Abstract
Groundwater is a finite resource in Davarzan region which is located between the ophiolite complex mountain in the north and salty playa at the south. The water samples were analyzed to assess the origin of groundwater pollution and explain links between the disturbed heavy metals composition of the earth’s surface and the human health risks. The main heavy metal pollutants in the groundwater are Cr, Fe, As and Pb ions. In general, the groundwater salinity and some elements such as Cr and As are increased along with surface topography and groundwater flow directions from the northern ophiolite highlands recharge area to the adjacent desert discharging zone in the south. Despite the ophiolite complexes being the most enriched in Cr element, the lowest Cr concentration in the groundwater was measured near the ophiolite area, which is in the range of its discharged springs. Based on the groundwater conceptual pollution model, bedrock geochemistry controls the composition of soil and hence that of groundwater. The Cr samples show a direct relation with the EC value indicating that intrusion of salinity from the salt pan is probably another reason for the increased Cr concentration. The results of health risk assessment indicated that the groundwater suffered from significant contamination and if used for long-term without pre-treatment may pose serious health risks to human population via drinking water and irrigation of agricultural fields. This is the first attempt to apply hydrogeological setting along with the source of pollution and its health risk in a desert-ophiolitic area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an important source in most arid and semiarid regions of the world and is the main source of water for various uses (Abiye, 2016; Healy & Scanlon, 2010; Kotchoni et al., 2019; Long et al., 2021; Manghi et al., 2009; Scanlon et al., 2006; Xing et al., 2013) Recently, with the increase in population and demand for water, a limited amount of the non-polluted water resources are available to humans (Burritt & Christ, 2018; Santana et al., 2020). The agriculture, energy production, industry and mining activity are the main agents of elevated heavy metals concentration in surface and groundwater (Santana et al., 2020). The groundwater contamination mainly contributes to the geological conditions, groundwater flow direction, topographic features and hydrological processes (Adewoyin et al., 2019).
Heavy metals with stable condition in the environment are toxic to the human body (Wang et al., 2021). The contamination of water resources with heavy metals due to toxicity, persistence and biological accumulation of these pollutants poses real risks to aquatic environments and human health (López et al., 2019; Reis et al., 2019; Santana et al., 2020). The heavy metals such as Cd, Cr and As can damage the human nervous system, digestive system and skin glands and may lead to diseases such as headache, joint pain, abnormal liver function, kidney and cancer (Mukherjee et al., 2021; Pratush et al., 2018; Singh et al., 2018; Wang et al., 2021; Xu et al., 2019; Yang & Massey, 2019). Many researchers have focused on the spatial distribution and hazards of heavy metal pollutants in groundwater (Alam et al., 2016; Ali et al., 2019; Emenike et al., 2018; Jain et al., 2010; Wu & Sun, 2016; Xie et al., 2012; Zhang et al., 2018).
The human activities, meteorological and hydrogeological conditions are controlled the groundwater quality in semiarid alluvial aquifer with a critical environmental concern (Kaur et al., 2020). The Davarzan plain is characterized by arid and semiarid climate where located in the west of Khorasan Razavi province in northeastern Iran. Groundwater resources are important as the main sources of drinking and irrigation water for local residents in the Davarzan area. In this geographical area, no research has been done so far on heavy metal contamination in groundwater and also no researches are available on the risk of heavy metals in groundwater to human health. The main reasons of the study in this area are the geological setting with ophiolitic complex near the salt pan and then most probable leaching of heavy metal from rock/topsoil into the groundwater.
Based on this, the objectives of this research are: (1) to investigate the content and special distribution characteristics of trace elements in the groundwater (2) to determine the origin of heavy metals and (3) to assess the potential human health risks of groundwater in the area. Multivariate statistical analysis was used to identify the sources of contaminations in the target areas of Davarzan plain, north of Iran. The results of this study can provide a scientific reference for groundwater resource management, heavy metal pollution control, and ensuring drinking water safety in the area.
Study area description
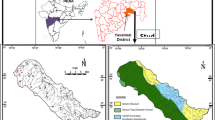
The Davarzan plain with an unconfined aquifer is located in the west of Khorasan Razavi province in northeastern Iran (Fig. 1). Davarzan city with a population of about 22,000 is the main residential area in this region. The alluvial deposits of the plain contain valuable groundwater resources, which is stretched in shape along the approximate trend of E–W (Fig. 1), covering an area of about 703 Km2. The iso-potential map of Davarzan aquifer shows that the highest groundwater levels are present in the northern part and the lowest level in the central and southwestern areas of the plain. The surface of the plain slopes from the northwest and west to the southeast, which is closely, aligned with surface and groundwater flow directions. The aquifer is mainly recharged from northern ophiolite highlands with maximum height of 2920 m above mean sea level. The general groundwater flow direction is from northeast to southwest of the area and discharges into the adjacent desert (Fig. 1). The northern heights of the Davarzan plain are part of Sabzevar ophiolite mélange (SOM), (Mazhari & Attar, 2015). The main lithological units in the Davarzan area can be divided into two groups of Eocene rocks and the main ophiolite unit. Eocene rocks are often composed of andesitic to basaltic lavas and alternating beds of marl, sandstone and tuff limestone.
Ophiolite units are distributed in the northern part of Davarzan plain and include various peridotites and serpentinites (Moghadam et al., 2015). The different springs are discharged from the ophiolite units with elevation of 1638 to 2261 m.a.s.l. Surface water is limited to seasonal rivers flowing from the northern heights during the rainy season (from winter to early spring) and the groundwater aquifer is the only source of water for various uses in this arid region. The aquifer is being discharged by 185 deep and semi-deep wells which are used for irrigated agriculture, industrial and drinking purposes. The average annual temperature, precipitation and evaporation for a period of 20 years (from 2002 to 2022) are 18.2 °C, 142 mm and 2824 mm, respectively.
Materials and methods
Sample collection and analysis
Groundwater was sampled from 26 representative pumping wells and 4 springs during a survey in August 2021 (Fig. 1). The electrical conductivity (EC), temperature (T) and pH were measured in situ by portable EC Meter-AQ Lytic SD-320 and pH Meter AQ-Lytic SD-300, respectively. Before sampling, the bottles were washed with distillated water. Water samples were collected in 1000-mL dark PVC bottles after pumping out the standing water in the casing of the well. All samples were immediately filtered through acid-treated Millipore filters (0.45 μm mesh, disposable not reusable) into pre-cleaned polyethylene-terephthalate (PET) bottles. The filtered samples were acidified to pH < 2 with ultra-purified 6 mol/L HNO3 to prevent metal precipitation. The samples were preserved at about 4 °C and immediately transferred to the Laboratory in Iran to determine the heavy metals concentration (As, Ba, Cr, Cu, Fe, Ni, Pb, Zn) by ICP-MS method. The limits of instrumental detection, accuracy, and precision of the data analysis (QC/QA measures) are provided in Table 1.
Geospatial analysis
Mapping and interpolation of groundwater parameters data was performed using ArcGIS software, using inverse distance weighted (IDW) option, based on a linear combination of closely related values. The weighted average in the IDW method is distance between the interpolation point and the discrete point (Gong et al., 2014). Before that, sampling wells and parameter concentrations were represented by point shape file (vector) and converted to raster through interpolation.
Heavy metal concentration data were also treated by multivariate statistical method of principal components analysis (PCA) in order to identify the main factors affecting groundwater pollution of the Davarzan aquifer. It is necessary to transform the compositions prior to standard statistical analysis such as PCA regarding the compositional nature of the data (Aitchison, 1986). The current study utilized the centered log-ratio transformation (clr), mathematically expressed as:
or in a compact way clr(x) = ln(x/g(x)), where the log ratio of the vector is applied component-wise (Van den Boogaart & Tolosana-Delgado, 2013). In this equation, x represents the composition vector, g(x) is the geometric mean of the composition x, and D is the number of parts of compositional data. All statistical calculations were conducted with software R (R Core Team, 2016) and its package “compositions” (Van den Boogaart et al., 2014). The generated compositional biplots were interpreted based on the rules recommended by Daunis-i-Estadella et al. (2006) and Van den Boogaart and Tolosana-Delgado (2013).
Heavy metal pollution index (HPI)
Heavy metal pollution index (HPI) was used to assess the total groundwater pollution with heavy metals which depends on numerous factors such as unit weight of a metal (Wi) and prescribed standard permissible limits (Si) for each metal. HPI can be calculated using the following equation:
where Wi denotes the unit weight of the ith parameter metal (computed as: 1/Si) (Hoaghia et al., 2019; Prasad & Bose, 2001), Qi is the sub-index of the ith parameter, and n is the number of heavy metals measured. Qi is expressed as follows:
where Mi is the value measured for the ith heavy metal and Ii is the ideal permissible limit for the ith heavy metal and Si is the standard permissible value.
According to HPI results, the pollution categories are classified in three grades as follows: low (HPI < 50), medium (HPI: 50–100), and high (HPI > 100) contamination (Bhuiyan et al., 2010).
Human health risk assessment
Health risk assessment is performed to assess the health risk of a person due to exposure to a factor by estimating the possibility of adverse effects on the human body (Qiao et al., 2020). The two groups of heavy metals of Cr, Cd, As and B, Mn, Fe, Co, Ni, Cu, Zn, Ba, and Pb ions are classified as carcinogenic and non-carcinogenic pollutants, respectively, according to the International Agency for Research on Cancer (IARC) (Agency, 1996). In this study, the carcinogenic risk assessments were used to determine Cd, Cr and As ion contents in samples, while non-carcinogenic risk assessments were applied to check other metals.
- Non-carcinogenic health risk assessment
The non-carcinogenic health risk assessments were calculated by evaluating the chronic daily intake (CDI) and the hazard quotient (HQ). The CDI was calculated as follows:
where CDI signifies the average dose contacted through ingestion, C is the mean value of the studied metals, IR denotes intake rate of water; 2, 1 and 0.75 L/day for an adult, child, and infant, respectively, the frequency to pollutants (EF) was taken as 365 days/year, ED represents the exposure duration, which was taken as 30 years (Adeyemi & Ojekunle, 2021), BW signifies the average body weight in kg; 60, 10 and 5 kg for an adult, child and infant, respectively, and AT is the time of exposure to the pollutants, which was taken as 30 years × 365 days/year (Ayedun et al., 2015). The hazard quotient (HQ) was calculated as follows:
where RfD is the oral reference dose. The HQ value higher than 1 is the probability of non-cancer causing impacts on human health. HQ value under 1 shows that the ingestion of groundwater would not possibly have any consequence on the occupants (Joel et al., 2018).
- Carcinogenic health risk assessment
Prolonged consumption of water contaminated with heavy metals increases the risk of cancer in humans. Therefore, assessing the carcinogenic risks to health is very important (Long et al., 2021). The carcinogenic potential of contaminated groundwater (C) was evaluated by multiplying the ingestion amount of each metal (CDI) by the cancer potency factor of that particular metal (SF) according to following equation (Kaur et al., 2020).
An index value beyond the maximum acceptable level of the carcinogenic health risk index recommended by ICRP of 5 × 10−5 indicates a high potential of carcinogenic health risk (Long et al., 2021). The flowchart of the study area is done to arrange and briefly explain all the main activities that have been carried out throughout the research. Figure 2 shows the flowchart of research methodology.
Results and discussions
Geochemical surveys of EC and heavy metals
The heavy metals concentrations, pH and electrical conductivity (EC) values in the groundwater samples of the Davarzan aquifer are listed in Table 2. The pH of the samples varies from 8 to 8.6. The pH values in the aquifer indicate the slightly alkaline nature of the water in general. The EC is used to characterize groundwater circulation. The EC values of the collected samples vary from 300 μS/cm at the recharge zone in the north to 3500 μS/cm in the southwest of the aquifer. In general, an increase of the EC is observed from the recharge area to the aquifer outlet in accordance with the direction of the groundwater flow (Fig. 3).
The average heavy metals concentration in the groundwater of the area was in the order of: Cr > Fe > As > Ba > Pb > Zn > Cu > Ni (Table 2). Among them, Cr is the main heavy metal, and its content ranges from 32 to 277 μg/L, with an average of 145 μg/L. Totally, the average value of heavy metals concentrations varies from less than 6 to more than 145 μg/L in the groundwater samples. The average concentration of As, Cr, Fe and Pb elements is higher than the WHO standard values for drinking waters.
The inverse distance weighted method (IDW) for spatial interpolation in ArcGIS was employed to determine the spatial distributions of heavy metals (Fig. 4). The Fe, Ba, Pb, Cu and Zn metals have an increasing trend from the east to the west of Davarzan aquifer, while As and Cr ions represent an increasing trend from north to the south region. Nickel metal has the lowest concentration compared to the other elements in groundwater samples and does not have a regular trend.
Correlations of the heavy metals were evaluated using Pearson correlation coefficient, and their significances were established at 95% confidence level (Table 3). The highest correlations are related to lead and zinc (0.60), lead and barium (0.58), arsenic and nickel (0.54), arsenic and chromium (0.50), arsenic and lead (0.46), copper and nickel (0.45) and chromium and nickel (0.42). The strong correlation values are aligned with the spatial distribution of heavy metals. In the following, the spatial distribution of the dominant elements and possible sources in the study area is discussed.
- Copper (Cu)
The Cu concentration varies from 2.86 in the northeast to 25.69 μg/L in the northwest of the aquifer, with an average concentration of 9.13 μg/L, which is much lower than WHO reference values. Copper is a chalcophile element found in ultramafic rocks, basalts, intermediate rocks and granites (Vincent, 1974). Among sedimentary rocks, black shale has the highest average of copper value (McLennan & Murray, 1999). In general, the concentration of copper element in all parts of the study area is very low compared to other elements, because of lack of copper-rich rocks in the recharge area.
- Iron (Fe)
Iron is one of the most abundant metals in earth’s crust. The range of iron element in natural fresh waters varies from 0.5 to 50 μg/L. The iron concentrations in the groundwater samples of Davarzan aquifer range from 20 to 547 μg/L with an average of 127.19. Its concentration is almost less than WHO standard, except in the western part of the aquifer (Fig. 4). The iron metal mainly originates from andesite-basalt rocks occurring in the northwest of the study area (Fig. 1).
- Nickel (Ni)
The Ni concentration (in μg/l) ranges from 0.5 to 13.7 in Davarzan aquifer (Table 2), which is less than WHO standard. Nickel element could exist in dissolved form in groundwater according to the pH and Eh conditions (Vallée, 1999). In natural environment, groundwater has generally very low nickel ion value (Bernard et al., 2008) and its main geological source is Ultramafic rocks (Kudelasek, 1971). In the Davarzan aquifer, there is a small amount of nickel that does not be dispersed regularly. However, most concentrations of Nickel are measured in the southeastern and northwestern parts of the aquifer (Fig. 4). The outcrop of Ophiolite complex in northern part of Davarzan plain can be the most important source of nickel element in the groundwater samples of the area.
- Lead (Pb)
The concentration of Pb ranges from 9.55 to 52.93 μg/l, and its average is 19.99 μg/l (Table 2). The average concentration of lead in groundwater samples is higher than the safe limit provided by WHO. The highest concentration of lead is measured in the western part of the aquifer, where the Davarzan city is located (Fig. 4). Therefore, due to existence of sewage wells (anthropogenic source) and also outcrop of the limestone and sandstone units (terrestrial source) (Fig. 1) in the northern part of the area can be the most important sources of enhancing lead concentration in the groundwater samples of this area.
- Zinc (Zn)
The Zn concentration varies from 1 in the east of aquifer to 25.72 μg/l at the west of aquifer (Table 2). The amount of zinc element in the all groundwater samples is much lower than WHO standard. Nevertheless, similar to lead ion, the highest amount of Zinc element was observed in the western part of Davarzan aquifer (Fig. 4). Since lead and zinc generally have the same geological source, the presence of limestone and sandstone units in the northwest of the aquifer (Fig. 1) probably leaches lower zinc concentration into the groundwater along with lead ion.
- Barium (Ba)
The Ba concentration ranges from 13.89 to 121 μg/L (Table 2) with an average of 39.30 μg/L. Barium concentrations in the all groundwater samples are less than WHO standard. The highest concentration of barium is measured in the western part of the aquifer (Fig. 4). Barium exists as a trace element in both the igneous and sedimentary rocks (Mokrik et al., 2009). The outcrop of andesite-basalt rocks in the northwest of the Davarzan aquifer (Fig. 1) can be the most probable source of increasing barium ion concentration in this area.
- Chromium (Cr)
The amount of Cr ranges from 32 to 277 μg/L, and its average is 145 μg/L (Table 2), which is the dominate heavy metal element in the area in comparison to the others. The concentration of chromium in the Davarzan aquifer is higher than WHO standard values, which is similar to other ultramafic and ophiolitic environment in the world (Chrysochoou et al., 2016; Emsley, 2011; Nriagu & Nieboer, 1988). Among geological formations, ultramafic rocks and serpentine in ophiolite complexes are the most enriched ones in Cr element (Ozeet al., 2007; Vasileiou et al., 2019). The measured Cr concentration in the ophiolite complex at the northern part of Davarzan plain was varied from 2340 to 7754 mg/kg (Shojaat et al, 2003). The higher concentration of hexavalent chromium (Cr(VI)) in soils, sediments, and groundwater is mainly attributed to weathering of ultramafic and ophiolitic rocks (Chrysochoou et al., 2016; Fantoni et al., 2002). The soil samples of Davarzan plain have geogenic Cr concentration of 700 to 1400 mg/kg. The sediment of salty pan at the southern part of the area also most probably has been linked to the occurrence of elevated concentrations elements such as Cr ion. Chromium is found in natural waters in both trivalent (Cr3+) and hexavalent (Cr6+) states. Cr3+ is insoluble and immobile in an alkaline and oxidative environment, while in comparison, Cr6+ is mainly soluble and mobile in such conditions (Sharma et al., 2008). Chromium in groundwater is usually present in hexavalent form (Cr6+), such as the study area (Kotaś & Stasicka, 2000; Sperling et al., 1992; Vasileiou et al., 2019). The distribution concentration map of Cr is shown in Fig. 4. High concentrations of Cr were distributed over the whole studied area. The ophiolite complex, leaching from topsoil and salt pan, is the main probable origin of elevated Cr ion in the area.
Chromium was demonstrated to occur naturally in waters and soils during dissolution and weathering of rocks, especially in ophiolitic zones. The Cr concentration of the discharged spring from the ophiolite complex of the area was about 35 μg/L. The lowest Cr concentration in the groundwater was measured near the ophiolite area in the northern part of the plain, which is in the range of the discharged springs. It can be concluded that the groundwater in the recharged area with lower residence time has the lower Cr concentration. Generally, an increasing trend of the Cr concentration is observed from the recharge area to the aquifer outlet in accordance with the groundwater flow direction (Fig. 5). The highest concentration of chromium is in the southern part of the Davarzan aquifer (Fig. 5).
The Cr can be released in the groundwater of the area mainly through dissolution of its minerals in the groundwater flow path and also the leaching from topsoil during the direct recharge and agricultural return water, too. They are the most important natural sources of chromium entry into bodies of groundwater.
The occurrence of salty playa in the southern margin of the plain may be the other possible source of a few elements in the groundwater samples of the area. Figure 6 shows the relationships between the Cr ion concentrations and the EC values of the groundwater samples. The Cr samples show a direct relation with the EC value indicating that salinity is probably the cause for the increased Cr concentration.
Salinity can affect the mobility of some heavy metals. An increase in ionic strength by any salts promoted a higher release of Cr in the groundwater. Due to the invasion of saline water from this area into the aquifer, there is a possibility of intrusion of some elements in this part of the aquifer. The hypothesis needs to be further investigated. Also, a few metal concentrations may enter the aquatic environment during the cation exchange process due to increasing salinity in the southern part of the plain.
- Arsenic (As)
The arsenic (As) concentration in the groundwater samples varied from 26.78 to 58.81 with a mean value of 44.331 μg/L (Table 2), which is higher than the WHO’s guideline value. The phosphate fertilizers, phosphate sediment and shale are the most common natural sources of dissolved arsenic element in the water (Jayasumana et al., 2015; Lin et al., 2016). As shown in Fig. 7, the highest As ion concentration is measured in the southern part of the Davarzan aquifer, where there is a focus on agricultural activities and pumping wells (Fig. 7). Therefore, agricultural return waters containing toxins and phosphate fertilizers can be the main cause of pollution in this area.
The principal component analysis (PCA)
In order to determine the origin of the studied elements, multivariate statistical method of principal component analysis (PCA) was used. Principal components analysis was used to determine the relationship between physicochemical parameters in the water samples. PCA is used to reduce complex and numerous datasets into a smaller number of components, while maintaining the information content. The compositional biplot generated from PCA on clr-transformed heavy metal data is presented in Fig. 8. The two main components have been determined based on the total variances. The first component with a justification of about 36% of the total variances is the most important component studied and influencing changes in heavy metal concentrations. The first feature that emerges from the biplot is the association of the variable vectors of As, Cr and Ni on one side, and Ba, Zn, Cu, Pb and Fe on the other part. The first group (As, Cr and Ni) is probably coherent with the geochemical reactions responsible for release of these elements into the groundwater along with the agricultural activity in the area. Short links between arrow heads of Zn, Cu and Fe represent proportional constituents commonly originating from weathering of ophiolite outcrops. Pb is also included in the second groups with geogenic origin. The results of the PCA analysis confirm the main role of anthropogenic and geogenic activities in Davarzan region.
Groundwater conceptual pollution model
Based on the above results, the conceptual model of the groundwater pollution in Davarzan plain was investigated (Fig. 9). The springs and adjacent alluvial aquifer is mainly recharged from a nearby ophiolitic complex area and to a lesser with direct recharge and agricultural return water. The heavy metal, especially Cr and As elements, is added to the groundwater of the area mainly through these recharged mechanisms and leaching from topsoil. They are the most important natural sources of the metals entry into bodies of the groundwater. Also, due to the invasion of saline water from this area into the aquifer, there is a possibility of intrusion of some elements in this part of the aquifer.
Heavy metal pollution index (HPI)
Heavy metal pollution index (HPI) is a method for ranking the combined effect of each heavy metal on the overall quality of water (Sheykhi & Moore, 2012). To calculate the HPI of the groundwater samples in the area, the concentration of selected metals (As, Br, Cr, Cu, Fe, Ni, Pb and Zn) is considered (Table 2). In the HPI index, weights (Wi) between 0 and 1 were assigned for each metals. Details of the calculations of HPI with unit weightage (Wi) and standard permissible value (Si) are shown in Table 2. According to the HPI values, the groundwater samples were classified in to two groups of medium (HPI = 50–100) and high (HPI > 100) contamination (Bhuiyan et al., 2010). The HPI value in the sample numbers of W1, W2, W3, W4, W5, W9, W10, W18, W19, W23, W24, W25 and W26 was less than 100, and in the remained samples, it was more than 100. The spatial distribution map of the HPI value is presented in Fig. 10. The HPI value of the collected samples varies from 50 to 100 at the recharge zone in the northern to more than 100 in the southwest of the aquifer. In general, an increase of the HPI is observed from the recharge area to the aquifer outlet aligned with the groundwater flow and increasing salinity trend. The higher HPI value in most parts of the aquifer is representative of high risk of groundwater that cannot be used for drinking. In other parts of the aquifer with HPI values below the critical pollution (100), it is indicative of low-risk water that is suitable for human consumption.
Human health risk assessment
Non-carcinogenic health risk assessment
The results of non-carcinogenic health risks assessment of metals across different age groups (adults, children, and infants) are summarized in Table 4. The non-carcinogenic health risk assessments were calculated by evaluating the chronic daily intake (CDI) and the hazard quotient (HQ). The HQ values for heavy metals except As and Cr ions were less than 1 in the adult age group.
HQ values for Cr and As ions in adults were 1.4 and 4.2, respectively. For the age class of children, in addition to the HQ of As and Cr metals, the HQ of Pb was more than 1 (14.8, 4.8 and 1.4 for As, Cr and Pb, respectively). HQ values for infants in As, Cr and Pb metals increase to 22.2, 7.2 and 2.1, respectively (Table 4). If the HQ is greater than 1, heavy metals may be associated with a potential non-carcinogenic risk (Giri & Singh, 2015) (Qiao et al., 2020). Thus, the high values of HQ observed in As, Cr and Pb can lead to non-cancerous diseases in the Davarzan area for all age groups (adults, children and infants).
Carcinogenic health risk assessment
Estimation of carcinogenic health impacts from As, Cr, Ni and Pb (Table 5) revealed that concentration of these metals, except Pb (which is only in adults), is relatively high to have carcinogenic health impacts on the consumers of groundwater in Davarzan area. According to Table 5, the arsenic risk index is 1.9E-03 for adults, 6.6E-03 for children and 1.0E-02 for infants. Chromium risk index for adults, children and infants is 1.7E-01, 6.1E-01 and 9.2E-01, respectively. The nickel risk index is 1.5E-04 for adults, 5.1E-04 for children and 7.6E-04 for infants. The lead risk index for adults, children, and infants is 2.4E-05, 8.4E-05, and 1.3E-04, respectively. Based on the above results, almost, the average groundwater cancer risk index in the Davarzan aquifer is more than the ICRP limit (5 × 10−5) for all age groups; except for Pb ion which is lower than the ICRP limit only in adults. An index value beyond the maximum acceptable level of the carcinogenic health risk index recommended by ICRP of 5 × 10−5 indicates a high potential of carcinogenic health risk (Long et al., 2021). Therefore, there is a potential for cancer in all age groups of groundwater users including adults, children and infants at the study area.
Conclusion
The present study was conducted to investigate the origin of heavy metals of the groundwater samples in Davarzan region, northeast of Iran. Furthermore, the study was aimed to ascertain potential health risk of heavy metal concentrations to local population. The average heavy metals concentration in the groundwater of the area was in the order of Cr > Fe > As > Ba > Pb > Zn > Cu > Ni, among which the average concentration of As, Cr, Fe and Pb elements exceeds standard limits recommended based on WHO’s guidelines. The Fe, Ba, Pb, Cu and Zn metals ions have an increasing trend from the east to the west of Davarzan aquifer, while As and Cr ions, aligned with the groundwater flow and salinity, have the north–south increasing trend. In general, an increase of the HPI is observed from the recharge area to the aquifer outlet, which is representative of high risk of groundwater that is not suitable for potable water. The northern heights of Sabzevar ophiolite and southern salty desert playa along with agricultural activities have most destructive effects on the quality setting of Davarzan crucial aquifer, which lead to increased health risks. Among carcinogenic substances, Cr, As and Pb metals have the highest carcinogenic risk and non-cancerous diseases in the Davarzan area for all age groups, which are close to the maximum acceptable risk value. It is necessary to pay high attentions to the dynamic changes of Cr, As and Pb contents in the groundwater. Thus, the groundwater sustainable management is an effective way to control and mitigate the risks of groundwater pollution in the future. It is recommended to apply the comprehensive methods developed in this study to crucial aquifers with a deteriorating water quality, in order to prevent and control salinity and heavy metal pollution to reduce the human health hazards associated with heavy metals in the groundwater that pose threats to the environment.
References
Abiye, T. (2016). Synthesis on groundwater recharge in Southern Africa: A supporting tool for groundwater users. Groundwater for Sustainable Development, 2, 182–189.
Adewoyin, O. O., et al. (2019). Risk assessment of heavy metal and trace elements contamination in groundwater in some parts of Ogun state. Cogent Engineering, 6(1), 1632555.
Adeyemi, A. A., & Ojekunle, Z. O. (2021). ‘Concentrations and health risk assessment of industrial heavy metals pollution in groundwater in Ogun state Nigeria. Scientific African, 11, e00666.
Agency, U. (1996). Proposed guidelines for carcinogen risk assessment. Federal Register, 61, 17960–18011.
Aitchison, J.,( 1986). The statistical analysis of compositional data. Monographs on statistics and applied probability. London: Chapman & Hall (Reprinted in 2003 with additional material by The Blackburn Press), 416 pp.
Alam, M. O., et al. (2016). Groundwater arsenic contamination and potential health risk assessment of Gangetic Plains of Jharkhand, India. Exposure and Health, 8(1), 125–142.
Ali, W., et al. (2019). ‘Vertical mixing with return irrigation water the cause of arsenic enrichment in groundwater of district Larkana Sindh Pakistan. Environmental Pollution, 245, 77–88.
Ayedun, H., et al. (2015). Toxic elements in groundwater of Lagos and Ogun States, Southwest, Nigeria and their human health risk assessment. Environmental Monitoring and Assessment, 187(6), 1–17.
Bernard, D., et al. (2008). Origin of nickel in water solution of the chalk aquifer in the north of France and influence of geochemical factors. Environmental Geology, 53(5), 1129–1138.
Bhuiyan, M. A. H., et al. (2010). Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. Journal of Hazardous Materials, 179(1–3), 1065–1077.
Van den Boogaart, K.G., Tolosana, R., Bren, M. (2014). compositions: Compositional data analysis. R package version 1.40–1. R Found. Stat. Comput. Vienna.
Burritt, R. L., & Christ, K. L. (2018). Water risk in mining: Analysis of the Samarco dam failure. Journal of Cleaner Production, 178, 196–205.
Chrysochoou, M., Theologoub, E., Bompotia, N., Dermatasb, D., & Panagiotakis, I. (2016). Occurrence, origin and transformation processes of Geogenic chromium in soils and sediments. Current Pollution Reports, 2(4), 224–135. https://doi.org/10.1007/s40726-016-0044-2
Daunis-i-Estadella, J., Barceló-Vidal, C., & Buccianti, A. (2006). Exploratory compositional data analysis. Geological Society, London, Special Publications, 264, 161–174.
Emenike, C. P., Tenebe, I. T., & Jarvis, P. (2018). Fluoride contamination in groundwater sources in Southwestern Nigeria: Assessment using multivariate statistical approach and human health risk. Ecotoxicology and Environmental Safety, 156, 391–402.
Emsley, J. (2011). Nature’s building blocks: An AZ guide to the elements. Oxford University Press.
Fantoni, D., Brozzo, G., Canepa, M., Cipolli, F., Marini, L., Ottonello, G., & Zuccolini, M. V. (2002). Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environmental Geology, 42, 871–882.
Giri, S., & Singh, A. K. (2015). Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environmental Monitoring and Assessment, 187(3), 1–14.
Gong, G., Mattevada, S., & O’Bryant, S. E. (2014). Comparison of the accuracy of kriging and IDW interpolations in estimating groundwater arsenic concentrations in Texas. Environmental Research, 130, 59–69.
Healy, R. W., & Scanlon, B. R. (2010). ‘Estimating Groundwater Recharge. Cambridge University Press’.
Hoaghia, M.-A., et al. (2019). Quality and human health risk assessment of metals and nitrogen compounds in drinking water from an urban area near a former non-ferrous ore smelter. Analytical Letters, 52(8), 1268–1281.
Jain, C. K., Bandyopadhyay, A., & Bhadra, A. (2010). Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environmental Monitoring and Assessment, 166(1), 663–676.
Jayasumana, C., et al. (2015). Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springerplus, 4(1), 1–8.
Joel, E. S., et al. (2018). Assessment of natural radioactivity in various commercial tiles used for building purposes in Nigeria. MethodsX, 5, 8–19.
Kaur, L., Rishi, M. S., & Siddiqui, A. U. (2020). ‘Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat Haryana, India. Environmental Pollution, 259, 113711.
Kotaś, J., & Stasicka, Z. (2000). Chromium occurrence in the environment and methods of its speciation. Environmental Pollution, 107(3), 263–283.
Kotchoni, D. O. V., et al. (2019). Relationships between rainfall and groundwater recharge in seasonally humid Benin: A comparative analysis of long-term hydrographs in sedimentary and crystalline aquifers. Hydrogeology Journal, 27(2), 447–457.
Kudelasek, V. (1971). ‘Las rocas ultrabásicas en condiciones de meteorización laterítica’.
Lin, T.-Y., et al. (2016). Both phosphorus fertilizers and indigenous bacteria enhance arsenic release into groundwater in arsenic-contaminated aquifers. Journal of Agricultural and Food Chemistry, 64(11), 2214–2222.
Long, X., et al. (2021). Estimation of spatial distribution and health risk by arsenic and heavy metals in shallow groundwater around Dongting Lake plain using GIS mapping. Chemosphere, 269, 128698.
López, R., et al. (2019). Heavy metal pollution in soils and urban-grown organic vegetables in the province of Sevilla, Spain. Biological Agriculture & Horticulture, 35(4), 219–237.
Manghi, F., et al. (2009). Estimating regional groundwater recharge using a hydrological budget method. Water Resources Management, 23(12), 2475–2489.
Mazhari, S. A., & Attar, R. S. (2015). ‘Rare earth elements in surface soils of the Davarzan area NE of Iran. Geoderma Regional, 5, 25–33.
McLennan, S. M. and Murray, R. W. (1999). ‘Geochemistry of sediments’, Encyclopedia of geochemistry, pp. 282–292.
Moghadam, H. S., et al. (2015). Arc-related harzburgite–dunite–chromitite complexes in the mantle section of the Sabzevar ophiolite, Iran: A model for formation of podiform chromitites. Gondwana Research, 27(2), 575–593.
Mokrik, R., et al. (2009). The origin of barium in the Cambrian-Vendian aquifer system, North Estonia. Estonian Journal of Earth Sciences, 58(3), 193.
Mukherjee, I., Singh, U. K., & Singh, R. P. (2021). An overview on heavy metal contamination of water system and sustainable approach for remediation. Water Pollution and Management Practices (pp. 255–277). Springer Singapore.
Nriagu, J. O., & Nieboer, E. (1988). Chromium in the natural and human environments. John Wiley & Sons.
Oze, C., Bird, D. K., & Fendorf, S. (2007). Genesis of hexavalent chromium from natural sources in soil and groundwater. Proceedings of the National Academy of Sciences, 104(16), 6544–6549.
Prasad, B., & Bose, J. (2001). Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environmental Geology, 41(1), 183–188.
Pratush, A., Kumar, A., & Hu, Z. (2018). Adverse effect of heavy metals (As, Pb, Hg, and Cr) on health and their bioremediation strategies: A review. International Microbiology, 21(3), 97–106.
Qiao, J., et al. (2020). Distributions of arsenic and other heavy metals, and health risk assessments for groundwater in the Guanzhong Plain region of China. Environmental Research, 181, 108957.
R Core Team, (2016). R: A language and environment for statistical computing [Computer software manual]. Vienna, Austria. URL http//www.R-project.org/.
Reis, M. M., et al. (2019). Metal contamination of water and sediments of the Vieira River, Montes Claros, Brazil. Archives of Environmental Contamination and Toxicology, 77(4), 527–536.
Santana, C. S., et al. (2020). Assessment of water resources pollution associated with mining activity in a semi-arid region. Journal of Environmental Management, 273, 111148.
Scanlon, B. R., et al. (2006). Global synthesis of groundwater recharge in semiarid and arid regions. Hydrological Processes: An International Journal, 20(15), 3335–3370.
Sharma, S. K., Petrusevski, B., & Amy, G. (2008). Chromium removal from water: A review. Journal of Water Supply: Research and Technology—AQUA, 57(8), 541–553.
Sheykhi, V., & Moore, F. (2012). Geochemical characterization of Kor River water quality, fars province, Southwest Iran. Water Quality, Exposure and Health, 4(1), 25–38.
Shojaat, B., Hassanipak, A. A., Mobasher, K., & Ghazi, A. M. (2003). Petrology, geochemistry and tectonics of the Sabzevar ophiolite, North Central Iran. Journal of Asian Earth Sciences, 21, 1053–1067.
Singh, U. K., Ramanathan, A. L., & Subramanian, V. (2018). ‘Groundwater chemistry and human health risk assessment in the mining region of East Singhbhum Jharkhand, India. Chemosphere, 204, 501–513.
Sperling, M., Xu, S., & Welz, B. (1992). Determination of chromium (III) and chromium (VI) in water using flow injection on-line preconcentration with selective adsorption on activated alumina and flame atomic absorption spectrometric detection. Analytical Chemistry, 64(24), 3101–3108.
Vallée, K. (1999). Le nickel dans les eaux alimentaires: application à des champs captants du bassin Artois-Picardie. Lille 1.
Van den Boogaart, K. G., & Tolosana-Delgado, R. (2013). Analyzing compositional data with R. Springer.
Vasileiou, E., et al. (2019). Expounding the origin of chromium in groundwater of the Sarigkiol basin, Western Macedonia, Greece: A cohesive statistical approach and hydrochemical study. Environmental Monitoring and Assessment, 191(8), 1–34.
Vincent, E. A. (1974). Wedepohl, editor. Handbook of Geochemistry, Volume II/3. Berlin, Heidelberg, and New York (Springer-Verlag), 1972. iv+ 845 pp., 161 figs. Loose-leaf binder: Price DM 258, US $81.80 (subscription price DM 206.40, US $65.50). Mineralogical Magazine, 39(305), 618–619.
Wang, Z., et al. (2021). Spatial distribution and health risk assessment of dissolved heavy metals in groundwater of eastern China coastal zone. Environmental Pollution, 290, 118016.
Wu, J., & Sun, Z. (2016). Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Exposure and Health, 8(3), 311–329.
Xie, Y., et al. (2012). The effects of sedimentary facies and palaeogeography on the formation and distribution of the deep groundwater of the Cretaceous strata in the Ordos Basin. Sediment Geol Tethyan Geol, 32(3), 64–73.
Xing, L., Guo, H., & Zhan, Y. (2013). Groundwater hydrochemical characteristics and processes along flow paths in the North China Plain. Journal of Asian Earth Sciences, 70, 250–264.
Xu, B., Zhang, Y., & Wang, J. (2019). Hydrogeochemistry and human health risks of groundwater fluoride in Jinhuiqu irrigation district of Wei river basin, China. Human and Ecological Risk Assessment: An International Journal, 25(1–2), 230–249.
Yang, F., & Massey, I. Y. (2019). Exposure routes and health effects of heavy metals on children. BioMetals, 32(4), 563–573.
Zhang, Y., Wu, J., & Xu, B. (2018). Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environmental Earth Sciences, 77(7), 1–12.
Acknowledgements
The authors would like to appreciate the continuous support of Shahrood University of Technology and the Khorasan Razavi Regional Water Organization in this research.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Peyman Sudegi. The first draft of the manuscript was written by Rahim Bagheri and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sudegi, P., Bagheri, R., Jafari, H. et al. Groundwater conceptual pollution model and related human health hazards, the main dilemma of a desert aquifer near ophiolite complex. Environ Geochem Health 45, 4025–4042 (2023). https://doi.org/10.1007/s10653-023-01482-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01482-2