Abstract
A mixture of geothermal waters having high ionic concentration to cold water aquifers is caused to deterioration at irrigation and drinking water quality. The aim of this study is determine spreading and extending of pollution in cold groundwater with the contribution of geothermal waters in the Sandıklı (Afyonkarahisar) basin. Hydrochemical facies of the waters is Ca–Mg–HCO3 and Ca–Mg–HCO3–SO4 in cold waters and Na–Ca–SO4–HCO3 in the geothermal waters, respectively. Ca2+ and HCO3 − are dominant ions in cold waters, whereas those in geothermal waters are Na+ and SO4 2−. Shallow groundwaters have low ionic concentrations, while deep circulation waters have high ionic concentrations. The increases of T, EC, Na+, SO4 2−, Cl−, B, Al, As, Fe, Mn, Ni, F, U and NO3 − parameters were determined in the cold groundwaters as locally due to hydrothermal contribution, water–rock interaction and anthropogenic effects in the Sandıklı basin. As a related to groundwater usage, there are two different risks in the basin, human health risk (drinking water usage) and environmental risk (irrigation water usage). From the results of hazard index, it is verified that As is the main contaminant, and toxic human risk can be caused by ingestion of water in the basin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Turkey has extensive geothermal resources given its location in the central part of the Alpine-Himalayan Mountain Belt. The geothermal systems are located mainly along recent, regional structural lines and are more frequent in regions of recent tectonism and Tertiary volcanism and/or metamorphism (Serpen et al. 2010). The distribution of geothermal fields in Turkey closely follows the tectonic patterns. Extensive tectonic activity and formation of E–W grabens have formed the shape of Western Anatolia (Simsek 2003; Mutlu and Gulec 1998; Vengosh et al. 2002).
Geothermal fluids from high temperature reservoirs can include a range of ions (e.g. chlorides, sodium, potassium, calcium, fluoride, magnesium, silicate, iodine, antimony, strontium, bicarbonates) and, of most concern, several toxic chemicals: boron, lithium, arsenic, hydrogen sulphide, mercury, rubidium, and ammonia. However, the low-to moderate temperature geothermal fluids used in most direct-use applications generally contain low levels of chemicals and the discharge of spent geothermal fluids is seldom a major problem. Contamination of surface and subsurface waters with toxic heavy metals is the most severe environmental impact of geothermal waters. These contamination problems are mainly attributed to flawed well construction, faulty reinjection applications and uncontrolled discharge of waste geothermal fluids to surface waters. As a consequence, surface and subsurface waters become chemically and thermally polluted.
Mixing is a dominant process at all scales in hydrogeological systems, and should be acknowledged as an important process in the interpretation of results. Mixing can explain hydrogeochemical and isotopic variations observed at a range of spatial and temporal scales, but it can also confound interpretations (Afsin et al. 2013). Relatively few studies have attempted to quantify mixing between deep hydrothermal systems and public supply aquifers. In Ethiopia’s central Rift Valley Region, Reimann et al. (2003) documented that the deepest drinking water wells have elevated concentrations of As, B, F, Ge, I, Li. Mo, Na, Rb, Sb, Ta, U and W due to a hydrothermal component in the groundwater. Aksoy et al. (2009) investigated shallow groundwater contamination in the Balçova Geothermal system in Izmir, Turkey, and found that the cold groundwater in the alluvial aquifer mixes with hydrothermal fluids, resulting in elevated concentrations of As, Sb and B. Near the Akarcay Basin in Turkey, Dogdu and Bayari (2005) investigated the impact of thermal water on ground and surface waters, and found that mixing with hydrothermal fluids results in higher electrical conductivity, warmer temperatures, and elevated concentrations of Na, Cl, Fe, Li, B, Br, Mn, Al, I and As (Forrest et al. 2013).
In order to assess the potential negative impacts, the groundwater pollution mechanisms of the specific geothermal field must be determined in order to propose possible mitigation measures. Sampling the cold water aquifers in the vicinity of geothermal fields provides valuable data on how the geothermal fluid mixes with cold waters and to what extent it influences the general water quality pattern (Aksoy et al. 2009).
The Sandıklı basin is selected as investigation area due to existing hydrothermal pollution. In this study, physical, hydrogeological and hydrochemical data from the groundwater system has been integrated and used to determine effects of thermal contribution to cold aquifer system. The potential impacts of these contaminants on the quality of drinking and irrigation water resources are investigated and possible mitigation measures are discussed. The results obtained are believed to be applicable to many geothermal fields in the Anatolia, Turkey that have similar geological characteristics and contamination patterns.
Site description
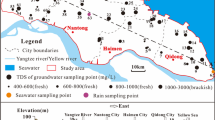
The Sandıklı basin is located in the Afyonkarahisar within the western Anatolian (Fig. 1). It is one of the largest agricultural areas in the inner Aegean region of Turkey. The basin is semi-closed basin due to discharge of groundwater and surface water to the Büyük Menderes River via Kestel creek. Groundwater is widely used for drinking, domestic and irrigation purposes in the basin. There are 1500 wells (shallow and deep) within the porous aquifer in the basin.
The Hüdai-Sandıklı geothermal field is located in the middle of the Sandıklı basin (Fig. 1). This field has 23 thermal productivity wells with a total discharge of 500 l/s, in addition has two re-injection wells with 780 m depth for geothermal waste waters. Temperature of thermal water and depth of thermal productivity wells are between 54–82 °C and 49–1053 m, respectively. The highest reservoir temperature of the geothermal waters is estimated as 107 °C for silica geothermometers. The overexploitation from the productive wells may have a negative effect on the sustainability of the geothermal waters in the future. The geothermal waters have been used for several purposes such as balneotherapy, heating and thermal tourism in recent years (Afsin et al. 2012, 2013).
Materials and methods
A total of 140 water samples were collected periodically for cold and thermal waters in four terms at wet and dry seasons in 2010/2012 years. All sample locations were situated with GPS equipment. Physical properties of the water samples such as pH, temperature (T; °C) and electrical conductivity (EC; μS/cm) were measured in situ with YSI Professional Plus handheld multiparameter instrument that were calibrated with standard solutions. Samples were collected in clean polyethylene bottles and dispatched for analysis to the laboratory in an ice-filled box. The samples for cation analysis were acidified (HNO3) to pH <2, while the samples for anion analysis were unacidified. Water chemistry analyses were carried out at the water chemistry laboratory at Hacettepe University (Ankara). The hydrochemical analyses were done according to APHA/AWWA/WPCF (1995) standards. Major cations (Ca2+, Mg2+, Na+ and K+) and heavy metals were analyzed by atomic absorption spectroscopy (AAS). SO4 2− was measured spectrophotometrically. Alkalinity was measured by standard titrimetry. Cl− was analyzed using an AgNO3 titrimetric method. Spectrophotometer (HCAH DR 2000) was used for determination of nitrate (NO3) concentrations. The isotope samples (δ18O, δ2H, 3H) were collected in double-capped 500 ml polyethylene bottles. 3H was analyzed by liquid scintillation at the environmental tritium laboratory at Hacettepe University (Ankara).
An ionic error balance was computed for each chemical sample and used as a basis for checking analytical results. In accordance with international standards, results with ionic balance error >5 % were rejected (Appelo and Postma 1993). Charge balances (CB) were calculated using Eq. (1):
where z is the ionic charge of cation (c) or anion (a) and M molar concentration of major solutes.
Results and interpretation
Geological and hydrogeological setting
Paleozoic, Mesozoic and Cenozoic aged rocks outcropped in the research area (Fig. 1). Sedimentary and volcanic rocks are located on the metamorphic base. Quaternary units are covered all of the units as discordant. Precambrian aged Kestel formation which is composed of metamorphic rocks is foundation unit of the research area. The metamorphic foundation is composed of quartz, sericite schist, albite, quartzite, calc-schist, phyllite and metabasalt. Paleozoic aged units are Kestel, Hüdai, Çaltepe and Seydişehir formations in the area. Hüdai formation is composed of quartzite and schist units, and it situated on the Kestel formation. Çaltepe formation is consists of dolomite and limestone. Seydişehir formation is composed of interbedded sandstone, siltstone, shale, quartz and is compatible with Çaltepe formation.
Mesozoic aged units are Karatepe, Derealanı and Akdağ formations in the basin. Permo-Triassic aged Karatepe formation which is composed of sandstone, conglomerate and siltstone is discordant with the Seydişehir formation. Jurassic aged Derealanı formation which is composed of sandstone, siltstone, claystone and clayey limestone is harmonic on the Karatepe formation. Akdağ formation which is consists of limestone, reef limestone and sandy limestone is also harmonic the Derealanı formation.
Cenozoic aged units are Bozoğlan formation, Oligocene conglomerate, Sandıklı lavas, Soğucak pyroclastic, Hamamçay formation, alluvium and travertine. Paleocene aged Bozoğlan formation which is consists of marly limestone, conglomerate, sandstone, siltstone and shale is crop out on the Akdağ formation. Oligocene conglomerate is situated on the Bozoğlan formation. The Sandıklı Lava is composed of andesite, trachyandesite and basalt. The Soğucak pyroclastic is also composed of tuff, tuffite and agglomerate. Miocene aged Sandıklı Lava is crop out as discordant on the Oligocene conglomerate formation. Pliocene aged Hamamçay formation is harmony on the Sandıklı Lava. The Hamamçay formation is intercalated gravel, clay, sand, silt, sandstone, claystone and loosely attached conglomerate. The youngest units are Quaternary aged travertine and alluvium. Alluvium which is composed of uncemented clay, sand, silt and gravel levels overlie above another units (Afsin 1991; Aksever 2011; Cakmakoglu 1986; Öngür 1973; Fig. 1).
The Seydişehir, Derealanı and Kestel formations are impermeable units. The Oligosen conglomerate, Sandıklı lavas, Bozoğlan formations, Soğucak pyroclastic are semipermeable. The Akdağ and Çaltepe formations are permeable and karstic aquifers. Quaternary alluvium and Pliocene aged Hamamçay units are important porous aquifers. The alluvium which is consists of sand, gravel, clay and silt levels and the Hamamçay unit which is composed of conglomerate having loose tissue and sand levels have a good aquifer character. The alluvium is covered an area of 174 km2 and the Hamamçay formation is also covered 366 km2. The Hamamçay formation is locally transitive and below of the alluvium. The well logs indicate that the thickness of the Quaternary and Pliocene units is between 200 and 300 m. The thickness of these units is lower in the Nuh, Serban and Akharım villages which is located northeast of the basin (Aksever 2011). The Hamamçay formation and alluvium units form unconfined and confined aquifers under suitable hydraulic conditions. Here, an unconfined aquifer has been considered. The unconfined aquifer system in the research area suggests an open system. This situation affects the groundwater flow system and residence time (Afsin 1991). The unconfined alluvial aquifer is extremely permeable and transmits significant amounts of water. The average annual yield of wells which are drilled on the alluvium and Hamamçay formation are between 4.78–51.1 and 7.44–50.46 l/s, respectively. The groundwater drawdown is 2.43–15.00 m approximately according to groundwater level measurements for three periods (2007, 2008 and 2009 year) in the study area (Aksever 2011).
Hüdai geothermal field is located in middle of the Sandıklı basin (Fig. 2). Thermal waters emerge in relation to the faults in the Hüdai geothermal field. The primary aquifers for the geothermal waters in the region are quartzite, dolomite and limestones (Akdağ formation). Seydişehir, Derealanı and Sandıklı formations which have low hydraulic conductivity and impermeable units are cap rocks. Heat sources of the geothermal waters may be suggested as geothermal gradient, volcanism and deep-sealed plutonic rocks (Afsin et al. 2012, 2013).
Hydrogeochemistry
In this study, groundwater samples were taken from 33 locations for cold water and from eight locations for thermal water at four periods (October-2010, May-2011, November-2011, May-2012). The values of pH and electrical conductivity (EC) are changed between 6.29 and 8.58 and 172 and 2193 µS/cm in cold groundwaters; whereas pH and EC are changed between 6.38 and 7.47 and 2642 and 5152 µS/cm in thermal waters, respectively (Table 1). EC value of water is directly related to the concentration of dissolved solids in the water. High EC value is (2193 µS/cm) measured at the Koçhisar well (sample 10; Fig. 3) which has 30 m depth and is located in the farm house. The temperatures of cold and thermal waters vary from 10.15 to 19.8 °C and from 56.70 to 82 °C, respectively. Temperature of cold waters increased in four locations (between 20.8 and 25.10 °C). These increases are also related to interference of deep thermal water with shallow cold water.
The ion content of groundwaters varies during the circulation due to contact with rocks. Therefore, the availability of these ions present in aquifers becomes a useful tool in the identification of geochemical processes (Nur et al. 2012). The interplay of precipitation and dissolution processes serves as an important determining factor of the overall chemical character of the groundwater coming from the unconfined aquifer. The major ion contents of groundwater in the research area are lower than dry period during the rainy period. Groundwaters which have the same reservoir, recharge area and origin showed similar peak values in the semi-logarithmic Schoeller diagrams (Fig. 4). But ion-abundance sequences of some groundwaters are different. For example, the HCO3 − and SO4 2− contents of thermal waters differs in rainy periods (October 2010 and November 2011) due to mixture/dilution processes with shallow waters in the groundwater flow systems.
Sodium concentrations in cold groundwater vary from 3.07 to 103.96 mg/l and from 2.69 to 76.84 mg/l in dry and wet seasons, respectively. The Na+ contents of thermal waters were determined as a range 211.96–291.36 mg/l in dry seasons and 205.08–288.29 mg/l in wet seasons. The Na+ content of cold groundwaters probably comes from the decomposition of albite within the volcanic rocks in the northeast of the Sandıklı basin and ion exchange processes between Na+ and Ca2+ ions. In the thermal waters, the origin of the Na+ probably related to sodic plagioclase-rich andesitic magma (Afsin and Canik 1996). K+ concentrations in cold groundwater vary from 0.64 to 13.25 mg/l and from 0.80 to 36.42 mg/l in dry and wet seasons, respectively. The K+ contents of thermal waters also vary from 33.28 to 47.35 mg/l and from 31.58 to 43.35 mg/l in dry and wet seasons, respectively.
The Ca2+ contents of cold waters were determined as a range 21.28–303.71 mg/l in dry seasons and 16.47–388.57 mg/l in wet seasons. The Ca2+ contents of thermal waters were determined as a range 165.25–182.45 mg/l in dry seasons and 171.43–203.94 mg/l in wet seasons. Ca2+ can be derived from dissolution of carbonate minerals (e.g. calcite, dolomite, aragonite) as well as carbonate cement within formations. Magnesium concentrations in cold groundwater vary from 4.87 to 65.74 mg/l in dry season and from 4.73 to 90.92 mg/l in wet season, respectively. The Mg2+ contents of thermal waters also vary from 26.97 to 29.98 mg/l and from 26.54 to 30.94 mg/l in dry and wet seasons, respectively. Mg2+ is significant component of most rock formations and an important ingredient in many petrogenic minerals, such as dark ferromagnesian minerals (amphiboles, olivine, pyroxenes), but also minerals like serpentinite, talc, brucite, chlorite, biotite, tourmaline, dolomite, magnesite and spinel (Hitchon et al. 1999). In a sedimentary environment, Mg largely occurs in association with the carbonate ion, predominantly as dolomite. The major source of Mg2+ in groundwater is probably Mg-bearing minerals such as dolomite and magnesium sulfate minerals in the research area.
The HCO3 − contents of cold waters were determined as a range 11.96–606.79 mg/l in dry seasons and 13.56–847.22 mg/l in wet seasons. The HCO3 − contents of thermal waters were determined as a range 460.49–571.47 mg/l in dry seasons and 547.42–687.94 mg/l in wet seasons. The HCO3 − derives mainly the dissolution of CaCO3 by CO2-rich meteoric water infiltrating along groundwater flow paths. The carbonate mineral dissolution may be diminished during the travel of groundwater from the recharge areas towards the thicker parts of the aquifer because of the lack of carbon dioxide (Afsin 1997). Cl− concentrations in cold groundwater vary from 1.51 to 152.69 mg/l in dry season and from 1.08 to 229.3 mg/l in wet season, respectively. The Cl− contents of thermal waters also vary from 76.42 to 99.20 mg/l and from 66.53 to 91.70 mg/l in dry and wet seasons, respectively. The increase of chloride concentration in the cold waters may be associated with anthropogenic activities and interaction of thermal water. The SO4 2− contents of cold waters were determined as a range 3.5–205.95 mg/l in dry seasons and 3.05–249.37 mg/l in wet seasons. The SO4 2− contents of thermal waters were determined as a range 446.92–589.86 mg/l in dry seasons and 401.53–519.87 mg/l in wet seasons. The enriched in SO4 2− anion of cold groundwater may be related to sulfide (pyrite) oxidation, the washing of coal levels within Upper Miocene aged unit (Sandıklı lava) and effects of mixing thermal water.
The major constituent chemical composition results are summarized in a Piper plot from which the main water types/hydrochemical facies were determined (Fig. 5). Three different water facies are determined in the research area according to IAH (1979) and Freeze and Cherry (1979). These are (1) Ca–Mg–HCO3, (2) Ca–Mg–HCO3–SO4; Ca–Mg–SO4–HCO3 and (3) Na–Ca–SO4–HCO3; Na–Ca–HCO3–SO4. (1) Facies is young cold waters, and originate from a rapid groundwater flow path (shallow system) and low residence time, and thus have low ionic concentrations. (2) Facies is mixing waters which have relatively longer circulation and high ion content. (3) Facies is old waters which have deep circulation within aquifers having low hydraulic conductivity and very high ion content due to prolonged contact with rocks. Cold groundwaters (spring and well waters) belong to first and second water types. Thermal waters belong to third type water in the research area. The thermal waters have the highest TDS values among all groundwaters samples; the cations are dominated by Na+ with lower relative concentrations of Ca2+ and Mg2+, and the anions are dominated by HCO3 − and SO4 2− (Afsin et al. 2013).
The Na/Cl ratios of all cold groundwaters in the basin are between 1.5 and 3.5. The ratios of all geothermal well waters are around 5. The plot for Na–Cl indicates that thermal, cold groundwater and mixing waters is drop out on the graph (Fig. 6). The Na/Cl ratios of 10 and 13 samples which are represented mixing water are <1. Sample 6 has the highest Na/Cl ratio (about 7). This well water has 23 °C and low EC values. The increases of Na/Cl ratios can be related to effects of rainfall, relationship with salty rocks during the groundwater flow and uplift to surface in the groundwater system and/or long circulating time.
A mixture of geothermal waters having high ionic concentration to cold water aquifers is caused to deterioration at irrigation and drinking water quality. The geothermal springs discharging from intersection points of faults N–S and E–W trending had been used in the Hudai geothermal field. Nowadays, deep drilling wells have been drilled in Hudai geothermal field due to obviation and reduction of discharges of these springs. The geothermal waters have been used the purposes of thermal tourism, greenhouse and domestic heating in the Sandıklı county. The increasing in T, EC, SO4, Cl, Al, As, Fe and B ionic values of cold waters is an important indicator of geothermal originated pollution. This situation is determined at wells in the Hüdai, Koçhisar and Gürsu surroundings.
Isotope geochemistry
The stable isotope analyses were made in four periods. There are some differences between cold groundwater and deep geothermal water isotope chemistry. The δ18O and δ2H contents of cold groundwater from the Sandıklı basin range from −10.44 to −7.41 and from −74.38 to −52.49, respectively. The δ18O and δ2H contents of thermal waters range from −10.80 to −10.06 and from −75.05 to −70.37, respectively. The relationship between the δ18O and δ2H values of cold and thermal water samples is plotted in Fig. 7, which also shows the Global Meteoric Water Line (δ2H = 8 δ18O + 10) of Craig (1961) with d-excess value of 10 % and the meteoric water line of Antalya in Turkey (δ2H = 8 δ18O + 16.37) of Sayın and Eyüpoğlu (2005) with d-excess value of 16.37 % and the meteoric water line of Koçbeyli (Isparta) in Turkey (δ2H = 8 δ18O + 12.16) of Sayın and Eyüpoğlu (2005) with d-excess value of 12.16 %. The stable isotope data of sampled sites lie generally above the global meteoric water line (GMWL) and above the Meteoric Water Line of Koçbeyli (KMWL) indicate its meteoric origin.
As a rule, the isotopic composition becomes more depleted in δ18O and δ2H at higher elevations. Groundwaters in the research area are separated to three groups. Thermal waters represented to recharging from higher altitudes. Generally wells which are located in the shallow depths are recharging from lower elevations. In addition, cold natural springs and deep wells are recharged from medium elevations in the basin.
The d-excess (=δ2H–8 δ18O) of the shallow groundwaters varies between 5 and 10 ‰, reflecting no substantial post-precipitation change of isotope composition (Fig. 8). The shallow groundwater with d-excess >5 ‰ and lower δ18O < −5 ‰ are representative of low evaporation and higher permeability (Gupta et al. 2005; Istvan et al. 2006). The higher tritium values are also in good consistence with this setting. Thus, the lower d-excess and higher δ18O values imply high evaporation and low permeability or a long residence time (Baba et al. 2009). The d-excess of the groundwaters in this region vary between 5 and 15 ‰, again reflecting inheritance from precipitation (Fig. 8). The groundwater with d-excess >5 ‰ and lower δ18O < −5 ‰ are representative of low evaporation and higher permeability (Baba et al. 2009; Gupta and Deshpande 2005).
Tritium, with a half-life of only 12.43 years, can be used to reveal the occurrence of modern groundwater recharge (that is, to detect recharge that has occurred over the past three decades). Tritium concentrations in groundwater may be used as indicators of groundwater age. The tritium level in young groundwater is about the same level as in precipitation. Since the mid-1980s the tritium values in rain water are 10 TU in the northern hemisphere (Mook 2001), except for some local anthropogenic releases of tritium from the nuclear industry. As the water moves downward and laterally, the tritium concentration decreases with time. Without monitoring tritium in the precipitation in the region, we used a mean value of 10 TU given by the IAEA (1981). The water samples with tritium contents between 2.59 and 4.53 TU indicate a mix of submodern and modern water. So, the groundwater included both circulating waters which represented water–rock interaction within the aquifer and a modern water contribution which can be included contamination parameters related to land use.
The 3H contents of thermal waters in the basin range from 0.07 to 1.31 TU. This low 3H values could be indicated that thermal waters fed by rainfall before 1950-year (Clark and Fritz 1997). The 3H contents of thermal waters decreased as related to deep circulation, long residence time of water and long time of interaction with rocks, recharging from higher altitude. The 3H contents of cold groundwaters and spring waters in the Sandıklı basin range from 0.5 to 9.61 TU and from 3.88 to 7.38 TU, respectively. These 3H values of cold waters could be represented that this waters recharged by local rainfall in the low altitude and are shallow aquifer waters and rapid circulation.
δ18O versus 3H graphs reflects the relationship between recharge elevations and residence times of waters (Fig. 9). It is shown that the altitude of recharge area increases to the closer to origin along horizontal axis of graph (δ18O) and the residence time in the aquifer also increases to the closer to origin along vertical axis of graph (3H). According to this, thermal waters of Hüdai (Sandıklı) geothermal area represented to deep and slow circulation. Cold waters in the basin have shallow and rapid circulation, and recharged from current precipitation. 3H contents of cold waters which are recharged from precipitation in the higher altitudes are more than thermal waters. The reason for this, residence time of thermal waters is longer than cold groundwaters and flow path of thermal water is deeper. The groundwaters corresponding to a mixture line have middle circulating.
Groundwater contamination
Groundwater is widely used for drinking, domestic and irrigation purposes in the basin. Most of the basin is covered by agricultural lands and residents. Hence, groundwater contamination by irrigation return flow, fertilizer and farm manure application, leaching of soil mineralized nitrogen, domestic sewage, runoff and leached rainfall water, etc., is also an important issue on groundwater contamination in the study area. In addition, the cold groundwater in the alluvial aquifer mixes with hydrothermal fluids in the Sandıklı basin. To determine contamination problem in the research area, nitrate and trace elements (B, Al, As, Fe, Mn, Ni, F, Cr, Cd, Hg, Se, U) were analyzed in the groundwater samples.
Aluminium (Al)
The Al contents in the cold and thermal water samples were determined between <1 to 1510 and 1 to <600 μg/l in dry season, respectively. In wet season, these are changed from <1–899 to 2–34 μg/l, respectively. The permissible limit value of Al for drinking water is 200 μg/l according to World Health Organization (WHO 2008) and Turkish Standards Institute (TSE-266 2005). The extreme value of Al is determined in sample 17. This spring discharged from the Sandıklı lavas at the Karacaören region in the north of the Sandıklı district. The increase of Al content is originated from geogenic sources with water–rock interaction as related to feldspar, kaolin and mica minerals within volcanic rocks (Eriksson 1981) and interference of thermal water in the basin. It has been hypothesized that Al exposure is a risk factor for the development or acceleration of onset of Alzheimer disease in humans (WHO 2011).
Arsenic (As)
As concentration of groundwaters is average 1–2 µg/l except for areas where affected volcanic rocks and sulphide minerals (Sahinci 1991). The concentration of the As in cold groundwater ranged from <0.5 to 102.1 µg/l in wet season and <0.5–126.6 µg/l in dry season. The As contents in the thermal water samples were determined between 514.4 and 866.9 µg/l. The permissible limit of As for drinking water is 10 µg/l according to WHO (2006, 2008), U.S. Environmental Protection Agency (EPA 2012) and TSE-266 (2005) standards. The increase of As in cold water associated with thermal water contribution at shallow groundwater system. High As concentrations in drinking waters is known to increase cancer risk in humans (Chatterjee and Mukherjee 1999).
Iron (Fe)
The Fe concentration of cold and thermal water samples are changed between <10–2301 and <10–10,000 µg/l in dry season and wet season, respectively. The permissible limit of Fe for drinking water is 300 µg/l according to EPA (2012) standards, but according to TSE-266 (2005), the permissible limit for Fe is 200 μg/l. The Fe contents of cold groundwater increased in the Gürsu and Koçhisar wells which are located around Hüdaihamamı spa. The increase in the Gökçealan region is originated from anthropogenic effects as related to agricultural activities.
Manganese (Mn)
The Mn contents in the cold groundwater samples were determined between <0.05 and 433.4 μg/l. The content of thermal water is changed from 14.13 to 220.13 μg/l. The permissible limit value of Mn for drinking water is 400 μg/l according to WHO (2008) standards, but according to TSE-266 (2005) and EPA (2012) permissible limit for Mn is 50 μg/l for drinking and domestic water, and is 20 μg/l for natural spring waters. As components of groundwater Fe and Mn are rarely anthropogenic, but rather are introduced to groundwater through natural interactions of water with rock (Daughney 2003). In the research area, the increase of Mn content which is determined in the Sandıklı, Karacaören and Koçhisar regions is originated from geogenic sources with water–rock interaction as related to magmatic rocks.
Nickel (Ni)
Ni contents in the cold groundwater samples were determined between <0.2 and 128.8 μg/l. The content of thermal water is changed from <0.2 to <300 μg/l. The permissible limit value of Ni for drinking water is 70 μg/l according to WHO (2008) standard, but is 20 μg/l according to TSE-266 (2005). In the research area, the increase of Ni is originated from water–rock interaction as related to magmatic rocks. Ni compounds are carcinogenic to humans and metallic nickel is possibly carcinogenic.
Boron (B)
Due to high B concentrations of thermal waters, environmental problems in ground and surface waters in some agricultural areas of western Anatolia have been observed (Gemici and Tarcan 2002). The B contents in the cold groundwater samples were determined between 5 and 341 μg/l. The increase of B is related to interference of the thermal aquifer. Boron has also been classified as a pollutant of drinking water in national and international drinking water legislation. Boron also affects the health and production of certain plants (Nable et al. 1997).
Nitrate (NO3 −)
Nitrate concentrations of cold and thermal waters vary from 0.10 to 261.52 mg/l in wet and 1.01 to 0.37 mg/l, respectively. The extreme value of NO3 − is determined in sample 10 at May-2012 with 440.51 mg/l due to anthropogenic activities. The increase of nitrate concentrations in groundwater demonstrated to effects of agricultural activities. The maximum contaminant level of nitrate is given to be 50 mg/l in TSE-266 (2005) and WHO (2008). However, if nitrate concentration is over 10 mg/l, it is indicated that groundwater is affected from anthropogenic factors. EPA’s (2012) maximum contaminant level for nitrate set to protect against blue-baby syndrome is 10 mg/l. About 37 % of the samples exceeded the recommended maximum allowable value for nitrate in potable water according to the WHO (50 mg/l NO3) and 61 % of the water samples were higher than 10 mg/l which was accepted as critical value for the NO3 pollution in drinking waters according to EPA (2012). A significant number of epidemiological studies have been carried out on the association of nitrate intake with primarily gastric cancers (WHO 2011).
Fluoride (F−)
The maximum tolerance limit of fluoride in drinking water specified by the WHO (2008) and TSE-266 (2005) is 1.5 mg/l. F contents in the cold water samples were determined between 0.04 and 3.47 mg/l in wet season. In dry season it is changed from <0.01 to 3.75 mg/l. These waters are used as drinking water in the basin. The F content of thermal water is changed from 3.53 to 4.70 mg/l in dry season and from 3.09 to 5.05 mg/l in wet season. Fluoride in natural waters in the study area originated from biotite within the trachi-andesite and tuff levels in the north of the Sandıklı biotite (Özkan et al. 1988; Afsin and Canik 1996). The most obvious sample that shows geothermal-based pollution is surface water sample (sample 12). This sample is taken from Hamamçay river which is pass through Hüdai spa. The waste waters of the Hüdai spa and domestic heating system in the Sandıklı disctrict are discharged Hamamçay river. Elevated fluoride intakes can have more serious effects on skeletal tissues. Low concentrations provide protection against dental caries, both in children and in adults (WHO 2011).
Uranium (U)
The U contents in cold water samples were determined between 0.02 and 190 µg/l in wet season. In dry season it is changed from 0.02 to 147.17 µg/l. The extreme value of U is determined in sample 10. The provisional guideline value of uranium in drinking water specified by the WHO (2005, 2011) is 30 µg/l. Uranium is present in the environment as a result of leaching from natural deposits, release in mill tailings, emissions from the nuclear industry, the combustion of coal and other fuels and the use of phosphate fertilizers that contain uranium (WHO 2011). The increase of U in sample 10 is originated from fertilizer usage in this region.
Selenium (Se)
The Se contents in cold water samples were determined between 0.5 and 6.1 µg/l in wet season. In dry season it is changed from 0.48 to 3.4 µg/l. The guideline value for selenium in drinking water is 40 µg/l (WHO 2011). Selenium is present in Earth’s crust, often in association with sulfur-containing minerals. Selenium is an essential trace element, and foodstuffs such as cereals, meat and fish are the principal source of selenium for the general population WHO (2008). Deficiencies of selenium in diets may cause a number of health effects, although few reports of clinical signs of deficiency are available. When ingested in excess of nutritional requirements in food and drinking water, selenium can cause a number of acute and chronic health effects (WHO 2003a).
Chromium (Cr)
The Cr contents in the cold groundwater samples were determined between <0.5 and 18.1 μg/l. The content of thermal water is changed from <0.3 to <300 μg/l. The health based guideline value for chromium in drinking water is 50 µg/l (WHO 2011; TSE-266 2005). Chromium is widely distributed in Earth’s crust. Chromium can be found in the environment in two valency states, Cr(III) and Cr(IV). The former predominates in soils, whereas the latter occurs exclusively as chromate (CrO4 2−) from antropogenic sources. Cr(IV) is the form which is of toxicological significance because of its easy uptake into cells together with SO4 2− and PO4 2− (Rivett et al. 2006).
In a long-term carcinogenicity study in rats given chromium(III) by the oral route, no increase in tumour incidence was observed. In rats, chromium(VI) is a carcinogen via the inhalation route, although an National Toxicology Program (USA) study has shown evidence for carcinogenicity via the oral route at high doses (WHO 2011).
Cadmium (Cd)
The Cd contents in cold water samples were determined between <0.05 and 0.58 µg/l in wet season. In dry season it is changed from <0.05 and 0.44 µg/l. The health based guideline value for cadmium in drinking water is 3 μg/l (WHO 2011). Cadmium minerals are usually created by the cadmium ions resulting from the decay of zinc deposits. Cadmium has +2 valency in natural waters (Sahinci 1991). Cadmium is released to the environment in wastewater, and diffuse pollution is caused by contamination from fertilizers and local air pollution. Contamination in drinking-water may also be caused by impurities in the zinc of galvanized pipes and solders and some metal fittings (WHO 2011). Cadmium has a high renal toxicity, which is not only due to its made of action but also to its irreversible accumulation in the kidney (Rivett et al. 2006).
Mercury (Hg)
The Hg contents in cold water samples were determined between <0.1 and 1 µg/l in wet season. In dry season it is changed from <0.1 and 0.2 µg/l. The guideline value for mercury in drinking water is 6 µg/l (WHO 2011). Mercury is chalcophile elements and is found at similar amount in Earth’s crust and whole magmatic rocks. It is generally found as cinnabar (reddish mercuric sulfide) in nature (Sahinci 1991). It is present in the inorganic form in surface water and groundwater at concentrations usually below 0.5 μg/l, although local mineral deposits may produce higher levels in groundwater.
Human health risk assessment
Risk assessment is the determination of quantitative or qualitative value of risk related to a concrete situation and a recognized threat (also called hazard). A human health risk assessment is the process to estimate the nature and probability of adverse health effects in humans who may be exposed to chemicals in contaminated environmental media, now or in the future. To explain this better, specific questions are determined by USEPA (2013). In this study, risk assessment of the research area was determined considering these questions which are proposed by USEPA (2013).
Q1: Who/what/where is at risk?
There are two different risks in the research area, drinking water usage (health risk) and irrigation water usage (environmental risk). In the research area, drinking water provided from some natural springs (sample nos.: 7, 8, 11, 16, 17, 18, 19) and shallow wells (sample nos.: 1, 2, 3, 9, 13). In addition, irrigation water requirement is largely supplied from groundwater in the basin.
Q2: What is the environmental hazard of concern?
The increases of T, EC, Na+, SO4 2−, Cl−, B, Al, As, Fe, Mn, Ni, F and NO3 − parameters were determined in the cold drilling wells as locally due to anthropogenic effects and hydrothermal contamination in the Sandıklı basin. The cold groundwater reserves of the alluvial aquifer are contaminated thermally and chemically.
Q3: Where do these environmental hazards come from?
Environmental hazards are originated from natural (thermal water interference, water–rock interaction) and anthropogenic (agricultural activities etc.) in the basin and are non-point sources.
Q4: How does exposure occur?
In the research area, health and environmental risks originated from groundwater contamination. These risks continue for many years and become by means of ingestion (water) and contact with skin.
Q5: What does the body do with the environmental hazard?, Q6: What are the health effects?
Environmental and health hazards were explained by taking into account B, Al, As, Mn, Ni, F and NO3 − parameters which are increased with anthropogenic activities, hydrothermal contamination and water–rock interaction in the basin.
Chronic noncancer hazard index (HI) approach
To assess the overall potential for noncarcinogenic effects posed by more than one chemical, a hazard index (HI) approach is developed based on EPA’s (1986) Guidelines for Health Risk Assessment of Chemical Mixtures. The method developed by USEPA was used in this study to estimate the lifetime health risk of pollutants through oral ingestion (USEPA 1989). The following are the basic equations used for lifetime cancer risk assessment and hazard index (HI) of pollutant i in drinking water:
where cancer risk i is the carcinogenic risk of pollutant i in drinking water (unitless); HI i is the hazard index of pollutant i in drinking water (unitless); SF i is the slope factor for pollutant i (kg day/mg); RfD i is the reference dose for pollutant i (mg/kg day); CDI i is the chronic daily intake (mg/kg day) for pollutant i (Li et al. 2007; Liu et al. 2009). The equation for CDI i is:
where C i is the concentration of pollutant i in drinking water (mg/l); L is the daily water ingestion rate (l/day), taken as 2 l/day; EF is the exposure frequency (days/year), taken as 365 days/year; ED is the exposure duration (year), taken as 30 years for non-carcinogens and 70 years for carcinogens; BW is the bodyweight (kg), taken as 70 kg; AT is the average exposure time (in day), 30 years × 365 days/year for non-carcinogens and 70 years × 365 days/year for carcinogens (Liu et al. 2009). RfD i and SF i values (Table 2) provided in the integrated risk information system (IRIS) at the web site of USEPA (2013).
It is generally accepted that HI below 1 is considered to mean no significant risk of non-carcinogenic effects, if the hazard index exceeds 1.0, it implies that the contaminants revealed toxicity. The non-cancerous hazard indexes (HI) of Al, Mn, Ni, Fe, NO3 − and U exceeded 1 as locally in the basin (Table 3). These locations are shallow wells which are observed contribution of thermal water and springs in the Karacaören region which are dominant water–rock interaction process. The HI of F, Cd, Cr, Hg, Se are below 1 all of the basin. The HI of As completely exceeded 1. This indicated that As posed serious health concerns to the local residents via oral intake, while other elements via oral intake and all the metals via dermal absorption had no or little health threat.
In the USEPA cancer classifications, arsenic and uranium belongs to Group A, and is an assured human carcinogen. The carcinogenic SF for arsenic and uranium through oral ingestion are 1.5 and 0.4 (mg/kg day)−1, respectively, as provided in the integrated risk information system (IRIS) at the web site of USEPA (2013). Carcinogenic risks were evaluated by Eq. (2). The estimated value was the incremental probability of an individual developing any type of cancer over a lifetime due to carcinogenic exposure. The range of carcinogenic risks acceptable or tolerable was 10−6–10−4 (USEPA 1992). Carcinogenic risk indexes of As were between 2.1 and 8.9 × 10−2 and exceeded the carcinogenic risk of EPA. Carcinogenic risk indexes of U were between 1.05 × 10−2 and 8.21 × 10−6. It is exceeded values of the carcinogenic risk of EPA as locally in the basin (Table 3). It is indicated that the ingestion of water over a long life time could increases the probability of cancer.
Assessment of irrigation water quality
The groundwaters mixing with thermal waters are also used as irrigation water in the research area. Therefore, irrigation water quality was also evaluated in this study. The suitability of groundwater for irrigation purposes depends upon the effect of mineral constituents of water on both plants and soils (Srinivasa Gowd 2005; Raju 2007).
Poor quality water may affect irrigated crops by causing accumulation of salts in the root zone, by causing loss of permeability of the soil due to excess sodium or calcium leaching, or by containing pathogens or contaminants which are directly toxic to plants or to those consuming them. Contaminants in irrigation water may accumulate in the soil and, after a period of years, render the soil unfit for agriculture. Water quality criteria for irrigation water generally take into account, amongst other factors, such characteristics as crop tolerance to salinity, sodium concentration and phytotoxic trace elements (Enderlein et al. 2003). According to FAO Irrigation Water Quality Guidelines (2006), certain parameters such as sodium absorption ratio (SAR), salinity (EC), specific ion toxicity (Na+, Cl−, B) and miscellaneous effects (NO3 −, HCO3 −, pH) are used to assess the suitability of groundwater for agricultural purposes.
Salinity is the amount of dissolved salt in water. All water used for irrigation contains some salt. This salt generally comes from weathering of soil, leaching of salts dissolved from geologic marine sediments into the soil solution or groundwater, and flushing of salts off of roads, landscapes and stream banks during and following precipitation events. Additionally, the amount of salt found in irrigation water generally is greater in arid and semi-arid areas than in humid and sub-humid areas (Bauder et al. 2008). The salinity of irrigation water can be determined directly by measurement of the concentration of all the dissolved salts in a water sample. The permissible limits for EC value is between 700 and 3000 μS/cm according to FAO (2006). It was observed from Table 1 that electrical conductivity of cold water for all stations ranged from 172 to 2193 μS/cm. These indicating slight to moderate degree of restriction on the use water that can be used moderate amount of leaching.
Sodium hazard is expressed in terms of sodium adsorption ratio (SAR), it is a measure of the suitability of water for use in agricultural irrigation. There is a significant relationship between SAR values of irrigation water and the extent to which sodium is absorbed by the soils. Continued use of water with high SAR value leads to a breakdown in the physical structure of the soil caused by excessive amounts of colloidally adsorbed sodium. The soil then becomes hard and compact when dry and increasingly impervious to water penetration (Nishanthiny et al. 2010; Khodapanah et al. 2009). SAR is calculated by the following formula (where the concentrations of all ions are in meq/l):
SAR in all stations ranged from 0.10 to 2.72 (Table 1), it is suitable for irrigation water usage. The plot of data on the US salinity diagram (Fig. 10), in which the EC is taken as salinity hazard and SAR as alkalinity hazard, shows that majority of the water samples fall in the category C2S1, indicating medium salinity and low alkali water. Such water can be used for irrigation in most soil and crops with little danger of development of exchangeable sodium and salinity.
In the research area, Na+, Cl− B, HCO3 − and pH concentrations of cold groundwaters are suitable for irrigation water usage according to FAO (2006) permissible limit values. But, NO3 concentrations of cold groundwater are exceeded to Irrigation Water Quality Guidelines of FAO (2006) as locally. Waters high in N can cause quality problems in crops such as barley and sugar beets and excessive vegetative growth in some vegetables. However, these problems can usually be overcome by good fertilizer and irrigation management (Bauder et al. 2008).
Conclusions
The Sandıklı basin is one of the important regions which is densely applied agricultural activities and is also one of the important geothermal fields of Turkey. In this study, the interference of a deep thermal system with a shallow aquifer in the Sandıklı basin was assessed with identification of the geochemical and isotopic results of water samples. Three different water facies are determined in the Sandıklı basin. Young cold waters have fast circulation and Ca–Mg–HCO3 hydrochemical facies. Mixing waters have relatively longer circulation and Ca–Mg–HCO3–SO4 and Ca–Mg–SO4–HCO3 hydrochemical facies. Old geothermal waters have deep circulation and Na–Ca–SO4–HCO3 hydrochemical facies. All the waters are found to be of meteoric origin based on δ18O and δ2H isotopes. The geothermal waters have long residence time, deep circulation and mixing features; whereas the cold waters have short residence time and shallow circulation. The environmental isotopes and major ion geochemistry have revealed the direct connection of the thermal waters with the cold groundwater in the basin. The contribution of thermal waters to cold groundwater aquifers causes contamination to aquifers and surface waters. Especially, this hydrothermal contribution is determined in the Koçhisar and Gürsu regions which are located in the surrounding of Hüdai spa and south of the Sandıklı district. The increases of T, EC, Na+, SO4 2−, Cl−, B, Al, As, Fe, Mn, Ni, F and NO3 − parameters were determined in the cold groundwater wells as locally due to hydrothermal contamination, water–rock interaction and anthropogenic effects in the basin. In addition, multivariate analyses were applied in order to elucidate constituent distribution patterns, determine which constituents are significantly associated with hydrothermal systems, and quantify the contribution of the hydrothermal system to groundwater used for drinking-water supply.
When compared to drinking water guidelines established by WHO, Turkey and the EPA, much greater attention should be paid to Al, As, Fe, Mn, Ni, U, NO3 and F though varied chemicals above the critical values in the different sampling time. The values of other toxic elements (Cd, Cr, Hg, Se) in cold groundwater are suitable to these drinking water guidelines. Hazard index method was used to assess the overall potential for noncarcinogenic effects of these parameters. The non-carcinogenic risk in the study area decreased in the following order: As > Al > NO3 > Mn > Ni > U. Carcinogenic risk indexes of As were between 2.1 and 8.9 × 10−2 and exceeded the carcinogenic risk of EPA. Carcinogenic risk indexes of U were between 1.05 × 10−2 and 8.21 × 10−6. It is exceeded values of the carcinogenic risk of EPA as locally in the basin. This indicated that As and U posed serious health concerns to the local residents via oral intake. But, other elements via oral intake had no or little health threat.
References
Afsin M (1991) Hydrogeological investigation of Afyon Sandıklı Kuruçay plain and Hüdai spa, Ankara University. Doctoral Thesis (in Turkish-unpublished)
Afsin M (1997) Hydrochemical evolution and water quality along the groundwater flow path in the Sandikli plain, Afyon, Turkey. Environ Geol 31(3/4):221–230
Afsin M, Canik B (1996) Hydrogeological and hydrochemical study of the Hudai (Sandikli, Afyon, Turkey) thermal mineral springs and interpretation of their genesis. J Geosound 28:69–86 (in Turkish)
Afsin M, Dağ T, Davraz A, Aksever F, Karakaş Z (2012) The origin and sustainability of Hudai geothermal waters, Sandıklı, Afyonkarahisar, Turkey. In: 39th IAH congress, 16–21 September 2012, Niagara Falls, Canada, pp 288–289
Afsin M, Davraz A, Karakas Z, Hınıs MA, Aksever F (2013) Hydrogeological, hydrogeochemical and isotopic of Hudai geothermal field and its vicinity, Sandikli-Afyonkarahisar, Turkey. TUBITAK report project no. CAYDAG-110Y034
Aksever, F. (2011). Hydrogeological investigations of the Sandıklı (Afyonkarahisar) Basin. Suleyman Demirel University, Doctoral thesis (in Turkish-unpublished)
Aksoy N, Simsek C, Gunduz O (2009) Groundwater contamination mechanism in a geothermal field: a case study of Balcova, Turkey. J Contam Hydrol 103:13–28
Appelo CAJ, Postma D (1993) Geochemistry, groundwater and pollution. AA Balkema, Rotterdam
Baba A, Yuce G, Deniz O, Ugurluoglu DY (2009) Hydrochemical and Isotopic composition of Tuzla geothermal field (Canakkale-Turkey) and its environmental impacts. Environ Forensics 10:146–163
Bauder JW, Bauder TA, Waskom RM, Thomas FS (2008) Assessing the suitability of water (quality) for irrigation-salinity and sodium, western fertilizer handbook
Cakmakoglu A (1986) Geology of Çivril-Banaz-Sandıklı-Dinar regions (K23—c1, c2, c3, c4, d3; K24—d3, d4; L23—a2, a3, b1, b2, b3, b4; L24—a1, a2, a3, a4). MTA report no. 8062 (in Turkish-unpublished)
Chatterjee A, Mukherjee A (1999) Hydrogeological investigation of ground water arsenic contamination in South Calcutta. Sci Total Environ 225:249–262
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1833–1834
Daughney CJ (2003) Iron and manganese in New Zealand’s groundwater. J Hydrol (NZ) 42(1):11–26
Dogdu MS, Bayarı CS (2005) Environmental impact of geothermal fluids on surface water, groundwater and streambed sediments in the Akarcay Basin, Turkey. Environ Geol 47:325–340
Enderlein UE, Enderlein RE, Williams P (2003) Water quality requirements. In: Helmer R, Hespanhol I (eds) Water pollution control. Taylor and Francis, London
Eriksson E (1981) Aluminium in groundwater possible solution equilibria. Nord Hydrol 12:43–50
FAO (2006) Irrigation water quality guidelines, Appendix 3, Food and Agriculture Organization of the United Nations
Forrest MJ, Kulongoski JT, Edwards MS, Farrar CD, Belitz K, Norris RD (2013) Hydrothermal contamination of public supply wells in Napa and Sonoma Valleys, California. Appl Geochem 33:25–40
Freeze RA, Cherry JA (1979) Groundwater, 1st edn. Prentice Hall, London
Gemici U, Tarcan G (2002) Distribution of boron in thermal waters of Western Anatolia, Turkey, and examples on their environmental impacts. Environ Geol 43:87–98
Gupta SK, Deshpande RD (2005) Groundwater Isotopic Investigations in India: what has been learned? Curr Sci 89(5):826–830
Gupta VB, Anitha S, Hegde ML, Zecca L, Garruto RM, Ravid R, Shankar SK, Stein R, Shanmugavelu P, Rao KSJ (2005) Aluminium in Alzheimer’s disease: are we still at a crossroad? Cell Mol Life Sci 62:143–158
Hitchon B, Perkins EH, Gunter WD (1999) Introduction to ground vlater geochemistry. Geosciene Publishing Ltd, Sherwood Park
IAEA (1981) International atomic energy agency, stable isotope hydrology. Technical report series 210
IAH (1979) International association of hydrogeologists, map of mineral and thermal water of Europe. Scale 1:500,000, United Kingdom
Istvan F, Toth T, Palcsu L, Barna G (2006) Small isotope effect of evaporation when the rate of evaporation is very high: soil salinization on Nyiro-lapos (Hortobagy, Hungary). In: International workshop on isotopic effects in evaporation, extended abstracts: area della Ricerca CNR Pisa, Italy
Khodapanah L, Sulaiman WNA, Khodapanah N (2009) Groundwater quality assessment for different purposes in Eshtehard District, Tehran. Iran. Eur J Sci Res 36(4):543–553 ISSN 1450-216X
Li J, Huang GH, Zeng G, Maqsood I, Huang Y, Lia J (2007) An integrated fuzzy-stochastic modeling approach for risk assessment of groundwater contamination. J Environ Manag 82:173–188
Liu Y, Zheng B, Fu Q, Meng W, Wang Y (2009) Risk assessment and management of arsenic in source water in China. J Hazard Mater 170:729–734
Mook WG (2001) Environmental isotopes in the hydrological cycle, principles and applications. International hydrological programme, vol 39, II. IAEA, Vienna
Mutlu H, Gulec N (1998) Hydrogeochemical outline of thermal waters and geothermometry applications in Anatolia (Turkey). J Volcanol Geotherm Res 85:495–515
Nable RO, Bañuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 193:181–198
Nishanthiny SC, Thushyanthy M, Barathithasan T, Saravanan S (2010) Irrigation water quality based on hydro chemical analysis, Jaffna, Sri Lanka. American-Eurasian J Agric Environ Sci 7(1):100–102
Nur A, Ishaku JM, Taiyib A (2012) Spatial distribution of chemical facies using geographical information system (GIS) in Michika, northeastern Nigeria. Res J Eng Appl Sci 1(2):102–109
Öngür T (1973) Geologic situation of Sandıklı (Afyon) geothermal investigation field and geothermal energy facilities. General Directorate of Mineral Research and exploration report no. 5520 (in Turkish-unpublished)
Özkan G, Koseoglu M, Bilgin A (1988) Relation with surrounding rocks of fluoride within the Isparta drinking water. I. National hydrogeology symposium, Ankara University, pp 247–257
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhara Pradesh, South India. Environ Geol 52:1067–1074
Reimann C, Bjorvatn K, Frengstad B, Melaku Z, Tekle-Haimanot R, Siewers U (2003) Drinking water quality in the Ethiopian section of the East African Rift Valley I—data and health aspects. Sci Total Environ 311:65–80
Rivett M, Drewes J, Barrett M, Chilton S, Appleyards S, Dieter HH, Wauchope D, Fastner J (2006) Chemicals: health relevance, transport and attenuation. In: Schmoll O, Howard G, Chilton J, Chonus I (eds) Protecting groundwater for health: managing the quality of drinking-water sources. IWA Publishing, London
Sahinci A (1991) Geochemistry of natural waters. The Reform Printing Office, Izmir (in Turkish)
Sayın M, Eyüpoğlu SO (2005) Determination of local meteoric water lines using stable isotope contents of precipitation in Turkey, II. Use of isotope techniques in hydrology symposium, Ankara
Serpen U, Aksoy A, Öngur T (2010) Present status of geothermal energy in Turkey. In: Proceedings of thirty-fifth workshop on geothermal reservoir engineering, Stanford University, Stanford, CA
Simsek S (2003) Geological study of Ihlara (Cappadocia) special environment region and hydrogeological and hydrogeochemical investigation of thermal springs and protection zone proposals, Coproject between Hacettepe University and The Directorate of Special Environment Protection of The Ministry of Environmental of Turkey (in Turkish-unpublished)
Srinivasa Gowd S (2005) Assessment of groundwater quality for drinking and irrigation purpose: a case study of Peddavanka watershed, Anantapur District, Andhra Pradesh, India. Environ Geol 48:702–712
TSE-266 (2005) Water intended for human consumption. Turkish Standards Institute, Ankara
USEPA (1986) Guidelines for the health risk assessment of chemical mixtures. Risk Assessment Forum U.S. Environmental Protection Agency, Washington
USEPA (1989) Risk assessment guidance for superfund. Human Health Evaluation Manual Part A, Interim Final, vol 1, EPA/540/1-89/002
USEPA (1992) Water quality standards—establishment of numeric criteria for priority toxic pollutants; states’ compliance; final Rule. Federal Register, Washington, 57/246, 60848–60923
USEPA (2012) Edition of the drinking water standards and health advisories, EPA 822-S-12-001. Office of Water US Environmental Protection Agency, Washington
USEPA (2013) Risk assessment IRIS (Integrated Risk Information System). http://www.epa.gov/risk_assessment
Vengosh A, Helvacı C, Karamanderesi IH (2002) Geochemical constraints for the origin of thermal waters from western Turkey. Appl Geochem 17:163–183
WHO (2003a) Selenium in drinking water background document for development of WHO Guidelines for drinking-water quality. World Health Organization, Geneva
WHO (2005) Uranium in drinking water background document for development of WHO Guidelines for drinking-water quality. World Health Organization, Geneva
WHO (2006) Guidelines for drinking-water quality [electronic resource]: incorporating first addendum, vol 1, Recommendations, 3rd edn. World Health Organization, WHO Library Cataloguing-in-Publication Data. ISBN 92 4 154696 4
WHO (2008) Guidelines for drinking-water quality, 1st and 2nd addenda, vol 1, Recommendations, 3rd edn. World Health Organization, WHO Library Cataloguing-in-Publication Data. ISBN 978 92 4 154761 1 (WEB version)
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, WHO Library Cataloguing-in-Publication Data, ISBN 978 92 4 154815 1
Acknowledgments
This study was financially supported by The Scientific and Technical Research Council of Turkey (TUBITAK) (Project No.: CAYDAG-110Y034—Project title: The study of hydrogeological, hydrogeochemical and isotopic of Hudai geothermal field and its vicinity, Sandıklı-Afyonkarahisar, Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davraz, A., Afsin, M., Aksever, F. et al. The interference of a deep thermal system with a shallow aquifer and health risk assessment: the case of Sandıklı (Afyonkarahisar) Basin, Turkey. Environ Earth Sci 75, 332 (2016). https://doi.org/10.1007/s12665-015-5144-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-5144-6