Abstract
Monitoring groundwater quality and analyzing geochemical processes are very crucial in dry environments, especially in areas with intense agriculture and inadequate irrigation management systems. A total of forty-one groundwater samples were collected from the study area and analyzed for major ions chemistry. The results of the geochemical analyses are used to investigate the geochemical processes and make groundwater assessments. The hydrogeochemical characteristics of the groundwater samples revealed a highly mineralized Na+–Cl− water type, implying that the majority of the samples are chemically unfit for human consumption. Based on the water quality parameters analyzed for the groundwater samples collected in this study, such as sodium adsorption ratio (SAR), soluble sodium percentage (SSP), residual sodium carbonate (RSC), magnesium hazard (MH), permeability index (PI), and Kelley's ratio (KR), the suitability of groundwater samples for irrigation purposes is good to moderately good. Also they may reflect some alkaline hazard threats to the soil as indicated by the SSP and KR. The salinity hazard is recognized as high and expected to increase with the expansion of irrigation; hence, special salinity control management for groundwater in the study area is required. Nitrate is widely recognized as a contaminant in the study area. The nitrate concentrations range from 0.1 to 256.8 mg/L, with more than 30% of the samples exceeding the safe drinking water limit of 50 mg/L. The main concern is that the average nitrate concentration will likely continue to increase in the groundwater of the study area. Groundwater monitoring, in terms of both quality and quantity, is essential for sustainable groundwater management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, Egypt has experienced serious water scarcity. The biggest challenge facing Egypt's water resources system is population growth and rapid economic development. The River Nile constitutes the principal permanent source of fresh water in Egypt and supplies nearly all of the country's water need, including those of agriculture and industry (Wahba et al. 2018). The current actual available water resources in Egypt are about 55.5 billion m3/year from the River Nile water, and supplies around 95% of the annual fresh water demand in Egypt (SEAM 2005; Omar and Moussa 2016). Rainwater is very limited in Egypt and is concentrated in a very narrow strip on the coastal parts of the country. Groundwater constitutes the second important source of freshwater in Egypt and represents about 12% of the total water supply in Egypt (Abo-El-Fadl 2013). The groundwater resource in Egypt comprises two main major aquifers, the transboundary Nubian Sandstone Aquifer System (NSAS) and the Nile Valley Aquifer System (NVAS). The Nubian Aquifer is the deepest and oldest sedimentary rocks, which are often early to late Cretaceous in age (Hess et al. 1987). The Nubian Aquifer covers about 82% of the area of Egypt as well as south-east Libya, north-east Chad, and north Sudan with a total area of about 2.2 million square kilometers (CEDARE 2001; Hesse et al. 1987). The Nubian Aquifer is heavily exploited in Egypt, particularly in the Western Desert, where deep drilling wells have been carried out in recent decades (El Tahlawi et al. 2008; Mohammed 2015). The Nubian Aquifer is located in the study area beneath the shallow Nile Valley Aquifer, but it is not widely utilized. The Nile Valley Aquifer is also one of the major aquifers in Egypt. It lies beneath the majority of the Nile valley, close to the River Nile banks, and constitutes a highly productive groundwater source. The Nile Valley Aquifer is shallow, and its deposits are often Tertiary to Quaternary deposits (El Tahlawi et al. 2008; Mohammed et al. 2016). The Nile Valley Aquifer is our main focus in this study.
For decades, Egypt has pursued a nationwide program of land reclamation and new settlements in order to address the overpopulation problem and to expand agricultural land (Wolff 1993; Adriansen 2009; Ashour et al. 2009; Mohammed et al. 2016). Converting arid or desert areas to agricultural land is referred to as land reclamation. This is primarily accomplished by extending water canals from existing agricultural land or drilling groundwater wells. Groundwater resources are viewed as the major sustainable water supply in many of the reclamation projects in Egypt. New lands are also reclaimed on a small scale by residents on the desert fringes outside the old lands. They mostly are relying on groundwater for irrigation and residential purposes because of the lack of water from the River Nile. As groundwater abstraction increases due to urban and agricultural expansion, changes in groundwater quantity and quality often occur, especially in the shallow aquifer systems in arid regions (Ambast et al. 2006; Ayotte et al. 2015; Li et al. 2015). The study area is an excellent example of a vast tract of reclaimed land that is held by the government, the business sector, and individuals.
Groundwater contamination due to over-exploitation, intensive irrigation, and human activities has been reported in many regions of the world (Han 1998; El-Naqa and Al-Shayeb 2009; Nel et al. 2009; McCallum et al. 2010; Rattray 2015; Li et al. 2016). Apparently, most groundwater contains dissolved salts and trace elements as a result of the natural weathering of the earth's surface and aquifer rock-forming minerals. Agriculture has become a critical cause and a source of groundwater pollution that might alter the soil physical properties and structures and leave the water unsuitable or less valuable for other water uses (Scanlon et al. 2007; Pastén-Zapata et al. 2014; Mohammed et al. 2016). Agricultural return flow water could be of a significant component contributing to the groundwater recharge particularly in the case of intensively irrigated areas. Irrigation return flow water could also transmit pollutants that produced from the frequently applied pesticides, fertilizers, and manure to the agricultural fields (Pulido-Bosch et al. 2000; Weitzman et al. 2021). Irrigation return flow significantly increases the salinity, nitrate, and total phosphorus concentrations in groundwater (Pearce and Schumann 2001; Widory et al. 2004; Foster et al. 2018; Park et al. 2018).
The hydrogeochemical characterization and assessment of groundwater quality have become a serious concern in recent decades as public awareness of the importance of groundwater quality protection rises. A various literature on groundwater quality has been published and recognized in many parts of the world (Li et al. 2013; Ogunfowokan et al. 2013; Alaya et al. 2014; Brindha et al. 2014; Wu et al. 2014; Shammi et al. 2016; Yehia et al. 2017; Tolera et al. 2020). Pollution of groundwater has an impact on water quality, human health, and economic development (Schiavo et al. 2006). In the developing countries, about 80% of diseases are directly related to poor drinking water quality and unsanitary conditions (UNESCO 2007; Das and Nag 2015). As a result, determining the quality of groundwater is critical for determining the acceptability of water for a specific use.
A basic knowledge of water quality is very useful for irrigation management and is an important consideration in the design and operation of the irrigation system in reclaimed lands. Therefore, water quality issues and their management need to be well studied. The objective of this paper is to employ hydrochemical approaches to get a broad picture of groundwater suitability for domestic and irrigation purposes. Also, being expected to provide information might be quite valuable in terms of groundwater sustainability in an intensive agricultural area.
Study area
Description
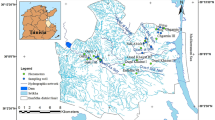
The study area is part of the Nile Valley and is located to the north of Qena city in Upper Egypt. The study area extends from the River Nile main channel to the high land of the limestone plateau in the Western Desert of Egypt. It is located between latitudes of 25°45′ to 26°20′ N and longitudes of 31°45′ to 32°30′ E as shown in Fig. 1. The study area includes both the old lands in the Nile Valley and the new reclaimed lands outside the Nile Valley on the desert fringes. The topography of the study area increases in elevation gradually from the River Nile main channel toward the limestone plateau (Mohammed 2005). The area is characterized by arid climatic conditions with extremely hot and dry summers and little to no rain in winter. Sporadic rain events occasionally occur, but are very rare. The average annual rainfall is about 0.01 mm (Qena Metrological Station 2001–2004).
Geology
The geomorphological units in the study area and throughout the Nile Valley can be distinguished into: (1) The young alluvial plain, which occupies the flood plain of the recent Nile, bordering the River Nile main channel on the east and west banks (Abdel-Samie 2000; Mohammed 2005). The young alluvial plain is almost flat and gently slopes from south to north. The surface of such plain is underlain by silt and clay deposits. (2) The old alluvial plain, which bounds the young alluvial plain on both sides of the Nile Valley and is underlain by mixed sands and gravels that have commonly developed into terraces that represent the former Nile levels during the Pleistocene period. The terraces are not typically preserved in a single location since they are frequently removed by water erosion (Ball 1910; Said 1962; Abdel-Samie 2000; Mohammed 2005). 3) The calcareous structural plateau, which is made up of tertiary limestone. The plateau has an irregular surface and rises to more than 450 m above sea level at the study area. The surface of the plateau is dissected by complex drainage patterns which drain toward the River Nile (Abdel-Samie 2000; Mohammed 2005).
The stratigraphic succession in the study area is generally composed of a variety of sedimentary rocks belonging to a wide range of ages. The top portion of the sedimentary section is dominated by carbonate rocks belonging to the Eocene age. The lower portion is mainly Mesozoic clastics, the Nubian Sandstone formation. The surface of the study area is overlain by Pliocene and Quaternary fluvial silty clay, sand, and gravel (Said 1991; Awad et al. 1997). Near the River Nile bank, the area is covered by the Nile mud and silt with little admixture of sand (Mohammed 2005).
The geological structure of the study area is part of the entire Nile Valley, which is mostly influenced by wrench faults that run more or less parallel to either the Gulf of Suez or the Gulf of Aqaba direction (Youssef 1968). The majority of the faults are normal fault-oriented NW − SE and NE − SW. They are notably affecting the Eocene limestone with downthrow toward the Nile Valley. They create the major graben, which contains the River Nile and its valley (Fig. 1) (Said 1962). There are also large joints associated with these faults (Said 1962). The existing complex structural elements may cause a hydraulic connection between deep aquifers and the quaternary aquifer in the study area.
Hydrogeology
Qena region represents an area of most extensive groundwater resources in Upper Egypt, where significant amounts of groundwater have been exploited and used in reclamation. In recent years, farmers in the old cultivated and new reclaimed lands have started to dig new wells to alleviate surface water shortages and the tail ends of irrigation canals. Tubewells have also been the only source of irrigation water in small-scale reclamation schemes. In most cases, wells are constructed on the initiative of farmers. Farmers share their wells either individually or in groups. In addition, large-scale desert reclamation projects that rely on groundwater have been expanded and developed (Awad et al. 1997; Mohammed 2013; Mohammed et al. 2016).
The Nile Valley Aquifer System represents the main source of groundwater in the Nile Valley plains of the Qena area. It mainly consists of graded sand and gravels, overlain in the majority of the region by clays and silts of the Holocene age. The sand and gravel water bearing unit varies in thickness from one place to another, with a range from 20 to 300 m, with maximum thickness near the center of the Nile Valley and decreasing towards its edges, and underlain by impermeable compact Pliocene plastic clays (Awad et al. 1997; Mohammed et al. 2016). The silt and clay layer has variable permeability and acts at many locations as a semi-confining layer. Its thickness ranges from 1 to 24 m, and it is missing at the desert fringes where sand and gravel constitute an unconfined aquifer (Idris and Nour 1990; Campos 2009; Mohammed et al. 2016). The hydraulic conductivity of this aquifer varies from 30 to 100 m/day with a regional average of 50 m/day (RIGW 1988; Awad et al. 1997). Recharge of the aquifer system is mainly by the vertical infiltration of the irrigation return flow water and seepage from irrigation canals. Recharge from rainfall precipitation is generally of minor significance. Another possible source of recharge is the vertical flow from deeper aquifers (most notably the Nubian Aquifer). Discharge of the aquifer system is either through the lateral outflow to the River Nile or vertically through pumping for irrigation (RIGW and IWACO 1986; Hamza et al. 1999; Mohammed et al. 2016). The regional groundwater flow of the Nile Valley Aquifer takes a longitudinal movement parallel to the River Nile flow. Transversal groundwater flow takes place from the reclaimed lands toward the cultivated lands in the flood plain, and then near to the River Nile channel, groundwater drains into the River Nile (RIGW 1986; Awad et al. 1997; Hamza et al. 1999; Mohammed et al. 2016). The groundwater levels were measured when possible in various producing wells in the study area. Under the cultivated lands, the groundwater depth is less than 10 m and increases toward the desert fringes where the reclaimed lands to reach about 46 m.
Agricultural activities
Agriculture is the primary use of lands within the study area. More than 81% of the inhabited Nile Valley is under cultivation in the Qena province, where the study area is part of (SEAM 2005). The study area is characterized by intensive agriculture activities, where multiple crops planted throughout the year. The principal crops grown are wheat, sugarcane, clover, corn, fava beans, and vegetables. The main agricultural challenges in the study area include the high cost of reclamation, limited water supplies, and a relatively rapid population growth rate. Groundwater is the major source for irrigation in the reclaimed fields, where the old cultivated lands are usually irrigated by irrigation canals that dissect the alluvial plains. No large irrigation schemes were constructed across the study area, while the irrigation habits comprise both traditional and modern irrigation practices. The vast majority of the agricultural lands are irrigated using the traditional surface (flood) irrigation system. Modern and high water use efficiency irrigation methods like drip and sprinkler are less widespread. The potential impacts of the surface irrigation practices include an increase in return flow rates. Irrigation return flow is the quantity of water which infiltrates back (returns) to the groundwater after irrigation of agricultural fields. Irrigation return flow water could be a significant source of groundwater recharge in shallow aquifers, but it could also transmit nutrients and pollutants, which may result in groundwater quality problems in the aquifer (Mohammed et al. 2016).
Large quantities of commercial fertilizer, together with manure, are applied during the growing seasons. The most regularly used commercial fertilizers are N, P, K, and S fertilizers, which include urea (46.5% N), ammonium nitrate (33.5% N), ammonium sulfate (20.6% N), calcium nitrate (15.5% N), superphosphate (15% P2O5), and potassium sulfate (50% K2O) (FAO 2005). However, N and P fertilizers are the most regularly utilized in the study area, and generally Egypt has one of the highest rates of nitrogen application in the world (FAO 2005). The application rates of these chemical fertilizers are described as extensive to moderate in the study area. Pesticides of various varieties are widely used in the agricultural activities in the study area. Pesticides are used to protect agricultural crops from insects, disease fungus, and weeds. Pesticides, on the other hand, could be easily leached from plants and soil into the shallow groundwater during irrigation.
In the recent few decades, the study area has experienced fast land cover changes (e.g., increased agricultural areas). We mapped the land-cover changes due to agricultural development and urban expansion using the Google Earth software platform. Google Earth enables users to freely examine satellite imagery with medium, high, and very high spatial resolution. Google Earth has enabled many users wishing to monitor land (foresters, conservationists, land cover, etc.) to bypass the need of remote sensing experts to monitor their land (Sidhu et al. 2018; Floreano and de Moraes 2021). Figure 2 depicts the change in land cover in the study area over a 35-year period. One can visually notice the extensive land-cover changes. A rough estimation of the land-cover changes in the study area indicates that the agricultural and urban areas increased from 33.17 Km2 in 1985 to 218.14 km2 in 2020. The continuous expansion in agricultural and urban areas in the study area is reliant on groundwater resources where the surface water (River Nile and irrigation canals) is not available. These agricultural developments, as well as high irrigation rates under arid conditions, are predicted to have an impact on groundwater quality in the study area.
Materials and methods
Sample collection and analysis
Forty-one groundwater samples were collected from the investigated area in March 2018. The sampling plan focused on the reclaimed lands. The locations (latitude and longitude) of the collected samples are located using a handheld global positioning system (GPS) device in the field and presented in Fig. 1. Samples were collected directly from tubewells after sufficient time of pumping to ensure that the water sample represented the original groundwater source rather than water retained in pipes. Samples were collected using new, dry, and clean polyethylene bottles of different sizes. Before collecting the samples, the bottles were washed thoroughly with well water. At each locality, one sample bottle was collected without headspace for alkalinity and anion measurements; second sample bottle was collected and filtered on site using a 0.45 micron filter and acidified with nitric acid (HNO3) to preserve dissolved metals. Cations were analyzed from these acidified bottle samples. Electrical conductivity (EC), hydrogen ion concentration (pH), and field temperature (°C) were measured in the field by using portable meters (JENWAY Model 430). All of the collected sample bottles were carefully labeled, tightly sealed, and stored in a refrigerator at 4 °C.
The chemical analyses of the collected samples were analyzed in the central laboratory of the Company for Drinking Water and Sanitation at Qena, Egypt. Titrations were used to determine alkalinity, hardness, and chloride. Flame photometer instrument (JENWAY PFP7) was used to analyze Na+ and K+ ions. Sulfate and nitrate levels were analyzed by using spectrophotometric methods (HACH DR 5000). The concentrations of the analyzed chemical constituents are reported in milligrams per liter (mg/L). As required, a milliequivalent per liter (meq/L) concentration is calculated.
As a basic and simple check of the quality of the chemical analysis in the data set, the charge balance error (CEB) is calculated. According to Freeze and Cherry (1979) and Kehew (2001), the percent charge-balance error is calculated based as follows (Eq. 1):
where the ion concentration unit is in milliequivalent per liter (meq/L). The % CBE value will have either a positive or negative charge, depending upon whether the cations or anions are larger. The groundwater chemistry results may be good if the CBE is < ± 5%, and if the CBE is > ± 5%, it will be considered as poor (Hounslow 1995; Rahman et al. 2021a). However, the CBE of up to ± 10% is acceptable (Rahman et al. 2021a). The minimum, maximum, and average charge balance errors are –6.7%, 4.9%, and –0.2%, respectively, in the collected samples in this study. Three samples only have charge balance errors of –6.7%, –6.0%, and –5.9%. The charge balance errors were considered to be reliable in this research work.
Water quality assessment methods
Water quality index
Various methodologies were used to better understand the data sets of the water quality parameters. The water quality index (WQI) is a simple and powerful tool for evaluating water quality status. The WQI aims to provide a single value of the water quality status by using the water constituents and their concentrations present in a sample (Abbasi and Abbasi 2012). The water quality indices (WQIs) have been used as a tool to assess the state of water quality since the 1960s (Horton 1965). Several WQIs have been produced by various researchers and agencies around the world. However, there is no WQI that has been globally preferred.
The WQI used in this work is the weighted arithmetic average method, developed by Brown et al. (1970). The method is one of the most effective tools for the evaluation of groundwater quality and is used for the evaluation of groundwater quality worldwide. This method has been used and described by various researchers throughout the world (Sadat-Noori et al. 2014; Batabyal and Chakraborty 2015; Sutadian et al. 2016; Gradilla-Hernández et al. 2020; Reyes-Toscano et al. 2020). Four common steps have been used in the WQI method:
In the first step: for the purpose of calculation, 11 water quality parameters have been selected (pH, TDS, Total Hardness (TH), Na+, K+, Ca2+, Mg2+, HCO3−, Cl−, SO42−, NO3−), and an individual weight (wi) was assigned to each of the 11 parameters based on its importance in the evaluation of water quality. The individual weight assigned ranges from 1 (lowest) to 5 (highest).
In the second step: a relative weight (Wi) of each given parameter is calculated using the following Eq. (2):
where Wi is the relative weight of the ith parameter, wi is the weight of each parameter, and n is the number of parameters. Table 1 lists the individual weight and the relative weight of the selected parameters.
In the third step: the quality rating for each parameter is calculated from the following Eq. (3):
where qi is the quality rating, Ci is the concentration of each parameter, and Si is the concentration of the permissible limits of the World Health Organization (WHO, 2011) (Table 1).
In the fourth step: the sub-index of ith parameter (SIi) is calculated for each parameter using the following Eq. (4):
where SIi is the sub-index of ith parameter, qi is the quality rating, and Wi is the relative weight. Then the WQI is calculated from the following Eq. (5):
The computed WQI values are classified into five categories: excellent water, good water, fair water, poor water and unacceptable water for human consumption (Table 2) (Sadat-Noori et al. 2014; Reyes-Toscano et al. 2020).
Irrigation purposes
In order to evaluate the suitability of groundwater in the study area for irrigation uses, multiple irrigation quality indices were calculated, including salinity hazard (EC), sodium adsorption ratio (SAR), soluble sodium percentage (SSP), residual sodium carbonate (RSC), magnesium hazard (MH), permeability index (PI), and Kelley's ratio (KR). These indices can be calculated as follows:
Sodium adsorption ratio (SAR) is computed using the absolute and relative concentrations of main cations (US Salinity Laboratory Staff 1954; Raghunath 1987; Abdel-Satar et al. 2017), with concentrations provided in milliequivalents per liter (Eq. 6).
Soluble sodium percentage (SSP), commonly known as sodium percentage (Na%), is an estimation of the sodium hazard in irrigation water that expresses the percentage of sodium out of the total cation concentrations (Wilcox 1955; Todd 1980; Reddy 2012). SSP is calculated using the following Eq. (7) where concentrations are reported in milliequivalents per liter.
Residual sodium carbonate (RSC) is used to evaluate the hazard effects of bicarbonate and carbonate on groundwater quality for irrigation usage (Eaton 1950; US Salinity Laboratory Staff 1954; Todd 1980). RSC is calculated using the following Eq. (8) where the concentrations are reported in milliequivalents per liter.
Magnesium hazard (MH), also known as magnesium adsorption ratio (MAR), is used to assess the impact of high magnesium concentrations on irrigation water (Szabolcs and Darab 1964; Todd 1980; Ayers and Westcot 1985; Raghunath 1987). It is calculated using the Eq. (9), with the concentrations expressed in milliequivalents per liter.
Permeability index (PI) measures how easily water flows through a medium. The following Eq. (10) is used to calculate PI (Doneen 1961, 1964), where the concentrations are in milliequivalents per liter.
Kelley's ratio (KR) is comparing the sodium ion to the calcium plus magnesium ions. KR is calculated using the following Eq. (11) (Kelley 1951, 1963), where the concentrations are in milliequivalents per liter.
Results and discussion
Hydrochemical characteristics
The physical and chemical parameters, including pH, the total dissolved solids (TDS), and major ions of the collected groundwater samples are summarized in Table 3. The physiochemical parameters of the groundwater samples in the study area showed considerable variability in close proximity to each other, which might be related to various geological origins and anthropogenic influences. The chemical composition of the natural groundwater is controlled by numerous factors, including precipitation, anthropogenic inputs, water–rock interaction, the evaporation process, residence time, and mixing (Clark and Fritz 1997; Mook 2000; Dotsika et al. 2010). The concentrations of cations and anions are represented in Fig. 3. The groundwater in the study area is alkaline/basic in character (pH from 7.39 to 8.22). The sources of the alkaline pH could be the levels of the hard water minerals and the effluents from agricultural activities. The TDS of the groundwater samples in the study area ranges from 620 to 5755 mg/L, with an average of 958 mg/L. TDS levels indicate the influence of the dissolution of the halite (NaCl) and gypsum (CaSO4.2H2O) of the adjacent carbonate plateau. The dissolution of limestone, dolomite, gypsum, and halite is also the principal source of the Ca2+, Mg2+, Na+, K+, CO32–, SO42–, and Cl– content in the groundwater (Somay and Gemici 2012; Pazand et al. 2018). Few boreholes distributed in the study area show a TDS of fresh water (TDS < 1000 mg/L).
Of most of the collected groundwater samples, the Na+ and Cl– ions had the highest concentrations of all the major elements. The concentration of Na+ ranges from 129 to 1370 mg/L, with an average of 428 mg/L, and the concentration of Cl– ranges from 110 to 2710 mg/L, with an average of 645 mg/L. Such a wide range of concentrations suggests that multiple sources generate that chemical composition. The Na+⎼Cl– relationship in the groundwater samples of the study area shows similarities in concentrations of both Na+ and Cl– ions. Figure 4 shows the value of Cl– as a function of Na+ in the groundwater samples in the study area, and there is a strong correlation between them (R2 = 0.92). The average Na+/Cl– ratio of the groundwater samples in the study area is 1.18, suggesting the same sources of both Na+ and Cl– ions, and illustrating the considerable influence of the dissolution of evaporates, such as halite, on groundwater chemistry. In addition, it indicates the less prevalent nature of the ion exchange interaction between divalent cations and sodium in the groundwater geochemical processes.
The major water type and dominating ionic concentrations of the collected groundwater samples are described and plotted on Chadha's diagram (Chadha 1999) as shown in Fig. 5. This diagram clearly shows that the majority of the groundwater samples (≈ 95%) are of Na+⎼Cl– water type. Few samples are of Ca2+⎼Mg2+⎼Cl– water type.
Chadha's classification diagram explaining the chemical groundwater type of the collected samples (Chadha 1999)
The Cl–/sum anions ratio is a valuable ratio for determining the source of groundwater (Hounslow 1995). When the ratio is > 0.8 and the TDS > 500 mg/L, then the source of groundwater is seawater, brine, or evaporates; when the ratio is > 0.8 and the TDS < 100 mg/L, then the source of groundwater is rainwater; and when the ratio is < 0.8, then the source of groundwater is rock weathering. This ratio is less than 0.8 in almost all of the collected groundwater samples of the study area, which indicate the weathering processes and mineral dissolution from the nearby sedimentary rocks.
The plot of (Ca2+ + Mg2+) versus (HCO3– + SO42–) (Fig. 6) shows that the majority of the samples are close to the 1:1 trend line, indicating that the dominant reactions in the system are the dissolutions of evaporates (Srinivasamoorthy et al. 2014; Pazand et al. 2018). The chloro-alkaline indices (CAIs) are used to assess the ion exchange reaction between groundwater and its host rock (Schoeller 1967). Two chloro-alkaline indices (CAI 1, 2) are used to identify the ion exchange processes and are calculated using the following Eqs. (12, 13) where the concentrations are in milliequivalents per liter.
When Na+ and K+ ions in groundwater are exchanged with Ca2+ or Mg2+ ions that occur in the aquifer materials (direct ion exchange), both indices will be positive and the dissolved concentrations of Ca2+ and Mg2+ will increase in the groundwater. If the indices are negative, a possible reverse ion exchange occurs between the Na+ and K+ ions contained in the aquifer materials and the Ca2+ or Mg2+ ions dissolved in the groundwater, and then, the concentrations of Na+ and K+ will increase in the groundwater. The CAI 1 and 2 indices are calculated for the collected groundwater samples from the study area, and both are similar in terms of positive and negative samples. The CAI values are scattered in a small range without any prevalence of direct or reverse ion exchange in the samples (Fig. 7). The CAI results in the study area might be an indication of the different sources of the dissolved ions in groundwater, including dissolution of the aquifer matrix, adjacent rock weathering, agricultural activities, urbanization, and industrial activities.
Water quality index
The WQI method is widely used for groundwater quality assessment around the world. In this study, the WQI calculated based on the hydrochemical data and the WHO (2011) standards for drinking purpose were considered for the calculation of the WQI. The calculated WQI values ranged from 53.3 to 447.5 with an average of 129.8. Table 4 represents the WQI categories and percentage of samples in each category. The results obtained from the groundwater samples from the study area showed that more than 50% of the samples are fair to poor water quality, while around of 39% are of good water quality (Table 4).
Drinking water quality
The suitability of the groundwater for domestic uses is directly related to different physiochemical parameters and reactions in groundwater. The natural baseline geochemistry of groundwater resulting from interaction with rocks, in addition to anthropogenic sources, could create widespread health and acceptability problems. In the present work, we evaluated the groundwater suitability for domestic uses in the study area through different parameters, as well as comparing the ion concentrations by the World Health Organization (WHO 2011) guideline values and the Egyptian guideline values (EMH 1995; EHCW 2007).
TDS is one of the main and important parameters in terms of drinking water. The quality of the taste is primarily related to the total amount of solids in groundwater. Based on the TDS classification given by Freeze and Cherry (1979) and Davis and Dewiest (1966), the groundwater samples of the study area are described as fresh and brackish groundwater types, as represented in Table 5. The TDS of brackish water is dominant in the study area, accounting for about 73% of the total samples (Fig. 8). The TDS values and the concentrations of the major ions in groundwater samples are compared with the WHO (2011) (Elumalai et al. 2020) and Egyptian (EMH 1995; EHCW 2007) drinking water guidelines and given in Table 3. High TDS indicates that groundwater may contain elevated levels of ions that are above the primary or secondary drinking water standards, such as elevated levels of nitrate and other trace constituents.
One commonly important aspect of water quality that is mostly caused by the presence of calcium and magnesium ions in water is the water hardness. Calcium carbonate is one of the main causes of hardness, so total hardness is usually expressed as equivalents of calcium carbonate, or mg/L as CaCO3. Total hardness (TH) can be calculated from individual concentrations of calcium and magnesium in mg/L using the following Eq. (14) (Kehew 2001):
The total hardness of the collected groundwater samples is calculated using Eq. (14) and is listed in Table 5. The hardness values in the collected groundwater samples from the study area range from 159 to 2291 mg/L as CaCO3, with an average of 504 mg/L as CaCO3. The degree of hardness in water is classified in terms of its calcium carbonate concentration according to Durfor and Becker (1964), and Todd and Mays (2005) after Sawyer and McCarty (1967). Based on the previous given classifications of TH, the groundwater samples in the study area range from hard to very hard groundwater (Table 5 and Fig. 9). The high levels of TH in the study area are attributed to the weathering of carbonate rocks from the nearby plateau and the excessive use of lime fertilizers during the agriculture activities. Long-term consumption of hard water can cause many diseases and health dysfunctions.
Livestock and poultry water quality
Domestic animals are an integral part of agricultural societies and provide a valuable source of food. Irrigation water supplies frequently serve as a drinking water source for livestock in the study area. For livestock, fundamentally the same water quality requirements hold as for human consumption. However, animals can drink water with moderately high dissolved solids (EC less than 5 dS/m) (Ayers and Westcot 1985). It is well known that excessively saline water can cause physiological distress or death in livestock. The National Academy of Sciences and National Academy of Engineering (NAS-NAE 1972) established a guideline for evaluating water for livestock and poultry production (Table 6). It is also recommended by NAS-NAE that water containing a TDS of 3000 mg/L or less should be satisfactory for livestock under almost any circumstance. Based on the preceding criteria, the groundwater in the study area is generally satisfactory for livestock (TDS < 5000 mg/L) (Fig. 10).
Irrigation water quality
The suitability of water for irrigation is determined by its mineral constituents and the type of the plant and soil to be irrigated. These minerals could be derived from natural processes and anthropogenic activities, and they might affect crop yields and soil fertility. It is therefore important to have detailed information concerning the quality of irrigation water and its impact on crops and soils. The suitability of water for irrigation is determined not only by the total amount of salt present but also by the kind of salt. The chemical parameters that are commonly used to determine the suitability of the water for irrigation, as well as their impact on crop productivity and soil quality are discussed below.
Salinity hazard (EC)
A salinity problem occurs when salt accumulates in the crop root zone to the point where the crop is unable to draw enough water from the salty soil solution, causing water stress for an extended period of time. When water uptake is significantly reduced, the plant slows its rate of growth (Ayers and Westcot 1985). Usually, water with a TDS of about 5,000 mg/L is unsuitable for irrigation (Pillsbury and Blaney 1966; Ayers and Westcot 1985). Water is divided into four classes based on the EC in µS/cm by the US Salinity Laboratory Staff (1954) (Table 7). As shown in Table 7, more than half of the collected groundwater samples from the study area exhibit conductivity values in the C4 class, which indicates a risk of salinity.
Sodium adsorption ratio (SAR)
The sodium adsorption ratio of a soil solution is simply related to the adsorption of sodium by soil. The main problem with high sodium concentration is its effect on soil permeability and water infiltration. Continued use of water with a high SAR leads to a breakdown in the physical structure of the soil. The sodium replaces calcium and magnesium adsorbed on the soil clay minerals and causes dispersion of soil particles. This dispersion results in the breakdown of soil aggregates and causes cementation of the soil under drying conditions, as well as prevents the infiltration of water and air into the soil (Ayers and Westcot 1985). In practice, the SAR value of the water in the soil increases due to an increase in the concentration of all salts (US Salinity Laboratory Staff 1954). The SAR can be calculated using Eq. (6).
Irrigation water is classified into four classes based on SAR values proposed by the US Salinity Laboratory Staff (1954) and shown in Table 7. According to the SAR classification, more than two-thirds (63%) of the groundwater samples collected from the study area fall into the low sodium hazard (S1 class), while the remaining samples (37%) fall into the S2 class, which groundwater could be used for irrigation with minor limitations for soils and plants (Table 7).
A diagram was proposed by Wilcox (1948) for the classification of irrigation water. This diagram has been widely used. The features of this diagram are based on the previously discussed salinity hazard (EC) and sodium adsorption ratio (SAR). Using the SAR and EC values as coordinates, locate the corresponding point on the diagram. The position of the point determines the quality classification of the water. The Wilcox diagram for the studied groundwater samples is shown in Fig. 11. In accordance with the Wilcox diagram, the groundwater samples in the study area belong to classes C3-S1 (17%), C3-S2 (27%), C3-S3 (5%), C4-S2 (10%), C4-S3 (36%), and C4-S4 (5%), indicating high salinity hazard and low, medium, and high alkali hazard, which might require special management for salinity control and good to very salt tolerance plants should be selected.
Soluble sodium percentage (SSP)
SSP is also referred to as sodium percentage (Na %). It is an estimation of the proportion of sodium ion in irrigation water like SAR, but it expresses the percentage of sodium out of the total cations. SSP is calculated by using Eq. (7). Todd (1980) classified the irrigation water according to the SSP into five classes (Table 7). According to this classification, the majority of the groundwater samples collected from the study area fall into permissible (27%) to doubtful (66%) categories (Table 7). Similar to SAR, high presence of sodium in irrigation water reacts with soil and causes a reduction in its permeability. Furthermore, frequent irrigation with high Na+ water makes the soil plastic and sticky in wet conditions and forms clods and crusts in dry conditions (Punmia and Lal 1981).
Residual sodium carbonate (RSC)
RSC compares the bicarbonate (HCO3–) and carbonate (CO32–) concentration (also known as carbonates) to Ca2+ and Mg2+ concentrations. Irrigation water containing high carbonates greatly complicates the management of excessive sodium. When water containing carbonates dries at the soil surface, Ca2+ and Mg2+ carbonates (lime) are formed. Since Ca2+ and Mg2+ are no longer dissolved, they do not counteract the negative effects of Na+, and problems related to high Na+ may occur. White lime deposits may also become visible on turf leaves during hot dry periods as carbonates are deposited during evaporation (James 2001). RSC is calculated using Eq. (8). The RCS equation indicates the potential for Ca2+ and Mg2+ precipitation in the soil and the removal of Ca2+ and Mg2+ from the soil solution. High carbonate water can have good infiltration properties if the Ca2+ and Mg2+ levels are also high (James 2001). If water that is high in RSC is repeatedly used, the soil becomes alkaline and is likely to become sodic over time if the water also contains significant amounts of Na+. The degree of sodium hazard in groundwater is classified into three classes based on RSC by the US Salinity Laboratory Staff (1954) (Table 7). According to the RSC classification, almost all the groundwater samples (40 samples) in the study area are safe for irrigation (RSC < 1.25).
Magnesium hazard (MH)
In a magnesium-dominated water (ratio of Ca2+/ Mg2+ < 1) or a magnesium soil (soil–water ratio of Ca2+/ Mg2+ < 1), the potential effect of sodium may be slightly increased. This means a given SAR value will show slightly more damage. The lower the Ca2+/ Mg2+ ratio, the more damaging is the SAR (Ayers and Westcot 1985). The magnesium hazard of irrigation water is calculated using Eq. (9) proposed by Szabolcs and Darab (1964) and could be classified into two classes (Table 7). Based on the MH classification, 41% of the collected water samples in the study area is suitable for irrigation (MH < 50%), and 59% of the samples are unsuitable for irrigation (Table 7). If the MH value is more than 50%, the soil becomes alkaline and its impact on crop yield will be observed (Chaudhary and Satheeshkumar 2018).
Permeability index (PI)
The SAR, SSP, and RSC are used to evaluate the effect of salts in irrigation water on soil permeability. As is well known, irrigation water with high sodium content is unsuitable for irrigation of agricultural lands. Excess sodium in water produces an undesirable effect by changing soil permeability and water infiltration. The concentrations of Ca2+, Na+, Mg2+, and HCO3– influence the permeability of the soil profile; therefore, these ions are used to calculate PI values of irrigation water. The PI could be explained as a measure of the extent to which soil structure deteriorates when water is applied, a high ratio indicating a high degree of deterioration (US Salinity Laboratory Staff 1954). Low soil permeability causes poor drainage by impeding the downward movement of water and often results in a surface crust formation that deters seed germination and emergence (Singh et al. 2020). PI is calculated using Eq. (10). PI is classified into three classes (Doneen 1964) as shown in Table 7. Based on the PI classification and the PI values, the entire groundwater samples collected from the study area fall under classes I and II (PI of 55–90%), which indicates that the water is overall good to moderately good for irrigation purposes.
Kelley's ratio (KR)
KR is an important index for evaluating irrigation water. It compares the Na+ ion concentration with the Ca2+ and Mg2+ ion concentrations (Kelley 1951, 1963). KR is calculated using Eq. (11). Water is classified into two categories: water with KR values > 1, which indicates an excess of Na+, and water with KR values < 1, which is suitable for irrigation (Das and Nag 2015). The groundwater samples in the study area show KR values of more than 1 (95% of samples). According to the KR values, the majority of the collected groundwater samples from the study area are unsuitable for irrigation purposes (Table 7).
Nitrate contamination
Nitrate pollution of groundwater is becoming more prevalent in many parts of the world, including most European and North American countries (Strebel et al. 1989; Korom 1992; Spalding and Exner 1993; Feast et al. 1998; Tesoriero et al. 2000), and poses a serious threat to groundwater-based drinking water supplies. Since the early 1960s, excessive fertilizers and manure application in agriculture have been the primary cause of increased shallow groundwater nitrate (Appelo and Postma 2005). The maximum-allowable nitrate concentration in drinking water is 50 mg NO3– and the recommended level is less than 25 mg/L NO3– (WHO 2011). Egypt is one of the countries suffering from the elevated nitrate content in their groundwater, which derived from a variety of sources. The Nile Valley Aquifer System, which this study is a part of, has been studied by many researchers, and they have reported the high nitrate levels that exceed the permissible limits of drinking water (Mohammed 2005; Ahmed et al. 2007; Gedamy et al. 2012; Ali and El-Aassar 2018; El-Sayed 2018; Salman et al. 2019; Redwan et al. 2020; Shamrukh and Akib 2021).
The nitrate (NO3–) concentrations in the groundwater samples in the study area range from 0.1 to 256.8 mg/L, with an average of 53 mg/L. Thirty percent (12 samples) of the groundwater samples had nitrate concentrations higher than the maximum acceptable nitrate concentration for drinking water of 50 mg/L set by WHO (2011). Sixty-three percent of the samples (25 samples) had nitrate levels higher than the recommended range of 25 mg/L. Elevated nitrate contents are found in the groundwater wells distributed in both agricultural and rural parts of the study area. The widespread use of chemical fertilizers, organic fertilizers (manure), and waste from livestock are considered to be the main sources of nitrate that leaches into groundwater in the agricultural areas, whereas septic systems are the primary source of high nitrate in rural sections of the study area. Another important factor for rising nitrate levels in reclaimed land is the lack of silty soils cover, which allows nitrate formed by the oxidation of N-fertilizers to easily infiltrate into the deeper part of the alluvial aquifer. This finding is in line with research from Egypt (Shamrukh et al. 2001; Mohammed 2005; Salman et al. 2019) as well as other parts of the world (Spalding et al. 1982; Baker 1992; Chowdary et al. 2005; Kundu et al. 2009; Gu et al. 2013; Rahman et al. 2021b). The nitrate contour map (Fig. 12) depicts the spatial distributions of nitrate concentrations in the study area and shows no notable trend of nitrate concentration changes. However, the increase in the nitrate concentrations in the eastern part of the study area is due to intensive agricultural activities in this area, such as newly reclaimed lands, as well as the presence of individual agriculturally related human activities, such as camel and poultry farms and other feedlots.
Health risk of NO3 – in groundwater
The presence of high nitrate levels in drinking water is believed to be a health risk to humans and animals (Sall and Vanclooster 2009; IARC 2010; WHO 2011). In humans, nitrate and nitrite are treated as probably carcinogenic (IARC 2010). Pregnant women are advised to avoid high nitrate water, because research shows possible connections to birth defects and miscarriages (IARC 2010). Infants who are given high nitrate water or infant formula mixed with high nitrate water can develop a condition called methemoglobinemia, also known as blue baby syndrome (Kross et al. 1992; WHO 2003; Greer and Shannon 2005). Cases have also been reported in babies who have been fed vegetables irrigated with high levels of nitrate water, particularly carrots and spinach (Simon 1966; Keating et al. 1973). Animals are affected by nitrate poisoning in the same manner as it affects humans and babies.
We conducted a preliminary human health risk assessment from the exposure to nitrate-contaminated groundwater following the USEPA methodology (Sadler et al. 2016; Zhai et al. 2017; Rahman et al. 2021b). In order to perform a health risk assessment, the nitrate intake from water is necessary to estimate. The health risk assessments for nitrate in drinking water are presented for adults and children. The average daily dose (ADD) as mg/kg/day was calculated using the following Eq. (15).
where Cf is the nitrate concentration in drinking water (mg/L), Cd is the average daily drinking water intake (L/day), and Bw is the body weight (kg). The ADD was calculated based on the NO3– concentrations obtained in the samples of the current study, and the average daily water intake, which is recommended to be 2.3 and 1.5 L/day for adults and children, respectively, was based on the literature (Su et al. 2013; Rahman et al. 2021b). Also, the average body weight used in the ADD calculation is 70 and 15 kg for adults and children, respectively, according to USEPA (2002) and Rahman et al. (2021b). The hazard quotient (HQ), which is the ratio of an exposure level to a reference level, is calculated from the following Eq. (16).
where ADD values are calculated from Eq. (15), and RfD is the acceptable daily intake (mg/kg/day) (the reference oral dose), where its value is 1.6 mg/kg/day as specified by USEPA (1991). It is presumed that there are no health concerns if the HQ value is less than one (Su et al. 2013).
The estimated ADD and HQ for adults and children in the groundwater samples from the study area are shown in Table 8. The higher the HQ value, the higher the health risks. The HQ value of < 1 often indicates low health risk in the exposed population (Sadler et al. 2016; Rahman et al. 2021b). The HQ value in the samples indicated a high level of risk (HQ > 1) for adults. HQ is more than one in more than 32% of the samples. We also found a significantly higher risk (HQ > 1) for children, with HQ greater than one in nearly 80% of the samples in the study area, implying that the children are more vulnerable to health risks of NO3– contamination in groundwater. The previous findings showed that the increase in the NO3– concentration in drinking water could be a potential health risk for humans, particularly infants and children. This study recommends proper management and monitoring of the agricultural activities in the study area to minimize the potential risk of nitrate in groundwater.
Water treatment
The groundwater in the study area is characterized by its high content of TDS (73% of the collected samples are brackish water) and other ion constituents, as described above. Furthermore, about 63% of the samples have nitrate concentrations higher than the recommended level. These constituents degrade water quality and are expected to cause problems with water use in a variety of ways. As a result, groundwater in many parts of the study area is unsuitable for a variety of uses (ex., drinking, irrigation, and livestock). In order to improve the groundwater quality and make it safe for usage, TDS has to be reduced, and dissolved nitrate should be reduced or removed. There is no simple method for lowering TDS and removing nitrate from water. However, to improve the water quality in the study area so that it is suitable for small-scale human activities or projects (e.g., poultry farms, camel farms, livestock, local brick industry, and medicinal and aromatic plants), mixing of water should be considered.
Mixing of water might offer an overall improvement in quality and reduce the potential toxicity problem if an alternative source of good quality water is available. The desired water salinity can be obtained by mixing two water of known salinity. You can select the parameters that will be included in the mixing calculations. The mixed water then generates solution concentrations as a result of the stepwise mixing of specified proportions of two selected water. For mixing, each of the input solutions is multiplied by its mixing fraction, and a new output solution is stoichiometrically calculated (Parkhurst and Appelo 1999).
In this section, a mixing model is conducted between water from different sources as a proposed solution for lowering the chemical content, particularly nitrate levels in the heavily contaminated wells to acceptable limits for usage in various purposes. The water of poor quality wells in the study area will be mixed with good quality water from nearby irrigation canals or the River Nile main channel. This also could be mixed with water from available nearby better quality wells. In this model, the water of wells Ar-6, Ar-22, and Ar-29 collected from the study area is mixed with irrigation canal water (S2) (Table 9). Mixing could take place in house cisterns, the roof tanks, and in pools. Table 10 summarizes the mixing percentages between the mixed sources and changes in the concentrations of the major ions and nitrate.
Conclusion
Forty-one groundwater samples were collected in this study from the investigated area in the Nile Valley, north of Qena city. The Nile Valley aquifer represents the major source of water for irrigation and domestic usage in the study area. The hydrochemical investigations show that groundwater in the study area is influenced by both natural processes and anthropogenic activities. The chemical weathering of the aquifer rock-forming minerals and the adjacent carbonate plateau is one of the most important processes influencing the groundwater chemistry in the study area. The groundwater samples in the study area are characterized by fresh to brackish water with Ca2+⎼Mg2+⎼Cl– and Na+⎼Cl– hydrochemical facies. The majority of the samples are chemically unsuitable for human consumption according to the permissible limits set by WHO, and they are classified as hard to very hard types.
Based on the water quality parameters examined for the groundwater samples collected in this study, the suitability of groundwater samples for irrigation purposes is good to moderately good. Also, it may reflect some prominent alkaline hazard to the soil as reflected by the SSP and KR. The salinity hazard is recognized as high, and it is expected to increase with the expansion of irrigation.
The results revealed that the NO3– concentrations of the samples exceed the safe limit for drinking water set by WHO of 50 mg/L. NO3– in the groundwater of the study area is derived from multiple sources, including synthetic N-fertilizers, manure, and rural septic tanks. The human health risk assessments indicate a high level of risk for adults, but a significantly higher health risk for children drinking NO3–-contaminated groundwater in the study area. The main concern is that the average NO3– concentration will likely continue to increase in the groundwater of the study area. In order to reduce nitrate pollution, excessive chemical fertilizer and manure applications should be prevented, as well as raise awareness of the negative impacts of excessive fertilizers use on the environment. Mixing of water from various sources was carried out in order to reduce the chemical content, particularly nitrate levels, in the highly contaminated wells to tolerable levels. Mixing, when it is possible, can be used as the most cost-effective water treatment method for decreasing chemical concentration below the limitations for various uses.
The salinity hazard is regarded as high, and special management for salinity control is required for groundwater in the study area. It is suggested that modern and high water use efficiency irrigation methods such as drip and sprinkler must be applied in the study area: first to prevent aquifer depletion and, second, to reduce the contamination that might be caused by return flow water. Otherwise, groundwater monitoring, both in terms of quality and quantity, is essential for long-term groundwater management.
References
Abbasi T, Abbasi SA (2012) Water quality indices. Elsevier, p 375
Abdel-Samie SG (2000) Estimation of the recharge sources and mixing percentage by using mathematical modeling for the groundwater in the Quaternary and Plio-Pleistocene aquifers in Nag-Hammadi area. Arab J Nucl Sci Appl 33:55–65
Abdel-Satar AM, Al-Khabbas MH, Alahmad WR, Yousef WM, Alsomadi RH, Iqbal T (2017) Quality assessment of groundwater and agricultural soil in Hail region, Saudi Arabia. Egypt J Aquat Res 43:55–64
Abo-El-Fadl MM (2013) Possibilities of groundwater pollution in some areas, east of Nile Delta. Int J Environ 1:1–21
Adriansen HK (2009) Land reclamation in Egypt: a study of life in the new lands. Geoforum 40:664–674. https://doi.org/10.1016/j.geoforum.2009.05.006
Ahmed MA, Aly AIM, Hussien RA (2007) Assessment of anthropogenic nitrate pollution in groundwater in Northeast Cairo using Nitrogen-15 Technique. Arab J Nucl Sci Appl 40:55–70
Alaya MB, Saidi S, Zemni T, Zargouni F (2014) Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ Earth Sci 71:3387–3421. https://doi.org/10.1007/s12665-013-2729-9
Ali MEA, El-Aassar A-HM (2018) The potential of nitrate removal from groundwater of Bani-Suif west area, Egypt using nanocomposite reverse osmosis membranes. J Basic Environ Sci 5:230–239
Ambast SK, Tyagi NK, Raul SK (2006) Management of declining groundwater in the Trans Indo-Gangetic Plain (India): some options. Agric Water Manag 82:279–296. https://doi.org/10.1016/j.agwat.2005.06.005
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. CRC Press, Taylor and francis Group, Leiden
Ashour MA, El Attar ST, Rafaat YM, Mohamed MN (2009) Water resources management in Egypt. J Eng Sci Assiut Univ 37:269–279
Awad MA, El Arabi NE, Hamza MS (1997) Use of solute chemistry and isotopes to identify sources of ground-water recharge in the Nile Aquifer System, Upper Egypt. Ground Water 35:223–228. https://doi.org/10.1111/j.1745-6584.1997.tb00078.x
Ayers RS, Westcot DW (1985) Water quality for agriculture. FAO irrigation and drainage paper, 29, Rev. 1. P 192
Ayotte JD, Belaval M, Olson SA, Burow KR, Flanagan SM, Hinkle SR, Lindsey BD (2015) Factors affecting temporal variability of arsenic in groundwater used for drinking water supply in the United States. Sci Total Environ 505:1370–1379. https://doi.org/10.1016/j.scitotenv.2014.02.057
Baker L (1992) Introduction to nonpoint source pollution in the United States and prospects for wetland use. Ecol Eng 1:1–26
Ball J (1910) IV.—on the origin of the Nile Valley and the Gulf of Suez. Geol Mag 7:71–76. https://doi.org/10.1017/S0016756800132753
Batabyal AK, Chakraborty S (2015) Hydrogeochemistry and water quality index in the assessment of groundwater quality for drinking uses. Water Environ Res 87:607–617. https://doi.org/10.2175/106143015X14212658613956
Brindha K, Neena Vaman KV, Srinivasan K, Sathis Babu M, Elango L (2014) Identification of surface water–groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai, Southern India. Appl Water Sci 4:159–174. https://doi.org/10.1007/s13201-013-0138-6
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index—do we dare? Water Sew Works 117:339–343
Campos EH (2009) A groundwater flow model for water related damages on historic monuments—case study West Luxor. Egypt Vatten 65:247–254
CEDARE (2001) Regional strategy for the utilisation of the nubian sandstone aquifer system, volumes 1 to 4 and annexes 1 to 2, center for environment and development of the Arab Region and Europe (CEDARE). Egypt, Cairo
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Chaudhary V, Satheeshkumar S (2018) Assessment of groundwater quality for drinking and irrigation purposes in arid areas of Rajasthan, India. Appl Water Sci 8:218. https://doi.org/10.1007/s13201-018-0865-9
Chowdary VM, Rao NH, Sarma PBS (2005) Decision support framework for assessment of non-point-source pollution of groundwater in large irrigation projects. Agric Water Manag 75:194–225. https://doi.org/10.1016/j.agwat.2004.12.013
Clark I, Fritz P (1997) Environmental Isotopes in Hydrogeology. Lewis Publishers. 328 pp
Das S, Nag SK (2015) Deciphering groundwater quality for irrigation and domestic purposes—a case study in Suri I and II blocks, Birbhum District, West Bengal, India. J Earth Syst Sci 124:965–992. https://doi.org/10.1007/s12040-015-0583-8
Davis SN, DeWiest RJM (1966) Hydrogeology. Wiley, New York, p 463
Doneen LD (1961) The influence of crop and soil on percolating waters. In Schiff, L., (Eds.), Proc. Biennial Conference on Ground Water Recharge, pp 156–163
Doneen LD (1964) Notes on water quality in Agriculture. Published as a Water Science and Engineering, Paper 4001, Department of Water Sciences and Engineering, University of California
Dotsika E, Poutoukis D, Raco B, Psomiadis D (2010) Stable isotope composition of Hellenic bottled waters. J Geochem Explor 107:299–304. https://doi.org/10.1016/j.gexplo.2010.07.002
Durfor CN, Becker E (1964) Public water supplies of the 100 largest cities in the United States, 1962. In: Geological Survey Water-Supply Paper 1812, U.S. Department of the Interior, U.S. Government Printing Office, Washington, pp 364. https://doi.org/10.3133/wsp1812
Eaton FM (1950) Significance of carbonates in irrigation water. Soil Sci 69:123–133
Egyptian Higher Committee for Water (EHCW) 2007 Egyptian standards for drinking water and domestic uses
Egyptian Ministry of Health (EMH) 1995 Decree of minister of health, No (108) and (301)/1995. Drinking-water source protection
El Tahlawi MR, Farrag AA, Ahmed SS (2008) Groundwater of Egypt: ‘“an environmental overview.”’ Environ Geol 55:639–652. https://doi.org/10.1007/s00254-007-1014-1
El-Naqa A, Al-Shayeb A (2009) Groundwater protection and management strategy in Jordan. Water Resour Manage 23:2379–2394. https://doi.org/10.1007/s11269-008-9386-x
El-Sayed SA (2018) Study of groundwater in Northeast Cairo Area, Egypt. J Geosci Environ Prot 6:229–251. https://doi.org/10.4236/gep.2018.64014
Elumalai V, Nethononda VG, Manivannan V, Rajmohan N, Li P, Elango L (2020) Groundwater quality assessment and application of multivariate statistical analysis in Luvuvhu catchment, Limpopo, South Africa. J Afr Earth Sc 171:103967. https://doi.org/10.1016/j.jafrearsci.2020.103967
Feast NA, Hiscock KM, Dennis PF, Andrews JN (1998) Nitrogen isotope hydrochemistry and denitrification within the chalk aquifer system of north Norfolk, UK. J Hydrol 211:233–252. https://doi.org/10.1016/S0022-1694(98)00245-5
Food and Agriculture Organization (FAO) (2005) Fertilizer use by crop in Egypt, (Rome) p 62
Floreano IX, de Moraes LAF (2021) Land use/land cover (LULC) analysis (2009–2019) with Google Earth Engine and 2030 prediction using Markov-CA in the Rondônia State, Brazil. Environ Monit Assess 193:239. https://doi.org/10.1007/s10661-021-09016-y
Foster S, Pulido-Bosch A, Vallejos A, Molina L, Llop A, MacDonald AM (2018) Impact of irrigated agriculture on groundwater-recharge salinity: a major sustainability concern in semi-arid regions. Hydrogeol J 26:2781–2791. https://doi.org/10.1007/s10040-018-1830-2
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs, NJ. USA
Gedamy YR, El-Aassar AM, Abdel-Gawad AM (2012) Pollutants detection in water resources at El Saff Area and their impact on human health, Giza Governorate, Egypt. Int J Environ 1:1–14
Gradilla-Hernández MS, de Anda J, Garcia-Gonzalez A, Montes CY, Barrios-Piña H, Ruiz-Palomino P, Díaz-Vázquez D (2020) Assessment of the water quality of a subtropical lake using the NSF-WQI and a newly proposed ecosystem specific water quality index. Environ Monit Assess 192:296. https://doi.org/10.1007/s10661-020-08265-7
Greer FR, Shannon M (2005) Infant Methemoglobinemia: the role of dietary nitrate in food and water. Pediatrics 116:784–786
Gu B, Ge Y, Scott XC, Luo W, Chang J (2013) Nitrate in groundwater of China: sources and driving forces. Glob Environ Chang 23:1112–1121. https://doi.org/10.1016/j.gloenvcha.2013.05.004
Hamza MS, Aly AIM, Awad MA, Nada AA, Abdel Samie SG, Sadek MA, Salem WM, Attia FA, Hassan TM, El Arabi NE, Froehlich K, Geyh MA (1999) Estimation of recharge from Nile Aquifer to the desert fringes at Qena area, Egypt. Isotope Techniques in Water Resources Development and Management Symposium 1999, International Atomic Energy Agency, Vienna, IAEA-CSP-2/C, Session 4. pp 34–46
Han Z (1998) Groundwater for urban water supplies in northern China—an overview. Hydrogeol J 6:416–420. https://doi.org/10.1007/PL00010968
Hesse KH, Hissene A, Kheir O, Schnacker E, Schneider M, Thorweihe U (1987) Hydrogeological investigations in the Nubian aquifer system, Eastern Sahara. Berliner Geowissenechaftliche Abhandlungen A 75(2):397–464
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–306
Hounslow AW (1995) Water quality data—analysis and interpretation. CRC Press, p 417
Idris H, Nour S (1990) Present groundwater status in Egypt and the environmental impacts. Environ Geol Water Sci 16:171–177
International Agency for Research on Cancer (IARC) (2010) Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins / IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2006: Lyon, France), Vol 94
James C (2001) Irrigation water quality. Clemson University, Turfgrass Program
Keating JP, Lell ME, Strauss AW, Zarkowsky H, Smith GE (1973) Infantile methemoglobinaemia caused by carrot juice. N Engl J Med 173:824–826. https://doi.org/10.1056/NEJM197304192881606
Kehew AE (2001) Applied chemical hydrogeology. Prentice Hall, Upper Saddle River, New Jersey 07458. 368 p
Kelley WP (1963) Use of saline irrigation water. Soil Sci 95:385–391
Kelley WP (1951) Alkali soils—their formation, properties and reclamation. Reinhold Publishing Corp., New York, U.S.A. P 56
Korom SF (1992) Natural denitrification in the saturated zone: a review. Water Resour Res 28:1657–1668. https://doi.org/10.1029/92WR00252
Kross BC, Ayebo AD, Fuortes LJ (1992) Methemoglobinemia: nitrate toxicity in rural America. Am Fam Phys 46:183–188 (PMID: 1631630)
Kundu MC, Mandal B, Hazra GC (2009) Nitrate and fluoride contamination in groundwater of an intensively managed agroecosystem: a functional relationship. Sci Total Environ 407:2771–2782. https://doi.org/10.1016/j.scitotenv.2008.12.048
Li P, Qian H, Wu J, Zhang Y, Zhang H (2013) Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environment 32:195–206. https://doi.org/10.1007/s10230-013-0234-8
Li P, Qian H, Howard KWF, Wu J (2015) Building a new and sustainable ‘“Silk Road economic belt.”’ Environ Earth Sci 74:7267–7270. https://doi.org/10.1007/s12665-015-4739-2
Li P, Wu J, Qian H (2016) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9:15. https://doi.org/10.1007/s12517-015-2059-1
McCallum JL, Crosbie RS, Walker GR, Dawes WR (2010) Impacts of climate change on groundwater in Australia: a sensitivity analysis of recharge. Hydrogeol J 18:1625–1638. https://doi.org/10.1007/s10040-010-0624-y
Mohammed AM (2013) Nile Valley Aquifer in Egypt: Qena Area. Lap Lambert Academic Publishing, Germany
Mohammed AM, Krishnamurthy RV, Kehew AE, Crossey LJ, Karlstrom KK (2016) Factors affecting the stable isotopes ratios in groundwater impacted by intense agricultural practices: a case study from the Nile Valley of Egypt. Sci Total Environ 573:707–715. https://doi.org/10.1016/j.scitotenv.2016.08.095
Mohammed AM (2005) Hydrogeological and Hydrochemical Evaluation of the Area Between Qena and Qus, Eastern Desert, Egypt. (Ms dissertation) South Valley University, Qena, Egypt
Mohammed AM (2015) Deep Fluid Inputs into the Continental-scale Nubian Sandstone Aquifer System Constrained by Hydrochemical, Stable Isotope, and Noble Gas Data (PhD dissertation) Western Michigan University, Kalamazoo, Michigan, USA
Mook WG (2000) Environmental isotopes in the hydrological cycle: principles and applications. VOL. 1, Introduction, Theory, Methods, Review. International Atomic Energy Agency, 185 pp
National Academy of Sciences and National Academy of Engineering (NAS-NAE) (1972) Water quality criteria, Washington, D.C., 613 p
Nel J, Xu Y, Batelaan O, Brendonck L (2009) Benefit and implementation of groundwater protection zoning in South Africa. Water Resour Manage 23:2895–2911. https://doi.org/10.1007/s11269-009-9415-4
Ogunfowokan AO, Obisanya JF, Ogunkoya OO (2013) Salinity and sodium hazards of three streams of different agricultural land use systems in Ile-Ife, Nigeria. Appl Water Sci 3:19–28. https://doi.org/10.1007/s13201-012-0053-2
Omar E-DM, Moussa AMA (2016) Water management in Egypt for facing the future challenges. J Adv Res 7:403–412. https://doi.org/10.1016/j.jare.2016.02.005
Park Y, Kim Y, Park S-K, Shin W-J, Lee K-S (2018) Water quality impacts of irrigation return flow on stream and groundwater in an intensive agricultural watershed. Sci Total Environ 630:859–868. https://doi.org/10.1016/j.scitotenv.2018.02.113
Parkhurst DL, Appelo CAJ (1999) User's guide to PHREEQC (Version 2) - a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water-Resources Investigations Report 99–4259, 312 p
Pastén-Zapata E, Ledesma-Ruiz R, Harter T, Ramírez AI, Mahlknecht J (2014) Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci Total Environ 470–471:855–864. https://doi.org/10.1016/j.scitotenv.2013.10.043
Pazanda K, Khosravib D, Ghaderic MR, Rezvanianzadehd MR (2018) Identification of the hydrogeochemical processes and assessment of groundwater in a semi-arid region using major ion chemistry: a case study of Ardestan basin in Central Iran. Groundw Sustain Dev 6:245–254. https://doi.org/10.1016/j.gsd.2018.01.008
Pearce MW, Schumann EH (2001) The impact of irrigation return flow on aspects of the water quality of the Upper Gamtoos Estuary, South Africa. Water SA 27:367–372. https://doi.org/10.4314/wsa.v27i3.4981
Pillsbury AF, Blaney HF (1966) Salinity problems and management in river-systems. J Irrig Drain Div 92:77–90
Pulido-Bosch A, Bensi S, Molina L, Vallejos A, Calaforra JM, Pulido-Leboeuf P (2000) Nitrates as indicators of aquifer interconnection. Application to the Campo de Dalias (SE-Spain). Environ Geol 39:791–799. https://doi.org/10.1007/S002540050495
Punmia BC, Lal PBB (1981) Irrigation and water power engineering. Standard Publishers Distributors, New Delhi, p 859
Qena Metrological Station (2001–2014) ID: 624020-99999, Qena, Egypt
Raghunath HM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi, India
Rahman A, Mondal NC, Tiwari KK (2021a) Anthropogenic nitrate in groundwater and its health risks in the view of background concentration in a semi arid area of Rajasthan, India. Sci Rep 11:9279. https://doi.org/10.1038/s41598-021-88600-1
Rahman Z, Ahmad S, Fidel R, Khalid S, Ahmad I, Humphrey OS, Khan H, Khan B (2021b) Faecal and nitrate contamination in the groundwater of Mardan district, Pakistan. Environ Geochem Health 43:3615–3624. https://doi.org/10.1007/s10653-021-00848-8
Rattray G (2015) Geochemical evolution of groundwater in the Mud Lake area, Eastern Idaho, USA. Environ Earth Sci 73:8251–8269. https://doi.org/10.1007/s12665-014-3988-9
Reddy TB, Ramana CV, Bhaskar C, Chandrababu JP (2012) Assessment of heavy metal study on ground water in and around Kapuluppada MSW site, Visakhapatnam, AP. Int J Sci Nat 3:468–471
Redwan M, Abdel Moneim AA, Mohammed NE, Masoud AM (2020) Sources and health risk assessments of nitrate in groundwater West of Tahta area, Sohag, Egypt. Episodes 43:751–760. https://doi.org/10.18814/epiiugs/2020/020048
Reyes-Toscano CA, Alfaro-Cuevas-Villanueva R, Cortés-Martínez R, Morton-Bermea O, Hernández-Álvarez E, Buenrostro-Delgado O, Ávila-Olivera JA (2020) Hydrogeochemical characteristics and assessment of drinking water quality in the urban area of Zamora. Mexico Water 12:556. https://doi.org/10.3390/w12020556
RIGW and IWACO (1986) Development and management of groundwater resources in the Nile valley and delta, Technical note. Cairo. Egypt
RIGW (1988) Development of groundwater for irrigation and drainage in the Nile valley and Delta. Technical note, Cairo, Egypt
Sadat-Noori SM, Ebrahimi K, Liaghat AM (2014) Groundwater quality assessment using the water quality index and GIS in Saveh-Nobaran aquifer, Iran. Environ Earth Sci 71:3827–3843. https://doi.org/10.1007/s12665-013-2770-8
Sadler R, Maetam B, Edokpolo B, Connell D, Yu J, Stewart D, Park MJ, Gray D, Laksono B (2016) Health risk assessment for exposure to nitrate in drinking water from village wells in Semarang, Indonesia. Environ Pollut 216:738–745. https://doi.org/10.1016/j.envpol.2016.06.041
Said R (1962) The Geology of Egypt. El Sevier. Amesterdam, New York
Said R (1991) The Geology of Egypt. Balkema AA: Rotterdam, Brookfield, 734 pp
Sall M, Vanclooster M (2009) Assessing the well water pollution problem by nitrates in the small scale farming systems of the Niayes region, Senegal. Agric Water Manag 96:1360–1368. https://doi.org/10.1016/j.agwat.2009.04.010
Salman SA, Arauzob M, Elnazera AA (2019) Groundwater quality and vulnerability assessment in west Luxor Governorate, Egypt. Groundw Sustain Dev 8:271–280. https://doi.org/10.1016/j.gsd.2018.11.009
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers. McGraw-Hill, New York
Scanlon SR, Jolly I, Sophocleous M, Zhang L (2007) Global impacts of conversions from natural to agricultural ecosystems on water resources: quantity versus quality. Water Resour Res 43:W03437. https://doi.org/10.1029/2006WR005486
Schiavo MA, Havser S, Gusimano G, Gatto L (2006) Geochemical characterization of groundwater and submarine discharge in the southeastern Sicily. Cont Shelf Res 26:826–834. https://doi.org/10.1016/j.csr.2005.12.001
Schoeller H (1967) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigation and development. Water Research, Series-33, UNESCO, pp 44–52
Shammi M, Rahman R, Rahman M, Moniruzzaman M, Bodrud-Doza M, Karmakar B, Uddin MK (2016) Assessment of salinity hazard in existing water resources for irrigation and potentiality of conjunctive uses: a case report from Gopalganj District, Bangladesh. Sustain Water Res Manag 2:369–378. https://doi.org/10.1007/s40899-016-0064-5
Shamrukh M, Akib S (2021) Trendline and Monthly variations of nitrate in water supply wells in Upper Egypt. Eng 2:43–53. https://doi.org/10.3390/eng2010004
Shamrukh M, Corapcioglu MY, Hassona FAA (2001) Modeling the effect of chemical fertilizers on groundwater quality in the Nile Valley Aquifer, Egypt. Groundwater 39:59–67
Sidhu N, Pebesma E, Camara G (2018) Using Google Earth engine to detect land cover change: Singapore as a use case. Eur J Remote Sens 51:486–500. https://doi.org/10.1080/22797254.2018.1451782
Simon C (1966) Nitrate poisoning from spinach. The Lancet 287(7442):872. https://doi.org/10.1016/S0140-6736(66)90205-4
Singh KKR, Tewari G, Kumar S (2020) Evaluation of groundwater quality for suitability of irrigation purposes: a case study in the Udham Singh Nagar, Uttarakhand. J Chem. https://doi.org/10.1155/2020/6924026
Somay MA, Gemici U (2012) Groundwater quality degradation in the Buyuk Menderes River Coastal Wetland. Water Air Soil Pollut 223:15–27. https://doi.org/10.1007/s11270-011-0834-5
Spalding RF, Exner ME (1993) Occurrence of nitrate in groundwater—a review. J Environ Qual 22:392–402. https://doi.org/10.2134/jeq1993.00472425002200030002x
Spalding RF, Exner ME, Lindau CW, Eaton DW (1982) Investigation of sources of groundwater nitrate contamination in the Burbank-Wallula area of Washington, U.S.A. J Hydrol 58:307–324
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub basin, Tamilnadu, India. J. King Saud Univ. Sci. 26:37–52. https://doi.org/10.1016/j.jksus.2013.08.001
Strebel O, Duynisveld WHM, Böttcher J (1989) Nitrate pollution of groundwater in western Europe. Agr Ecosyst Environ 26:189–214. https://doi.org/10.1016/0167-8809(89)90013-3
Su X, Wang H, Zhang Y (2013) Health risk assessment of nitrate contamination in groundwater: a case study of an agricultural area in Northeast China. Water Resour Manage 27:3025–3034. https://doi.org/10.1007/s11269-013-0330-3
Support for environmental assessment and management (SEAM) (2005) Qena Governorate, Environmental Action Plan. Ministry of State for Environmental Affairs, Egyptian Environmental Affairs Agency, Entec UK Limited and ERM. p 69
Sutadian AD, Muttil N, Yilmaz A, Perera C (2016) Development of river water quality indices—a review. Environ Monit Assess 188:58. https://doi.org/10.1007/s10661-015-5050-0
Szabolcs I, Darab C (1964) The Influence of Irrigation Water of High Sodium Carbonate Content of Soils. Proceedings of 8th International Congress of ISSS, 2, 803–812
Tesoriero AJ, Liebscher H, Cox SE (2000) Mechanism and rate of denitrification in an agricultural watershed: electron and mass balance along groundwater flow paths. Water Resour Res 36:1545–1559
Todd DK (1980) Groundwater Hydrology, 2nd edn. Jhon Wiley and Sons, New York, p 535
Todd DK, Mays L (2005) Ground Water Hydrology. John Wiley and Sons Inc, Hoboken, p 652
Tolera MB, Choi H, Chang SW, Chung II-M (2020) Groundwater quality evaluation for different uses in the lower Ketar Watershed, Ethiopia. Environ Geochem Health 42:3059–3078. https://doi.org/10.1007/s10653-019-00508-y
U.S. Salinity Laboratory Staff, U.S. Department of Agriculture (1954) Diagnosis and improvement of saline and alkali soils. Agriculture Handbook No. 60, Government Printing Office, Washington 25, DC, 166 p
UNESCO (2007) Water portal newsletter no. 161: Water related diseases; http://www.unesco.org/water/news/newsletter/161.shtml
USEPA (United States Environmental Protection Agency) (1991) Nitrate; CASRN 14797–55–8. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0076_summary.pdf. Accessed August 2021
USEPA (United States Environmental Protection Agency) (2002) Child-specific exposure factors handbook. National Center for Environmental Assessment, Washington, DC; EPA/600/P-00/002B
Wahba SM, Scott K, Steinberger JK (2018) Analyzing Egypt’s water footprint based on trade balance and expenditure inequality. J Clean Prod 198:1526–1535. https://doi.org/10.1016/j.jclepro.2018.06.266
Weitzman JN, Brooks JR, Mayer PM, Rugh WD, Compton JE (2021) Coupling the dual isotopes of water (δ2H and δ18O) and nitrate (δ15N and δ18O): a new framework for classifying current and legacy groundwater pollution. Environ Res Lett 16:045008. https://doi.org/10.1088/1748-9326/abdcef
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
WHO (2003) Nitrate and Nitrite in Drinking-water. Background Document for Development of WHO Guidelines for Drinking-water Quality (Report No. WHO/SDE/WSH/04.03/56), Geneva
Widory D, Kloppmann W, Chery L, Bonnin J, Rochdi H, Guinamant J-L (2004) Nitrate in groundwater: an isotopic multi-tracer approach. J Contam Hydrol 72:165–188. https://doi.org/10.1016/j.jconhyd.2003.10.010
Wilcox LV (1948) The quality of water for irrigation. US Department of Agricultural Technical Bulletin No 962:1–40
Wilcox LV (1955) Classification and Use of Irrigation Waters. Vol 969. United States Department of Agriculture, circular No 969, Washington DC
Wolff P (1993) Land reclamation in Egypt. Tropenlandwirt 94:103–117
Wu J, Li P, Qian H (2014) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: a case study in Laoheba phosphorite mine in Sichuan, China. Arab J Geosci 7:3973–3982. https://doi.org/10.1007/s12517-013-1057-4
Yehiaa M, Baghdadyb A, Howaric FM, Awadb S, Gadb A (2017) Natural radioactivity and groundwater quality assessment in the northern area of the Western Desert of Egypt. J Hydrol Reg Stud 12:331–344. https://doi.org/10.1016/j.ejrh.2017.06.002
Youssef M (1968) Structure pattern of Egypt and its interpretation. Am Assoc Pet Geol Bull 52:601–614. https://doi.org/10.1306/5D25C44D-16C1-11D7-8645000102C1865D
Zhai Y, Lei Y, Wu J, Teng Y, Wang J, Zhao X, Pan X (2017) Does the groundwater nitrate pollution in China pose a risk to human health? A critical review of published data. Environ Sci Pollut Res 24:3640–3653. https://doi.org/10.1007/s11356-016-8088-9
Acknowledgements
The authors would like to thank South Valley University in Qena, Egypt, for providing logistical support during the field work and sampling, as well as laboratory facilities during the research work. We are grateful to the critical reviews by three anonymous reviewers that helped improve the manuscript considerably.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they have no conflicts of interest.
Data availability
All data used in this study are available from the corresponding author upon request.
Ethical approval
No animals were involved in this study. No human participants were involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohammed, A.M., Refaee, A., El-Din, G.K. et al. Hydrochemical characteristics and quality assessment of shallow groundwater under intensive agriculture practices in arid region, Qena, Egypt. Appl Water Sci 12, 92 (2022). https://doi.org/10.1007/s13201-022-01611-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-022-01611-9