Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, also regarded as “type 3 diabetes” for the last few years because of the brain insulin resistance (IR) and dysregulation of insulin signaling in the brain, which can further promote pathological progression of AD. IRS-1/PI3K/Akt insulin signaling pathway disorder and its downstream cascade reaction are responsible for cognitive decline in the brain. In recent years, a growing number of studies has documented that dysregulation of insulin signaling is a key feature of AD and has crucial correlations with serine/tyrosine (Ser/Tyr) phosphorylation of insulin receptor substance-1(IRS-1). Phosphorylation of this protein has been identified as an important molecule involved in the process of amyloid-β (Aβ) deposition into senile plaques (SPs) and tau hyperphosphorylation into neurofibrillary tangles (NFTs). In this paper, we review the links between IRS-1 and the PI3K/Akt insulin signaling pathway, and highlight phosphorylated IRS-1 which negatively regulated by downstream effector of Akt such as mTOR, S6K, and JNK, among others in AD. Furthermore, anti-diabetic drugs including metformin, thiazolidinediones, and glucagon-like peptide-1 (GLP-1) analogue could modulate IRS-1 phosphorylation, brain IR, PI3K/Akt insulin signaling pathway, and other pathologic processes of AD. The above suggest that anti-diabetic drugs may be promising strategies for AD disease-modifying treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of individuals living with Alzheimer’s disease (AD) is rapidly increasing, mainly as populations continue to age, which poses a growing problem for families and societies worldwide (Nichols et al. 2019). According to the World Alzheimer Report 2019, approximately over 50 million people worldwide are living with dementia and this number is projected to increase to 152 million by 2050 (Alzheimer's Disease International 2019). AD, one of the most pressing epidemics of our time, is a progressive, age-related neurodegenerative disease that is characterized clinically by cognitive deterioration, behavioral changes, memory loss, and executive function impairments. Pathologically, AD is characterized by neuropathologic hallmarks, amyloid-β (Aβ), which forms extracellular senile plaques (SPs), and intracellular neurofibrillary tangles (NFTs) consisting of aggregated hyperphosphorylated tau proteins, along with neuroinflammation, synaptic dysfunction, neuronal loss and dystrophy, among others (Arranz and De Strooper 2019; De Strooper and Karran 2016). Nevertheless, despite our understanding the advanced molecular pathogenesis of AD from the “amyloid cascade hypothesis” perspective, the prevention and/or clearance of Aβ plaques from the brain has failed to translate into effective therapies for AD patients and has not alleviated cognitive degeneration (Blanchard and Tsai 2019; Weller and Budson 2018). It has been suggested that underlying pathogenic mechanisms influence the modulation of the “amyloid cascade hypothesis”.
Over the past 2 decades, a growing body of research has revealed that dysregulation of insulin signaling and brain insulin resistance (IR) are associated with sporadic AD, and that both exert a fundamental role in the progression of AD pathogenesis (Akhtar et al. 2020a, b; Eric Steen et al. 2005; Ferreira et al. 2014; Spinelli et al. 2020; Talbot et al. 2012). Indeed, the insulin signaling pathway is central to neuronal survival, regulation of synapse number, dendritic plasticity, and glial functions (Chiu et al. 2008; McNay and Recknagel 2011; Van der Heide et al. 2005). In post-mortem AD brain tissue, insulin and insulin receptor expression is strikingly decreased, and alterations in downstream insulin signaling molecules are observed, including diminished IRS-1/2, IRS-associated PI3K, and p-Akt levels (Eric Steen et al. 2005; Moloney et al. 2010). Moreover, aberrantly distributed insulin receptor proteins are concentrated within neurons in AD (Moloney et al. 2010). In AD animal models using 3xTg-AD and Tg2576 mice, for example, brain IR has been observed along with perturbed brain levels of IRS-1, p-PI3K, p-Akt, and GSK-3β, among other proteins, which suggests an impaired insulin signaling pathway (Velazquez et al. 2017). Brain insulin receptor sensitivity and IRS-1/2 expression levels are reduced in association with increased levels of IRS-1 phosphorylation at Ser-312 (pSer312-IRS-1) (Kleinridders 2016; Moloney et al. 2010); this suggests that brain IR is compromised in AD. Furthermore, Aβ42 is the main neurotoxic isoform composition of Aβ plaques, and defective insulin signaling drives the accumulation of Aβ42 neurotoxic isoform but not Aβ40 in the brain of APP/PS1 mice (Chua et al. 2012), the formation of hyperphosphorylated tau proteins and dystrophic neurites (Yarchoan et al. 2014), impairs hippocampal long-term potentiation (LTP) and learning (Grillo et al. 2015), and stimulates neuroinflammation and the accumulation of reactive oxygen species (de la Monte 2014).
Type 2 diabetes mellitus (T2DM) is a multifactorial disease characterized by IR, which is associated with disturbances in insulin signaling in cellular level, and has been demonstrated as a substantial risk factor for AD developing. Compared with the general population, individuals with T2DM have a more than 1.5 times greater risk of developing AD, especially in Eastern populations (Zhang et al. 2017). Besides, further clinical studies support a link between T2DM and AD (Beeri and Bendlin 2020). Results of some studies assessing the effect of anti-diabetic drugs involving metformin, thiazolidinediones, and glucagon-like peptide-1 (GLP-1) analogue on individuals diagnosed as mild cognitive impairment (MCI) or AD, in some extend, pose a notable cognitive benefit (Kellar and Craft 2020; Munoz-Jimenez et al. 2020; Rotermund et al. 2018). Above these suggesting that a considerable overlap in pathogenesis exists across these two conditions, AD and T2DM. This association may due in large part to dysregulation of insulin signaling pathway which mediates IR (Boccardi et al. 2019). Brain IR is simply defined as inactivated insulin signaling pathway, especially the IRS/PI3K/Akt pathway, which is crucial for maintaining synaptic plasticity and cognitive functions (Bedse et al. 2015; Boucher et al. 2014). IR, the linking mechanism between T2DM and AD, which potentiates the formation of Aβ plaques by reducing the degradation and clearance of Aβ, and especially impairs the downstream insulin signaling pathway PI3K/Akt, leading to enhanced production of Aβ and hyperphosphorylated tau in the brain with AD (Diehl et al. 2017; Zlokovic 2011) (Fig. 1). Furthermore, the impaired insulin signaling pathway PI3K/Akt downstream effectors involving mTOR, S6K, JNK, and GSK3, among others could elicit defect in energy metabolism, oxidative stress, neuroinflammation, mitochondrial dysfunction, and autophagy dysfunction (Boccardi et al. 2019; Chen et al. 2021; Khan et al. 2019) (Fig. 1). Results of experimental studies also suggested that anti-diabetic drugs might act in the brain to mitigate IR, modulate dysfunction of insulin signaling, and other mechanisms including Aβ deposition, tau hyperphosphorylation, neuroinflammation, and oxidative stress (Boccardi et al. 2019; Chen et al. 2021; Escribano et al. 2010; Khan et al. 2019). Given the important role of IR in the brain for learning and memory, it is essential to further understand the insulin signaling pathway that implicated in AD pathophysiology, as well as how its crucial molecule–insulin receptor substrate-1 (IRS-1) related to brain IR in AD.
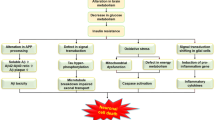
Schematic representation of the shared mechanisms between AD and T2DM. IR could stimulate amyloid deposition by reducing the degradation of Aβ by IDE and clearance of Aβ by impairing BBB. Furthermore, IR makes insulin signaling pathway conduction abnormal, leading to PI3K/Akt signaling pathway and its downstream molecules dysfunction. The impaired insulin signaling pathway elicits oxidative stress, energy metabolism dysfunction, neuroinflammation, mitochondrial dysfunction, autophagy dysfunction, and neuronal death ultimately, all of which promote cognitive impairment in AD. AD Alzheimer’s disease, T2DM Type 2 diabetes mellitus, IR Insulin resistance, Aβ amyloid-β peptide, IDE insulin-degrading enzyme, BBB blood–brain barrier
The most important representative of the IRS protein family (termed IRS1-6), IRS-1, is by definition one of the crucial molecules in the insulin signaling pathway that is involved in brain IR. It is appreciated that aberrant phosphorylation of IRS-1 is also extensively associated with brain IR in addition to with skeletal muscle, adipose tissue, and liver (Mullins et al. 2017a). Moreover, research on the relationship between brain insulin signaling and AD pathogenesis has shown that low levels of IRS-1 expression are associated with phosphorylated tau proteins and that aberrant hyperphosphorylation of IRS-1 is related to tau hyperphosphorylation (De Felice 2013; Moloney et al. 2010; Mullins et al. 2017a). One study recently revealed that AD brain atrophy is associated with IRS-1 expression, which indicates a positive relationship with IRS-1pan-Tyr phosphorylation and a negative relationship with pSer312-IRS-1, in a spatial pattern (Mullins et al. 2017b). Overall, decreasing levels of IRS-1 and increasing levels of pSer-IRS-1 are convincing changes in insulin signaling pathway disorder, and accumulating evidence suggests that pSer-IRS-1 is an indicator of IR in both the peripheral and the brain (Hirosumi et al. 2002; Kleinridders 2016; Moloney et al. 2010; Talbot et al. 2012). In contrast to previous reviews (Akhtar and Sah 2020; Candeias et al. 2012; Daisuke et al. 2019; Dineley et al. 2014), here, we outline the insulin signaling pathway crucial molecule, IRS-1, and highlight its actions with respect to PI3K/Akt insulin signaling pathway. We also summarize downstream effector of Akt involving mTOR, S6K, JNK, and GSK3 which could negatively regulate pSer-IRS-1 and how it affects hyperphosphorylated tau protein and Aβ plaques development in AD (Fig. 2), and the role of anti-diabetic drugs including metformin, thiazolidinediones, and GLP-1 analogue treating individuals with AD.
The feedback regulation between IRS-1 phosphorylation and IRS-1/PI3K/Akt insulin signaling pathway in AD. Insulin binds to insulin receptor which stimulates autophosphorylation of itself and subsequently activates tyrosine residues of IRS-1 triggering cascade events. IRS-1 recruits and activates PI3K complex, which then phosphorylates and activates Akt. Activated Akt ultimately leads to the translocation of GLUT4 to the cell membrane for uptake of glucose into neurons. Akt-mediated activation of mTOR and the downstream targets of mTOR, like S6K and 4EBP1, serve to regulate protein synthesis, lipid synthesis and autophagy, among other processes. Phosphorylation of GSK3 by Akt inhibits the constitutive activity of this kinase. Activated Akt also can directly phosphorylate JNK/IKK. IR results in increased pSer-IRS-1 phosphorylation which further enhance PI3K/Akt signaling pathway and its downstream molecules dysfunction, leading to pathological process of AD involving hyperphosphorylated tau protein, Aβ plaques, autophagy dysfunction, glucose metabolism dysfunction, neuroinflammation, and oxidative stress. Dark green solid arrows represent activation upon insulin stimulation and blocked arrow shows inhibition. Red dotted arrows represent downstream molecules that can phosphorylate Ser residues in IRS-1 leading to inactivation of IRS-1/PI3K/Akt signaling pathway by feedback inhibition loop (Arnold et al. 2018; Stanley et al. 2016). PI3K phosphoinositide 3 kinase, GLUT4 Glucose transporter 4, mTOR mammalian target of rapamycin, TSC1/2 tuberous sclerosis ½, S6K ribosomal protein S6 kinase, 4EBP1 4E-binding protein 1, GSK3 glycogen synthase kinase 3, FOXO fork-head family box O, IKK inhibitor of nuclear factor-κB kinase, JNK c-Jun-N-terminal kinase, IR insulin resistance, AD Alzheimer’s disease, Aβ amyloid-β peptide
The molecular structure of IRS
IRS-1 and IRS-2 are widely distributed, and are the most well-characteristic protein among the IRS1-6; IRS-1 has a central role in the cerebral cortex and skeletal muscle, whereas IRS-2 is expressed primarily in the hypothalamus and liver (Arnold et al. 2018). The N terminus of IRS proteins contains pleckstrin-homology domains (PH domains) and phosphotyrosine-binding domains (PTB domains) that bind to the activated IR-β subunit. Near the C terminus tail of IRS proteins are as many as 20 tyrosine-phosphorylation binding sites for Src-homology-2 (SH2)-containing proteins (Shc), such as the regulatory subunit p85 PI3K and the adaptor molecule growth factor receptor-bound protein 2 (Grb2). These binding sites are also specific for SH2 domain-containing tyrosine phosphatase-2 (Shp2) proteins and for cytoplasmic tyrosine kinases (Fig. 3) (Copps and White 2012; Taniguchi et al. 2006). Shc activates the RAS-mitogen-activated protein kinase (MAPK) pathway, which activates extracellular signal-regulated kinase1/2 (ERK1/2); ERK1/2 in turn regulates cell proliferation, survival and gene transcription (Ferreira et al. 2014; Kleinridders et al. 2014). In contrast, phosphorylation at tyrosine sites in IRS mostly activates the phosphoinositide 3‑kinase (PI3K)/Akt (also known as PKB) cascade, which largely mediates metabolism, protein/lipid/glycogen synthesis, glucose transport, and autophagy, among others processes (Arnold et al. 2018; Boucher et al. 2014). Besides, the PI3K/Akt insulin signaling pathway is implicated in promoting learning and memory, which occurs by regulating synaptic plasticity and improving memory consolidation (Chiang et al. 2010; Horwood et al. 2006), and thus, most of the discussion below focuses on the PI3K/Akt insulin signaling pathway regulating IRS-1 phosphorylation.
A schematic representation of the IRS-1 protein in mice/rats (1233/1235 amino acid sequences) and human (1242). The IRS-1 sequence has 2 major functional structural regions near the N-terminus portion. A pleckstrin-homolog (PH) domain (light blue) spans between amino acids 12 and 115, and a phosphotyrosine-binding (PTB) domain (dark blue) between 155 and 259. The serine residues (S) and tyrosine residues (Y) are also shown. Red circles represent sites of negative regulation, whereas green circles represent sites of positive regulation. The black line shows a series of binding sites for PI3K kinase, and some black arrows indicate exact binding sites Y891-893 for Grb2 and Y1179/1222 for SHP2. More than one protein phosphorylates S307 site such as IKK, JNK, PKC θ, and S6K, S612 site such as Akt/PKB, ERK, and mTOR, and S632 site such as ERK and mTOR (Copps and White 2012; Gual et al. 2005; Taniguchi et al. 2006). PI3K phosphoinositide 3 kinase, Grb2 growth factor receptor-bound protein-2, SHP2 Src-homology-domain-containing tyrosine phosphatase-2, GSK3β glycogen synthase kinase 3β, IKK inhibitor of nuclear factor-κB kinase, JNK c-Jun-N-terminal kinase, PKC θ protein kinase C θ, S6K ribosomal protein S6 kinase, mTOR mammalian target of rapamycin
IRS and the PI3K/Akt insulin signaling pathway
Insulin receptor is a tetramer protein composed of extracellular α subunits and transmembrane β subunits. Ligand such as insulin or insulin-like growth factors (IGF1) binds to insulin receptor α subunits that regulates the activity of intracellular tyrosine kinase on β subunits, and then insulin receptor undergoes a conformational change: the dimerization of intracellular β subunits. Subsequently, tyrosine autophosphorylation in the β subunits activates intrinsic tyrosine kinases and recruits intracellular effectors (mainly IRS-1 and IRS-2). The activated insulin receptor phosphorylates multiple tyrosine residues of IRS-1 or/and IRS-2, which elicits IRS-1 or/and IRS-2 self-activation leading to the recruitment and activation of the lipid kinase PI3K complex. Notably, IRS plays a pivotal switch in insulin signaling pathway, the phosphorylation of IRS-1 on serine (Ser) residues can inhibit IRS-1 activity, resulting in insulin resistance (Yarchoan et al. 2014). PI3K phosphorylates 3 phosphoinositide dependent protein kinase (PDK1), which then directly phosphorylates and activates threonine (Thr) residues in Akt; this initiates the PI3K/Akt insulin signaling pathway, and consequently, triggers many downstream effects (Chatterjee and Mudher 2018; Diehl et al. 2017).
Glucose transporter 4 (GLUT4) mainly expressed in neurons in hippocampus and cortex, and it is often co-expressed with GLUT3 (Apelt et al. 1999). GLUT4 functions primarily to uptake glucose into some neurons, muscle and adipose cells for energy (Fernando et al. 2008); Akt phosphorylates AS160, the 160 kDa substrate of Akt, which consequently induces the translocation of GLUT-4 to the cell membrane. Results of patients with AD demonstrated that downregulation of GLUT1 and GLUT3 proteins are detected in cerebral cortex and hippocampus (Liu et al. 2008; Mooradian et al. 1997; Yan et al. 2020). The downregulation of GLUT1 and GLUT3 proteins as an early pathogenetic mechanism of AD aggravate the deposition of Aβ and hyperphosphorylation of tau (Zhu et al. 2014). Glycogen synthase kinase 3β (GSK3β) is also mediated by Akt, which inhibits its constitutive activity through serine phosphorylation. GSK3β also plays a vital role in the regulation of microtubule-associated proteins including tau, which can form NFTs, one of the features of AD (Pardeshi et al. 2017; Stanley et al. 2016). Activated Akt also phosphorylates tuberous sclerosis 1/2 (TSC1/2) protein, which ultimately activates the mammalian target of rapamycin (mTOR) and its downstream factors ribosomal protein S6 kinase (S6K) and 4E-binding protein 1 (4EBP1); this in turn modulates protein synthesis. Additionally, inhibitor of nuclear factor-κB kinase (IKK) and c-Jun N-terminal kinase (JNK) are also directly activated by Akt kinase, as well as fork-head family box O (FOXO) transcription factors, which can regulate mitochondrial function (Fernandez and Torres-Aleman 2012). More importantly, several kinases, S6K, JNK, mTOR and GSK3 themselves, for example, permit the inhibition of IRS-1 activity through the negative feedback regulation of IRS-1 site-specific serine phosphorylation, thus contributing to inactivation of the insulin signaling pathway (Fig. 2) (Copps and White 2012).
IRS-1 phosphorylation in AD
The tail regions of IRS proteins are enriched in Ser/Thr/Tyr residues, and multiple phosphorylations of IRS Ser/Thr/Tyr residues regulate IRS function, which further influences downstream molecules such as IRS-1/PI3K/Akt insulin signaling either positively or negatively. It has been previously demonstrated that the phosphorylation pattern of IRS-1 dictates signal capacity, as well as its capacity to bind to receptors (Gual et al. 2005), which suggests that aberrant IRS-1 phosphorylation at tyrosine and serine residues may evoke a pathologic state of insulin receptor; this is associated with the IRS-1/PI3K/Akt insulin signaling pathway (Copps and White 2012; Hancer et al. 2014; Herschkovitz et al. 2007; Samuel and Shulman 2012). Indeed, phosphorylation of IRS-1 at tyrosine residues contributes to signaling functions, whereas phosphorylation at serine residues inhibits the dissociation of IRS-1 from the insulin receptor and diminishes tyrosine phosphorylation, which results in insulin signaling dysregulation (White 2003). Homeostasis between tyrosine and serine residues phosphorylation in IRS-1 is therefore important in the IRS-1/PI3K/Akt insulin signaling pathway.
In neural-derived blood exosomes from preclinical subjects (asymptomatic amyloidosis subjects who are cognitively intact at 1–10 years before their diagnosis of AD) or AD patients, the total IRS-1 level is decreased to a lesser extent compared with the extent of phosphorylation of pan-Tyr in IRS-1, which is significantly diminished (Kapogiannis et al. 2015). In contrast, the pSer312-IRS-1 level is apparently increased; the IR index means the ratio of pSer312-IRS-1 to P-pan-Tyr-IRS-1 in preclinical subjects or AD patients that is also higher than that in age- and gender-matched control (Kapogiannis et al. 2015). On the other hand, because IRS-1 phosphorylation or IR index could predict the development of AD up to 10 years prior to clinical onset, and some suggest, in some extend, IRS-1 phosphorylation or IR index may serve as biomarkers of AD (Kapogiannis et al. 2015). The levels of pSer612-IRS-1 and pSer636-IRS-1, which play a pivotal role in IR, are significantly elevated. This has been demonstrated in the brains of AD transgenic mice (APP/PS1 mice) as well as in the brains of individuals with AD with increased levels of pSer616-IRS-1and pSer636/639-IRS-1, and this phosphorylation pattern is positively associated with Aβ oligomer levels (Bomfim et al. 2012; Mao et al. 2016; Talbot et al. 2012). Increased IRS-1 phosphorylation in serine sites ultimately lead to IRS-1 inhibition, which has been shown in the brains of AD transgenic mice, in that IRS-1 phosphorylation at serine residues may disrupt the IRS-1/PI3K/Akt insulin signaling pathway and further accelerate AD progression (Bomfim et al. 2012). In hippocampal neurons stimulated by Aβ oligomers, the pSer307-IRS-1 and pSer312/616-IRS-1 levels are prominently elevated, whereas IRS-1 phosphorylation at tyrosine-465(pTyr465-IRS-1) is inhibited, which triggers defective brain insulin signaling (Bomfim et al. 2012). Chenodeoxycholic acid (CDCA) lowers pSer307-IRS-1 levels and increases Akt activation and GLUT4 levels, which helps mitigate IR in the hippocampus (Bazzari et al. 2019). Taken together, IRS-1 phosphorylation at tyrosine and serine residues is crucial to the IRS-1/PI3K/Akt insulin signaling pathway in AD pathology and to the negative feedback control process. Importantly, the IRS-1 downstream components mTOR, S6K, GSK3, IKK and JNK, play a negative regulatory role in IRS-1 Ser/Tyr phosphorylation, and the detailed phosphorylation site is presented in Table 1.
mTOR and S6K
mTOR, a major downstream effector of IRS-1/PI3K/Akt insulin signaling, acts as two functional complexes, mTORC1 and mTORC2, which are implicated in the regulation of protein and lipid synthesis, mitochondrial function, insulin signaling, and autophagy (Butterfield and Halliwell 2019). Research indicates that IRS-1 tyrosine phosphorylation increases when the PI3K/Akt/mTOR pathway is suppressed by PI3K inhibition, Akt or mTOR inhibition, whereas phosphorylation of IRS-1 at Ser302, 307, 318 decreases (Hancer et al. 2014). mTOR enhances the phosphorylation of serine residues in IRS-1. Moreover, aberrant mTOR activation could further exacerbate brain IR in both AD and MCI patients after induction with Aβ monomers and/or soluble oligomers through phosphorylation of S6K proteins, a downstream target of mTOR; this includes an approximate twofold increase in advanced phosphorylation of the IRS-1 inhibitory Ser residue (Ser307) by a negative feedback process (Tramutola et al. 2015). Moreover, activated mTOR signaling contributes to the buildup of SPs and NFTs, two hallmarks of AD neuropathology, through inhibition of autophagy as well as activation of downstream targets S6K and 4EBP1, which contribute to hyperphosphorylation of tau proteins (Di Domenico et al. 2018; Tramutola et al. 2015).
S6K, a critical signaling molecule in IR development, is a downstream target of mTOR that also induces IR by mediating IRS-1 Ser1101 phosphorylation (Tremblay et al. 2007). Another study indicated that activating mTORC1/S6K leads to increased IRS-1 Ser636 phosphorylation and diminished IRS-1 tyrosine phosphorylation, which are part of a feedback inhibition loop of insulin signaling (Gao et al. 2015). In SH-SY5Y cells, biliverdin reductase A inactivation is concomitant with increased p-mTOR and IRS-1 Ser307 phosphorylation levels; this is also observed in 3xTg AD mice, which suggests that elevated levels of p-mTOR parallels either IR or impaired biliverdin reductase A (Barone et al. 2016). On the contrary, hyperactivation of the mTORC1/S6K pathway instigates aberrant IRS-1 serine phosphorylation and degradation, which disrupts insulin signaling (Shah et al. 2004). Overall, in MCI and AD brains, this is a negative feedback mechanism by which overactivated mTOR induces IRS-1 serine residue phosphorylation to promote IRS-1 inactivation, which halts the normal activation of downstream targets in the insulin signaling pathway (Gupta and Dey 2012; Perluigi et al. 2015; Tramutola et al. 2015).
JNK/IKK
JNK, a serine/threonine protein kinase, inhibits insulin signaling by regulating phosphorylated IRS, and growing evidence indicates the role of elevated p-JNK in the induction of IRS-1 serine phosphorylation in AD brains (Zick 2005). Moreover, one study that investigated post-mortem brain tissues and cerebrospinal fluid (CSF) samples from AD patients showed that increased JNK and p-JNK levels are associated with Aβ42 levels, which reflects the degree of cognitive decline (Gourmaud et al. 2015). Aβ peptide forms abnormal plaques due to its abnormal aggregation, which can promote the release of tumor necrosis factor-α (TNF-α), a proinflammatory factor, by activating microglial cells; subsequently, this results in elevated levels of TNF-α that can activate the JNK and/or IKK signaling pathways (Park and Bowers 2010; Ribe and Lovestone 2016). Aβ triggers IRS-1 serine phosphorylation via JNK activation by TNF-α, which ultimately leads to the dysfunction of insulin signaling.
A technique revealing the interaction between IRS proteins and insulin receptor/insulin-like growth factor receptor demonstrated that, by altering the interaction kinetics between IRS and insulin receptor, JNK phosphorylates serine residues of IRS, which contributes to IR (Lanzerstorfer et al. 2015). In addition, JNK/TNF-α is activated by Aβ oligomers, leading to the phosphorylation of multiple serine residues within IRS-1 and the physiologic inhibition of IRS-1tyrosine phosphorylation, which also contributes to IR in primary hippocampal neurons and AD transgenic mouse models (Bomfim et al. 2012). Further research in vivo demonstrated that intracerebroventricular injection of Aβ oligomers causes IRS-1 serine phosphorylation and JNK activation in cynomolgus monkeys hippocampi (Bomfim et al. 2012). On the contrary, as Aβ oligomers enhance JNK pathway activation, IRS-1Ser616 phosphorylation and Tau Ser422 phosphorylation are increased (Yoon et al. 2012). Additionally, Aβ oligomers induce endoplasmic reticulum stress (ERS), which interferes with insulin signaling by JNK-dependent IRS-1 serine phosphorylation in AD pathogenesis (Zhang et al. 2016). Moreover, IRS-1 Ser307,318,612 phosphorylation is enhanced and accompanied by increased tau Thr181 hyperphosphorylation (Zhang et al. 2016). Those findings are concordant with those in previous studies (Ma et al. 2009). Furthermore, inhibition of JNK activation can diminish SPs formation and hyperphosphorylation of tau proteins and is thus able to mitigate cognitive deficits (Zhang et al. 2016; Zhou et al. 2015). Therefore, JNK could modulate IRS-1 Ser/Tyr phosphorylation by elevating TNF-α levels or by ERS, which interferes with insulin signaling and contributes to inactivation of IRS-1/PI3K/Akt downstream molecules through the feedback mechanism.
Akt/GSK3
GSK3 refers to two isoforms, GSK3α and GSK3β, and is a multifunctional serine/threonine kinase that, when phosphorylated, exerts its effects through Akt. GSK3 regulates microtubule-associated proteins and the phosphorylation and buildup of tau proteins through the IRS-1/PI3K/Akt pathway (Arnold et al. 2018; Hanger et al. 1992). GSK3 activity is diminished through the phosphorylation of Ser9 in the GSK3β N-terminal as well as by phosphorylation of Ser21 in GSK3α. In AD pathogenesis, brain IR evokes GSK3β overactivation, which partly exacerbates tau hyperphosphorylation (includes misfolded tau and fibril aggregation) (Bhat et al. 2003). In autopsied frontal cortex brain tissue from AD patients, decreased levels of IRS-1, p-Akt, and p-GSK3β (Ser9) have been observed, which suggests that GSK3β activity is increased and results in tau phosphorylation; GSK3β expression also colocalizes with NFTs (Liu et al. 2011). It has been documented that overexpression of GSK3 in AD results in diminished LTP induction, which leads to learning and memory deficits that present early in AD (Salcedo-Tello et al. 2011). Additionally, activated GSK3 triggers IRS-1 serine phosphorylation and suppresses phosphorylation of tyrosine residues within IRS-1, which indicates that a role for GSK3 is the promotion of IR; this in turn leads to IRS-1/PI3K/Akt insulin signaling deficiency (Eldar-Finkelman and Krebs 1997). GSK3, as the first identified physiological target of PI3K/Akt, is overactivated on account of aberrant IRS-1/PI3K/Akt insulin signaling, which results in tau hyperphosphorylation and LTP inhibition (Dubey et al. 2020; Liu et al. 2011; Salcedo-Tello et al. 2011). This leads to diminished synaptic plasticity, which suggests a crucial role for GSK3 in AD pathogenesis (Jaworski et al. 2019).
Therapeutic approaches to brain IR/insulin signal pathway in AD
Results of epidemiologic, clinical and experimental compellingly support a link between T2DM and AD that bear interrelated disease mechanisms involving insulin resistance and dysfunction of insulin signaling, and moreover antidiabetic drugs could modify the pathological and clinical progression of AD and improve cognition in some extend (Munoz-Jimenez et al. 2020; Rotermund et al. 2018; Yarchoan and Arnold 2014). Abnormal Ser/Tyr phosphorylation of IRS-1 is observed in the brain and neural-derived exosomes extracted from the blood of AD patients, and notably, is related to brain atrophy, all of which indicate a correlation between abnormal Ser/Tyr phosphorylation of IRS-1 and cognitive dysfunction (Kapogiannis et al. 2015; Mullins et al. 2017b; Rahman et al. 2019). Research on AD patients documented that the deposition of Aβ promotes Ser phosphorylation of IRS-1, which further elicits the impairment of downstream insulin signaling pathway, leading to brain IR, and these processes in turn further expedite Aβ accumulation and tau hyperphosphorylation (Mullins et al. 2017a; Talbot et al. 2012). Antidiabetic drugs involving sulfonylureas, metformin, thiazolidinediones, and GLP-1 analogues improve brain IR, impaired insulin signaling, neuroinflammation, oxidative stress, Aβ accumulation, tau hyperphosphorylation and other pathological processes in AD experimental and clinical research (Table 2). Despite the controversial findings of clinical studies, a number of literature and several compelling hypotheses still suggest that antidiabetic therapies hold potential as treatments for dementia (Bendlin 2019). We mainly summarize the effects of metformin, thiazolidinediones, and GLP-1 analogues on AD from experimental and clinical studies, and discussed below.
Metformin is a biguanide derivative that could increase insulin sensitivity and glucose uptake in individuals with T2DM, and also can cross the blood brain barrier (BBB) and has an impact on the brain biochemical pathways (Chaudhari et al. 2020; Pasquale et al. 2016). One study showed that metformin exerts a beneficial effect on both Aβ and tau pathologies in APP/PS1 mice (Chen et al. 2021). Findings from the study showed that metformin ameliorated microglial autophagy impairment, promoted the phagocytosis of pathological Aβ and tau proteins, and then limited the propagation of Aβ and tau aggregates in dystrophic neurites surrounding Aβ plaques. Additionally, metformin increased the protein levels of p-AMPK and insulin-degrading enzyme (IDE) in the brain of APP/PS1 mice, restored the antioxidant status, reduced the neuroinflammation, thus improving the cognitive decline (DiTacchio et al. 2015; Garg et al. 2017; Lu et al. 2020). In vitro, metformin is neuroprotective against Aβ-induced cytotoxicity and can enhance excitatory synaptic transmission in hippocampal CA1 neurons, increase glycolytic lactate production, and improve neuronal insulin resistance (Blumrich and Dringen 2019; Chen et al. 2016a, b; Chen et al. 2020; Gupta et al. 2011). However, results of other studies indicate that metformin exerts paradoxical effects on tau pathology, possibly leading to increased tau aggregation, and metformin induce mitochondrial dysfunction and promote the aggregation of toxic amyloid pre-fibrillar in brain cortex region (Barini et al. 2016; Pasquale et al. 2016). Results from clinical studies should that long-term treatment with metformin may decrease the risk of cognitive decline in individuals with T2DM (Hsu et al. 2011; Tze et al. 2014). Notably, however, the results of another study suggested that individuals with T2DM treated with metformin for a long-term had a slightly higher risk of developing AD than the T2DM patients treating with sulfonylureas or thiazolidinediones (Imfeld et al. 2012). Besides, in a pilot randomized placebo controlled clinical trial comparing placebo individuals, metformin improves total recall of the selective reminding test (SRT) in amnestic mild cognitive impairment patients (Luchsinger et al. 2016). Results of the other randomized placebo-controlled crossover study suggest that metformin was associated with improved executive functioning, and trends suggested improvement in learning/memory and attention in AD (Aaron et al. 2017). Overall, the results from experimental and clinical studies assessing the effect of metformin on cognitive decline and AD are mostly promising, and the further study, such as a 2-year metformin clinical trial (ClinicalTrials.gov NCT04098666) results should be expected (Munoz-Jimenez et al. 2020).
Thiazolidinediones, also known as glitazones, that include rosiglitazone and pioglitazone, are peroxisome proliferator-activated receptor gamma (PPARγ) agonists. Recent progress indicated that PPARγ agonists could modulated different cellular targets in AD and improve cognitive impairments. Results of several experimental studies indicated that rosiglitazone could promote the phagocytic ability of microglia, reduce the expression of proinflammatory factors, decrease Aβ and tau pathology in the brain of AD transgenic mice, and restore neural networks compromised by AD (Denner et al. 2012; Escribano et al. 2010). Pioglitazone also reversed behavioral deficits in AD model mice by decreasing hippocampal Aβ and tau proteins deposits, enhancing short- and long-term plasticity, attenuating neuroinflammation, activating phosphorylated ERK (p-ERK) during memory consolidation (Jahrling et al. 2014; Mandrekar-Colucci et al. 2012; Searcy et al. 2012). Furthermore, pioglitazone successfully reverts metabolic dysfunction in cortex, restore the energy metabolism, lower Aβ levels and deposition in AD model mice (Chang et al. 2019; Wong et al. 2020). Besides, the initial clinical results with rosiglitazone for AD patients were positive influence, but the later clinical trials evidence from larger patient groups and from the systematic review and meta-analysis are insufficient to support the use of rosiglitazone in MCI and AD patients to improve cognitive performance (Liu et al. 2015; Risner et al. 2006; Tzimopoulou et al. 2010; Watson et al. 2005). Moreover, rosiglitazone only has a neutral effect on the risk of dementia in T2DM, and individual patient level data suggest that treated with rosiglitazone is associated with a potential risk of cardiovascular disease (Tseng 2019; Wallach et al. 2020) risk. By contrast, pioglitazone seems to be promising therapeutic approach to AD patients. Results of clinical trials showed that pioglitazone improves memory and cognitive performance in mild AD patients, reduces dementia risk in patients with T2DM, and indicated the greatest efficacy compared to placebo (Cao et al. 2018; Cheng et al. 2016; Sato et al. 2011; Tseng 2018). However, the another several studies also demonstrated that pioglitazone has no beneficial effect on cognitive performance in patients with AD or MCI (Geldmacher et al. 2011; Hildreth et al. 2015). Overall, the controversial effects on MCI or AD patients exerted by thiazolidinediones are worthy of more investigation, and the possible explanations for the difference results of the research could be the unblind selection of patients, the small samples, the status of ApoE4(−/ +), and the brain bioavailability of the drugs (Chang et al. 2015; Hildreth et al. 2015; Iketani et al. 2018). Thus, the efficacy of thiazolidinediones as a disease-modifying drug on individuals with MCI or/and AD needs to be further confirmed by rigorous well-designed with large-scale randomized controlled trials.
GLP-1 analogue, such as liraglutide and exenatide (synthetic form of exendin-4) facilitate insulin signaling, and can cross the BBB reaching the brain to target GLP-1 receptors, which alleviate brain IR and insulin signaling pathway disorders, decrease the levels of hippocampal pSer-IRS-1 in AD model mice, thus improving cognitive dysfunction (Bomfim et al. 2012; Hunter and Hölscher 2012; Salameh et al. 2020; Talbot and Wang 2014). Liraglutide could improve learning and memory impairments in AD models by decreasing Aβ plaque load and modulating tau hyperphosphorylation, as well as regulating brain IR, PI3K/Akt pathway and insulin signal transduction (Batista et al. 2018; Jantrapirom et al. 2020; Liu et al. 2016; Qi et al. 2016). Exenatide administration prevented cognitive decline through alleviating Aβ deposition, tau hyperphosphorylation, improving brain glucose metabolism, mitigating mitochondrial toxicity by PI3K/Akt-mediated pathway as well as regulating IRS-1 phosphorylation (An et al. 2019; Bomba et al. 2013; Bomfim et al. 2012; Garabadu and Verma 2019). Overall, the in vivo and in vitro studies of GLP-1 analogues treating AD demonstrate an effect of this treatment on amyloid and tau pathologies as well as brain IR, abnormal insulin signaling pathway, oxidative stress, synaptic plasticity, apoptosis, and other core neuronal functions (Hansen et al. 2015; Liu et al. 2016; McClean et al. 2015; McClean et al. 2011; Perry et al. 2002, 2003; Qi et al. 2016). The multiple mechanism of action of liraglutide and exenatide for the treatment or prevention of AD progression are detailed presented in Table 2. Indeed, treatment with liraglutide or exenatide has consistently been associated with improvements in cognition and memory in preclinical model of AD.
Several more recent studies indicated that GLP-1 analogues such as liraglutide and exenatide, are potential candidate for AD disease-modifying treatment (Ballard et al. 2020; Talbot 2014). A randomized, placebo-controlled, double-blind clinical study in individuals with AD indicated that, compared with placebo, liraglutide treatment prevented the decline of glucose metabolism in the brain, which is associated with cognitive degeneration and synaptic dysfunction, and declining brain glucose metabolism often indicate dysfunction in brain activities (Gejl et al. 2016). Further research indicated that the underlying mechanism for this effect was an increased blood–brain glucose transport at the BBB (Gejl et al. 2017). Besides, a double-blind randomized placebo-controlled study which included only 21 participants, indicated that exenatide could lower plasma neuronal extracellular vesicles (EV) Aβ42 level, a biomarker in clinical trials in AD, and, however, only marginally improve cognitive outcomes in AD patients (Mullins et al. 2019). Given the very limited power of this study, early termination, small sample size as well as at a single-center study, these observations may underpowered and cannot be meaningfully interpreted (Ballard et al. 2020; Mullins et al. 2019). Clinical trial of Parkinson’s disease (PD) treatment with exenatide demonstrated that, compared with the control group, exenatide improved motor function and cognitive measures in individuals with PD, which was identified as potential disease-modifying treatment in neurodegenerative disease (Athauda et al. 2017; Aviles-Olmos et al. 2013). Thus, these results of GLP-1 analogues are promising and provide increasing evidence that these drugs are potential for the treatment of AD, and the further results of Evaluating Liraglutide in Alzheimer’s disease (ELAD) trial are eagerly awaited (Femminella et al. 2019).
Conclusion
In recent years, cumulative studies have elucidated that brain IR, which is a crucial pathological feature of AD, is associates with cognitive dysfunction, Aβ plaques, hyperphosphorylated tau protein and impaired cerebral glucose metabolism. Here, we highlight a key molecule in brain IR, IRS-1, which is phosphorylated at Ser/Tyr residues and is related to neuropathologic hallmarks of AD such as Aβ plaques and hyperphosphorylated tau proteins, and we present their potential mechanisms. In conclusion, dysregulation of IRS-1 Ser/Tyr phosphorylation could exacerbate disturbances in the IRS-1/PI3K/Akt insulin signaling pathway and the pathway’s interaction with mTOR, S6K, JNK/IKK and Akt/GSK3, among others. Anti-diabetic drugs could modulate the insulin signaling pathway, brain IR and other pathological process of AD, which provide a potential strategy for AD disease-modifying treatments, and future studies will contribute to the precise mechanism of Ser/Tyr phosphorylation in IRS-1 in the regulation of IRS-1/PI3K/Akt insulin signaling pathway in AD.
References
Aaron MK et al (2017) Effects of the insulin sensitizer metformin in Alzheimer disease pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord 31:107–113. https://doi.org/10.1097/WAD.0000000000000202
Akhtar A, Sah SP (2020) Insulin signaling pathway and related molecules: role in neurodegeneration and Alzheimer’s disease. Neurochem Int 135:104707. https://doi.org/10.1016/j.neuint.2020.104707
Akhtar A, Bishnoi M, Sah SP (2020a) Sodium orthovanadate improves learning and memory in intracerebroventricular-streptozotocin rat model of Alzheimer’s disease through modulation of brain insulin resistance induced tau pathology. Brain Res Bull 164:83–97. https://doi.org/10.1016/j.brainresbull.2020.08.001
Akhtar A, Dhaliwal J, Saroj P, Uniyal A, Bishnoi M, Sah SP (2020b) Chromium picolinate attenuates cognitive deficit in ICV-STZ rat paradigm of sporadic Alzheimer’s-like dementia via targeting neuroinflammatory and IRS-1/PI3K/AKT/GSK-3β pathway. Inflammopharmacology 28:385–400. https://doi.org/10.1007/s10787-019-00681-7
An J et al (2019) Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5xFAD mouse model of Alzheimer’s disease. Behav Brain Res 370:111932. https://doi.org/10.1016/j.bbr.2019.111932
Apelt J, Mehlhorn G, Schliebs R (1999) Insulin-sensitive GLUT4 glucose transporters are colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J Neurosci Res 57:693–705
Arnold SE et al (2018) Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 14:168–181. https://doi.org/10.1038/nrneurol.2017.185
Arranz AM, De Strooper B (2019) The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol 18:406–414. https://doi.org/10.1016/s1474-4422(18)30490-3
Athauda D et al (2017) Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390:1664–1675. https://doi.org/10.1016/s0140-6736(17)31585-4
Aviles-Olmos I et al (2013) Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest 123:2730–2736. https://doi.org/10.1172/JCI68295
Bahrami F, Asgari A, Hosseinmardi N, Janahmadi M (2019) Peroxisome proliferator-activated receptor (PPAR)-gamma modifies abeta neurotoxin-induced electrophysiological alterations in rat primary cultured hippocampal neurons. Iran J Pharm Res 18:1403–1418. https://doi.org/10.22037/ijpr.2019.1100783
Ballard C et al (2020) Drug repositioning and repurposing for Alzheimer disease. Nat Rev Neurol 16:661–673. https://doi.org/10.1038/s41582-020-0397-4
Barini E et al (2016) Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener 11:16. https://doi.org/10.1186/s13024-016-0082-7
Barone E et al (2016) Impairment of biliverdin reductase-a promotes brain insulin resistance in Alzheimer disease: a new paradigm. Free Radic Biol Med 91:127–142. https://doi.org/10.1016/j.freeradbiomed.2015.12.012
Batista AF et al (2018) The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol 245:85–100. https://doi.org/10.1002/path.5056
Bazzari FH, Abdallah DM, Elbhar HS (2019) Chenodeoxycholic acid ameliorates AlCl3-induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules 24:1992. https://doi.org/10.3390/molecules24101992
Bedse G, Di Domenico F, Serviddio G, Cassano T (2015) Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front Neurosci 9:204. https://doi.org/10.3389/fnins.2015.00204
Beeri MS, Bendlin BB (2020) The link between type 2 diabetes and dementia: from biomarkers to treatment. Lancet Diabetes Endocrinol 8:736–738. https://doi.org/10.1016/s2213-8587(20)30267-9
Bendlin BB (2019) Antidiabetic therapies and Alzheimer disease. Dialogues Clin Neurosci 21:83–91. https://doi.org/10.31887/DCNS.2019.21.1/bblendin
Bhat R et al (2003) Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem 278:45937–45945. https://doi.org/10.1074/jbc.M306268200
Blanchard JW, Tsai LH (2019) Unraveling the paradox of statins with human neurons: new leads in Alzheimer’s disease. Cell Stem Cell 24:347–349. https://doi.org/10.1016/j.stem.2019.02.003
Blumrich EM, Dringen R (2019) Metformin accelerates glycolytic lactate production in cultured primary cerebellar granule neurons. Neurochem Res 44:188–199. https://doi.org/10.1007/s11064-017-2346-1
Boccardi V, Murasecco I, Mecocci P (2019) Diabetes drugs in the fight against Alzheimer’s disease. Ageing Res Rev 54:100936. https://doi.org/10.1016/j.arr.2019.100936
Bomba M et al (2013) Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell Death Dis 4:e612. https://doi.org/10.1038/cddis.2013.139
Bomba M et al (2018) Exenatide exerts cognitive effects by modulating the BDNF-TrkB neurotrophic axis in adult mice. Neurobiol Aging 64:33–43. https://doi.org/10.1016/j.neurobiolaging.2017.12.009
Bomfim TR et al (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease—associated Aβ oligomers. J Clin Invest 122:1339–1353. https://doi.org/10.1172/JCI57256
Boucher J, Kleinridders A, Kahn CR (2014) Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6:a009191. https://doi.org/10.1101/cshperspect.a009191
Butterfield DA, Halliwell B (2019) Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20:148–160. https://doi.org/10.1038/s41583-019-0132-6
Candeias E et al (2012) The impairment of insulin signaling in Alzheimer’s disease. IUBMB Life 64:951–957. https://doi.org/10.1002/iub.1098
Cao B et al (2018) Comparative efficacy and acceptability of antidiabetic agents for Alzheimer’s disease and mild cognitive impairment: a systematic review and network meta-analysis. Diabetes Obes Metab 20:2467–2471. https://doi.org/10.1111/dom.13373
Chang KL, Pee HN, Yang S, Ho PC (2015) Influence of drug transporters and stereoselectivity on the brain penetration of pioglitazone as a potential medicine against Alzheimer’s disease. Sci Rep 5:9000. https://doi.org/10.1038/srep09000
Chang KL, Wong LR, Pee HN, Yang S, Ho PC (2019) Reverting metabolic dysfunction in cortex and cerebellum of APP/PS1 mice, a model for alzheimer’s disease by pioglitazone, a peroxisome proliferator-activated receptor gamma (PPARgamma) agonist. Mol Neurobiol 56:7267–7283. https://doi.org/10.1007/s12035-019-1586-2
Chatterjee S, Mudher A (2018) Alzheimer’s disease and type 2 diabetes: a critical assessment of the shared pathological traits. Front Neurosci 12:383. https://doi.org/10.3389/fnins.2018.00383
Chaudhari K et al (2020) Determination of metformin bio-distribution by LC-MS/MS in mice treated with a clinically relevant paradigm. PLoS ONE 15:e0234571. https://doi.org/10.1371/journal.pone.0234571
Chen B, Teng Y, Zhang X, Lv X, Yin Y (2016a) Metformin alleviated abeta-induced apoptosis via the suppression of JNK/MAPK signaling pathway in cultured hippocampal neurons. Biomed Res Int 2016:1421430. https://doi.org/10.1155/2016/1421430
Chen Q, Wang YP, Pan XD, Liu XY, Chen Zh, Liu L (2016b) Exendin-4 protects Aβ(1–42) oligomer-induced PC12 cell apoptosis. Am J Transl Res 8:3540–3548 (Collection 2016)
Chen WB, Chen J, Liu ZY, Luo B, Zhou T, Fei EK (2020) Metformin enhances excitatory synaptic transmission onto hippocampal CA1 pyramidal neurons. Brain Sci 10:706. https://doi.org/10.3390/brainsci10100706
Chen Y et al (2021) Metformin attenuates plaque-associated tau pathology and reduces amyloid-beta burden in APP/PS1 mice. Alzheimers Res Ther 13:40. https://doi.org/10.1186/s13195-020-00761-9
Cheng H, Shang Y, Jiang L, Shi TL, Wang L (2016) The peroxisome proliferators activated receptor-gamma agonists as therapeutics for the treatment of Alzheimer’s disease and mild-to-moderate Alzheimer’s disease: a meta-analysis. Int J Neurosci 126:299–307. https://doi.org/10.3109/00207454.2015.1015722
Chiang HC, Wang L, Xie Z, Yau A, Zhong Y (2010) PI3 kinase signaling is involved in abeta-induced memory loss in Drosophila. Proc Natl Acad Sci USA 107:7060–7065. https://doi.org/10.1073/pnas.0909314107
Chiu SL, Chen CM, Cline HT (2008) Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58:708–719. https://doi.org/10.1016/j.neuron.2008.04.014
Chua LM, Lim ML, Chong PR, Hu ZP, Cheung NS, Wong BS (2012) Impaired neuronal insulin signaling precedes Aβ42 accumulation in female AβPPsw/PS1ΔE9 mice. J Alzheimers Dis 29:783–791. https://doi.org/10.3233/JAD-2012-111880
Copps KD, White MF (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55:2565–2582. https://doi.org/10.1007/s00125-012-2644-8
Daisuke T, Wataru F, Taguchi A (2019) Involvement of insulin receptor substrates in cognitive impairment and Alzheimer’s disease. Neural Regen Res 14:1330–1334. https://doi.org/10.4103/1673-5374.253535
De Felice FG (2013) Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest 123:531–539. https://doi.org/10.1172/JCI64595
De Strooper B, Karran E (2016) The cellular phase of Alzheimer’s disease. Cell 164:603–615. https://doi.org/10.1016/j.cell.2015.12.056
de la Monte SM (2014) Type 3 diabetes is sporadic Alzheimers disease: mini-review. Eur Neuropsychopharmacol 24:1954–1960. https://doi.org/10.1016/j.euroneuro.2014.06.008
Denner LA et al (2012) Cognitive enhancement with rosiglitazone links the hippocampal PPARgamma and ERK MAPK signaling pathways. J Neurosci 32:16725–16735a. https://doi.org/10.1523/JNEUROSCI.2153-12.2012
Di Domenico F, Tramutola A, Foppoli C, Head E, Perluigi M, Butterfield DA (2018) mTOR in down syndrome: role in Aβ and tau neuropathology and transition to Alzheimer disease-like dementia. Free Radic Biol Med 114:94–101. https://doi.org/10.1016/j.freeradbiomed.2017.08.009
Diehl T, Mullins R, Kapogiannis D (2017) Insulin resistance in Alzheimer’s disease. Transl Res 183:26–40. https://doi.org/10.1016/j.trsl.2016.12.005
Dineley KT, Jahrling JB, Denner L (2014) Insulin resistance in Alzheimer’s disease. Neurobiol Dis 72(Pt A):92–103. https://doi.org/10.1016/j.nbd.2014.09.001
DiTacchio KA, Heinemann SF, Dziewczapolski G (2015) Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. J Alzheimers Dis 44:43–48. https://doi.org/10.3233/JAD-141332
Duarte AI et al (2020) Liraglutide protects against brain amyloid-beta1-42 accumulation in female mice with early Alzheimer’s disease-like pathology by partially rescuing oxidative/nitrosative stress and inflammation. Int J Mol Sci 21:1746. https://doi.org/10.3390/ijms21051746
Dubey SK et al (2020) Insulin mediated novel therapies for the treatment of Alzheimer’s disease. Life Sci 249:117540. https://doi.org/10.1016/j.lfs.2020.117540
Eldar-Finkelman H, Krebs EG (1997) Phosphorylation of insulin receptor substrate 1 by glycogen synthase kinase 3 impairs insulin action. Proc Natl Acad Sci USA 94:9660–9664
Escribano L et al (2010) Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology 35:1593–1604. https://doi.org/10.1038/npp.2010.32
Farr SA, Roesler E, Niehoff ML, Roby DA, McKee A, Morley JE (2019) Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer’s disease. J Alzheimers Dis 68:1699–1710. https://doi.org/10.3233/JAD-181240
Femminella GD et al (2019) Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study). Trials 20:191. https://doi.org/10.1186/s13063-019-3259-x
Fernandez AM, Torres-Aleman I (2012) The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 13:225–239. https://doi.org/10.1038/nrn3209
Fernando RN, Albiston AL, Chai SY (2008) The insulin-regulated aminopeptidase IRAP is colocalised with GLUT4 in the mouse hippocampus–potential role in modulation of glucose uptake in neurones? Eur J Neurosci 28:588–598. https://doi.org/10.1111/j.1460-9568.2008.06347.x
Ferreira ST, Clarke JR, Bomfim TR, De Felice FG (2014) Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement 10:S76-83. https://doi.org/10.1016/j.jalz.2013.12.010
Gao S, Duan C, Gao G, Wang X, Yang H (2015) Alpha-synuclein overexpression negatively regulates insulin receptor substrate 1 by activating mTORC1/S6K1 signaling. Int J Biochem Cell Biol 64:25–33. https://doi.org/10.1016/j.biocel.2015.03.006
Garabadu D, Verma J (2019) Exendin-4 attenuates brain mitochondrial toxicity through PI3K/Akt-dependent pathway in amyloid beta (1–42)-induced cognitive deficit rats. Neurochem Int 128:39–49. https://doi.org/10.1016/j.neuint.2019.04.006
Garg G, Singh S, Singh AK, Rizvi SI (2017) Anti-aging effect of metformin on brain in naturally aged and accelerated senescence model of rat. Rejuvenation Res 20:173–182. https://doi.org/10.1089/rej.2016.1883
Gejl M et al (2016) In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci 8:108. https://doi.org/10.3389/fnagi.2016.00108
Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A (2017) Blood-brain glucose transfer in Alzheimer’s disease: effect of GLP-1 analog treatment. Sci Rep 7:17490. https://doi.org/10.1038/s41598-017-17718-y
Geldmacher DS, Fritsch T, McClendon MJ, Landreth G (2011) A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol 68:45–50. https://doi.org/10.1001/archneurol.2010.229
Gold M et al (2010) Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord 30:131–146. https://doi.org/10.1159/000318845
Gourmaud S et al (2015) Increased levels of cerebrospinal fluid JNK3 associated with amyloid pathology: links to cognitive decline. J Psychiatry Neurosci 40:151–161. https://doi.org/10.1503/jpn.140062
Grillo CA et al (2015) Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 64:3927–3936. https://doi.org/10.2337/db15-0596
Gual P, Le Marchand-Brustel Y, Tanti JF (2005) Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87:99–109. https://doi.org/10.1016/j.biochi.2004.10.019
Gupta A, Dey CS (2012) PTEN, a widely known negative regulator of insulin/PI3K signaling, positively regulates neuronal insulin resistance. Mol Biol Cell 23:3882–3898. https://doi.org/10.1091/mbc.E12-05-0337
Gupta A, Bisht B, Dey CS (2011) Peripheral insulin-sensitizer drug metformin ameliorates neuronal insulin resistance and Alzheimer’s-like changes. Neuropharmacology 60:910–920. https://doi.org/10.1016/j.neuropharm.2011.01.033
Hancer NJ, Qiu W, Cherella C, Li Y, Copps KD, White MF (2014) Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation. J Biol Chem 289:12467–12484. https://doi.org/10.1074/jbc.M114.554162
Hanger DP, Hughes K, Woodgett JR, Brion JP, Anderton BH (1992) Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci Lett 147:58–62. https://doi.org/10.1016/0304-3940(92)90774-2
Hansen HH et al (2015) The GLP-1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence-accelerated mouse model of Alzheimer’s disease. J Alzheimers Dis 46:877–888. https://doi.org/10.3233/JAD-143090
Harrington C et al (2011) Rosiglitazone does not improve cognition or global function when used as adjunctive therapy to AChE inhibitors in mild-to-moderate Alzheimer’s disease: two phase 3 studies. Curr Alzheimer Res 8:592–606. https://doi.org/10.2174/156720511796391935
Herschkovitz A, Liu YF, Ilan E, Ronen D, Boura-Halfon S, Zick Y (2007) Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J Biol Chem 282:18018–18027. https://doi.org/10.1074/jbc.M610949200
Hildreth KL et al (2015) Effects of pioglitazone or exercise in older adults with mild cognitive impairment and insulin resistance: a pilot study. Dementia Geriatr Cogn Disord Extra 5:51–63. https://doi.org/10.1159/000371509
Hirosumi J et al (2002) A central role for JNK in obesity and insulin resistance. Nature 420:333–336. https://doi.org/10.1038/nature01137
Horwood JM, Dufour F, Laroche S, Davis S (2006) Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci 23:3375–3384. https://doi.org/10.1111/j.1460-9568.2006.04859.x
Hsu CC, Wahlqvist ML, Lee MS, Tsai HN (2011) Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 24:485–493. https://doi.org/10.3233/JAD-2011-101524
Hunter K, Hölscher C (2012) Drugs developed to treat diabetes, liraglutide and lixesenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13:33. https://doi.org/10.1186/1471-2202-13-33
Iketani R, Ohno K, Kawasaki Y, Matsumoto K, Yamada H, Kishino S (2018) Apolipoprotein E gene polymorphisms affect the efficacy of thiazolidinediones for Alzheimer’s disease: a systematic review and meta-analysis. Biol Pharm Bull 41:1017–1023. https://doi.org/10.1248/bpb.b17-00929
Imfeld P, Bodmer M, Jick SS, Meier CR (2012) Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 60:916–921. https://doi.org/10.1111/j.1532-5415.2012.03916.x
International AsD (2019) World Alzheimer report 2019: attitudes to dementia.
Jahrling JB, Hernandez CM, Denner L, Dineley KT (2014) PPARgamma recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J Neurosci 34:4054–4063. https://doi.org/10.1523/JNEUROSCI.4024-13.2014
Jantrapirom S et al (2020) Liraglutide suppresses tau hyperphosphorylation, amyloid Beta accumulation through regulating neuronal insulin signaling and BACE-1 activity. Int J Mol Sci. https://doi.org/10.3390/ijms21051725
Jaworski T, Banach-Kasper E, Gralec K (2019) GSK-3β at the intersection of neuronal plasticity and neurodegeneration. Neural Plast 2019:4209475. https://doi.org/10.1155/2019/4209475
Jia XT et al (2016) Exendin-4, a glucagon-like peptide 1 receptor agonist, protects against amyloid-beta peptide-induced impairment of spatial learning and memory in rats. Physiol Behav 159:72–79. https://doi.org/10.1016/j.physbeh.2016.03.016
Kapogiannis D et al (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 29:589–596. https://doi.org/10.1096/fj.14-262048
Kellar D, Craft S (2020) Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol 19:758–766. https://doi.org/10.1016/s1474-4422(20)30231-3
Khan MA, Alam Q, Haque A, Ashafaq M, Khan MJ, Ashraf GM, Ahmad M (2019) Current progress on peroxisome proliferator-activated receptor gamma agonist as an emerging therapeutic approach for the treatment of Alzheimer’s disease: an update. Curr Neuropharmacol 17:232–246. https://doi.org/10.2174/1570159X16666180828100002
Kleinridders A (2016) Deciphering brain insulin receptor and insulin-like growth factor 1 receptor signalling. J Neuroendocrinol. https://doi.org/10.1111/jne.12433.10.1111/jne.12433
Kleinridders A, Ferris HA, Cai W, Kahn CR (2014) Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63:2232–2243. https://doi.org/10.2337/db14-0568
Kong J, Wan L, Wang Y, Zhang H, Zhang W (2020) Liraglutide attenuates Abeta42 generation in APPswe/SH-SY5Y cells through the regulation of autophagy. Neuropsychiatr Dis Treat 16:1817–1825. https://doi.org/10.2147/NDT.S260160
Lanzerstorfer P, Yoneyama Y, Hakuno F, Muller U, Hoglinger O, Takahashi S, Weghuber J (2015) Analysis of insulin receptor substrate signaling dynamics on microstructured surfaces. FEBS J 282:987–1005. https://doi.org/10.1111/febs.13213
Liberman Z, Eldar-Finkelman H (2005) Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling. J Biol Chem 280:4422–4428. https://doi.org/10.1074/jbc.M410610200
Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX (2008) Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett 582:359–364. https://doi.org/10.1016/j.febslet.2007.12.035
Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2011) Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol 225:54–62. https://doi.org/10.1002/path.2912
Liu J, Wang LN, Jia JP (2015) Peroxisome proliferator-activated receptor-gamma agonists for Alzheimer’s disease and amnestic mild cognitive impairment: a systematic review and meta-analysis. Drugs Aging 32:57–65. https://doi.org/10.1007/s40266-014-0228-7
Liu XY, Wang LX, Chen Z, Liu LB (2016) Liraglutide prevents beta-amyloid-induced neurotoxicity in SH-SY5Y cells via a PI3K-dependent signaling pathway. Neurol Res 38:313–319. https://doi.org/10.1080/01616412.2016.1145914
Lu XY et al (2020) Metformin ameliorates abeta pathology by insulin-degrading enzyme in a transgenic mouse model of Alzheimer’s disease. Oxid Med Cell Longev 2020:2315106. https://doi.org/10.1155/2020/2315106
Luchsinger JA et al (2016) Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J Alzheimers Dis 51:501–514. https://doi.org/10.3233/JAD-150493
Ma QL et al (2009) Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci 29:9078–9089. https://doi.org/10.1523/JNEUROSCI.1071-09.2009
Mandrekar-Colucci S, Karlo JC, Landreth GE (2012) Mechanisms underlying the rapid peroxisome proliferator-activated receptor-gamma-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J Neurosci 32:10117–10128. https://doi.org/10.1523/JNEUROSCI.5268-11.2012
Mao YF et al (2016) Intranasal insulin alleviates cognitive deficits and amyloid pathology in young adult APPswe/PS1dE9 mice. Aging Cell 15:893–902. https://doi.org/10.1111/acel.12498
McClean PL, Holscher C (2014) Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer’s disease. Neuropharmacology 76Pt A:57–67. https://doi.org/10.1016/j.neuropharm.2013.08.005
McClean PL, Parthsarathy V, Faivre E, Holscher C (2011) The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci 31:6587–6594. https://doi.org/10.1523/JNEUROSCI.0529-11.2011
McClean PL, Jalewa J, Holscher C (2015) Prophylactic liraglutide treatment prevents amyloid plaque deposition, chronic inflammation and memory impairment in APP/PS1 mice. Behav Brain Res 293:96–106. https://doi.org/10.1016/j.bbr.2015.07.024
McNay EC, Recknagel AK (2011) Brain insulin signaling: a key component of cognitive processes and a potential basis for cognitive impairment in type 2 diabetes. Neurobiol Learn Mem 96:432–442. https://doi.org/10.1016/j.nlm.2011.08.005
Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C (2010) Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 31:224–243. https://doi.org/10.1016/j.neurobiolaging.2008.04.002
Mooradian AD, Chung HC, Shah GN (1997) GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging 18:469–474
Mullins RJ, Diehl TC, Chia CW, Kapogiannis D (2017a) Insulin resistance as a link between amyloid-beta and tau pathologies in Alzheimer’s disease. Front Aging Neurosci 9:118. https://doi.org/10.3389/fnagi.2017.00118
Mullins RJ, Mustapic M, Goetzl EJ, Kapogiannis D (2017b) Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer’s disease. Hum Brain Mapp 38:1933–1940. https://doi.org/10.1002/hbm.23494
Mullins RJ et al (2019) A pilot study of exenatide actions in Alzheimer’s disease. Curr Alzheimer Res 16:741–752. https://doi.org/10.2174/1567205016666190913155950
Munoz-Jimenez M, Zaarkti A, Garcia-Arnes JA, Garcia-Casares N (2020) Antidiabetic drugs in Alzheimer’s disease and mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord 49:423–434. https://doi.org/10.1159/000510677
Nichols E et al (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 18:88–106. https://doi.org/10.1016/s1474-4422(18)30403-4
Panagaki T, Michael M, Holscher C (2017) Liraglutide restores chronic ER stress, autophagy impairments and apoptotic signalling in SH-SY5Y cells. Sci Rep 7:16158. https://doi.org/10.1038/s41598-017-16488-x
Pardeshi R et al (2017) Insulin signaling: an opportunistic target to minify the risk of Alzheimer’s disease. Psychoneuroendocrinology 83:159–171. https://doi.org/10.1016/j.psyneuen.2017.05.004
Park KM, Bowers WJ (2010) Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal 22:977–983. https://doi.org/10.1016/j.cellsig.2010.01.010
Pasquale P et al (2016) Biological and biophysics aspects of metformin-induced effects cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging (Albany NY). 8:1718–1734. https://doi.org/10.18632/aging.101004
Perluigi M, Di Domenico F, Butterfield DA (2015) mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 84:39–49. https://doi.org/10.1016/j.nbd.2015.03.014
Perry TA, Lahiri DK, Chen DM, Zhou J, Shaw KTY, Egan JM, Greig N (2002) A novel neutrotrophic property of glucagon-like peptide 1 a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther 300:958–966. https://doi.org/10.1124/jpet.300.3.958
Perry TA, Lahiri DK, Sambamurti K, Chen DM, Mattson MP, Egan JM, Greig N (2003) Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 72:603–612. https://doi.org/10.1002/jnr.10611
Prakash A, Kumar A (2014) Role of nuclear receptor on regulation of BDNF and neuroinflammation in hippocampus of β-amyloid animal model of Alzheimer’s disease. Neurotox Res 25:335–347. https://doi.org/10.1007/s12640-013-9437-9
Qi L et al (2016) Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase-3beta pathway in an amyloid beta protein induced alzheimer disease mouse model. Eur J Pharmacol 783:23–32. https://doi.org/10.1016/j.ejphar.2016.04.052
Rahman SO, Panda BP, Parvez S, Kaundal M, Hussain S, Akhtar M, Najmi AK (2019) Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Abeta peptides in animal model of Alzheimer’s disease. Biomed Pharmacother 110:47–58. https://doi.org/10.1016/j.biopha.2018.11.043
Ribe EM, Lovestone S (2016) Insulin signalling in Alzheimer’s disease and diabetes: from epidemiology to molecular links. J Intern Med 280:430–442. https://doi.org/10.1111/joim.12534
Risner ME et al (2006) Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J 6:246–254. https://doi.org/10.1038/sj.tpj.6500369
Rotermund C, Machetanz G, Fitzgerald JC (2018) The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol (Lausanne) 9:400. https://doi.org/10.3389/fendo.2018.00400
Salameh TS, Rhea EM, Talbot K, Banks WA (2020) Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem Pharmacol 180:114187. https://doi.org/10.1016/j.bcp.2020.114187
Salcedo-Tello P, Ortiz-Matamoros A, Clorinda A (2011) GSK3 function in the brain during development, neuronal plasticity, and neurodegeneration. Int J Alzheimers Dis 2011:189728. https://doi.org/10.4061/2011/189728
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871. https://doi.org/10.1016/j.cell.2012.02.017
Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T (2011) Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 32:1626–1633. https://doi.org/10.1016/j.neurobiolaging.2009.10.009
Searcy JL et al (2012) Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer’s disease. J Alzheimers Dis 30:943–961. https://doi.org/10.3233/JAD-2012-111661
Shah OJ, Wang Z, Hunter T (2004) Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol 14:1650–1656. https://doi.org/10.1016/j.cub.2004.08.026
Solmaz V, Cinar BP, Yigitturk G, Cavusoglu T, Taskiran D, Erbas O (2015) Exenatide reduces TNF-alpha expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats. Eur J Pharmacol 765:482–487. https://doi.org/10.1016/j.ejphar.2015.09.024
Spinelli M, Fusco S, Grassi C (2020) Brain insulin resistance impairs hippocampal plasticity. Vitam Horm 114:281–306. https://doi.org/10.1016/bs.vh.2020.04.005
Stanley M, Macauley SL, Holtzman DM (2016) Changes in insulin and insulin signaling in Alzheimer’s disease: cause or consequence? J Exp Med 213:1375–1385. https://doi.org/10.1084/jem.20160493
Steen E et al (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes. J Alzheimers Dis 7:63–80. https://doi.org/10.3233/jad-2005-7107
Talbot K (2014) Brain insulin resistance in Alzheimer’s disease and its potential treatment with GLP-1 analogs. Neurodegener Dis Manag 4:31–40. https://doi.org/10.2217/nmt.13.73
Talbot K, Wang HY (2014) The nature, significance, and glucagon-like peptide-1 analog treatment of brain insulin resistance in Alzheimer’s disease. Alzheimers Dement 10:S12-25. https://doi.org/10.1016/j.jalz.2013.12.007
Talbot K et al (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122:1316–1338. https://doi.org/10.1172/JCI59903
Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7:85–96. https://doi.org/10.1038/nrm1837
Tramutola A et al (2015) Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133:739–749. https://doi.org/10.1111/jnc.13037
Tremblay F et al (2007) Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA 104:14056–14061
Tseng CH (2018) Pioglitazone reduces dementia risk in patients with type 2 diabetes mellitus: a retrospective cohort analysis. J Clin Med. https://doi.org/10.3390/jcm7100306
Tseng CH (2019) Rosiglitazone has a neutral effect on the risk of dementia in type 2 diabetes patients. Aging (Albany NY) 11:2724–2734. https://doi.org/10.18632/aging.101944
Tze PN, Liang F, Keng BY, Tih ShL, Chay HT, Winblad B (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41:61–68. https://doi.org/10.3233/JAD-131901
Tzimopoulou S et al (2010) A multi-center randomized proof-of-concept clinical trial applying [(1)(8)F]FDG-PET for evaluation of metabolic therapy with rosiglitazone XR in mild to moderate Alzheimer’s disease. J Alzheimers Dis 22:1241–1256. https://doi.org/10.3233/JAD-2010-100939
Van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM (2005) Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem 94:1158–1166. https://doi.org/10.1111/j.1471-4159.2005.03269.x
Velazquez R, Tran A, Ishimwe E, Denner L, Dave N, Oddo S, Dineley KT (2017) Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer’s disease. Neurobiol Aging 58:1–13. https://doi.org/10.1016/j.neurobiolaging.2017.06.003
Wallach JD et al (2020) Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses. BMJ (Clin Res ed) 368:l7078. https://doi.org/10.1136/bmj.l7078
Wang X, Wang L, Jiang R, Xu Y, Zhao X, Li Y (2016) Exendin-4 antagonizes Abeta1-42-induced attenuation of spatial learning and memory ability. Exp Ther Med 12:2885–2892. https://doi.org/10.3892/etm.2016.3742
Watson GS et al (2005) Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry 13:950–958. https://doi.org/10.1176/appi.ajgp.13.11.950
Weller J, Budson A (2018) Current understanding of Alzheimer's disease diagnosis and treatment. F1000Res 7 doi:https://doi.org/10.12688/f1000research.14506.1
White MF (2003) Insulin signaling in health and disease. Science 302:1710–1711
Wong LR, Wong P, Ho PC (2020) Metabolic profiling of female Tg2576 mouse brains provides novel evidence supporting intranasal low-dose pioglitazone for long-term treatment at an early stage of Alzheimer’s disease. Biomed. https://doi.org/10.3390/biomedicines8120589
Yan X, Hu Y, Wang B, Wang S, Zhang X (2020) Metabolic dysregulation contributes to the progression of Alzheimer’s disease. Front Neurosci 14:530219. https://doi.org/10.3389/fnins.2020.530219
Yarchoan M, Arnold SE (2014) Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes 63:2253–2261. https://doi.org/10.2337/db14-0287
Yarchoan M et al (2014) Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol 128:679–689. https://doi.org/10.1007/s00401-014-1328-5
Yoon SO et al (2012) JNK3 perpetuates metabolic stress induced by Aβ peptides. Neuron 75:824–837. https://doi.org/10.1016/j.neuron.2012.06.024
Zhang X, Tang S, Zhang Q, Shao W, Han X, Wang Y, Du Y (2016) Endoplasmic reticulum stress mediates JNK-dependent IRS-1 serine phosphorylation and results in Tau hyperphosphorylation in amyloid beta oligomer-treated PC12 cells and primary neurons. Gene 587:183–193. https://doi.org/10.1016/j.gene.2016.05.018
Zhang J, Chen C, Hua S, Liao H, Wang M, Xiong Y, Cao F (2017) An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract 124:41–47. https://doi.org/10.1016/j.diabres.2016.10.024
Zhou Q et al (2015) Inhibition of c-Jun N-terminal kinase activation reverses Alzheimer disease phenotypes in APPswe/PS1dE9 mice. Ann Neurol 77:637–654. https://doi.org/10.1002/ana.24361
Zhu Y, Shan X, Yuzwa SA, Vocadlo DJ (2014) The emerging link between O-GlcNAc and Alzheimer disease. J Biol Chem 289:34472–34481. https://doi.org/10.1074/jbc.R114.601351
Zick Y (2005) Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE 2005:pe4. https://doi.org/10.1126/stke.2682005pe4
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12:723–738. https://doi.org/10.1038/nrn3114
Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (NO.81573927) and The Scientific Research and Graduate Training Project of Beijing Municipal Commission of Education (2016, 2017) for providing support.
Author information
Authors and Affiliations
Contributions
MCZ designed and wrote the manuscript. PWW critically reviewed the manuscript and did the required editing and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the preparation of manuscript.
Rights and permissions
About this article
Cite this article
Zheng, M., Wang, P. Role of insulin receptor substance-1 modulating PI3K/Akt insulin signaling pathway in Alzheimer’s disease. 3 Biotech 11, 179 (2021). https://doi.org/10.1007/s13205-021-02738-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02738-3