Abstract
A hydrogeochemical study was conducted in the Dongsheng Coalfield, Ordos Basin, China, to identify the mechanisms responsible for the chemical compositions of the shallow groundwater and to document water quality with respect to agricultural and drinking supply standards, prior to mining. Tri-linear diagrams, principal component analysis, and correlation analysis were used to reveal the hydrogeochemical characteristics of the shallow groundwater, and the potential water–rock interactions. In general, the major cations and anions were present at low concentrations, but were relatively higher around Jiushenggong than elsewhere in the study area. Groundwater around Jiushenggong has a long residence time and is also subject to extensive evapotranspiration. The dominant hydrochemical facies are HCO3-Ca, HCO3-Na, and mixed HCO3-Ca·Na·Mg types. Increases in major ion concentrations along the flow path, including Na, Cl, and SO4, coincide with increases in total dissolved solids. The predominant mechanism controlling groundwater chemistry proved to be the dissolution of carbonates, gypsum, and halite. Cation exchange and mixing with local recharge water are also important factors. The shallow groundwater quality in the study area is suitable for agricultural and drinking purposes.
Zusammenfassung
Es wurde ein hydrochemische Studie im Kohlefeld Dongsheng (Ordos Becken, China) durchgeführt, um die Mechanismen aufzuklären, die für die chemische Zusammensetzung des flachen Grundwassers verantwortlich sind und um die Grundwasserqualität vor Beginn des Bergbaus zu dokumentieren, gemessen an Standards für die Wassernutzung in der Landwirtschaft und für die Trinkwasserversorgung. Dreiecksdiagramme, Hauptkomponentenanalyse und Korrelationsanalyse wurden benutzt, um die hydrochemische Charakteristik des flachen Grundwassers und die möglichen Wechselwirkungen Wasser-Gestein aufzuklären. Grundsätzlich waren die Konzentrationen der Hauptkationen und –anionen niedrig. Um Jiushenggong waren die Konzentrationen jedoch höher als im restlichen Untersuchungsgebiet. Das Grundwasser um Jiushenggong hat eine lange Aufenthaltszeit und ist außerdem durch hohe Evapotranspiration beeinflusst. Die dominierenden Grundwassertypen sind HCO3-Ca, HCO3-Na und gemischte HCO3-Ca*Na*Mg Typen. Die Zunahme der Konzentrationen der Hauptionen entlang des Fließweges, einschließlich Na, Cl und SO4, treten zusammen mit einem Anstieg der Gesamtkonzentration gelöster Stoffe auf. Die dominierenden Prozesse, die den Grundwasserchemismus bestimmen, sind die Auflösung von Karbonaten, Gips und Halit. Kationenaustausch und die Mischung mit der lokalen Grundwasserneubildung sind ebenfalls wichtige Faktoren. Die Qualität des flachen Grundwassers ist für landwirtschaftliche Nutzung und die Trinkwassergewinnung geeignet.
Resumen
Se realize un studio hidrogeoquímico en Dongsheng Coalfield, Ordos Basin, China, para identificar los mecanismos responsables de las composiciones químicas del agua subterránea superficial y documentar la calidad de agua con respecto a los estándares de agua potable y de agua para agricultura anteriores a la minería. Diagramas trilineales, análisis de componentes principales y análisis de correlación fueron usados para revelar las características hidrogeoquímicas del agua subterránea superficial y de las interacciones potenciales agua-roca. En general, los principales cationes y aniones estuvieron presentes en bajas concentraciones pero fueron relativamente mayores cerca de Jiushenggong. El agua subterránea cerca de Jiushenggong tiene un largo tiempo de residencia y está sujeto a evapotranspiración extensiva. Las fases hidroquímicas dominantes son HCO3-Ca, HCO3-Na y mezclas tipo HCO3-Ca*Na*Mg. Incrementos en las concentraciones de los iones mayoritarios a lo largo del flujo, incluyendo Na, Cl y SO4, coinciden con el total de sólidos disueltos. La disolución de carbonatos, yeso y halita provó ser el mecanismo que controla la química del agua subterránea. El intercambio de cationes y la mezcla con agua de recarga, son otros factores de importancia. La calidad del agua subterránea superficial en el área de estudio es adecuada para la agricultura y también para el consumo.
摘要
为了研究鄂尔多斯盆地东胜煤田煤矿开采前浅层地下水的水化学形成机制及其农业用水和饮用水水质状况,采用水文地球化学的理论,通过三线图、主成分分析和相关分析研究了浅层地下水的水文地球化学特征及其潜在的水岩作用。研究表明:总体上研究区地下水主要阴阳离子含量较低,但在九盛宫附近这些主要阴阳离子的含量相对较高。这主要是由于九盛宫附近浅层地下水径流缓慢,滞留时间较长且蒸发强烈。研究区浅层地下水主要以HCO3-Ca, HCO3-Na和HCO3-Ca·Na·Mg为主,主要离子浓度(包括Na+、Cl−和SO4 2−)沿流向不断增大,引起溶解性总固体也沿流向逐渐增大。碳酸盐、石膏和岩盐的溶解为控制地下水化学成分演化的主要机制。阳离子交换和局部补给水的混合也是影响地下水化学成分变化的重要因素。研究区浅层地下水水质适用于农业灌溉和饮用。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over one-third of China’s mainland has an arid to semi-arid climate. These arid to semi-arid areas are mostly distributed in northwest China where groundwater is a major source of water. The quantity and quality of groundwater resources have been affected by the rapid development of coal mining in northwest China over the past several decades. Since 2011, groundwater conservation and protection measures must be implemented for all new construction projects in China in accordance with the technical guidelines of the PRC Ministry of Environmental Protection (2011). This is especially important in mining areas, since water is an essential component of the mining process (Agartan and Yazicigil 2012). Prior to mining, major ions in groundwater are usually controlled by slow, natural processes. However, mining can lower shallow groundwater levels, influence groundwater dynamics, and increase reaction kinetics. Thus, water resource protection requires an understanding of water quality and natural processes that control major ion chemistry before adverse effects occur.
The Ordos Basin, covering a total of 28.2 × 104 km2, contains the second largest coal reserves in China (Dai et al. 2006). It was approved as a national energy base in 1998 by the former State Planning Commission (Hou et al. 2008). The groundwater resources and hydrogeology in the Ordos Basin have been studied by many Chinese scholars and institutes because they play a key role in regional economic development and natural resources exploitation. The most important research on this basin’s groundwater resources has been conducted by China’s Geological Survey Bureau since 1999. This research, which has cost a hundred million CNY, provides a solid basis for the present study. However, regional groundwater studies may not provide much precise information on the groundwater flow properties in small basins (Tóth 1963). Therefore, it is also important to carry out local groundwater resources investigations.
The Dongsheng Coalfield, located in Inner Mongolia, is an important part of the Ordos Energy Base in China. It is the largest explored coalfield with a vast potential for future development. The coal is produced from Jurassic formations and exploited mostly by subsurface mining. The Abuhai investigation area is a sub-basin of the Dongsheng coalfield; residents there rely on groundwater due to the insufficient supply of surface water. However, expanded mining activities could significantly influence water chemistry and their water supply. The aims of the present study were to describe the geochemical factors controlling major ion chemistry and assess groundwater quality for agricultural and domestic purposes. The results will be useful in defining the hydrogeologic setting of the area prior to extensive mining, and providing important information such as the status of groundwater quality and the suitability of groundwater for domestic and agricultural purposes to local water suppliers and decision makers.
Study Area
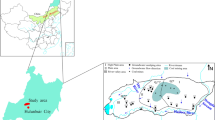
The study area is located in the northern part of the Ordos Basin about 15 km southwest of Ordos City. It ranges within latitudes 39°46′30″–39°52′22″N and longitudes 109°38′19″–109°52′27″E, covering 210 km2 (Fig. 1). It is administratively governed jointly by Dongsheng District and Yijinhuoluo County of Ordos City. The study area lies within the continental arid to semiarid climate region of the northern temperature zone, and is characterized by a long, cold, and windy winter, a dust-laden spring, a short, hot, sometimes humid summer, and a very short autumn (Li et al. 2010a, 2011a). In winter, the temperature falls to a minimum of −31.4 °C, while in summer it reaches a maximum of 36.6 °C. The average annual temperature of the area is 5.2 °C, and the annual average rainfall is 357.5 mm. The precipitation is mostly concentrated in June to September, accounting for 62 % of the annual rainfall. The least precipitation is usually measured in January. The annual potential evapotranspiration is 2,530 mm and is most intense in May and June. There are no large rivers within the study area; the only surface water body is a small intermittent stream in the Abuhai Valley.
Surrounding the Abuhai Valley, three highlands can be observed around Qingchunshan, the southern area of Jiushenggong, and the eastern area of Ganjiata. Three types of landforms can be classified in the study area: a gully region, a flat hill ridge region, and an alluvial-eolian plain. The gully region includes most of the study area, and is underlain by a Cretaceous formation. The flat hill ridge region is mainly distributed around Jiushenggong and Qingchunshan, where the topography is flat and most villages are located. The alluvial-eolian plain can only be observed along the Abuhai Valley, where the land surface elevation decreases from north to south.
Hydrogeology
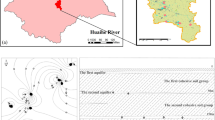
A Cretaceous formation composed of sedimentary sandstones and conglomerates can be widely observed in the study area. Quaternary sediments composed of alluvial and eolian sediments are distributed along the Abuhai Valley, while the Tertiary formation only crops out around Qingchunshan (Fig. 2). Older formations from which coal is produced, such as the Jurassic deposits, are not exposed at the surface, and are not suitable for groundwater development because of the great depths to an aquifer. Dolomite, calcite, gypsum, halite, albite, potassium feldspar, and illite are major minerals in the Cretaceous and Quaternary aquifers (Hou et al. 2008).
The thickness of the Quaternary phreatic aquifer can be over 50 m (Fig. 3). It can be subdivided into a Quaternary alluvial aquifer and an eolian aquifer. The alluvial aquifer is mainly distributed at the bottom of the Abuhai Valley and its tributaries. Its lithology includes Holocene alluvial sands and gravels. The depth to water level in this aquifer is usually within 3–5 m and its water is preferable for domestic use. The eolian aquifer was formed by windblown sand and fine sand, and is distributed along the left bank of the Abuhai Valley. The thickness of the eolian aquifer ranges from 3 to 10 m. Groundwater dynamics are controlled by climatic conditions and local landforms.
The Cretaceous aquifer is the most widespread and important water supply aquifer in the area, with a thickness exceeding 200 m. Five lithologic cycles exist in this formation; the uppermost cycle with a thickness of about 80 m forms the phreatic aquifer. The depth to groundwater in this aquifer is usually about 10 m. It is viewed as the most promising aquifer for supplying water for domestic and agricultural uses because of its great thickness and high permeability. The groundwater levels in the Quaternary and Cretaceous phreatic aquifers, which form an integrated shallow groundwater system in the area, are similar, according to borehole data.
Shallow groundwater levels were contoured to show the general flow pattern in the area (Fig. 2). Groundwater levels were measured during August 2007 from 31 wells and 40 boreholes screened at the phreatic aquifer. Controlled by topography, the groundwater flows mainly from north to south and from highlands to valleys (Fig. 2). The groundwater flows from the Cretaceous aquifer to the Quaternary aquifer due to the low topographic position of the Quaternary aquifer. These shallow aquifers are recharged mainly by lateral inflow. Besides sparse precipitation, leakage from seasonal surface water bodies and irrigation water can also provide a small proportion of groundwater recharge. The main discharge patterns include lateral outflow, evaportranspiration, and artificial extraction.
Materials and Methods
Sample Collection and Analysis
A total of 15 shallow groundwater samples were collected during August and September 2007. The sampling locations are shown in Fig. 1. Samples were collected in pre-cleaned plastic polyethylene bottles for physicochemical analysis. Prior to sampling, all the sampling containers were rinsed thoroughly with the groundwater to be taken for analysis. The groundwater samples were analyzed in the laboratory of the Xi’an Institute of Geology and Minerals Resources using standard procedures recommended by Chinese Ministry of Water Resources. For each sample, ten indices including major cations and anions, total dissolved solids (TDS), pH, and total hardness (TH) were analyzed. The general information of sampled wells and physiochemical analysis results are listed in Table 1. As electric conductivity (EC) was not measured, it was calculated in Aq·QA® (RockWare Inc. 2004) for later use. It is a temperature-corrected conductivity calculated according to Standard Method 2510-A (APHA 1995), accounting for ionic strength effects.
Principal Component Analysis (PCA)
Correlations among water-quality parameters are very useful in understanding the major hydrochemical processes in a groundwater system. In the present study, Pearson correlation coefficients and PCA were calculated with statistical package SPSS 13.0 for Windows® (SPSS Inc. 2004). PCA was used to transform the correlated variables into a smaller number of uncorrelated parameters, thus reducing the dimensionality of the data matrix (Jolliffe 2002; Kaufman 1990; Menció and Mas-Pla 2008). According to Niu et al. (2011), there are six steps for a complete PCA: selection of variables and data input, data standardization, calculation of correlation coefficient matrix, calculation of the eigenvalue of the matrix, calculation of the principal component loading matrix, and finally calculation of the parameter scoring weights.
Results and Discussion
Hydrogeochemical Characteristics
All major ions were within China’s acceptable limits for Groundwater Quality (Bureau of Quality and Technical Supervision of China 1994) and the highest permissible limits of the WHO for drinking water [WHO (World Health Organization) 2011]. The concentration of TDS varied from 217 to 632 mg/L, with an average of 406 mg/L, indicating fresh water. TH measured as CaCO3 varied between 132 and 388 mg/L, suggesting soft to hard water that is suitable for drinking. Generally, the groundwater in the up-gradient samples contained mostly HCO3 − and Ca2+, and the concentrations of Na+, SO4 2−, and Cl− increase down-gradient due to mineral dissolution along groundwater flow paths (Qian and Li 2011). Ca2+ and Na+ were the dominant cations in this study, followed by Mg2+, whereas HCO3 − and SO4 2− were the dominant anions, followed by Cl− (Table 2).
The spatial distribution of TH varies (S-Fig. 1; supplementary figures are published in the on-line version of this journal and can be downloaded for free by all subscribers). Slightly hard water is distributed across most of the study area, while hard water is mainly observed in the areas around Jiushenggong and northeast of Ganjiata, as well as near Dashunhao. Soft water can be observed in the south of the study area, which may be due to local recharge from rainwater or local surface water bodies with low concentrations of Ca2+ and Mg2+.
The Na+ concentration increased from the middle to the outer parts of the study area (S-Fig. 2a). Anomalously high concentrations were found around Dashunhao, Jiushenggong, and Ganjiata, but low concentrations were observed around Qingchunshan. Ca2+ concentrations increased from north and south to the middle, and the lowest concentration was observed in the southern corner of the area, while the highest was seen between Jiushenggong and Ganjiata (S-Fig. 2b). The spatial distribution of Mg2+ and Cl− was similar (S-Figs. 2c and 2d), with increasing concentrations from southwest to northeast, and high concentrations near Jiushenggong. Interestingly, the concentrations of SO4 2− and HCO3 − have a similar distribution pattern (S-Fig. 2e and 2f), gradually increasing from northwest to the southeast and from southwest to northeast, forming a high concentration area near Jiushenggong.
It can be seen that nearly all of the major ions had a high concentration area near Jiushenggong, which may have been caused by similar hydrogeochemical processes. The topography around Jiushenggong is much flatter than in any other places, and therefore groundwater flow in this area is slower, indicating a longer groundwater residence (and water–rock interaction) time, which may produce more dissolution of minerals. Furthermore, evaporation in the area is intense; the annual evaporation is seven times that of the rainfall (Li et al. 2010a). The decrease of ion concentrations down-gradient of this zone may be attributed to mixing of surface water with very low concentrations of dissolved ions. Local human activities, such as sewage disposal and agricultural irrigation, may induce additional local recharge to the shallow groundwater.
Tri-linear diagrams such as the Piper diagram (Piper 1953) are perhaps the most commonly used techniques for finding hydrochemical patterns in major ion data. A Piper diagram drawn with Aq·QA® (RockWare Inc. 2004) is shown in Fig. 4. With respect to cations, samples are mostly plotted in zones A, B, and D of the lower left delta-shaped region, suggesting that some samples are calcium-type water, some are sodium-type water, and some are mixed-type, while for anions, most samples are located in zone E of the lower right delta-shaped region (Fig. 4), indicating dominance of bicarbonate-type water. Carbonate hardness is represented by Ca2+ and Mg2+ that can be balanced by the amount of carbonate (i.e. HCO3 −, CO3 2−, and CO2) in solution (RockWare Inc. 2004). The carbonate hardness of most samples exceeds 50 % (Fig. 4, area 5), which means that the total hardness of most water samples can be decreased by water softening technology. It is also suggested by Fig. 4 that alkaline earth and weak acids exceeded the alkali earths and strong acids in most groundwater samples. The dominant hydrochemical facies are HCO3-Ca, HCO3-Na, and mixed HCO3-Ca·Na·Mg types. It can be inferred from the hydrochemical facies that the groundwater chemistry in the area is influenced by the dissolution/precipitation of carbonate minerals and Na-containing minerals such as halite and albite. The dissolution of halite is probably an important source of Na+, but there must be other factors influencing the concentration of Na+, because the proportion of Cl− concentration in anions is not as great as that of Na+ in cations. Na+ could also be derived from cation exchange with Ca2+ or Mg2+.
Major Ion Variation Along Flow Path
Physiochemical parameters for sample points along a flow path can demonstrate changes in solutes that occur with time and distance of travel as water flows down-gradient (Lee 1981). Generally speaking, the regional evolutionary trend of the predominant anions along the groundwater flow path is: HCO3 − → HCO3 − + SO4 2− → SO4 2− + HCO3 − → SO4 2− + Cl− → Cl− + SO4 2− → Cl− (Chebotarev 1955; Qian and Li 2011). With the groundwater flowing from the upper to lower reaches of a region, Ca2+ and HCO3 − tend to decrease while Na+ + K+, SO4 2−, and Cl− tend to increase (Lee 1981). There are two different groundwater evolution patterns in the study area. Figure 5a illustrates the most general hydrochemical evolution pattern along flow path (from W201 to W203). Concentrations of Na+ + K+, Cl−, and SO4 2− increase down-gradient while Ca2+ and HCO3 − decrease, resulting in the transition of hydrochemical type from HCO3-Ca to HCO3·SO4-Ca·Na type. However, groundwater evolution pattern may be different if there is local recharge along the flow path. It can be seen from Fig. 5b that along the flow path from W204 to W117, Ca2+, Mg2+, SO4 2−, and HCO3 − decrease significantly, whereas Na+ + K+ and Cl− remain relatively constant. The second groundwater evolution pattern indicates that recharge water with very low concentrations of dissolved Ca2+, Mg2+, SO4 2−, and HCO3 − could have mixed with shallow groundwater between W204 and W117. The two different groundwater evolution patterns show that water quality in the study area is influenced by regional groundwater flow and local recharge.
Correlations of Parameters
Correlations among water-quality parameters are shown in Table 3. The major anions and cations, except for K+ and Na+, are significantly correlated with TDS, which suggests that the continuous addition of these cations and anions into groundwater has promoted increased TDS. The concentration of Cl− is correlated with Na+ and Mg2+, with correlation coefficients of 0.795 and 0.707, respectively. Cation exchange could possibly explain the correlation between Cl−, Na+, and Mg2+, as explained later.
The concentration of Ca2+ is correlated with HCO3 − and TDS. Furthermore, Ca2+ exhibits a positive correlation with TH with a correlation coefficient of 0.918, since hardness is an approximate measure of Ca2+ and Mg2+. TH is positively correlated with HCO3 −, with a correlation coefficient of 0.907. As calcite and dolomite dissolve, the concentrations of Mg2+, Ca2+, and HCO3 − increase, resulting in increased TH and TDS.
The concentration of SO4 2− is correlated with those of Mg2+ and Na+, suggesting the possible dissolution of gypsum, followed by cation exchange, as explained in more detail later. Although the correlation between SO4 2− and Ca2+ is not significant, it is logical to assume the dissolution of gypsum as a source of SO4 2− and Ca2+. According to Hou et al. (2008), pyrite is not present in these strata, while gypsum is, so it is reasonable to conclude that gypsum is the source of the SO4 2−. Additional reactions involving Ca2+, such as carbonate dissolution/precipitation and cation exchange, would account for the lack of correlation with SO4 2−. The pH is negatively correlated with Ca2+, presumably because at higher pH, hydrogen ion concentrations in water decreases, which constrains carbonate mineral dissolution.
It should be noted that the chemical reactions that happen in a groundwater system are very complex and many reactions are involved. Correlation analysis, though very useful, can only provide a general insight into water–rock interactions. If one wants to know more about the reactions taking place in a groundwater system, more comprehensive analysis, such as aquifer mineralogy, are needed.
Principal Component Analysis (PCA)
Most parameters are strongly correlated with more than one other constituent (Table 3). In PCA, the correlations among the variables were used to estimate principal components (PCs) with common associations and associated constituent loadings on the principal component axis (Lucas and Jauzein 2008). The total variance explained by each PC and the loading matrix of PCs are shown in Table 4. Three PCs with eigenvalues greater than 1 were extracted, accounting for 90.8 % of the total variance (Table 4). The first two PCs explain 50.4 and 29.5 % of the variance, respectively, accounting for most of the variance in the original dataset. Principal component three (PC3) explains 10.9 % of the total variance.
PC1 has significant loadings by Ca2+, Mg2+, SO4 2−, HCO3 −, TDS, and TH (Table 4), which suggests that PC1 represents the dissolution/precipitation processes of carbonate and sulfate minerals. Major ions, such as Ca2+, Mg2+, SO4 2− and HCO3 −, are important components of TDS and TH. PC2 has positive loadings by Na+, Cl− and pH, and a negative loading by Ca2+, representing dissolution of halite and cation exchange between Na+ and Ca2+. PC3 represents the dissolution of K-containing minerals because it has a strong loading by K+. S-Fig. 3 illustrates individual sample scores generated by PCA. The water chemistry of W112 and W211 (plotted in the upper right of S-Fig. 3a) was predominately influenced by both PC1 and PC2, namely, dissolution/precipitation of carbonate and sulfate minerals, halite dissolution, and ion exchange. 5 out of 15 were influenced predominately by halite dissolution and ion exchange, and five were mainly influenced by carbonate and sulfate minerals dissolution/precipitation. The other three were not influenced significantly neither by PC1 nor PC2. The hydrochemistry of 60 % of the samples was influenced either by the dissolution/precipitation of carbonate and sulfate minerals or by dissolution of K-containing minerals, or by both (S-Fig. 3b). Overall, PCA reveals that water–rock interactions are the main factor influencing the major ion concentrations in the area.
Sources of Ions
The correlation analysis and the PCA have indicated that the dissolution/precipitation of carbonate and sulfate minerals, halite, and K-containing minerals are important sources of ions. In this section, some of these processes will be discussed in detail.
Dissolution/Precipitation of Carbonate Minerals and Gypsum
The dissolution of carbonate minerals such as calcite and dolomite is probably the most common weathering reaction. Theoretically, the dissolution of calcite will introduce Ca2+ and HCO3 − into groundwater at a ratio from 1:1 to 1:2, depending on the amount of atmospheric CO2 involved in the reactions (Han et al. 2012; Li et al. 2012). All the samples are plotted below the 1:2 line (S-Fig. 4a), suggesting that the dissolution of calcite is not the sole source of Ca2+ and HCO3 −. Most samples are located between the 1:1 and 1:2 lines (S-Fig. 4b), which indicates the dissolution of calcite and dolomite are important factors in explaining the sources of Ca2+ and Mg2+.
Dissolution of gypsum can contribute equal moles of Ca2+ and SO4 2− in groundwater. The plot of Ca2+ against SO4 2− shows that most of the groundwater samples are located above the 1:1 line (S-Fig. 4c), indicating that the dissolution of gypsum is not the sole source of Ca2+. Additional Ca2+ results from the dissolution of carbonate minerals.
Weathering of Na-Containing Minerals
Weathering of Na-containing minerals is responsible for high concentration of Na+ (Marghade et al. 2012). The dissolution of halite is one of the most common sources of Na+ and Cl−. Theoretically, the dissolution of halite will release equal amounts of Na+ and Cl− into the solution. Nevertheless, all samples deviate from the theoretical 1:1 line (S-Fig. 4d). Greater Na/Cl ratios may be attributed to the dissolution of other Na-containing minerals and cation exchange. The dissolution of Na-silicate minerals such as albite (NaAlSi3O8) can introduce Na+ into groundwater, leading to the deviation of sample points from the expected 1:1 line (Eq. 1). Cation exchange between Ca2+ or Mg2+ and Na+ can also explain the high Na+ concentration in the groundwater, as discussed later.
Due to the short contact time with the aquifer minerals and/or insufficient mineral sources, groundwater in recharge areas or up-gradient areas of a regional flow system is usually unsaturated with calcite, dolomite, and gypsum, while groundwater in recharge areas or down-gradient areas may become saturated with these minerals (Han et al. 2012). Saturation indices (SI) with respect to calcite, dolomite, and gypsum were calculated using the following equation (Li et al. 2010b):
where IAP is the relevant ion activity product in a mineral dissolution reaction, which can be obtained by multiplying the ion activity coefficient γ i and the composition concentration m i , and K is the equilibrium constant of mineral dissolution at the sample temperature. The saturation index of gypsum varies from −1.878 to −1.187 (S-Fig. 5a), suggesting that the groundwater would tend to dissolve gypsum presenting in the aquifer. The SI of calcite and dolomite range from −0.035 to 1.165 and from −0.560 to 1.139, respectively, indicating that the groundwater is saturated to oversaturated with the two major carbonate minerals (S-Figs. 5b and c).
Dissolution/precipitation of gypsum, calcite, and/or dolomite is also implied through the quadratic proportional relationships in the plots of (Ca2+ + SO4 2−) and (Ca2+ + HCO3 −) against the SI of waters with respect to the referred minerals (Dassi 2011). S-Fig. 5 shows that with an increased SI, Ca2+ + SO4 2− and Ca2+ + HCO3 − increase as a quadratic style, indicating that the dissolution of these minerals are a feasible source of Ca2+, SO4 2−, and HCO3 −. Thus, it is believed that the groundwater in the study area is mainly recharged by lateral inflow. However, local recharge is also observed in the study area, especially in the Abuhai Valley where seasonal surface water is available. Local recharge water gets mixed with shallow groundwater, resulting in variations of major ions (Lee 1981). Saturation of calcite and dolomite can be achieved quickly due to the abundance of carbonate minerals in the aquifer.
Cation Exchange
Cation exchange is a process that commonly modifies the major ion chemistry of groundwater (Xiao et al. 2012). It is of great significance in the evolution of hydrochemical compositions (Li et al. 2011b). The negative Pearson correlation coefficient between Na+ and Ca2+ (Table 3) may be explained by cation exchanges between Na+ and Ca2+ or Mg2+, where the Ca2+ or Mg2+ have exchanged places in clays with, in this case, Na+.
The ion exchange can be studied through chloro-alkaline indices proposed by Schoeller (Li et al. 2012; Marghade et al. 2012). The Schoeller indices, such as CAI-I and CAI-II are calculated by the following formulas:
where all ions are expressed in meq/L. If negative values for the Schoeller indices are obtained, Ca2+ and Mg2+ have been removed from solution, and Na+ and/or K+ have taken their place. If the indices are positive, then the inverse reactions have taken place. In the present study, all samples had negative Schoeller index values (Table 5).
Groundwater Chemistry Formation Mechanisms
Gibbs (1970) proposed two diagrams to assess the functional sources of dissolved chemical constituents. The diagrams represent the weight ratios of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3 −) as a function of TDS, and the mechanisms include precipitation dominance, rock dominance, and evaporation dominance (Gibbs 1970). These diagrams have been widely referred and used in hydrogeochemical studies (Marghade et al. 2012; Naseem et al. 2010; Subba Rao 2002, 2006). In the present study, the distribution of samples in Gibbs diagrams (Fig. 6) shows that chemical weathering of rock-forming minerals is the main causative factor in the evolution of the chemical compositions of groundwater in the study area. The spreading of the weight ratios of cations Na+/(Na+ + Ca2+) from low to high without great variation of TDS suggests that cation exchange also influences the rock dominance by increasing Na+ and decreasing Ca2+. During the cation exchange process, 1 mmol/L of Ca2+ in the water will be exchanged by 2 mmol/L of Na+. This process does not change the TDS content significantly because the weight of 1 mmol/L of Ca2+ (40 mg/L) is approximately equal to that of 2 mmol/L of Na+ (23 × 2 = 46 mg/L).
Water Quality Assessment
Water Quality for Agricultural Purpose
Shallow groundwater is an important source for agricultural use in the area because surface water is limited. It is mostly extracted manually or with low power water pumps for irrigating vegetables and corns in the gardens. The sodium adsorption ratio (SAR) and soluble sodium percentage (%Na) are universally used to assess the sodium hazard, and are calculated by:
where all the ionic concentrations are expressed in meq/L. The SAR values of groundwater samples range from 0.55 to 3.14 and the highest %Na is 56.3 %, suggesting a low sodium hazard and its suitability for agricultural use. Wilcox (1948) and the US Salinity Laboratory Staff (USSL 1954), considering alkalinity and salinity simultaneously, proposed irrigational specifications for evaluating the suitability of water for irrigation (Singh et al. 2010). The Wilcox diagram (S-Fig. 6a) shows that most samples plotted in the zone of excellent to good and only a few samples plotted in the zone of good to permissible, suggesting general suitability of the groundwater for agricultural uses. The USSL diagram relates SAR and EC (S-Fig. 6b). Most samples are plotted in C2S1 and a few in C3S1, suggesting good to acceptable quality for irrigation. Thus, the groundwater in the study area is fit for agricultural use.
Drinking Water Quality
The shallow groundwater in the area is also used in households. The major ions or parameters affecting water quality such as pH, Na+, Cl−, SO4 2−, TDS, and TH were compared with China’s permissible limits for Drinking Water (Ministry of Health of the PRC and Standardization Administration of the PRC 2006) and WHO standards (WHO 2011). The comparison (Table 2) shows that the parameters are within the acceptable limits of both, which indicates that the water quality, with regard to the selected parameters, is suitable for drinking.
Conclusions
Shallow groundwater is an important source for various uses in the Dongsheng Coalfield. This paper investigated the major ion chemistry of shallow groundwater in the area with various methods such as correlation analysis, principal component analysis, saturation index and tri-linear diagrams. The water quality for agricultural and drinking purposes was also assessed. The following conclusions can be reached.
-
The concentrations of major cations and anions were low in the shallow groundwater in the area, indicating good water quality. The concentrations of major chemical parameters were relatively higher around Jiushenggong than in other areas. The hydrochemical facies of the shallow groundwater in the study area are dominantly HCO3-Ca, HCO3-Na, and mixed HCO3-Ca·Na·Mg types.
-
The shallow groundwater in the study area is controlled by chemical weathering of rock-forming minerals. This is confirmed by the results of correlation analysis, principal component analysis, and saturation index. The main hydrogeochemical processes include the dissolution/precipitation of halite, gypsum, calcite, and dolomite. Cation exchange and dissolution of other minerals, such as albite, also took place. Mixing with local recharge water can also explain some of the observed variation of ions.
-
The shallow groundwater quality in the study area, revealed by SAR, %Na, Wilcox, and USSL diagrams, is generally suitable for agricultural use, with low alkalinity but medium to high salinity. The concentrations of the main chemical parameters suggest that groundwater in the study area is fit for human consumption.
References
Agartan E, Yazicigil H (2012) Assessment of water supply impacts for a mine site in western Turkey. Mine Water Environ 31:112–128. doi:10.1007/s10230-011-0167-z
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington
Bureau of Quality and Technical Supervision of China (1994) National standard of the People’s Republic of China: quality standard for groundwater, GB/T 14848-93 [in Chinese]
Chebotarev II (1955) Metamorphism of natural waters in the crust of weathering. Geochim Cosmochim Acta 8(1–2):22–48. doi:10.1016/0016-7037(55)90015-6
Dai SF, Ren DY, Chou CL, Li SS, Jiang YF (2006) Mineralogy and geochemistry of the no. 6 coal (Pennsylvanian) in the Junger Coalfield, Ordos Basin, China. Int J Coal Geol 66:253–270. doi:10.1016/j.coal.2005.08.003
Dassi L (2011) Investigation by multivariate analysis of groundwater composition in a multilayer aquifer system from North Africa: a multi-tracer approach. Appl Geochem 26:1386–1398. doi:10.1016/j.apgeochem.2011.05.012
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090. doi:10.1126/science.170.3962.1088
Han Y, Wang GC, Cravotta CA III, Hu WY, Bian YY, Zhang ZW, Liu YY (2012) Hydrogeochemical evolution of Ordovician limestone groundwater in Yanzhou, North China. Hydrol Process. doi:10.1002/hyp.9297
Hou GC, Zhang MS, Liu F, Wang YH, Liang YP, Tao ZP, Zhao ZH, Hu FS, Su XS, Lyu Y, Xie Y, Wang WK, Wang XY, Yang YY, Lu Q, Jiao YQ, Yang XC, Cui XD, Ma JL, Yin LH (2008) Groundwater investigation in ordos basin. Geologic Publishing House, Beijing [in Chinese]
Jolliffe IT (2002) Principal component analysis. Springer, New York City
Kaufman L (1990) Finding groups in data: an introduction to cluster analysis. Wiley, New York City
Lee RW (1981) Geochemistry of water in the Fort Union Formation of the Northern Powder River Basin, Southeastern Montana. USGS Water-Supply Paper 2076. US Government Printing Office, Washington
Li PY, Wu JH, Qian H (2010a) Groundwater quality assessment and the forming mechanism of the hydrochemistry in Dongsheng Coalfield of Inner Mongolia. J Water Resour Water Eng 21(1):38–41 [in Chinese]
Li PY, Qian H, Wu JH, Ding J (2010b) Geochemical modeling of groundwater in southern plain area of Pengyang County, Ningxia, China. Water Sci Eng 3(3):282–291. doi:10.3882/j.issn.1674-2370.2010.03.004
Li PY, Qian H, Wu JH (2011a) Application of set pair analysis method based on entropy weight in groundwater quality assessment—a case study in Dongsheng City, Northwest China. E-J Chem 8(2):851–858. doi:10.1155/2011/879683
Li PY, Qian H, Wu JH (2011b) Hydrochemical formation mechanisms and quality assessment of groundwater with improved TOPSIS method in Pengyang County Northwest China. E-J Chem 8(3):1164–1173. doi:10.1155/2011/251918
Li PY, Wu JH, Qian H (2012) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci. doi:10.1007/s12665-012-2049-5
Lucas L, Jauzein M (2008) Use of principal component analysis to profile temporal and spatial variations of chlorinated solvent concentration in groundwater. Environ Pollut 151:205–212. doi:10.1016/j.envpol.2007.01.054
Marghade D, Malpe DB, Zade AB (2012) Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ Monit Assess 184:2405–2418. doi:10.1007/s10661-011-2126-3
Menció A, Mas-Pla J (2008) Assessment by multivariate analysis of groundwater–surface water interactions in urbanized Mediterranean streams. J Hydrol 352:355–366. doi:10.1016/j.jhydrol.2008.01.014
Ministry of Health of the PRC and Standardization Administration of the PRC (2006) Standards for drinking water quality (GB 5749–2006). China Standard Press, Beijing [in Chinese]
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78:1313–1321. doi:10.1016/j.chemosphere.2010.01.010
Niu CC, Wang Q, Wen XY, Guo Y, Zhagn PL, Zhu RY, He XH (2011) Application of principal component analysis to evaluation of black soil degradation in Jilin. Global Geol 14(1):54–58. doi:10.3969/j.issn.1673-9736.2011.01.07
Piper AM (1953) A graphic procedure in the geochemical interpretation of water analysis. USGS, Washington
PRC Ministry of Environmental Protection (2011) Technical guidelines for environment impact assessment-groundwater environment. China Environmental Science Press, Beijing
Qian H, Li PY (2011) Hydrochemical characteristics of groundwater in Yinchuan Plain and their control factors. Asian J Chem 23(7):2927–2938
RockWare Inc. (2004) A user’s guide to RockWare® Aq·QA®, version 1.1. RockWare Inc., Golden
Singh SK, Mahato MK, Neogi B, Singh KK (2010) Quality assessment of mine water in the Raniganj coalfield area, India. Mine Water Environ 29:248–262. doi:10.1007/s10230-010-0108-2
SPSS Inc. (2004) SPSS base 13.0 user’s guide. SPSS Inc., New York City
Subba Rao N (2002) Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41(5):552–562. doi:10.1007/s002540100431
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49(3):413–429. doi:10.1007/s00254-005-0089-9
Tóth J (1963) A theoretical analysis of groundwater flow in small drainage basin. J Geophys Res 68(16):4795–4812
United States Salinity Laboratory (USSL) (1954) Diagnosis and improvement of saline and alkali soils. US Dept of Agriculture (USDA), Agriculture Handbook 60, Washington, pp 69–81
Wilcox LV (1948) The quality of water for irrigation use. US Dept of Agriculture Tech Bull 1962, Washington
World Health Organization (WHO) (2011) Guidelines for drinking-water quality, 4th edn. http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf. Accessed 24 Nov 2012
Xiao J, Jin ZD, Zhang F, Wang J (2012) Solute geochemistry and its sources of the groundwaters in the Qinghai Lake catchment, NW China. J Asian Earth Sci 52:21–30. doi:10.1016/j.jseaes.2012.02.006
Acknowledgments
The research was supported by the Doctor Postgraduate Technical Project of Chang’an University (CHD2011ZY025 and CHD2011ZY022), the Special Fund for Basic Scientific Research of Central Colleges (CHD2011ZY020 and CHD2011TD003), the National Natural Science Foundation of China (41172212, 51009009, and 41130753), and the special Funds for Scientific Research on Public Interest of the Ministry of Water Resources (201301084). Special thanks to Dr. Huang Wenfeng from Dalian University of Technology for his assistance in the field investigation. Sincere thanks to Dr. Charles A. Cravotta III, the associate editor of Mine Water and the Environment, for his editing of our manuscript, and Dr. Roger Lee, Dr. Wang Guangcai, and an anonymous reviewer for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

10230_2013_234_MOESM1_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM2_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM3_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM4_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM5_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)

10230_2013_234_MOESM6_ESM.jpg
Correlation coefficients of growth indices with precipitation and temperature data corresponding to August (Year prior to growth) to December (Year of growth) period, for Prades (a) and Arcalís (b). Climate and growth indices data are from the period 1952 to 2008. Asterisks indicate significant relationships (p < 0.05) (JPEG 1.15 MB)
Rights and permissions
About this article
Cite this article
Li, P., Qian, H., Wu, J. et al. Major Ion Chemistry of Shallow Groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ 32, 195–206 (2013). https://doi.org/10.1007/s10230-013-0234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-013-0234-8