 15, 20, 25, 30 and 35 °C. Colony diameters were measured at daily intervals and growth rates (mm/day) for each strain determined at each temperature. Regression curves were fitted to values of daily growth rate versus temperature, and optimum growth temperatures established for each strain based on relationships described by a third order polynomial (Sánchez et al. 2003).
Representative cultures of each fungal strain reported in this paper are maintained in the collection at the National Wine and Grape Industry Centre (NWGIC), Wagga Wagga, New South Wales (NSW), Australia, and were deposited in the Plant Pathology Herbarium (DAR), at the Orange Agricultural Institute, Department of Primary Industries (DPI), Orange, NSW, Australia (Table 1). Where available dry specimens (fruiting bodies on dead wood) were also deposited at DAR.
DNA extraction, PCR amplification and sequencing
Colonized agar plugs from pure cultures were transferred to 50 mL Falcon tubes containing 20 mL of potato dextrose broth (Oxoid Ltd., Basingstoke, Hampshire, England). Broth cultures were incubated on a Sartorius Certomat BS-1 (Goettingen, Germany) orbital shaker revolving at 90 rpm for 7 days at 25 °C. Mycelia were harvested by filtration, lyophilized and total genomic DNA extracted using the Qiagen Plant Mini Kit according to the manufacturer’s instructions (Qiagen Pty Ltd, Clifton Hills, Victoria, Australia).
Molecular identification was achieved via PCR amplification and comparison of DNA sequences from the ITS, TEF and BT genes to those deposited in GenBank. ITS PCR reactions contained 0.1 volume of 10× buffer (15 mM MgCl2), 200 mM each of dNTPs, 0.15 mM each of primers ITS1 and ITS4 (White et al. 1990), 1 unit of HotStar Taq DNA polymerase (Qiagen), and ~50 ng of DNA template, and were adjusted with sterile nanopure water to a total volume of 50 μL. Amplification was achieved through an initial step of 15 min at 95 °C, followed by 40 cycles of 30 s at 94 °C, 45 s at 55 °C, and 1.5 min at 72 °C, with a final extension of 5 min at 72 °C. For TEF PCR, reaction components were as described above, but reactions were made up to a total volume of 40 μL and comprised instead 0.5 mM each of primers, EF1-728F and EF1-986R (Carbone and Kohn 1999). After an initial step of 15 min at 95 °C, amplification comprised 35 cycles of 30 s at 95 °C, 40 s at 58 °C, and 1 min at 72 °C, with a final extension of 5 min at 72 °C. For BT PCR, reactions were performed as per ITS PCR, but comprised instead primers Bt2a and Bt2b (Glass and Donaldson 1995). Amplification was accomplished by an initial step of 2 min at 94 °C, followed by 35 cycles of 1 min at 94 °C, 1 min at 58 °C, and 1.5 min at 72 °C, with a final extension of 5 min at 72 °C. All PCR reactions were performed in an Eppendorf Master Thermocycler (Eppendorf AG, Hamburg, Germany).
ITS, TEF and BT PCR products were separated by electrophoresis on 1 % agarose gels containing 0.5× Tris-acetate-EDTA buffer. Positive amplifications were confirmed by photography under UV light following staining with ethidium bromide (0.5 mg/L). PCR products were purified using the QIAquick PCR Purification Kit (Qiagen). Both strands of the ITS, TEF and BT regions were sequenced by the Australian Genome Research Facility (University of Queensland, St Lucia, Queensland, Australia).
Phylogenetic analyses
Sequences of Dothiorella and Spencermartinsia species from grapevine and other hosts, including ex-type specimens identified in previous studies and available in GenBank, were included in phylogenetic analyses (Table 1). All new sequences were checked manually and nucleotides at ambiguous positions clarified with sequences from both strands. All terminal primer sequences were removed and sequences aligned using ClustalW (Thompson et al. 1994). Manual adjustments to the alignment were carried out using BioEdit (Hall 1999).
Separate phylogenetic analyses were performed on combined datasets; ITS-TEF and ITS-TEF-BT, and tree topologies were compared. Phylogenetic analyses were performed with MEGA 5.1 (Tamura et al. 2011) by Neighbor-joining (NJ) (Saitou and Nei 1987) using the maximum composite likelihood method (Tamura et al. 2004) and by Maximum Parsimony (MP) using the heuristic search option with 1000 random addition sequence replicates and the tree bisection-reconnection (TBR) branch-swapping algorithm (Nei and Kumar 2000). Gaps were treated as missing data and all characters were unordered and equally weighted. In the MP method branches corresponding to partitions reproduced in less than 50 % of trees were collapsed and all equally parsimonious trees were saved. Tree consistency index (CI), retention index (RI) and composite index were calculated. Trees were rooted to Botryosphaeria dothidea (Moug.: Fr.) Ces. & De Not. and statistical support to assess robustness of inferred groups was estimated using 1000 bootstrap replicates (Felsenstein 1985). All new ITS, TEF and BT sequences generated from representative fungal strains used in this study were deposited in GenBank (Table 1), and the alignment and MP tree in TreeBase (S14035).
Results
Morphological characterization
Cultures (Figs. 1 and 2) arising from isolations from grapevine cankers collected from vineyards in NSW and South Australia (SA) bore pycnidia and conidia of a coelomycete similar to descriptions of Dothiorella iberica, D. sarmentorum and D. viticola (Phillips et al. 2005; Luque et al. 2005). Conidiogenesis was holoblastic, with conidiogenous cells giving rise to periclinal thickenings (Fig. 3). Conidia were brown, thick-walled and 1-septate prior to release from conidiogenous cells (Fig. 3), with rounded apex and rounded or occasionally truncate base (Fig. 4) and were most similar in length and width to D. sarmentorum (21.6 × 9.8 μm), but slightly shorter and narrower than D. iberica (23.2 × 10.9 μm), and significantly longer and broader than D. americana (15 × 6.1 μm). Strains grew at temperatures between 5 °C and 35 °C with optimum temperatures of 22.5 °C for DAR78994, 22.9 °C for DAR78992 and 23.1 and 23.2 °C for DAR78995 and DAR78993, respectively (Fig. 5).
Another collection from discarded pruned canes from a vineyard in WA bore an ascomycete containing brown, 1-septate ascospores. Cultures obtained from single ascospores of this latter collection produced pycnidia and conidia that were similar to those produced by the coelomycete cited above (Figs. 6 and 10). Ascomata (Fig. 7) were rarely found. Clavate bitunicate asci contained eight, thick-walled, brown, 1-septate ascospores (Fig. 8). Conidiogenesis was holoblastic, with conidiogenous cells giving rise to periclinal thickenings (Fig. 9). Conidia were brown, thick-walled and 1-septate prior to release from conidiogenous cells (Fig. 9), with rounded apex and rounded or occasionally truncate base (Fig. 10). While conidia of the unidentified ascomycete were consistent with Spencermartinsia viticola based on morphological characters and dimensions, ascospores were shorter and narrower than previously described, and lacked the terminal apiculi that typify the genus. Strains grew at temperatures between 5 °C and 35 °C with an optimum of 24 °C.
Spencermartinsia ‘viticola’. 6. 28 day-old colony of DAR80529 on PDA. 7. Ascomata partially erumpent through host bark. 8. Clavate bitunicate asci at various stages of development, containing eight brown, 1-septate ascospores arranged biseriately. 9. Conidia developing on conidiogenous cells with periclinal thickenings. 10. Brown, 1-septate conidia. Bars = 20 μm
Phylogenetic analyses
Because the phylogenetic placement within the Botryosphaeriaceae of genera such as Diplodia, Lasiodiplodia, and Neofusicoccum is well established (Crous et al. 2006; Phillips et al. 2008), with the exception of ex-type cultures of Botryosphaeria dothidea to which trees were rooted, only species in clades of interest, namely Dothiorella and Spencermartinsia were included in this study. Analysis of combined ITS-TEF and ITS-TEF-BT datasets reflected the same underlying phylogeny, so the most comprehensive dataset combining all three genes was used for the final analysis.
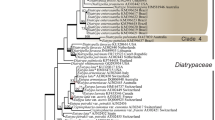
Sequence alignment of 37 taxa including two outgroup strains (Table 1), contained 1174 characters, 495 for the ITS region, 281 for the TEF gene, and 398 for the BT gene. However, several regions, including segments of the ITS1 region (characters 63–112), TEF gene (characters 496–553 and 641–664) and BT gene (883–938) could not be aligned unambiguously and were excluded from the analysis. Thus 986 characters were included in the final dataset, of which 786 were conserved, 200 were variable and 5 parsimony uninformative. Maximum parsimony (MP) analysis of the remaining 195 parsimony informative characters resulted in 16 equally parsimonious trees, with similar clade topologies, of which one is shown (Fig. 11). The 16 trees differed only in the position of the isolates in the terminal clades, and exhibited low homoplasy as indicated by a consistency index of 0.7333, a retention index of 0.9115 and a composite index of 0.6729 (0.6685) for all sites (and parsimony informative sites). NJ analysis produced trees with the same topology (tree not illustrated).
One of 16 equally parsimonious trees obtained from the combined ITS, TEF and BT sequence data of Spencermartinsia and Dothiorella species (Botryosphaeriaceae). Accessions in bold signify holotype cultures linked morphologically to the type material. Bootstrap support values of 70 or greater (percentage of 1000 replications) are shown above branches in bold font for maximum parsimony (MP) and below branches in regular font for neighbor-joining (NJ). Bar represents 10 changes
In phylogenetic analyses, Dothiorella strains DAR78992, DAR78993, DAR78994 and DAR78995 identified as D. iberica in a previous study (Pitt et al. 2010), clustered with ex-type specimens of D. iberica (CBS115041), D. americana (UCD2252MO) and D. sarmentorum (IMI63581b) with 78 % bootstrap support, but remained distinct from these and other species, forming a separate sub-clade at the apex of the tree with 88 % of bootstrap support (Fig. 11). Most closely related were Diplodia coryli Fuckel (CBS242.51) and D. juglandis Fr. (CBS188.87), which shared a common ancestor in 87 % of trees. However, DNA sequence comparisons of ITS, TEF and BT regions revealed significant divergence between the three species with both D. coryli and D. juglandis differing from putative Dothiorella iberica strains by a total of 10 nucleotide substitutions, 5 within the TEF gene and a further 5 within the BT gene. More distantly related were D. casuarini J. de Wet et al., D. moneti K. Taylor et al. D. santali K. Taylor et al., D. brevicollis Jami et al., and D. dulcispinae Jami et al. Combined with morphological data, the phylogenetic placement of the Dothiorella strains cited above provides sufficient evidence to justify introduction of a new species, which we describe and refer to hereafter as, Dothiorella vidmadera W.M. Pitt et al.
Ascomycete strains, DAR80529–DAR80531, derived from hyphal-tip transfer of ascospores from ascomata found on dead wood of grapevines in WA clustered tightly with the ex-type strain of Spencermartinsia viticola (CBS117009) and two others, DAR78870 and DAR78868, identified in previous work, forming a strongly supported cluster with 94 % bootstrap support. Strains from WA differed from the ex-type by a total of 3 nucleotide substitutions, all within in the TEF gene. Two additional strains, DAR78869 and DAR78872, also from previous work, formed a second group with S. viticola (CBS117006) with 99 % support. Together the two branches formed a single clade with 100 % bootstrap support.
Taxonomy
Dothiorella vidmadera W.M. Pitt, J.R. Úrbez-Torres & Trouillas sp. nov.
Mycobank: 803533.
Etymology: Spanish for the host (grapevine, ‘vid’) and substrate (wood, ‘madera’) from which it was first isolated.
Cultural characteristics: Pycnidia 2 mm diameter, solitaria vel aggregatis micelio albus velum. Pycnidia globosa cum ostiolo. Cellulae conidiogenae holoblasticae. Conidia primo hyalina unicellularia initialis, fuscus unum septum postea, apice rotundata basi truncata (17.9–) 21.2–21.9 (−24.2) × (8.3–) 9.6–9.8 (−10.6) μm (statura media 21.6 ± 1.2 × 9.7 ± 0.5 μm) (longitudo/latitudo = 2.2 ± 0.1 μm). Mycelio griseo-lazulinus initialis, lazulino-ardesiacus postea. Micelio temperatio optimus 22.9 °C.
On PDA colonies were initially greyish-blue (47”’) with suppressed mycelium after 7 days (Fig. 1), turning slate-blue (49”’i) with sparse peripheral mycelium within 28 days (Fig. 2) and forming spherical to globose, stromatic, white mycelial covered pycnidia up to 2 mm in diameter with a central ostiole, solitary or aggregated into clusters within 14 days at 25 °C. Conidiogenesis holoblastic, with conidiogenous cells giving rise to periclinal thickenings (Fig. 3). Conidia initially hyaline, thin-walled, unicellular (non-septate), becoming thick-walled, brown 1-septate prior to release from the conidiogenous cells (Fig. 3), with rounded apex and rounded or occasionally truncate base measuring (17.9–) 21.2–21.9 (−24.2) × (8.3–) 9.6–9.8 (−10.6) μm, with a mean length and width of 21.6 ± 1.2 × 9.7 ± 0.5 μm, and an average length to width ratio of 2.2 ± 0.1 μm (n = 50) (Fig. 4).
Cardinal temperatures for growth: Between 5 °C and 35 °C, with an optimum of 22.9 °C (Fig. 5).
Habitat: Vitis vinifera cultivars Chardonnay, Cabernet Sauvignon and Gamay.
Known distribution: New South Wales and South Australia, Australia.
Material examined: Australia, Eden Valley, South Australia, W.M. Pitt & A. Loschiavo, Chardonnay (holotype culture DAR78992); Australia, Loxton, South Australia, W.M. Pitt & A. Loschiavo, Cabernet Sauvignon (DAR78993); Australia, Barossa Valley, South Australia, W.M. Pitt & A. Loschiavo, Gamay (DAR78994); Australia, Adelaide Hills, South Australia, W.M. Pitt & A. Loschiavo, Chardonnay (DAR78995).
Spencermartinsia ‘viticola’ (A.J.L. Phillips & J. Luque) A.J.L. Phillips et al., Persoonia 21: 51 (2008)
≡Botryosphaeria viticola A.J.L. Phillips & J. Luque, Mycologia 97: 1118 (2005)
Cultural characteristics: On PDA colonies were dark slate-blue (41””k) with sparse aerial mycelium, forming solitary, stromatic, spherical to globose, greenish-grey (33””) mycelial covered pycnidia up to 2 mm in diameter with single central or multiple ostioles within 28 days at 25 °C (Fig. 6). Ascomata (Fig. 7) from wood samples collected from vineyards in Western Australia bore an ascomycete with clavate bitunicate asci, containing eight ascospores arranged biseriately (Fig. 8). Ascospores, initially hyaline, thin-walled, unicellular (non-septate), becoming thick-walled, brown 1-septate with age, and slightly constricted at the septum, measuring (13.6–) 14.8–15.2 (−17.3) × (6.6–) 7.5–7.7 (−8.3) μm, with mean length and width of 15 ± 0.7 × 7.6 ± 0.4 μm, and an average length to width ratio of 2 ± 0.1 μm (n = 50) (Fig. 8). Conidiogenesis holoblastic, with conidiogenous cells giving rise to periclinal thickenings (Fig. 9). Conidia, initially hyaline, thin-walled, unicellular (non-septate), becoming thick-walled, brown 1-septate prior to release from the conidiogenous cells (Fig. 9), with rounded apex and rounded or occasionally truncate base measuring (16–) 18.8–19.7 (−23.3) × (8–) 9.8–10.3 (−11.9) μm, with a mean length and width of 19.3 ± 1.5 × 10.1 ± 0.9 μm, and an average length to width ratio of 1.9 ± 0.2 μm (n = 50) (Fig. 10).
Cardinal temperatures for growth: Between 5 °C and 35 °C, with an optimum of 24 °C.
Habitat: Vitis vinifera.
Known distribution: Western Australia, Australia.
Material examined: Australia, Upper Swan, Western Australia, F.P. Trouillas (DAR80529); Australia, Upper Swan, Western Australia, F.P. Trouillas (DAR80530); Australia, Upper Swan, Western Australia, F.P. Trouillas (DAR80531).
Discussion
Dothiorella are unique within the Botryosphaeriaceae. In early phylogenetic studies these species clustered with Neofusicoccum (Phillips et al. 2005), a genus introduced by Crous et al. (2006) to accommodate Fusicoccum-like species with Botryosphaeria-like teleomorphs. However, the thick-walled, brown 1-septate conidia typical of Dothiorella do not conform to, and cannot be accommodated in Neofusicoccum (Crous and Palm 1999). Similarly, Dothiorella closely resemble Diplodia (Wollenweber 1941; Laundon 1973), but phylogenetically do not belong in this genus either, and morphologically remain separate from this and most other genera within the Botryosphaeriaceae based on the onset of conidial pigmentation and septation, both of which occur prior to release from conidiogenous cells (Phillips et al. 2005).
In previous studies Dothiorella strains DAR78992, DAR78993, DAR78994 and DAR78995, now recognized as D. vidmadera, were closely related to D. iberica and D. sarmentorum and consistently grouped together with ex-type specimens of these species with >99 % bootstrap support both in phylogenetic analyses of DNA sequences of the ITS region and partial sequences of the TEF gene (Pitt et al. 2010). However, D. vidmadera were clearly separate from D. iberica and D. sarmentorum, and in both analyses occupied a discrete branch within the Dothiorella clade, supported by >97 % of bootstrap replicates.
In phylograms produced in this study, based on combined analysis of ITS, TEF and BT sequences, Dothiorella vidmadera also formed a highly supported group with ex-type strains of D. iberica and D. sarmentorum, and along with D. americana recently described by Úrbez-Torres et al. (2012) clustered together with 78 % bootstrap support (Fig. 11). However, D. vidmadera were again distinct from ex-type strains of D. iberica and D. sarmentorum, as well as from D. americana and other species of recent description, namely D. casuarini (de Wet et al. 2009), D. moneti, D. santali (Taylor et al. 2009), D. brevicollis and D. dulcispinae (Jami et al. 2012), and formed a discrete cluster at the apex of the tree that was supported by 88 % of bootstrap replications.
The inclusion of additional strains and species from recent studies showed that Dothiorella vidmadera were most closely related to Diplodia coryli CBS242.51 and D. juglandis CBS188.87 which formed a sub-clade immediately basal to Dothiorella vidmadera, and with which a broader cluster supported by 87 % of bootstrap replicates was formed. Unfortunately, Phillips et al. (2008) considered neither strain authentic, and since Stevens (1936) reduced Diplodia coryli to synonymy with D. sarmentorum Fr. (≡Dothiorella sarmentorum (Fr.) A.J.L. Phillips et al.), the placement of CBS242.51 in the phylogram separate from ex-type strains of D. sarmentorum effectively confirmed Phillips et al. (2008) suspicions rendering this strain and Diplodia juglandis useless as markers for the identity and relationships of other isolates.
While no formal descriptions were made in conjunction with prior work (Pitt et al. 2010), phylogenetic differences identified previously, and supported by this work show that Dothiorella vidmadera are closely related, but clearly distinct from D. iberica, D. americana and D. sarmentorum. Morphologically, conidia of D. vidmadera also differ from those of their two closest relatives, being slightly shorter and narrower than D. iberica (Phillips et al. 2005), and significantly longer and broader than D. americana (Úrbez-Torres et al. 2012).
While Dothiorella spp. are frequently isolated from diseased wood and grow readily in culture, fruiting bodies of Dothiorella were encountered infrequently, and to our knowledge have not previously been observed in Australia. Sexual Dothiorella morphs are unusual in that many species with connections in the Botryosphaeriaceae comprise ascospores that remain hyaline and aseptate, as opposed to pigmented and 1-septate. Historically, these characteristics placed teleomorphic Dothiorella in Dothidotthia Hohn. (Barr 1989), but more recent studies of the Dothideomycetes have shown that Dothidotthia is unrelated to the Botryosphaeriaceae (Schoch et al. 2006), and instead Phillips et al. (2008) adopted Dothiorella as a holomorphic genus for pleomorphic Dothiorella, and subsequently transferred D. viticola to Spencermartinsia, a genus that now accommodates Dothiorella possessing brown 1-septate ascospores with terminal appendages known as apiculi (Phillips et al. 2008).
Phylogenetic analyses of combined sequences of the ITS, TEF and BT genes of Spencermartinsia viticola and closely related strains and species downloaded from GenBank showed that strains DAR80529-DAR80531, derived by hyphal-tip transfer of ascospores from ascomata found on dead wood of grapevines in WA were closely related to S. viticola, and clustered with the ex-type (CBS117009) and other strains of this species identified in prior work (Pitt et al. 2010), forming a strongly supported clade with 100 % bootstrap support (Fig. 11). However, within this clade, two branches were apparent, both highly supported, one containing the ex-type, and the other, an additional isolate, CBS117006, that by comparison differed from CBS117009 by one substitution and one deletion in ITS region and nine substitutions in TEF gene. While some morphological differences were also noted, Luque et al. (2005) regarded these differences as reasonable intraspecific variation.
In contrast, strains of Spencermartinsia ‘viticola’ from WA differed from the ex-type, CBS117009, by only 3 nucleotides, all in the TEF gene, and conidia were similar both in length and width. However, some morphological differences from published descriptions were apparent in the teleomorph, with ascospores being shorter and narrower than those of the type, and lacking terminal apiculi (Luque et al. 2005; Phillips et al. 2008). Given the high degree of sequence homology between these strains and the ex-type, the fact that ITS and TEF sequence data closely reflect morphological differences in the teleomorphs of Dothiorella (Luque et al. 2005; Phillips et al. 2005), and that conidia are similar in size, shape, and are Dothiorella-like, insomuch as they are 1-septate and pigmented prior to dehiscence from conidiogenous cells, it would seem appropriate to record Spencermartinsia viticola for the first time in Australia. However, we would need to assume that the differences outlined here similarly reflect reasonable intraspecific variation. We are reluctant to do so given the morphological disparity we have observed in the teleomorph. Instead, we provide here a description of this species for future reference.
To date, only two other species have been described in this genus, namely Spencermartinsia uruguayensis C.A. Pérez et al. and S. pretoriensis Jami et al. (Perez et al. 2010; Jami et al. 2012). Both were placed in Spencermartinsia based on phylogenetic studies conducted by these authors. However, this is curious given that Phillips et al. (2008) introduced Spencermartinsia to accommodate teleomorphic Dothiorella possessing apiculi, a feature that along with the perfect state, has yet to be observed in either species. What’s more, no BT sequences are available to allow for inclusion of Spencermartinsia uruguayensis in this study, and in an ITS-TEF neighbor-joining tree provided by the authors and similar reconstructions by us, S. uruguayensis was more closely related to Dothiorella iberica and D. sarmentorum, than to the type, Spencermartinsia viticola.
Similarly, in their ITS-TEF-BT-LSU multigene phylogeny, Jami et al. (2012) included four strains of Spencermartinsia (CMW25404, CMW25405, CBS121760 and CBS121761) from van der Walt (2008) and made detailed sequence comparisons to CMW25404 and CMW25405 to support the introduction of S. pretoriensis as a novel species. But no nucleotide sequences for the BT and LSU genes were provided, nor archived by van der Walt (2008), and no accession numbers for these sequences were given by Jami et al. (2012), nor do any appear in the GenBank database. Furthermore, in phylograms presented here as well as reconstructions based on sequences selected by Jami et al. (2012), S. pretoriensis consistently grouped not with the type, but with Dothiorella santali and D. moneti. Given that these authors then went on to provide a different placement for S. pretoriensis in their succeeding paper (Jami et al. 2013), which by their own admission is based on DNA sequences from the same gene regions, the classification of S. pretoriensis in Spencermartinsia also seems increasingly inappropriate.
To date, three species of Dothiorella, namely D. iberica, D. sarmentorum and D. americana have been associated with the decline of grapevines (Úrbez-Torres et al. 2007; Martin and Cobos 2007; Gramaje et al. 2009; Úrbez-Torres et al. 2012). While all three are closely related to Spencermartinsia viticola, itself a pathogen of grapevine in the United Sates and Australia (Úrbez-Torres et al. 2007; Pitt et al. 2010), they remain distinct from Spencermartinsia both morphologically and phylogenetically (Phillips et al. 2008). Recently, pathogenicity studies have shown that Dothiorella vidmadera, as D. iberica, is also pathogenic to grapevines, causing necrotic lesions in the trunks of 15 year-old Chardonnay (Pitt et al. 2013). But whereas D. vidmadera was only moderately virulent when compared to the more aggressive members of the Botryosphaeriaceae, namely Lasiodiplodia theobromae and Neofusicoccum spp., in prior surveys of southeastern Australia Dothiorella were second in abundance only to Diplodia and unlike their more virulent counterparts were found throughout most viticultural regions (Pitt et al. 2010). Hence, Dothiorella spp. remain a threat to the prosperity and longevity of grapevines in Australia, if not through virulence, then by shear prevalence and distribution.
References
Barr ME (1989) The genus Dothidotthia (Botryosphaeriaceae) in North America. Mycotaxon 34:517–526
Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91:553–556
Crous PW, Palm ME (1999) Reassessment of the anamorph genera Botryodiplodia, Dothiorella and Fusicoccum. Sydowia 51:167–175
Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Phillips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253
de Wet J, Slippers B, Preisig O, Wingfield BD, Tsopelas P, Wingfield MJ (2009) Molecular and morphological characterization of Dothiorella casuarini sp. nov. and other Botryosphaeriaceae with diplodia-like conidia. Mycologia 101:503–511
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330
Gramaje D, Munoz RM, Lerma ML, Garcia-Jimenez J, Armengol J (2009) Fungal grapevine trunk pathogens associated with Syrah decline in Spain. Phytopathol Mediterr 48:396–402
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Sym Ser 41:95–98
Jami F, Slippers B, Wingfield MJ, Gryzenhout M (2012) Five new species of the Botryosphaeriaceae from Acacia karroo in South Africa. Crypt Mycol 33:245–266
Jami F, Slippers B, Wingfield MJ, Gryzenhout M (2013) Greater Botryosphaeriaceae diversity in healthy than associated diseased Acacia karroo tree tissues. Australas Plant Pathol. doi:10.1007/s13313-013-0209-z
Laundon GF (1973) Botryosphaeria obtusa, B. stevensii and Otthia spiraeae in New Zealand. Trans Br Mycol Soc 6:369–374
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat JD, Romero AI, Zhuang WY, Monkai J, Jones EBG, Chukeatirote E, Ko Ko TW, Zhao YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Luque J, Martos S, Phillips AJL (2005) Botryosphaeria viticola sp. nov. on grapevines: a new species with a Dothiorella anamorph. Mycologia 97:1111–1121
Martin MT, Cobos R (2007) Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathol Mediterr 46:18–25
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York, p 352
Perez CA, Wingfield MJ, Slippers B, Altier NA, Blanchette RA (2010) Endophytic and canker-associated Botryosphaeriaceae occurring on non-native Eucalyptus and native Myrtaceae trees in Uruguay. Fungal Divers 41:53–69
Phillips AJL, Alves A, Correia A, Luque J (2005) Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 97:513–529
Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW (2008) Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia 21:29–55
Pitt WM, Huang R, Steel CC, Savocchia S (2010) Identification, distribution and current taxonomy of Botryosphaeriaceae species associated with grapevine decline in New South Wales and South Australia. Aust J Grape Wine Res 16:258–271
Pitt WM, Huang R, Steel CC, Savocchia S (2013) Pathogenicity and epidemiology of Botryosphaeriaceae species isolated from grapevines in Australia. Australas Plant Pathol. doi:10.1007/s13313-013-0221-3
Rayner RW (1970) A mycological colour chart. Commonwealth Mycological Institute & British Mycological Society, Kew
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sánchez ME, Venegas J, Romero MA, Phillips AJL, Trapero A (2003) Botryosphaeria and related taxa causing oak canker in southwestern Spain. Plant Dis 87:1515–1521
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041–1052
Stevens NE (1936) Two species of Physalospora in England. Mycologia 36:330–336
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Taylor K, Barber PA, St J, Hardy GE, Burgess TI (2009) Botryosphaeriaceae from tuart (Eucalyptus gomphocephala) woodland, including descriptions of four new species. Mycol Res 113:337–353
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Úrbez-Torres JR (2011) The status of Botryosphaeriaceae species infecting grapevines. Phytopathol Mediterr 50:S5–S45
Úrbez-Torres JR, Gubler WD, Luque J (2007) First Report of Botryosphaeria iberica and B. viticola Associated with Grapevine Decline in California. Plant Dis 91:772
Úrbez-Torres JR, Leavitt GM, Voegel TM, Gubler WD (2006) Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California. Plant Dis 90:1490–1503
Úrbez-Torres JR, Peduto F, Striegler RK, Urrea-Romero KE, Rupe JC, Cartwright RD, Gubler WD (2012) Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers 52:169–189
van der Walt FJJ (2008) Botryosphaeriaceae associated with Acacia species in southern Africa with special reference to A. mellifera. Masters Thesis. Faculty of Natural and Agricultural Sciences, Department of Microbiology and Plant Pathology, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa.
van Niekerk JM, Fourie PH, Halleen F, Crous PW (2006) Botryosphaeria spp. as grapevine trunk disease pathogens. Phytopathol Mediterr 45:S43–S54
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: A guide to methods and applications. Academic Press, San Diego, pp 315–322
Wollenweber HW (1941) Diplodia sarmentorum Fries und ihre Vernreitung. Zentralbl Bakteriol Parasitendk 103:347–357
Acknowledgments
This work was funded by the Winegrowing Futures Program, a joint initiative between the NWGIC and the Grape and Wine Research and Development Corporation (GWRDC). F.P. Trouillas was the recipient of a GWRDC travel grant for the duration of these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pitt, W.M., Úrbez-Torres, J.R. & Trouillas, F.P. Dothiorella vidmadera, a novel species from grapevines in Australia and notes on Spencermartinsia . Fungal Diversity 61, 209–219 (2013). https://doi.org/10.1007/s13225-013-0244-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-013-0244-7