Abstract
In Algeria, groundwater is an important source of irrigation. In order to evaluate the quality of groundwater in study area, 24 groundwater samples were collected and analyzed. Physical and chemical major parameters of groundwater such as electrical conductivity (EC), pH, total dissolved solids, Na, K, Ca, Mg, Cl, \(\hbox {HCO}_{3}\), \(\hbox {SO}_{4}\), and \(\hbox {NO}_{3}\) were determined. The important constituents that influence the water quality for irrigation such as sodium percentage, sodium adsorption ratio, soluble sodium percentage, and residual sodium bi-carbonate, and permeability index, Magnesium adsorption ratio, and Kelly’s ratio were calculated. Assessment of water samples from various methods indicated that groundwater in south sector is chemically suitable for agricultural uses, but the groundwater in the coastal sector is unsuitable for irrigation. The highest values of groundwater samples (EC, Cl, and Na) were consistently recorded in the coastal area. This shows that the mineralization of the groundwater is dominated by these two ions (sodium chloride facies). We know that the main source of these ions is likely the sea. The overexploitation of this aquifer without any awareness by farmers for irrigation crops was caused salinization of the portion of the aquifer closest to the coast and made the water unsuitable for the desired uses of either drinking water supply or crop irrigation. It will badly affect crop yield and reduces land fertility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an important source of water for domestic, agricultural, and industrial purposes. Assessing its quantity and quality is most important for sustainable development of regions. Its exploitation and utilization have become important and its use in agriculture is increasing day by day. Monitoring water quality by determining the levels of contaminants in the groundwater has direct relevance to human health. The quality of groundwater is the resultant of all the processes and reactions that have acted on the water from the moment it condensed in the atmosphere to the time it is discharged by a well. Hence, it varies from place to place, seasonally with the depth of water table and dissolved solids present in it (Ben Moussa et al. 2011). The majorities of groundwater quality problems are caused by contamination, overexploitation, or combination of the two and making them difficult to detect and hard to resolve. The solutions are usually very expensive, time consuming, not always effective, and somewhat hit and miss by nature (Appelo and Postma 2005; Mondal et al. 2011; Suribabu et al. 2012). Recent years have witnessed a growing awareness of the need for increased understanding of the various effects which man’s activities have upon the natural environment. Today an excessive increase in salinity of coastal groundwater aquifers has been observed in many locations along the coast of Algeria. The aquifers are in geological continuity with the sea water. Agricultural land development in the coastal area can introduce salinity problems. In the north of Algeria, the alluvial aquifers are generally vulnerable to seawater intrusion and domestic and agricultural pollution. In this study, physical and hydrochemical data from the groundwater will be used to determine the main factors and mechanisms controlling the chemistry of groundwater in the area. The groundwater quality refers to the characteristics of a water source, which will influence its suitability for a specific use. The evaluation of water quality is related to soil problems, which is interrelated to salinity, toxicity, water infiltration rate, and other miscellaneous problems. This study assessed the quality of groundwater in Nador coastal aquifer with respect to domestic and agricultural purposes
Climatological, Geological, and Hydrogeological Settings
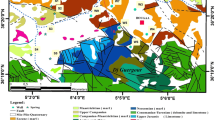
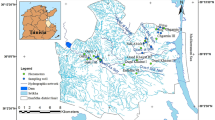
The climate of the study area can be classified as semi-arid and is characterized by hot summers and mild winters. Temperatures commonly exceed \(25\,^{\circ }\hbox {C}\) during June, July, and August and fall below \(10\,^{\circ }\hbox {C}\) for a few days in the winter months. The average annual temperature at Nador plain is about \(18\,^{\circ }\hbox {C}\). The long-term (1988–2012) average annual precipitation at the region is 540.4 mm. During the winter, the rainfall is more uniformly distributed than the rest of the year. When the evaporation is the most significant water loss from the catchment area of the region. The Nador plain belongs to a great littoral unit called the Sahel of Algiers. It is located at 75 km approximately in the West of Algiers. This study area is limited to North by the Mediterranean Sea, the South, and the East by the slopes of the Sahel, and to the West by the massif of Chenoua (Fig. 1). The area is situated between the latitudes \(36^{\circ }33', 36^{\circ }36'\) and the longitudes \(2^{\circ }21', 2^{\circ }27'\). This plain borrows a synclinal of NE–SW orientation whose main axis is occupied by the Wadi Nador, which gave its name to study area.
The outcropping formations of the study area are represented by the pliocene and the quaternary series (Ayme and Flandrin 1965).
-
The Pliocene is represented by Plaisancian clayey marls overlying Astian limestones and sandstones. Of a great thickness and a very low permeability, the clayed marl forms the overlying substratum of the aquifer. This is characterized by high permeability values reaching \(6,83.10^{-4}\hbox { m}/\hbox {s}\), a saturated thickness of 55 m and a transmissivity value of about \(2.10^{-2}\hbox { m}^{2}/\hbox {s}\). All these characteristics make aquifer astien, the most important water tank and more solicited on all area study.
-
The quaternary formations are visible with the center of the plain with a thickness which can reach 30 m. They rest in discordance on the astien calcareous sandstone and are formed by sands, clay, and gravel and conglomerate. These deposits have an important permeability of interstices (about \(10^{-3}\hbox { m}/\hbox {s}\)) and a transmissivity of about \(10^{-2}\hbox { m}^{2}/\hbox {s}\). They are surmounted by argillaceous grounds far from permeable.
Materials and Methods
The hydrogeochemistry of the Nador aquifer has been considered in terms of the major ionic constituents \(\hbox {Ca}^{2+}\), \(\hbox {Mg}^{2+}\), \(\hbox {Na}^{+}\), \(\hbox {K}^{+}\), \(\hbox {Cl}^{-}\), \(\hbox {SO}_{4}^{2-}\), \(\hbox {HCO}_{3}^{-}\), \(\hbox {NO}_{3}^{-}\) and of the physical parameters (pH, EC, and temperature). The physico-chemical parameters have been collected from 24 monitoring wells tapping the aquifer in April during the year 2012. Samples from all wells were collected in 500-mL polyethylene bottles, following the standard guidelines (Schoenleber 2005). Total dissolved solids (TDS), electrical conductivity (EC), and pH were analyzed in situ using a multi-parameter measurement WTW 3410 IDS. Major ions like chlorides, bicarbonates were analyzed using volumetric methods. Calcium, magnesium, sodium, and potassium were analyzed using atomic adsorption spectrometry. Nitrates were measured by a colorimetric method. Sulfates were estimated by the UV–visible spectrophotometer. The ion-balance-error computation, taking the relationship between the total cations and the total anions for each water sample, is observed to be within the range of acceptability (\(\pm 5\,\%\)) used in most laboratories (APHA 2005). The analysis of depths of the wells used in this study revealed that all of the measurement points are in the same hydrogeological context, with depths ranging from 8 to 40 m. The analytical methods used are summarized in Table 1.
Results and Discussion
Piezometric Water Level Fluctuation
The analysis of the piezometric map related to the period of high water (April 2012) had allowed characterizing the flow directions of the water table and the evolution of the hydraulic gradient of the aquifer (Fig. 2). The map shows that the lines of groundwater flows converge toward the axis of the synclinal furrow, which constitutes the principal drainage axis of the Plio-quaternary aquifer. This map also indicates the existence of the piezometric water level ‘0 m’ inside the plain. This is explained by a reversal in the direction of the water table flow. This change of direction is related to groundwater overexploitation from pumping near the sea, which generates an important mixture between marine water and groundwater. These mixtures are a consequence of groundwater salinization, in which the degree depends on the effective precipitation rate and pumping.
Water Quality Evaluation for Irrigation Purpose
The major ion chemistry of groundwater from Nador area was analyzed and the suitability of the groundwater for irrigation purpose was discussed by the following basic criteria. The analytical results have been evaluated to ascertain the suitability of groundwater of the study area for agricultural uses. Salinity and indices such as, sodium percentage (Na %), sodium absorption ratio (SAR), soluble sodium percentage (SSP), residual sodium bi-carbonate (RSBC), and permeability index (PI) are important parameters for determining the suitability of groundwater for agricultural uses. All the ions are expressed in (meq/l).
Electrical Conductivity (EC) The most influential water quality guideline on crop productivity is the water salinity hazard as measured by electrical conductivity (Ahmed et al. 2002). Water used for irrigation can vary greatly in quality depending upon type and quantity of dissolved salts. The primary effect of high EC water on crop productivity is the inability of plant to compete with ions in the soil solution for water leading to physiological drought. The higher the EC, the lesser the water available to plants, even though the soil may appear wet. Since plants can only transpire “pure” water, usable plant water in the soil solution decreases dramatically as EC increases (Kumar et al. 2014). The results of the geochemical analysis of groundwater in avril 2012 are given in Table 2. The pH values are in the range of 6.7–7.7 indicating groundwater of the study area is slightly acidic to alkaline in nature. The EC value of the samples varies from 1,510 to \(5,500\,\upmu \hbox {S}/\hbox {cm}\) in the study area. The TDS value varies from 1,035 to 3,638 mg/L. These results indicate that 75 % of groundwater samples are of high salinity (\(1,510 < \hbox {EC }< 2,250\,\upmu \hbox {S}/\hbox {cm}\)) and 75 % of samples are very high salinity water (\(5,500 >\hbox { EC }> 2,250\,\upmu \hbox {S}/\hbox {cm}\)).
Chlorides Certain ions are taken up with the soil-water and accumulate in the leaves during water transpiration to an extent that result in damage to plants. The magnitude of damage depends upon time, concentration, crop sensitivity, and crop water use and if damage is severe enough, crop yield is reduced. The usual toxic major ions in irrigation water are chloride and sodium. Chlorides is not absorbed or held back by soils, therefore, it moves readily with the soil-water, is taken up by the crops, moves in the transpiration stream and accumulates in the leaves. If the chloride concentration in the leaves exceeds the tolerance of the crop, injury symptoms develop such as leaf burn or drying of the leaf tissue, yellowing of leaf and spotting on the leaf. The limits for chloride toxicity for some fruit crop cultivars are given by Ayers and Westcot (1985). In the present study, 33.33 % of the water samples are unsuitable for irrigation with more than 20 meq/L (Tables 2, 3).
Sodium Percentage (% Na) The sodium percent (% Na) called also soluble sodium percentage (SSP) is obtained by the Equation:
Groundwater always contains measurable quantities of dissolved substances, which are called salts. The salts present in the water, besides affecting the growth of the plants directly, also affect the soil structure, permeability and aeration, which indirectly affect the plant growth.
Water classification according to Wilcox (1955)
The sodium percentage (% Na) in the study area varies from 23.47 to 64.04 (Tables 2, 3). As per the world health organization, 2011 standards, the sodium percentage of 60 is the maximum recommended limit for irrigation water (Fig. 3). The high sodium saturation in the water samples directly causes calcium deficiency in human beings. This according to Wilcox is good to unsuitable water quality for irrigation (58.34 % of the water samples are good to permissible, 8.33 % are doubtful to unsuitable and 33.33 % are unsuitable).
Sodium Adsorption Ratio (SAR) Sodium adsorption ratio (SAR) is calculated by the following equation:
Sodium adsorption ratio (SAR) is an important parameter for determining the suitability of groundwater for irrigation because it is a measure of alkali/sodium hazard to crops. A very low SAR (less than 2) indicates no danger from sodium; low SAR (2–12) indicates little danger from sodium; medium hazards are indicated between 12 and 22; high hazards are between 22 and 32, and very high hazards more than that. SAR can indicate the degree to which irrigation water tends to enter into cation-exchange reactions in soil. Sodium replacing adsorbed calcium and magnesium is a hazard as it causes damage to the soil structure owing to dispersion of the clay particles and becomes compact and impervious. The values of SAR in the groundwater samples of the study area ranges from 1.77 to 15.7 (Tables 2, 3), and according to the Richards classification based on SAR values and EC (Fig. 4), the groundwater of the study area falls under the categories of low and medium sodium hazards (66.67 %) for upstream sector, and of high and very high salinity for the coastal samples (33.33 %) which may be a danger from sodium hazard. High sodium water may produce harmful levels of exchangeable sodium in most soils and will require special soil management like good drainage, high leaching, and organic matter additions.
Water classification according to Richard (1954)
Residual Sodium Bi-Carbonate (RSBC) The concentration of carbonate and bicarbonate in water has a major effect on hardness and the alkalinity. The relative amounts of carbonate, bicarbonate, and carbonic acid in water are known to be related to the pH. Under normal water pH conditions (\(\hbox {pH }< 9\)), bicarbonate predominates. Bicarbonate concentrations in natural water range from less than 25 mg/L in areas of non-carbonate rocks to over 400 mg/L where carbonate rocks are present. Carbonate concentrations in surface and groundwater by contrast are usually low and nearly always less than 10 mg/L (Kumar et al. 2014). The RSBC is calculated as follows:
Richard has also determined the hazardous effect of carbonate and bicarbonate on water quality in terms of residual sodium carbonate or residual sodium bi-carbonate. The high bicarbonate contents in the water are due to the biological activities of plant roots; from the oxidation of organic matter included in the soils and in the rock, and from various chemical reactions. The groundwater samples have RSBC values between 4.0 and 0.01 meq/l (Tables 2, 3). All the samples were satisfactory (RSBC value \(< 5\) meq/l) according to criteria set by Gupta and Gupta (1987).
Permeability Index (PI) The permeability index (PI) is calculated according to the equation as
The soil permeability is affected by long-term use of irrigation water. It is influenced by sodium, calcium, magnesium, and bicarbonate contents of soil (Raju and Janardhana 2007). Doneen (1964) has evolved a formula, PI to measure the soil permeability for assessing the suitability of water for irrigation purposes. The PI values of groundwater samples range from 32.33 to 66.83 (Tables 2, 3). It revealed that 100 % of the groundwater samples fall in Class I. These waters are not likely to create any permeability problem.
Magnesium Adsorption Ratio (MAR) It is calculated as follows:
In natural waters, excess of magnesium in groundwater samples affects the quality of soils, which causes poor in yield of crops (Nagaraju et al. 2014; Joshi et al. 2009). The MAR of irrigation water is defined by Raghunath (1987). The MAR values exceeding 50 are considered harmful and unsuitable for irrigation use. In the analyzed groundwater samples, it is found between 22.94 and 74.9 (Tables 2, 3). In the present area, 41.67 % of the groundwater samples exceed value limit (MAR \(> 50\)).
Kelly’s Ratio The Kelly Ratio (KR) is calculated using the following equation:
Kelly (1940) have suggested that the sodium problem in irrigational water could very conveniently be worked out on the basis of the values of Kelley’s ratio. A Kelly’s ratio of more than one indicates excessive sodium in water. Therefore, water with a Kelly’s ratio less than one are suitable for irrigation, while those with a ratio more than one are unsuitable. The KR has been calculated for all the water samples of the study area. It can be suggested that the KR varies from 0.3 to 1.78. In the present study, 33.33 % of the water samples are unsuitable for irrigation with more than one of Kelly’s ratio (Tables 2, 3).
Hydrogeochemical Facies
The data obtained from the chemical analyses can be used to evaluate the geochemical processes and mechanisms in the aquifer system. Piper diagrams are one of the most useful ways of representing and comparing water quality. Piper plots are commonly used to evaluate the hydrogeochemical evolution of groundwaters. Based on the Piper trilinear plot (Fig. 5), Na–Cl and mixed Ca–Mg–Cl types waters are the most commonly found waters in the area, with 37.5 and 62.5 %, respectively. Low concentrations of chloride in groundwater normally originate from dilution via precipitation, while high chloride concentrations can have several reasons including dissolution of halite, intrusion of sea water, and influx of sewage or waste water (Sujatha and Reddy 2003). It should be noted that the Na–Cl-type groundwater samples are close to the coast; however, ion exchange occurs when this saline water comes into contact with a fresh groundwater system where the clays are dominated by Calcium. When seawater intrudes into a coastal fresh groundwater aquifer, the following exchange reaction takes place. Ca–Mg–Cl-mixed water is caused by the due to dissolution of carbonate minerals and replacement of \(\hbox {Ca}^{2+}\) and/or \(\hbox {Mg}^{2+}\) by \(\hbox {Na}^{+}\) through cation exchange processes.
Origin of Salts in Groundwater
To identify geochemical processes responsible for the chemical evolution observed in the groundwater of the aquifer, bivariate diagrams of major elements (\(\hbox {Na}^{+}\), \(\hbox {Mg}^{2+}\) and \(\hbox {Ca}^{2+})\) versus \(\hbox {Cl}^{-}\) values are used. These identify geochemical processes that contribute to the groundwater salinization in this aquifer. The state of saturation minerals that react in the system during the period of high water in 2012 was calculated from the analytical data sampling. The mineral saturation indices (SI) indicate the degree of saturation in a particular mineral phase compared to the aqueous solution with which it is in contact. Based on this SI value, the trend of precipitation or dilution of the mineral phases can be deduced. The simulation was performed using the thermodynamic software PHREEQC. The calculation of the saturation index was performed by the formula SI = [log (Q)] / [log (Ksp)]. The values of the saturation indices for halite, calcite, anhydrite, dolomite, and gypsum are collected in Table 4. The values were projected against the percentage of seawater calculated for each sample, and the line representing the theoretical SI for this percentage mixed freshwater–seawater was added. It is postulated that mineral phases that are undersaturated (SI \(\le - 0.1\)) will tend to dissolve, and mineral phases that are oversaturated (SI \(\ge 0.1\)) will precipitate these mineral phases out of solution. Equilibrium is taken to be between SI \(= -\)0.1 and SI \(=\) 0.1.
Hydrochemical correlations have been realized with the relationship of \(\hbox {Na}^{+}\), \(\hbox {Mg}^{2+}\) and \(\hbox {Ca}^{2+}\) versus \(\hbox {Cl}^{-}\) values. The relationship between \(\hbox {Na}^{+}\) and \(\hbox {Cl}^{-}\) shows a good correlation between these elements (Fig 6a), indicating probably the same origin. The plot of \(\hbox {Ca}^{2+}\) and \(\hbox {Mg}^{2+}\) versus \(\hbox {Cl}^{-}\) (Fig. 6b, c) shows a moderate correlation; these cations have the most distinctive distribution pattern in the mixing water sample and exhibit concentrations especially of \(\hbox {Ca}^{2+}\) that exceed the theoretical concentrations. This indicates the dissolution of the scalcite, anhydrite, dolomite, and gypsum present in the catchment area and dispersed in the aquifer. The positive correlations of \(\hbox {Na}^{+}\), \(\hbox {Mg}^{2+}\), and \(\hbox {Ca}^{2+}\) versus \(\hbox {Cl}^{-}\) indicate the contribution of these elements to the groundwater mineralisation in this aquifer. It also shows the effect of exchange between the groundwater and the seawater for the coastal wells, along with the interactions between the groundwater and the geological formations for the upstream wells.
a \(\hbox {Na}^{+}{-}\,\hbox {Cl}^{-}\) relationship (meq/L); b \(\hbox {Mg}^{2+}{-}\,\hbox {Cl}^{-}\) relationships (meq/L); c \(\hbox {Ca}^{2+}{-}\,\hbox {Cl}^{-}\) relationships (meq/L); d \(\Delta \hbox {Na}^{+},\,\Delta \hbox {Mg}^{2+}\) and \(\Delta \hbox {Ca}^{2+}\) relationships with \(\hbox {Cl}^{-}\); e SI of gypsum relationships with % seawater, f SI of halite relationships with % seawater; g SI of dolomite relationships with % seawater and h SI of calcite relationships with % seawater of the Nador aquifer samples (April 2012). SW seawater pole, FW freshwater pole
The ionic deltas of the major elements (\(\Delta \hbox {Na}^{+}\), \(\Delta \hbox {Mg}^{2+}\), \(\Delta \hbox {Ca}^{2+}\)) versus chloride increase in order of the concentration of chlorides, regardless of their geographical position (Fig. 6d). Generally, it is seen that \(\Delta \hbox {Mg}^{2+}\), \(\Delta \hbox {Ca}^{2+}\) are positive. However, the \(\Delta \hbox {Na}^{+}\) is negative. This indicates the depletion of \(\hbox {Na}^{+}\) and the enrichment of \(\hbox {Ca}^{2+}\) and \(\hbox {Mg}^{2+}\) essentially in the coastal samples. The loss of \(\hbox {Na}^{+}\) and the gain of \(\hbox {Ca}^{2+}\) and \(\hbox {Mg}^{2+}\) suggest a direct cation exchange usually observed in similar situations when the seawater is replacing freshwater. The figure also shows that the values of \(\Delta \hbox {Na}^{+}\) range between \(-\)4 and \(+\)1.5 mq/L, while the values of \(\Delta \hbox {Ca}^{2+}\) are positive, varying from 0 to \(+\)5 mq/L. However, the ionic delta of \(\Delta \hbox {Mg}^{2+}\) becomes more positive with the increase of the chloride ion, considerably more positive in the most salinized water, with values of \(\Delta \hbox {Mg}^{2+}\) exceeding 10 mq/L. This can be supported by processes of interaction between water and rock. That is, by a process of ion exchange between water salinized by the intrusion of seawater and the abundant layers of Plio-quaternary clay in the aquifer. (Cardona et al. 2004; Appelo and Postma 2005; Yaouti et al. 2009). The result also shows that this behavior is even more noticeable as the chloride ion content increases, which is more noticeable in the areas salinized by seawater intrusion. The gypsum and halite saturation indices show undersaturation. Indeed, this dissolution is confirmed by the negative saturation indices of groundwater with respect to gypsum and halite (Fig. 6e, f). These salts are then leached, under rainfall, toward the groundwater. The SI of dolomite and calcite shows oversaturation in these minerals, confirming that the \(\hbox {Ca}^{2+}\) and \(\hbox {Mg}^{2+}\) enrichment is due to cation exchange dependent on the residence time, and not due to carbonate dissolution (Fig. 6g, h). Thus, dolomite or calcite precipitation is likely to occur in the plio-quaternary aquifer. Another explanation for the increase in the SI of dolomite in coastal wells is probably the exchange between freshwater and seawater.
The seawater fraction in the groundwater is often estimated using chloride concentration, since this ion has been considered as a conservative tracer not affected by ion exchange (Custodio 1987). We used data from the analysis of 1974, which was conducted before the intrusion processes were initiated: the average chloride level was 160 mg/L and the concentration of seawater was 18,700 mg/L. For conservative mass balance of the mixture, the equation used is
where \(f\) is the mixing ratio expressed as percentage seawater; \(\hbox {Cl}_\textrm{{mix}}.\) is the Cl concentration in groundwater of the mixture; \(\hbox {Cl}_\textrm{{freshwater}}\) is the idem in fresh groundwater; \(\hbox {Cl}_\textrm{{seawater}}\) is the idem in seawater.
The resulting seawater fraction in all observation wells using this equation shows that the average mixing rate of seawater intrusion reached 12 % (Table 4), confirming the process of marine intrusion for each well in the aquifer. The highest value achieved, 12 %, decreased as we moved away from the coastal area, being less than 1 % in the upstream of Nador plain.
Conclusions
This paper presents the groundwater quality assessment carried out for the coastal area of Nador plain. In this area, the groundwater was evaluated for their chemical composition and suitability for irrigation on the basis of various guidelines. According to classification of water based on EC and Cl reveals that the groundwater in study area is doubtful or unsuitable for the irrigation in coastal sector (EC \(>\) 2250 \(\upmu \)S/cm and Cl \(>\) 20 mq/L) and the groundwater is permissible for the irrigation in upstream sector (EC \(<\) 2250 \(\upmu \)S/cm and Cl \(<\) 12 mq/L). Irrigation water classified based on SAR and sodium percentage has indicated that 12 % of samples belong to doubtful category; remaining samples belong to permissible or good category for irrigation purpose. The PI and RSBC indicate that this groundwater is not likely to create any permeability problem; hence continued use of water with low RSBC water has not affected on crop growing conditions. The Mg adsorption ratios and Kelly’s ratios indicate than more 33 % were unsuitable water for irrigation.
According to in the present study, it is evident that high salinity of groundwater persists at the coastal area near the sea for 8 wells. Hence, for high to very high salinity of waters, soil must be permeable with adequate drainage facilities for satisfactory crop growth. The results also show that the composition of groundwater is affected by seawater intrusion, especially in the north part. Na and Cl were the dominating cations and anions in wells near the coast, which is also evident from the Na–Cl facies observed with high EC in majority of the coastal samples. Therefore, recharge area (south sector) was characterized by low salinity; the groundwater can used without any problem.
References
Ahmed SS, Mazumdar QH, Jahan CS, Ahmed M, Islam S (2002) Hydrochemistry and classification of groundwater, Rajshahi city corporation area, Bangladesh. J Geol Soc India 60:411
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. A.A. Balkema Publishers, Amsterdam 0–41-536428-0
Ayers RS, Westcot DW (1985) Water quality for agriculture. FAO irrigation and drainage paper 29(Rev. 1):1–144
Ayme A, Flandrin J, (1965) Notice explicative de la carte géologique de Tipaza 1/50,000
Ben Moussa A, Zouari K, Marc V (2011) Hydrochemical and isotope evidence of groundwater salinization processes on the coastal plain of Hammamet-Nabeul, northeastern Tunisia. Phys Chem Earth 36(5–6):167–178
Cardona A, Carrillo-Rivera J, Huizar-Alvarez R, Garniel-Castro E (2004) Salinization in coastal aquifers of arid zones: an example from Santo Domingo, Baja California Sur, Mexico. Environ Geol 45:350–366
Custodio E (1987) Hydrogeochemistry and tracers. In: Custodio E (ed) Groundwater problems in coastal areas studies and reports in hydrology, vol 45. UNESCO, Paris
Doneen LD (1964) Notes on water quality in agriculture. Published as a Water Science and Engineering Paper 4001, Department of Water Science and Engineering, University of California
El Yaouti F, El Mandour A, Khattach D, Benavente J, Kaufmann O (2009) Salinization processes in the unconfined aquifer of Bou-Areg (NE Morocco). Appl Geochem 24:16–31
Gupta SK, Gupta IC (1987) Management of Saline Soils and Water. Oxford and IBH Publication Coy, New Delhi, p 399
Joshi DM, Kumar A, Agarwal N (2009) Assessment of the irrigation water quality of river Ganga in Haridwar District. Rasayan J Chem 2:285–292
Kelly WP (1940) Permissible composition and concentration of irrigated waters. In: Proceedings of the A.S.C.F., p. 607
Kumar R, Singh K, Singh B, Aulakh SS (2014) Mapping groundwater quality for irrigation in Punjab, North-West India, using geographical information system. Environ Earth Sci 71:147–161
Mondal NC, Singh VP, Singh S, Singh VS (2011) Hydrochemical characteristic of coastal aquifer from Tuticorin, Tamil Nadu, India. Environ Monit Assess 175:531–550
Nagaraju A, Sunil Kumar K, Thejaswi A (2014) Assessment of groundwater quality for irrigation: a case study from Bandalamottu lead mining area. Guntur District, Andhra Pradesh, South India, Appl Water Sci, 12p
Raghunath IIM (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi, pp 344–369
Raju N, Janardhana A (2007) Hydrogeochemical parameters for assessment of ground water quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Predesh, South India. Environ Geol 52:1067–1074
Richard LA (1954) Diagnosis and improvement of saline and alkali soils. Agric Handbook, vol 60. USDA, Washington D.C
Sathish S, Elango L, Rajesh R, Sarma VS (2011) Assessment of seawater mixing in a coastal aquifer by high resolution electrical resistivity tomography. Int J Environ Sci Technol 8:483–492
Schoenleber JR (2005) Field sampling procedures manual. Department of environmental protection, New Jersey, p 574
Sujatha D, Reddy BR (2003) Quality characterization of groundwater in the south-eastern part of the Ranga Reddy district, Andhra Pradesh, India. Environ Geol 44(5):579–586
Suribabu CR, Ravindar S, Ananadapadmanaban S, Seshadri S, Bhaskar J (2012) Tracing the evidence of sea water intrusion in the coastal aquifer of Adhirampattinam, India, using hydro-chemical analysis. J Hydraul Eng 18(2):90–100
Wilcox LV (1955) Classification and use of irrigation water. Agric circ 969. USDA, Washington D.C., p 19
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bouderbala, A. Assessment of Groundwater Quality and its Suitability for Agricultural Uses in the Nador Plain, North of Algeria. Water Qual Expo Health 7, 445–457 (2015). https://doi.org/10.1007/s12403-015-0160-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-015-0160-z