Abstract
The plant matter, lignocellulosic biomass, is a renewable and inexpensive abundant natural resource in the world. The development of inexhaustible energy rehabilitated from agricultural waste is an alternative for fossil fuel to reduce CO2 emission and prevent global warming. The amount of waste generated has a direct correlation with the human population. Thus, the waste generated by the community is being added to the environment as the municipal, agricultural waste, and waste produced from forest-based industries. Moreover, there are high possibilities of having environment-friendly valuable bio-based products, including biofuels, biogas, enzymes, and biochar from biomass without competing with the food supply chain. However, only a few or limited kinds of products are produced industrially. This review highlights the significance of lignocellulosic biomass. It describes the different valuable products like biochemicals, biochar, enzymes, single-cell protein, dye dispersant, and bioplastic from lignocellulosic biomass, emphasizing their applications briefly. Besides, this review also highlights the pretreatment of biomass, mainly focusing on biological pretreatment. Natural biomass utilization would lead to solving the energy shortage, food security issues, and obstacles for developing technological solutions in agriculture, agro-processing, and other related manufacturing sectors.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
There have been several studies regarding the bioconversion of lignocellulosic biomass into value-added products. However, almost all articles review and include the hemicellulose, cellulose, and lignin. This review article aims to have pectin as a constituent of biomass because some lignocellulosic biomasses are rich in pectin. Though pectin is present in a low concentration in many plants’ biomasses, it is accountable in fruits and vegetable wastes, and pectin has diverse applications. Thus, to include pectin as the cell wall component of plant biomass in this review article is the novelty of the article. Also, this article describes the different possible value-adding products produced from biomass. It is also necessary to know about additional steps to produce various products from biomass, including the pretreatment methods, to reduce production cost. With the utilization of biomasses, pollutions and disposal problems caused by those wastes will be decreased.
Introduction
The organic matter which is found readily and abundantly in the earth like wood, grasses, crop residues, kitchen waste, forestry waste, animal manures, and wastes produced by different industries including paper, agriculture, food, and municipal are Lignocellulosic biomass (LB) [1, 2]. Agricultural and fruit waste are also used as feed for animals or used to make organic fertilizer [3] or were burned out [4]. However, LB can be used as resources in paper industries, animal feed, biomass fuel production [5], and are convertible into different value-added products [6, 7]. The burning of LB increases air and environmental pollution, which indirectly affects the health of humans and living things [4]. LB is easily obtainable, can be renewed, recycled, is an excellent economical and eco-friendly alternative of fossil carbon source for biofuel and other bio-based products like biochemicals production [8, 9].
Additionally, a carbon-neutral renewable source can reduce greenhouse gases (GHG) emissions and environmental pollution [9, 10]. The report of Bioproducts production and development survey of 2015 mentioned that about 21 million metric tons of agricultural and forestry biomass in Canada per year is used for bioproducts production [11]. Similarly, the report has stated that bio-based products are expected to make 11% of global chemical sales and bio-based sales of $375–$441 billion by 2020 [12]. When biofuel is produced from starch and sugar crops, there may arise the question of sustainability and may lead to a struggle with food production [13]. However, lignocellulosic feedstocks have crucial advantages over other biomass supplies because they are the non-edible portion of plants and do not interfere with food supplies [9]. Moreover, the utilization of biomass prevents different environmental severe problems; for example, forestry, agricultural and agro-industrial wastes which accumulate every year in large quantities will be used resulting in fewer disposals of these wastes to the soil or landfill [8, 9]. Agriculturally important biofuel feedstocks include corn starch, soybeans, and sugar cane, which can be cultivated for energy purposes in a short duration at a lower cost than others. Huber has described that these biofuel feedstocks are significantly cheaper than crude oil [14]. When these feedstocks are cultivated in the land, the production per unit land area increases and land-use efficiency also increases [15]. In Bio-based Chemicals- IEA Bioenergy, it is mentioned that the statement on biomass use as “Indeed, its use in green chemistry and green materials is saving more CO2. It is more resource-efficient and leads to more employment than using the equivalent land area to produce bioenergy” [16].
Thus, LB has a hopeful future as a predictable, feasible, and maintainable resource for biofuels and other value-added products [9, 15, 17]. In general, the bioconversion process includes the different steps such as pretreatment and hydrolysis of biomass that help biomass to break down into simple sugars, which further undergo fermentation and other methods to produce additional value-adding products. Although LB is abundant and usually low-priced, and the conversion process seems simple, there is a big challenge to convert LB into fine chemicals and polymers at an economical cost [18, 19]. This challenge is due to the resistant of LB against enzymatic and chemical degradation [13]. Another challenge is to decrease high oxygen content from biomass and produce low-value, high-volume biofuel with high energy density having physical and chemical properties like fossil fuel and need to develop the integrated biocatalyst technology [20]. Pretreatment can enhance low-value high-volume biofuels and other high-value low-volume chemical production from LB on an industrial scale. However, their pretreatment is not easy, and there is a debate about the pretreatment of those materials [20]. Pretreatment of LB is expensive, relating to both cost and energy but is essential for changing the physical and chemical properties of the lignocellulosic matrix [21, 22]. More about pretreatment is explained later in section “Pretreatment of LB”. Biorefinery and biofuel technologies are developed for renewable oil and green monomers production from LB compatible with petrochemistry [23]. This review article highlights the significance of LB, the structure of LB, the pretreatment method, different hydrolytic enzymes, valuable products from the degradation of LB and their applications in the sections below.
Structure and Sources of Lignocellulosic Biomass
LB is mainly composed of cellulose, hemicelluloses, and lignin as the main component of plant cell walls [1, 2]. In some plant biomass, pectin like carbohydrate polymer is also present [4, 24,25,26]. In addition, other components like acetyl groups, minerals, phenolic substituents [27], and ash are present in a minimal amount [28]. The composition of LB differs between the species and their sources, such as hardwoods, softwoods, and grasses (Table 1). Moreover, the composition also varies with age, stage, and conditions of plant growth even in a single species. Depending on the type of LB, these carbohydrate polymers are organized into complex non-uniform three-dimensional structures to different degrees and varying relative composition [27, 29]. The resistance in degradation and toughness or recalcitrance of LB is due to the crystallinity of cellulose, hydrophobicity of lignin, encapsulation of cellulose [2, 30, 31], and complex structure of pectin [26].
Cellulose
Cellulose is the major component of LB, fibrous, insoluble, and has high molecular weight constituting 35–50% of LB. The homopolymer of anhydrous glucose units are linked by β-1,4-glycosidic linkages in cellulose that is the primary structural component of plants [31]. The crystalline region of cellulose is formed by unbranched long polysaccharide chains arranged parallelly, whereas, in the amorphous part of cellulose, the polysaccharide chain is less orderly arranged [32, 33]. The cross-polarization/magic angle spinning (CP/MAS) study reveals the crystalline structure of cellulose has two polymorphs Iα and Iβ [34]. The cellulose chain length or degree of polymerization varies from 250 to 10,000 sugar units per molecule depending on the source of material and treatment methods. The chain length also impacts the physiological, mechanical, and biological properties of the cellulose [35, 36]. Cellulose is regarded as the best saccharide for fuel production due to its environment-friendly characteristics, such as renewability, biocompatibility, and biodegradability [36].
Hemicellulose
Hemicellulose is a heterogeneous polymer of pentoses (including xylose and arabinose), hexoses (mainly mannose, less glucose, and galactose), and sugar acids [27, 37], resulting in a complex, randomly branched and amorphous structure. Typically, hemicellulose is composed of five different sugars; L-arabinose, D-galactose, D-glucose, D-mannose, and D-xylose, along with other components, such as acetic, glucuronic, and ferulic acids. These sugar units are linked together by β-1,4-glycosidic and sometimes by β-1,3-glycosidic bonds [27]. The configuration of different sugars varies with other plant sources, wood, and cultivation conditions [38]. Hardwood hemicelluloses mostly consist of xylans, whereas softwood hemicelluloses mainly consist of glucomannans [37, 38]. Usually, hemicelluloses comprise 15–35% of LB and the degree of polymerization ranges from 100 to 200 sugar units per molecule [35, 38]. Hemicelluloses are entrenched in the plant cell walls to form a complex network (crosslinked network) of bonds providing structural strength by linking cellulose fibers into microfibrils [27, 30].
Lignin
Lignin is an abundant heterogeneous polymer of LB constituting up to 30% of LB and is a complex amorphous hetero biopolymer. Lignin has a three-dimensional polymer of phenylpropanoid units joined by carbon-carbon and aryl-ether linkages. The complex heteropolymer provides rigidity and comprehensive strength to the plant tissue and the individual fibers, stiffness to the cell wall, resistant to water, insects, pathogens, and chemicals [9, 38]. Lignin, along with cellulose, is considered the most abundant biopolymer in nature [39] and formed by three phenylpropanoid units; p-coumaryl, sinapyl, and coniferyl alcohol [9, 39]. The various aromatic chemicals produced due to the different structural and chemical properties of lignin varies with the plant source, type of plants, and wood. Softwood is constituted of more than 90% of coniferyl alcohol, while hardwood is composed of varying degrees of coniferyl and sinapyl alcohol [38].
Pectin
Pectin is the complex heteropolysaccharides and contains galacturonic acids (70%), rhamnose, xylose, arabinose, and galactose. It is acidic and negatively charged polysaccharides. Pectin has a vital role in the growth of the plant, development of plant morphology, the defense system of the plant, and has the gelling and stabilizing properties [38]. Due to these gelling and stabilizing properties, pectin used in diverse food and outstanding product production significantly affects human health and has biomedical uses [26]. Although the amount of pectin is low in many biomasses, it plays an essential role in secondary wall development in addition to primary wall synthesis and modification [40]. It is highly accountable in fruits and vegetable wastes. Dragon fruit (Hylocereus spp.) peel and pulp contain 38–47% water soluble pectic substance [41], murta fruit (Ugni molinae Turcz) 30% by dry weight [42], pomegranate peels between 6.8–10.1% [43], grape berries pulp represents 8–20% [44], and passion fruit peel 14.8 g/100 g of dried peel [45]. Similarly, pectic polysaccharides in the edible flesh of the loquat fruit contribute up to 70% of total cell wall polysaccharides [46], orange peel 23% pectin, and mandarin peel 16% [47]. Hilz et al. [48] mentioned the cell wall pectin contents in black currants and bilberries vary between 0.20–1.79 g/100 g, and 0.10–0.78 g/100 g respectively. The yield of pectin extracted from creeping fig seed (Ficus pumila Linn.) ranged from 5.25–6.07% (w/w) dry weight [49].
It is reported that sugar beet pulp, apple pomace, and citrus waste, like high pectin biomasses, possess about 12% to 35% pectin by dry weight and grass and woody biomass, which are not regarded as high pectin biomass contain 2–10% and 5% of pectin respectively [50]. The pectin content in wood ranges from 10 to 40 mg/g of wood and eucalyptus wood, about 15.2–25.8 mg/g [51]. Likewise, when there is less pectin in biomass, lipid concentration is high and vice versa, showing the non-reciprocal relation between pectin and lignin [50, 52]. Biosynthesis of pectin takes place in the Golgi apparatus and secreted in apoplast. It is suggested the pectin might block other enzymes to degrade cellulose and/or hemicellulose present in different biomasses [53]. Pectin-rich biomasses are used as bioenergy, biofuel feedstocks [25]. It can act as a determinant of cell wall porosity because it has cross-linking and water complexation properties [54]. Pectinase, a group of enzymes, degrade pectin through depolymerization and de-esterification reactions [55] and also leads for other enzymes for the degradation of other polysaccharides present in biomasses [53].
Besides, pectin has some drawbacks like the gelation of highly methylated pectin in high sucrose concentration; therefore, it is not appropriate for diabetes patients. The reduction in the ability to effectively regulate the release of drugs is due to the hydration, swelling, and water-solubility properties of pectin. However, these kinds of drawbacks can be overcome by pectin modifications and derivatives formation using different techniques as amidation, sulfation, grafting, cross-linking, β-elimination, hydrolysis, degradation, etc. The pectin amendment has increased its application more widely [56].
Pretreatment of LB
Different components present in LB are complex and impart their role differently in different types of biomass in converting LB to other value-adding products. The major constituents of biomass are cellulose, hemicellulose, lignin, and pectin. Among these various constituents, lignin acts as the protective covering, and it is responsible for the delay in the degradation of hemicellulose and cellulose [57, 58]. Also, pectin prevents the degradation of other polysaccharides present in biomass [53]. This kind of action makes pretreatment more important so that lignin, pectin, and other polysaccharides disintegrate efficiently into smaller fragments and simple sugars [53, 57, 58].
The pretreatment of LB is the fundamental step that affects the efficiency of conversion and downstream processes to produce different products. The goal of pretreatment is (i) to disrupt the structure matrix of LB and make smaller fragments (ii) to increase the surface area and pore volume (iii) to reduce crystallinity and recalcitrant nature of LB (iv) to increase the yield of simple fermentable sugars, improving hydrolysis [21, 58, 59]. There are different types of pretreatment methods developed, and the techniques are expensive. For considering the effective pretreatment method, the method need to have different advantages like the method should use low energy and cost effective, should be applicable for different kinds of biomass, should depolymerize hemicellulose, reduce crystallinity and recalcitrant properties of LB, should not produce the inhibitors resisting the hydrolysis and growth of microorganisms, and should be able to recover all the lignocellulosic components [30, 58]. In addition, the pretreatment process should have low sugar disintegration, less chemical consumption, should be safe to operate, and less risky [59]. The factors like cost, disposal, and toxicity need to be focused before planning or choosing the pretreatment method [60].
Classification of Pretreatments
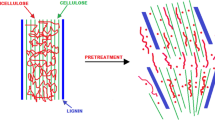
The pretreatment methods are broadly classified into physical, chemical, biological, and combination or multiple or hybrid methods (Fig. 1). Nowadays, the combination pretreatment method is commonly employed because it is more effective in breaking down biomass [21, 57, 59].
Physical Pretreatment
This method includes grinding, chipping, milling, etc. like processes that generally reduce the size of biomass, increasing surface area, reduce the crystallinity of cellulose, and reduce the degree of polymerization [59]. The physical treatment is also known as mechanical treatment. Radiation, ultrasonication, heat treatment, microwave, and sonication are included in physical pretreatment methods. Physical pretreatment enhances hydrolysis by increasing surface area and transferring heat and mass [21]. In physical treatment, high temperatures with high mechanical shearing are applied. However, when treated biomass is taken out from the chamber, explosion or damage in lignin may occur due to a rapid decline in temperature and pressure. The disadvantages of the physical pretreatment are high energy requirements and operational cost, not suitable for lignin removal, and a high chance of equipment devaluation [57].
Chemical Pretreatment
In this process, varieties of chemicals such as alkali, acids, organic solvents, ozone, ionic liquids are used for treatment. The chemical pretreatment process has been useful for various biomass, although there is a chance of less production of sugars from softwoods [60]. Sodium, calcium, potassium, and ammonium hydroxides are commonly used chemicals in alkaline pretreatment. In such a pretreatment process, swelling of biomass occurs, surface area increases, polysaccharides are exposed by breaking down intramolecular linkages, and sometimes the crystalline structure of cellulose as well [21]. Alkali pretreatment is suitable for those biomasses containing less lignin [30]. In acidic pretreatment, commonly hydrochloric acid, phosphoric acid, sulphuric acid, etc. are used. Dilute acids are more preferred because strong acids are corrosive and non-environment friendly. However, dilute acid may degrade carbohydrates and form humins, which further impact sugar yield and produce unwanted byproducts [60]. Organic solvents such as ethanol, methanol, acetone, oxalic acid, glycerol, ethylene, and salicylic acid are also applied in the pretreatment process. These organic solvents enhance retention and enzymatic digestibility of cellulose and help to remove lignin and hemicellulose. However, some organic solvents are inflammable and may cause fire and explosion. Moreover, organic solvent increases the cost and impacts the environment [57]. Ionic solvents treatment is a new and expensive method. However, these solvents are nonvolatile, environmentally friendly, thermostable, non-toxic, non-explosive, and can be recovered and reused in various ways. In the ionic solvent treatment process, cation interacts with lignin, whereas anions interact with cellulose. Thus, the combined action of cations and anions effectively degrade LB. Ionic solvents such as 1-butyl-3-methylimidazonium chloride, triethylammonium hydrogen sulfate dissolve biomass [21, 60].
Biological Pretreatment
This method uses different microorganisms, mostly fungi like brown-rot fungi, white-rot fungi, soft-rot fungi, and some actinomycetes and bacteria are used for hydrolysis of LB. Those microorganisms produce different enzymes so that degradation and hydrolysis of different cell wall components occur [21, 57, 60]. Other enzymes useful in the degradation of biomasses are described in section “Lignocellulolytic enzymes”. Since biological pretreatment uses different microorganisms, the conditions applied should be favorable for microorganisms. The size of particles, temperature, aeration, pH, organic compounds, or inorganic compound, a ratio of carbon and nitrogen source, and strain of bacteria or fungi, etc. should be optimized for efficient and effective biological treatment [60]. Here, degradation and fermentation co-occur, resulting in hydrolysis of cellulose, hemicellulose, lignin, and pectin producing organic acids, biofuels, enzymes, etc. [57]. This method requires less energy, environment friendly, sustainable, cost-effective, no production of inhibitors, and no chemical is required. However, this method needs a long time to take a few days to months, large space, and careful growth conditions [30, 60].
In biological pretreatment, bacteria such as Clostridium sp., Cellulomonas sp., Streptomyces sp., Bacillus sp., Azospirillum lipoferum, Mucilaginibacter sp., etc. and fungi like Trichoderma reesei, Aspergillus niger, Aspergillus nidulans, Fusarium gramineum, Neurospora crassa, etc. are used. Many studies have also shown that microbial consortia are being more effective. The consortium may be of more than two bacteria or more than two fungi or a combination of bacteria and fungus. Some insects, ruminant animals, gastropods, and worms also can degrade lignocellulose in their specific way [57].
Hybrid Pretreatment
This pretreatment class includes the combination of other pretreatment methods, for example, steam explosion, ammonia fiber explosion, CO2 explosion, etc. which enhance the degradation and hydrolysis of LB [57]. This class of pretreatment exploits the chemicals and the use of conditions that affect the physical and chemical properties of biomass [30]. Such type of pretreatment method consumes less chemical and energy, degrades hemicellulose and cellulose, addition of chemicals enhances hydrolysis and is applicable in industries. However, this method needs high pressure and produces inhibitory products, there is a chance of low yield, high energy consumption, and chemical threat [57].
Lignocellulolytic Enzymes
Lignocellulolytic enzymes are a complex array of microbial enzymes that are required for the degradation of a complex structure of LB. Those lignocellulolytic enzymes are found in bacteria, fungi, yeast, plants, and actinomycetes [2]. These enzymes are categorized into ligninolytic, cellulolytic, and hemicellulolytic enzymes. Some biomass also contains pectin, and pectinolytic enzymes degrade pectin. These enzymes help to convert LB into value-added commodities, bio-scorning, and bio-polishing of jeans, improving the efficacy of detergents, maceration, and color extraction from juices. Also, those enzymes are employed for enzymatic deinking, pulping, wastewater treatment, improving the nutritional properties of animal feed, retting of flax, producing oligosaccharides, clarifying juices, treating dyes and other organic pollutants, bioethanol production, and developing biosensors, etc. [2, 61,62,63]. Studies have demonstrated that when different lignocellulolytic enzymes are applied together, they are more effective and work better. Agrawal et al. analyzed the synergistic relation between cellulase and accessory enzymes (xylanase, pectinase, glucosidase, etc.) for hydrolysis of wheat straw. The study revealed the enzyme cocktail or multiple enzymes were more effective and is a sustainable approach for efficient hydrolysis of biomass [64].
Cellulolytic Enzymes
The cellulolytic enzymes are essential industrial enzymes, and they hydrolyze cellulose into fermentable sugars, which can be used for further applications. These cellulolytic enzymes are used in various industries, including pulp and paper, textile, laundry, biofuel production, food and feed industry, brewing, and agriculture [62].
Cellulase consists of endo-glucanase, exo-glucanases, or cellobiohydrolase (CBH), and β-glucosidase, which are hydrolytic enzymes, act synergistically, and belong to glycosyl hydrolase (GH) family [65]. The endo-glucanase hydrolyzes the glycosidic bonds on amorphous sites in between the chain randomly and produces small fibers with free reducing and non-reducing ends. In contrast, exo-glucanase hydrolyzes on chain ends of cellulose to release cellobiose and some glucose [66, 67]. So far, cellobiose has inhibitory activities during cellulose hydrolysis, and the β-glucosidase is essential to break the final glycosidic bonds of cellobiose and produce enough glucose molecules. In some cellulase complexes, β-glucosidase is absent or present in less amount resulting in incomplete hydrolysis [66, 68, 69]. Some cellulase producing microbes are; Aspergillus niger, A. nidulans, A. oryzae, Penicillium brasilianum, P. occitanis, P. occitanis, P. fumigosum, Neurospora crassa, Trichoderma atroviride, Sporotrichum thermophile, Trametes versicolor, Agaricus arvensis, Pleurotus ostreatus, Phlebia gigantea, Acinetobacter junii, A. amitratus, Bacillus subtilis, B. pumilus, B. amyloliquefaciens, B. licheniformis, B. circulan, B. flexus, Bacteriodes sp., Cellulomonas biazotea, Paenibacillus curdlanolyticus, Pseudomonas cellulose, Streptomyces drozdowiczii, and S. lividans [62]. Recently, cellulolytic cocktail enzymes, which are specific to feedstocks, are being studied for reducing the production cost of cellulolytic enzymes that are used in converting biomass into biofuel and different value-adding products [70].
Hemicellulolytic Enzymes
Hemicellulases include a group of enzymes; xylanases, β-mannanases, β-D galactanases, β-xylosidases, and arabinofuranosidases involved in the breakdown and hydrolysis of xylans, mannans, galactans, xylobiose, and arabans [71]. Xylan and mannan are the most copious component of the hemicelluloses. In hardwood hemicellulose, xylan is a significant component, whereas, in softwood, mannan is a considerable component. These different hemicellulases are interdependent [71] and act synergistically in the hydrolysis of hemicellulose to form several monomeric sugars, and aid in exposing the surface for cellulase activities [39, 72]. Thermobifida halotolerans, Actinomadura sp., Cellulomonas flavigena, Streptomyces cyaneus, Cellulosimicrobium cellulans, Enterobacter sp., Penicillium sp., Bacillus pumilus like microbes produce hemicellulases [73].
Xylanases catalyze the hydrolysis of internal β-1,4-xylosidic linkages of xylan to oligomers. The enzymes like endo- and exo-xylanases hydrolyze the cross-linked hemicelluloses that cleave the xylene to generate oligosaccharides [39].
Mannanases are the second important hemicellulases after xylanases. Mannanases hydrolyze β-D-1,4-mannopyranosyl linkages randomly in mannose containing polysaccharides such as glucomannans and galactomannans to produce short β-1,4-manno-oligomers. These short oligomers are finally hydrolyzed into mannose by β-mannosidase. The side group connected to xylan and glucomannan chains can be cleaved by α-glucuronidase, α-arabinosidase, and α-D-galactosidase [74, 75].
Galactanases hydrolyze the D-galactans and L-arabino D-galactans. Endo-galactanases degrade D-galactans randomly at the β-1,4-D-galactosyl linkage and produce D-galactose and galactose oligosaccharides. Another type of endo-galactanases degrade β-1,3-D-linked galactosyl bonds of arabinogalactans and produce D-galactose, L-arabinose and other oligosaccharides [75].
Β-xylosidase hydrolyzes xylobiose and small xylooligosaccharides to xylose and facilitates the hydrolysis of xylan. Arabinofuranosidase catalyzes the removal of arabinose substituents and promotes an increase in the access points of xylanase to the xylan [75].
Ligninolytic Enzymes
The ligninolytic enzymes generally comprise three principal enzymes; lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase. These enzymes are concerned with the degradation of lignin into low molecular weight compounds [2, 76]. Other enzymes like aryl alcohol dehydrogenase, vanillate hydroxylate, dioxygenase, catalase, aromatic aldehyde oxidase, etc. act as mediators of lignin degradation. Those mediator enzymes either help by producing H2O2 required for the activity of peroxidases or catalyze the breakdown products of lignin degradation [76]. Ligninolytic enzymes are produced by several bacteria, fungi, and actinomycetes particularly white-rot fungi having ligninolytic function [77, 78]. Ligninolytic microbes are Pseudomonas fluorescens, Pseudomonas putida, Enterobacter lignolyticus, Escherichia coli, Streptomyces viridosporus, S. paucinobilis, Rhodococcus jostii [79], Panaeolus papilionaceus, and Coprinopsis friesii [80]. The white-rot fungus species like Bjerkandera adusta, Cyathus stercoreus, Dichmitus squalens, Phanerochaete chrysosporium produce multiple isoenzymes of lignin and manganese peroxidases but does not produce laccase [81].
Laccase is a polyphenol and multicopper oxidase having four copper atoms per molecule at their active site. It acts as an oxidizing agent and cofactor; thus, it involves in an efficient oxidation, cleavage, and polymerization of several biological as well as synthetic phenolic and non-phenolic compounds [2, 77, 82]. Laccases do not require H2O2 to oxidize different substrates because it has four copper ions [76]. Laccase converts polyphenol of the biomass to yield phenoxy radicals and quinines [83].
LiP, also known as ligninase, is a member of the oxidoreductase’s family. LiP oxidizes the compounds having high redox potential in the presence of hydrogen peroxide and can oxidize both phenolic and non-phenolic compounds. LiP also catalyzes the oxidation of a wide range of aromatic substrates. Thus, LiP having a wide range of substrate specificity and high redox potential are utilized in various industrial applications [84].
MnP, another critical enzyme for lignin degradation, is also one of the oxidoreductases. MnP has heme peroxidases with low redox potential and requires hydrogen peroxide for the activity. This enzyme is not able to oxidize nonphenolic compounds and can be manganese dependent or versatile peroxidases [76, 82, 85]. MnP oxidizes Mn2+ to highly reactive Mn3+ that catalyzes the oxidation of phenolic structure to phenoxy radicals. MnP also depolymerizes natural and synthetic lignin as well as lignocelluloses in free form. The molecular weight of MnP ranges from 38 to 62.5 kDa, and 11 different isoforms in Ceriporiopsis subvermispora were observed [76, 78, 86, 87].
Pectinolytic Enzymes
The pectinolytic enzyme is a complex enzyme consisting of a mixture of enzymes for the hydrolysis of pectin substances [88]. These pectinolytic enzymes are classified into two major types; esterase and depolymerase. Esterase randomly hydrolyzes the pectin and produces pectic acid and methanol, whereas depolymerase breaks pectin by hydrolysis and trans-elimination processes [89, 90].
Esterase enzyme is commonly known as pectin methylesterase, which catalyzes the de-esterification of methyl ester linkage of pectin and produces methanol and acidic pectin. Depolymerizing enzymes like polygalacturonase (PG), and pectin lyases (PL) break the main pectin chain. PG catalyzes the breakdown of α-1,4-glycosidic bonds between galacturonic acid chains while PL transeliminates pectin molecules, producing an unsaturated product [89, 90]. Pectinases are produced from Bacillus sp., Pseudomonas sp., Aspergillus niger, Aspergillus oryzae, Aspergillus flavus, Penicillium chrysogenum, Penicillium expansum, Trichoderma viride, Mucor piriformis, Yarrowia lipolytica, Saccharomyces sp., Candida sp., Actinomycetes sp., Lactobacillus sp., Aeromonas cavi [91, 92].
Valuable Products from LB
For the production of useful, high value-added products from LB multi-steps as mentioned are usually required: (i) pretreatment (mechanical, chemical or biological) [93], (ii) hydrolysis of the polymers to produce readily metabolizable molecules (e.g. hexose or pentose sugars), (iii) bio-utilization of these molecules to support microbial growth or to produce chemical products and (iv) the separation and purification [94]. Various sources of LB need to be considered separately since the different constituents like cellulose, hemicelluloses, lignin, and pectin vary in different resources. The pretreatment method and depolymerization process play an essential role in other feedstocks to produce different types of products; chemicals, enzymes, single-cell protein, polysaccharides, bioactive compounds, bioenergy, etc. [95,96,97]. Some of the value adding products produced from different biomass are listed in Table 2 with their references.
Platform Chemicals
From biomass, the first possible chemicals in the biorefinery can be sugar compounds (C5 and C6 sugars) and further these C5 and C6 sugar compounds can subsequently be converted to any chemicals or materials. The degradation of these C5 and C6 compounds also depends on the depolymerization step [98]. The report of the US Department of Energy (DOE) describes sugar derivable building block chemicals that can be transformed into new useful molecules. These C5 and C6 sugar derived platform chemicals include 1,4-diacids (succinic acid, fumaric acid, and malic acid), 2,5-furan dicarboxylic acid (2,5-FDCA), 3-hydroxy propionic acid (3-HPA), aspartic acid, glucaric acid, glutamic acid, itaconic acid, levulinic acid, 3-hydroxybutyrolactone (3-HBL), glycerol, sorbitol, and xylitol/arabinitol [99]. Bozell and Petersen [100] explained that all these platform chemicals can be produced from biomass-derived carbohydrate sources except glycerol. The bio-based products sales are expected to increase by 4% annually and reach 11% making bio-based products of $3401 billion in the global chemical market by 2020 [12].
Succinic Acid
Succinic acid, C4 dicarboxylic acid, is a useful chemical in the pharmaceutical, agricultural, and food industries. From succinic acid, different high-value derivatives like adipic acid, 1,4-butanediol, methyl ethyl ketone, 1,3-butadiene, ethylene diamine disuccinate can be produced. Polyethylene succinate (PES), polypropylene succinate (PPS) and polybutylene succinate (PBS) are the most studied polyesters of succinic acid. Through hydrogenation of succinic acid, 1,4-butanediol (BDO), γ-butyrolactone (GBL), and tetrahydrofuran (THF) are obtained [10, 101]. Thus, Succinic acid can be used to produce succinate esters, which are precursors for BDO, THF, and GBL. Succinic acid produces succinic anhydride under dehydrogenative cyclization and acts as a precursor of fumaric acid and maleic acid synthesis [102]. In the food market, they are used as pH modifiers, flavoring agents, anti-microbial agents, health-related agents, and biodegradable plastic [10, 98, 103].
Lactic Acid
Lactic acid is one of the most versatile organic acids produced from the fermentation of carbohydrates. It is transformed into other valuable chemicals like lactate ester, lactide, acetaldehyde, 2,3-pentanedione, pyruvic acid, lactate, oxalic acid, propylene oxide, propanoic acid, acrylates via esterification, hydrogenolysis, dehydration, oxidation, and reduction processes [10, 104]. Lactic acid and its derivatives are applicable to produce biodegradable polymers in the food and beverage sectors, pharmaceutical, and personal care products. It is also useful in making cellulose-acetate-propionate polymers used as a composite, adsorbent, coating material for furniture and automobile seating, bedding, and carpet underlay thermal insulation [105, 106]. Lactic acid has moisturizing, pH regulating, and skin lightening properties. Thus, lactic acid is a common ingredient in personal care products. Lactic acid can also be used as a herbicides and pesticides, in textile and tanning industries, fermentation of food in food industries, production of dairy products like yogurt, buttermilk, acidophilus milk, cottage cheese, etc. Polylactic acid is biodegradable and produced from the polymerization of lactic acid, used in food packaging [107].
Levulinic Acid (LA)
LA is a linear C5 keto acid produced from the hydration of 5-HMF [98]. Traditionally, LA was made from maleic acid in high cost and low volume, but now LA is produced at a lower price and large volume from lignocellulose [10]. LA can replace bisphenol A (BPA) as a plasticizer [12] and is the foundation of the levulinic family. The presence of two reactive functional groups in LA helps to produce many valuable new compounds with novel applications. The most important compounds obtained from LA are levulinic esters, 5-aminolevulinic acid (δ-aminolevulinic acid, DALA), angelica lactones (AnLs), 2-butanone, 4-hydroxypentanoic acid or its esters, γ-valerolactone (GVL) and 1,4-pentanediol [10, 108]. LA and its derivatives have a range of applications in the preparation of pharmaceuticals and textile products, plasticizers, animal feed, coating material, and antifreeze. They also serve as a valuable chemical constituent from almost all sugars manufactured in the biorefinery [109]. DALA has agricultural value as it can be used as herbicide and to increase photosynthesis resulting in the growth of plants [98].
Furfural
Furfural can be produced from various renewable agricultural resources like corn stalk, sugarcane bagasse, and eucalyptus wood [110]. Conventionally, xylose is used to make furfural, which in turn as a chemical feedstock to produce furfuryl alcohol [111], furoic acid, maleic acid, 5-membered oxygen-heterocycles, succinic acid, and levulinic acid [112]. Reduction of furfural produces tetrahydrofurfuryl alcohol which can be further converted into 1,5-pentanediol, a precursor to polyesters and polyurethanes [113]. Furfural has been extensively used in plastics, pharmaceutical and agrochemical industries, adhesives, and flavor enhancers [113].
Hydroxymethylfurfural (HMF)
5-HMF is C6 sugars aromatic compound and is produced by dehydration of hexoses in acidic media. Various furan derivatives like 2,5-diformylfuran, 2,5-dimethylfuran, 2,5-bis(hydroxymethyl)furan, 2,5-bis(aminomethyl)furan, 2,5-dihydroxymethyltetrahydrofuran, 1,2,6-hexanetriol, 1,6-hexanediol, 2,5-bis(aminomethyl) tetrahydrofuran, caprolactone, caprolactam are formed after hydrogenation, condensation, reduction, and oxidation of 5-HMF [20]. Some of the derivatives of HMF including 2,5-furandicarboxylic acid (FDCA), and 2,5-bis(hydroxymethyl)furan can be used as polyester [10, 98], other derivatives such as 2,5-dimethylfuran, 5-ethoxymethylfurfural, ethyl levulinate, and γ-valerolactone are upcoming biofuels [114]. 1,6-hexanediol is used in the preparation of polycarbonatediols to yield polyurethanes. Polyurethanes are applicable in coatings, elastomers, and adhesives. 1,6-hexanediamine and ε-caprolactone in the synthesis of various polymers. FDCA has many potential applications in polyesters, polyamides, and plasticizers [10].
Glycerol
Glycerol is a simple polyol (the simplest trihydric alcohol) compound and not a carbohydrate, but it has a mini sugar like structure. Glycerol is regarded as important material because many lipids contain glycerol backbone [100]. There are many derivatives formed from glycerol after undergoing fermentation, reduction, and rehydration. Some of them are; diglycerol, mannitol, 1,3-propanediol, propylene glycol, glycerol carbonate, ethylene glycol, glycidol, dihydroxyacetone, propane, acrolein, acrylic acid, glyceraldehyde, allyl alcohol, 3-methoxy-1-propene, glyceric acid, acetol [10, 100]. Glycidol is a derivative of glycerol, prospective for producing other industrially valuable chemicals like epoxy resins, polyurethanes, and polyglycerol esters [115]. Some microorganisms produce 1-butanol, 2,3-butanediol, 1,3-propanediol, ethanol, lactic acid, succinic acid, propionic acid, and dihydroxyacetone by utilizing glycerol [116]. Another derivative like glycerol carbonate has been used in the synthesis of industrially important chemicals such as glycidol, polymers, polyurethane foams, coatings, adhesives, and lubricants. Glycerol has also been utilized in the commercial production of epichlorohydrin (used as polymer and resins) [100, 117].
Sorbitol
Sorbitol is a sugar alcohol produced by the hydrogenation of glucose. Many compounds formed from sorbitol like isosorbide, sorbitan, sorbose, 2-ketogulonic acid, and vitamin C [10, 98]. Sorbitol and its derivatives are extensively used as sweetener, thickener, dispersant in food, cosmetics, toothpaste, polyurethane coatings, biocomposites, and hydrophilic interaction chromatography. Sorbitol prevents food by preventing the denaturation of protein, oxidation of fat, and retrogradation of starch. By fermentation, sorbitol is converted to L-ascorbic acid (vitamin C) forming 2-keto-L-gulonic acid as intermediate. This ascorbic acid can be used as an antioxidant for foods [118], also has nutritional value to prevent scurvy and other pharmacological matters [98]. Dehydration of sorbitol produces isosorbide and sorbitan. Isosorbide is used differently in medical fields like in regulating pressure in brain tumor (decreasing intracerebral pressure) and glaucoma (increasing intraocular pressure), and as medicine for Meniere’s disease and angina pectoris [98, 119]. By hydrogenolysis, sorbitol can be converted into lower alcohol, such as glycerol, propylene glycol, ethylene glycol, ethanol, and methanol. These lower alcohols further can be utilized in producing other value-added products [120].
3-Hydroxypropionic Acid (3-HPA)
3-HPA is formed by fermentation of glycerol. On catalytic hydrogenation of 3-HPA produces 1,3-propanediol, whereas heating of aqueous 3-HPA produces acrolein and acrylic acid. They are polymerized and used as an absorbent in diapers, hygiene products, coatings, adhesives, carpets, and fabrics. Traditionally, 3-HPA was produced from propylene (product of crude oil refining) oxidation, but now it is made as bio-based production [12, 100].
Xylitol
Xylitol is a five-carbon sugar alcohol, produced commercially by catalytic hydrogenation of xylose [100] found naturally in some fruits and vegetables with fewer calories [121]. Xylitol metabolism is not dependent on insulin; thus, it can be used in the therapeutic sector like sugar substitute for diabetes individuals [122]. Microbial production (from yeast) of xylitol is becoming very attractive because the high-quality product is produced and is cost-efficient. In addition, high pressure and temperature, and xylose purification are not needed to make xylitol from yeast [123]. Xylitol can be converted into ethylene glycol and propylene glycol. Ethylene glycol is further used as an anti-freeze and a precursor of polyester. Similarly, propylene glycol can be used as an anti-freeze, brake fluid, cosmetic and food additive, and food emulsifier [98].
Ethanol
From LB, ethanol is produced by the thermal gasification process and biochemical fermentation process following different steps like pretreatment, saccharification and hydrolysis, fermentation, and ethanol recovery [2, 124, 125]. The thermal gasification process includes biomass conversion into syngas (hydrogen and carbon monoxide) via partial oxidation at high temperature (500–800 °C), purification of syngas as the impurities affect the process and conversion of syngas into ethanol [126, 127] as shown in equations below.



From eqs. (1) and (2), carbon monoxide and hydrogen are produced in the same ratio, but methanol formation does not require the same ratio of carbon monoxide and hydrogen. Thus, carbon monoxide reacts with steam and adjusts the proportion of them [126] as in eq. (4).

Ethanol can be produced from syngas either by the chemical catalysis process [110] or the biological fermentation process [112]. In the biological process, the syngas produces ethanol, as shown in eqs. 5 and 6 [126].


Biological fermentation process is more specific and produces a higher yield of the desired product compared to the chemical catalytic process [100] and benefits from operating at ambient pressures and temperatures [126]. Dehydration and polymerization of ethanol can produce polyethylene, which further can produce polyvinylchloride (PVC) [100]. Bioethanol can act as renewable building blocks to produce ethylene, propylene, and butadiene synthesis. Additionally, bioethanol can also be converted into other chemical products such as acetaldehyde, ethyl acetate, and acetic acid. The rapidly increasing production of bioethanol is an important replacement for fossil fuel [113].
Enzymes
Industrially essential and highly demanded enzymes are cellulases, xylanases, ligases, pectinases, and proteases. Different substances present in LB favors different enzymes of microorganisms during solid-state fermentation or submerged fermentation. Different enzymes and their functions have already been described briefly in the section “Lignocellulosic enzymes”. Those enzymes are applied by various industries such as fuel, food, wine and brewery, animal feed, textile and laundry, pulp and paper, agriculture, etc. [61,62,63]. Hence to congregate the increasing demand for industrially essential enzymes and to comprehend the enzymes potentials in different fields, different integrative researches on fundamental and applied aspects is crucial and must be continued [62].
Bioactive Compounds
Fruit and vegetable biomass contain various health beneficial natural compounds known as bioactive compounds. These bioactive compounds are extracted by different methods, like solvent-based extraction, solid-liquid extraction, liquid-liquid extraction, ultrasound-assisted extraction, microwave-assisted extraction, enzyme-assisted extraction, etc. Biocatalyst, such as cellulase, pectinase, and hemicellulase, improve the extraction and recovery of these bioactive compounds like carotenoids, flavonoids, phenolic acids [128]. Such bioactive compounds are used as the antioxidant, anti-inflammatory, anti-allergic agents, and in the production of beauty products, prevention, and treatment of various diseases [2, 128].
The carotenoids group includes zeaxanthin, lutein, cryptochrome, lycopene β-carotene, β-cryptoxanthin, α-carotene, and β-carotene. They are found commonly in colored fruits and vegetables like tomato, different citrus peels, carrot waste, and shellfish. Also, these compounds are found in other photosynthetic organisms like cyanobacteria, algae, higher plants, some non-photosynthetic bacteria, yeast, and fungi [129, 130].
Flavonoids include luteolin, sinensetin, naringin, hesperidin, neohesperidin, diosmin, rutin, kaempferol, quercetin, which are present in different citrus peels [131]. Different flavonoids have other functions like protecting from ultraviolet radiation and phytopathogens, signaling during nodulation, male fertility, auxin transport, coloration of flower, attracts pollinators, etc. [132].
Phenolics include gallic acid, sinapic acid, ferulic acid, hydroxybenzoic acid, caffeic acid-O-glucoside, tryptophane, anthocyanins, flavonol glycosides, catechin, myricetin, which are present in different peels and seeds of fruits [133]. The source of phenolics are fruits, vegetables, and beverages like coffee, tea, wine, and fruit juices. Phenolics protect human tissue against oxidative stress, have different health benefits, and also avoid the development of chronic diseases [134].
Bioplastic
Using lignin bioplastic can be produced because lignin has glass transition temperatures, is renewable, and has thermoplastic properties. Bioplastic is competent with petroleum-based plastics in both cost and performance and sustainable, cost-effective, and biodegradable. However, lignin is rigid in nature, has relatively low molecular weight and high polydispersity that hinder the development of high-performance thermoplastic materials. Plasticizers or polymers having low glass transition temperatures can be added as addictive to improve the thermoplasticity of lignin, i.e., flexibility, processability, and durability [135].
Bioenergy/Electricity
The fossil energy is depleting continuously; thus, renewable energy source development is becoming essential. Potocnik [136] mentioned solar energy and biomass energy are two important energy sources. Technologies like solid oxide fuel cells and microbial fuel cells can convert biomass to electricity [137]. Photocatalytic conversion of LB can also generate electricity. It is mentioned that the International Energy Agency suggests bioenergy has the potential of providing 10% of the world’s energy supply by 2035, and biofuel can replace 27% of world transportation fuel by 2050 [138]. Microbial fuel cells (MFCs) are bio-electrochemical systems for electric power generation. MFCs can function at low temperatures, low electric power output, harsh reaction conditions, and limited lifetime hinder applications [139]. MFC is composed of anode and cathode. Organic compounds are oxidized at the anode, and electrons are liberated, which move through an external circuit to the cathode. At the cathode, electrons combine with an electron acceptor to generate electricity [138, 139].
Algae biomass has the potential to produce sustainable and low-cost electricity [140]. Activated sludge is reported to contain various types of electricity-producing bacteria such as Alcaligenes faecalis, Enterococcus gallinarum, Pseudomonas aeruginosa, and Shewanella sp. Thus, these bacteria help in waste treatment and electricity generation [141]. Electricity production from biomass using bio-electrochemical systems is also challenging because a single organism may not hydrolyze the biomass. However, binary culture or mixed culture of microbes like Clostridium cellulolyticum and Geobacter sulfurreducens can produce electricity [142].
Biodiesel
Biodiesel is another product that can be produced from LB. It is non-toxic, safe to use, eco-friendly, cost-effective, has excellent lubricity, and exhibits a higher flash point than petroleum diesel. The waste residues can be feed to oleaginous microbes such as Myxozyma melibiose, Lipomyces sp., Candida freyschussii, Aspergillus sp., Rhizopus sp., Mycobacterium sp., Haematococcus pluvialis, Euglena gracilis, Crypthecodinium cohnii, etc. to produce biodiesel [143]. Those microbes produce oil which is also known as single cell oil containing fatty acid very similar to vegetable oil. This prospect is beneficial as it can be potential alternatives for petroleum diesel. However, the production of biodiesel from LB can be impressive economically and technically. The biomasses such as maize cobs, rice bran, and sugar cane bagasse are used as raw material for microbial oil production, which is finally used for biodiesel production [144]. Thushari and Babel produced biodiesel by utilizing coconut waste and nonedible used oil employing solid acid catalyst one step procedure using different types of reactors [145].
Lignin Derivable Polymers
Lignin is considered as the key aromatic resource of the bio-based economy. Lignin contains different functional groups like methoxy, phenolic, hydroxyl, and aldehyde, thus can produce different chemicals particularly aromatic compounds and fuels. Some compounds derived from lignin are syringic acid, naphthalin methylnapthaline, p-coumaric acid, ferulic acid, caffeic acid, methanol, syringaldehyde, 4- hydroxybenzaldehyde, BTX (benzene, toluene, xylene), phenol and phenolics, benzoic acid, terephthalic acid, styrene, 4-hydroxybenzoic acid, eugenol, vanillin, vanillic acid, cinnamaldehyde, cinnamic acid, catechol, muconic acid, etc. [10, 146, 147]. These polymers are used for the production of aromatic monomers and polymers, as the bio-absorbents for heavy metal ions in wastewater purification, a roughage or fiber in food to prevent colon cancer, the anti-viral, antioxidant, additives, chemical fuels, the vehicles for gene delivery into human cells, a dispersing agent while using pesticides and herbicides [147, 148].
Single-Cell Protein
The world would need to produce millions of tons of meat and dairy products per year by 2050 to meet global demand for animal-derived protein as per current consumption levels [149]. The developing countries in the world reportedly have a significant protein deficiency concern, and hence there is a need for an increased protein in animal feed and human food supply. The protein demand can be fulfilled by single-cell protein (SCP) as an alternative to global food problems [150]. SCP can be produced from inexpensive waste materials like food and beverage processing industries, as well as directly from forestry and agricultural sources [151]. Algae, fungi, including filamentous fungi, yeast, and bacteria can all be used as SCP [151]. SCP derived from fungi provide vitamins primarily from the B-complex group (thiamine, riboflavin, biotin, niacin, pantothenic acid, pyridoxine, choline, glutathione, folic acid, and p-aminobenzoic acid). The cell walls of fungi being rich in glucans also provide fiber to the diet. Low-density lipoprotein cholesterol has been reduced when mycoprotein from Fusarium venenatum was consumed [152].
Syngas
Syngas is the synthetic gas produced when biomass is heated over 430 °C/860 ° F in the presence of oxygen or gas. This process is also known as gasification. Syngas thus produced from a renewable source like biomass can be used as the source for power production or can be converted into lower alcohol; methanol, ethanol, fuel, and chemical products; ammonia, dimethyl ether [153]. Polyhydroxyalkanoates (PHA), like biodegradable polymer, can be produced from the fermentation of syngas by microbes, and this can replace petrochemical plastics since this process is economical and feasible [128].
Biochar
LB can also be converted into carbonaceous solid material, i.e., biochar or activated carbon upon different processes like slow, intermediate and fast pyrolysis, gasification, hydrothermal carbonization, or flash carbonization [154] and various activation processes (chemical or physical treatments) [155]. The properties of biochar vary with the type of biomass, temperature differences for example living, colorful biomass having low surface area produces black biochar with a high surface area and no cellular structure. Biochar is used to mitigate greenhouse gas emissions, climate change, and mostly use in carbon sequestration. In the agricultural view, they are used to increase soil pH, decrease aluminum toxicity, remove pollutants, decrease soil tensile strength, improve soil conditions for earthworm populations, and improve the fertilizer use efficiency [156].
Dye Dispersants
There are different dyes like direct dyes, reactive dyes, disperse dyes, sulfur dyes, basic dyes, acid dyes, and solvent dyes. All those dyes are difficult to disperse in water without dispersants [157]. Dye dispersants have good thermal stability, are environment- friendly, can be renewed, and improve the dispersion performance. Therefore, the dye dispersants are attracting great attention. Dye dispersants are synthesized from lignosulfonate and alkali lignin, which are byproducts produced from the lignin industry [158]. Sodium lignosulfonate, naphthalene sulfonate formaldehyde condensates, sulfonated alkali lignin, and hydroxypropyl sulfonates alkaline lignin are four types of lignin-based dye dispersants. Among these four lignin-based dye dispersants, hydroxypropyl sulfonated alkaline lignin is the best due to its high molecular weight (110.2 kDa), higher temperature stability, and high dye uptake (85.3%). Sulfonated alkali lignin is used as a dye dispersant, has a dark color and severe staining problem, which is the main obstacle for its application. This staining problem was reduced effectively by removing the chromophores and made applicable as dye dispersant [159].
Nanocellulose
LB contains high fiber cellulose-rich waste material that can be converted into a novel and advanced material known as nanocellulose. Nanocellulose has unique properties like high specificity, high surface area, liquid crystalline behavior, barrier properties, surface chemical reactivity, biocompatibility, biodegradability, and lack of toxicity. Depending upon morphology, dimension and size, nanocellulose are grouped into three types; cellulose nanocrystals (CNC), cellulose nanofibrils (CNF), and bacterial cellulose (BC) [95, 96]. CNC and CNF are produced from cellulosic biomass after pretreatment and purification, whereas BC is made mainly from bacteria (Gluconacetobacter xylinus) that can use the variety of carbon sources [96]. Nanocellulose has a wide range of application; it can be used as a capacitor in the energy storage device, for controlled drug delivery, repairing connective tissue and congenital heart defects, constructing contact lenses and protective barriers, thermal insulation and fire retardation, ultrafiltration and packaging etc. [160].
Conclusion
In this review, the properties of LB and their components (cellulose, hemicelluloses, lignin, and pectin) are discussed. Different valuable products such as biochemicals, single-cell protein, biochar, nanocellulose, bioactive compounds, dyes dispersants, bioplastics, and electricity production have briefly been discussed, and their applications have been listed. In literature, it is evident that lignocellulosic materials contain several high-value substances such as sugars, minerals, and protein. When these materials are discarded in the environment, this may cause serious environmental problems and loss of these value-added substances to the soil or landfill. Therefore, emphasis should be made to reuse these organic wastes as raw materials to produce industrially relevant compounds like enzymes via fermentation, single-cell proteins, and many other products with appropriate technologies. Researchers need to be encouraged in a detailed study about the potentials of LB and different products, different efficient and versatile technologies for utilizing wide varieties of biomass, and more. The technologies applied need to be environment-friendly, non-hazardous, economically feasible, and easy for waste management. However, more extensive research is required for the commercial production of various valuable products by a resourceful, assimilated, friendly, and low-cost biotransformation process.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dashtban, M., Schraft, H., Qin, W.: Fungal bioconversion of lignocellulosic residues, opportunities and perspectives. Int. J. Biol. Sci. 5, 578–595 (2009)
Saini, A., Aggarwal, N.K., Sharma, A., Yadav, A.: Actinomycetes: a source of lignocellulolytic enzymes. Enzyme Res. 2015, 1–15 (2015)
Sabiiti, E.N.: Utilizing agricultural waste to enhance food security and conserve the environment. Afr. J. Food Agric. Nutr. Dev. 11, 1–9 (2011)
Ezcurra, A.I., De-Zarate, O., Dhin, P.V., Lacaux, J.P.: Cereal waste burning pollution observed in the town of Vitoria (northern Spain). Atmos. Environ. 35, 1377–1386 (2001)
Sanchez, C.: Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol. Adv. 27, 185–194 (2009)
Kumar, A., Gautam, A., Dutt, D.: Biotechnological transformation of lignocellulosic biomass into industrial products: an overview. Adv. Biosci. Biotechnol. 7, 149–168 (2016)
Iqbal, H.M.N., Kyazze, G., Keshavarz, T.: Advances in valorization of lignocellulosic materials by biotechnology: an overview. BioResources. 8, 3157–3176 (2013)
Gupta, V.K., Kubicek, C.P., Berrin, J.G., Wilson, D.W., Couturier, M., Berlin, A., Filho, E.X.F., Ezeji, T.: Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem. Sci. 41, 633–645 (2015)
Soccol, C.R., Faraco, V., Karp, S.G., Vandenberghe, L.P.S., Thomaz-Soccol, V., Woiciechowski, A.L., Pandey, A.: Lignocellulosic Bioethanol: Current Status and Future Perspectives. Biofuels: Alternative Feedstocks and Conversion Process for the Production of Liquid and Gaseous Biofuels, 2nd edn, pp. 331–354. Academic press / Elsevier Inc, New York (2019)
Isikgor, F.H., Becer, C.R.: Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 6, 4497–4559 (2015)
Rancourt, Y., Neumeyer, C., Zou, N.: Results from 2015 Bio-Products Production and Development Survey. A report by statistics Canada, Ottawa (2017)
Biotechnology Innovation Organization: Advancing the bio-based economy: Renewable chemical biorefinery commercialization, progress and market opportunities, 2016 and beyond. http://www.bio.org/sites/default/files/BIO_Advancing _the _Biobased _Economy _2016.pdf (2016)
Cherubini, F.: The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energ. Convers. Manage. 51, 1412–1421 (2010)
Huber, G.W.: Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: Next Generation Hydrocarbon Biorefineries, pp. 23–25. National Science Foundation, New York (2008)
Larson, E.: Biofuel Production Technologies: Status, Prospects and Implications for Trade and Development, p. 41. United Nations Conference on Trade and Development, Geneva (2008)
De Jong, E., Higson, A., Walsh, P., Wellissch, M.: Bio-based chemicals, value added products from biorefineries. In: IEA Bioenergy task 42 report. https://www.ieabioenergy.com/wpcontent/uploads/2013/10/Task-42-Biobased-Chemicals value-added-products-from biorefineries.pdf (2013)
Saritha, M., Arora, A.: Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol. 52, 122–130 (2012)
Zhou, C.H., Xia, X., Lin, C.X., Tonga, D.S., Beltraminib, J.: Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 40, 5588–5617 (2011)
Alonso, D.M., Wettstein, S.G., Dumesic, J.A.: Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 15, 584–595 (2013)
Melero, J.A., Iglesias, J., Garcia, A.: Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ. Sci. 5, 7393–7420 (2012)
Rajendran, K., Drielak, E., Varma, V.S., Muthusamy, S., Kumar, G.: Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production–a review. Biomass Conver. Biorefin. 8, 471–483 (2017)
Saha, B.C.: Enzymes as Biocatalysts for Conversion of Lignocellulosic Biomass to Fermentable Sugars, in Handbook of Industrial Biocatalysis, C.R.C. Press, pp. 1–12 (2005)
Stocker, M.: Biofuels and biomass-to-biofuels in the biorefinery: catalytic conversion of lignocellulosic biomass using porous materials. Angew. Chem. Int. Ed. Engl. 47, 9200–9211 (2008)
Doran-Peterson, J., Cook, D.M., Brandon, S.K.: Microbial conversion of sugars from plant biomass to lactic acid or ethanol. Plant J. 54, 582–592 (2008)
Edwards, M.C., Henriksen, E.D., Yomano, L.P., Gardner, B.C., Sharma, L.N., Ingram, L.O., Peterson, J.D.: Addition of genes for Cellobiase and Pectinolytic activity in Escherichia coli for fuel ethanol production from pectin-rich Lignocellulosic biomass. App. Environ. Microbio. 77, 5184–5191 (2011)
Mohen, D.: Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277 (2008)
Scheller, H.V., Ulvskov, P.: Hemicelluloses. Annu. Rev. Plant Biol. 61, 263–289 (2010)
Bajpai, P.: Pretreatment of lignocellulosic biomass for biofuel production: Structure of lignocellulosic biomass, pp 17–70. Springer eBooks (2016)
Vassilev, S.V., Baxter, D., Andersen, L.K., Vassileva, C.G., Morgan, T.J.: An overview of the organic and inorganic phase composition of biomass. Fuel. 94, 1–33 (2012)
Agbor, V.B., Cicek, N., Sparling, R., Berlin, A., Levin, D.B.: Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 29, 675–685 (2011)
Morais, S., Morag, E., Barak, Y., Goldman, D., Hadar, Y., Lamed, R., Shoham, Y., Wilson, D.B., Bayer, E.A.: Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes. Microbiology. 3, 1–11 (2012)
Gardner, K., Blackwell, J.: The structure of native cellulose. Biopolymers. 13, 1975–2001 (1974)
Li, X., Hua-jun-Yang, H., Roy, B., Wang, D., Yue, W., Jiang, L., Park, E.Y., Miao, Y.: The most stirring technology in future: Cellulase enzyme and biomass utilization. Afr. J. Biotechnol. 8, 2418–2422 (2009)
VanderHart, D.L., Atalla, R.H.: Studies of microstructure in native celluloses using solid-state carbon-13 N.M.R. Macromolecules. 17, 1465–1472 (1984)
Kuhad, R.C., Singh, A., Eriksson, K.E.L.: Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Adv. Biochem. Eng. Biotechnol. 57, 45–125 (1997)
Klemm, D., Heublein, B., Fink, H.P., Bohn, A.: Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 44, 3358–3393 (2005)
Sorieul, M., Dickson, A., Hill, S.J., Pearson, H.: Plant fibre: molecular structure and biomechanical properties, of a complex living material, influencing its deconstruction towards a biobased composite. Materials. 9, 1–36 (2016)
Saha, B.C.: Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30, 279–291 (2003)
Pérez, J., Muñoz-Dorado, J., De-la-Rubia, T., Martínez, J.: Biodegradation and biological treatments of cellulose, hemicelluloses and lignin: an overview. Int. Microbiol. 5, 53–63 (2002)
Xiao, C., Anderson, C.T.: Roles of pectin in biomass yield and processing for biofuels. Frontiers in plant sci. 4(67) (2013)
Liaotrakoon, W., Van Buggenhout, S., Christiaens, S., Houben, K., De Clercq, N., Dewettinck, K., Hendrickx, M.E.: An explorative study on the cell wall polysaccharides in the pulp and peel of dragon fruits (Hylocereus spp.). European Food Res. Technol. 237, 341–351 (2013)
Taboada, E., Fisher, P., Jara, R., Zúñiga, E., Gidekel, M., Cabrera, J.C., Pereira, E., Gutierrez-Moraga, A., Villalonga, R., Cabrera, G.: Isolation and characterization of pectic substances from murta (Ugni molinae Turcz) fruits. Food Chem. 123, 669–678 (2010)
Abid, M., Cheikhrouhou, S., Renard, C.M., Bureau, S., Cuvelier, G., Attia, H., Ayadi, M.A.: Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 215, 318–325 (2017)
Saulnier, L., Thibault, J.F.: Extraction and characterization of pectic substances from pulp of grape berries. Carbohydr. Polym. 7, 329–343 (1987)
Kulkarni, S.G., Vijayanand, P.: Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Sci. Technol. 43, 1026–1031 (2010)
Femenia, A., Garcia-Conesa, M., Simal, S., Rosselló, C.: Characterisation of the cell walls of loquat (Eriobotrya japonica L.) fruit tissues. Carbohydr. Polym. 35, 169–177 (1998)
Boluda-Aguilar, M., García-Vidal, L., del Pilar González-Castañeda, F., López-Gómez, A.: Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Bioresour. Technol. 101, 3506–3513 (2010)
Hilz, H., Bakx, E.J., Schols, H.A., Voragen, A.G.: Cell wall polysaccharides in black currants and bilberries—characterisation in berries, juice, and press cake. Carbohydr. Polym. 59, 477–488 (2005)
Liang, R.H., Chen, J., Liu, W., Liu, C.M., Yu, W., Yuan, M., Zhou, X.Q.: Extraction, characterization and spontaneous gel-forming property of pectin from creeping fig (Ficus pumila Linn.) seeds. Carbohydr. Polym. 87, 76–83 (2012)
Ridley, B.L., O’Neill, M., Mohen, D.: Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochem. 57, 929–967 (2001)
Coetzee, B., Schols, H.A., Wolfaardt, F.: Determination of pectin content of eucalyptus wood. Holzforschung. 65, 327–331 (2011)
Edwards, M.C., Doran-Peterson, J.: Pectin-rich biomass as feedstock for fuel ethanol production. Appl. Microbiol. Biotechnol. 95, 565–575 (2012)
Marcus, S.E., Verhertbruggen, Y., Herve, C., Ordaz-Ortiz, J.J., Farkas, V., Pedersen, H.L., Willats, W.G., Knox, J.P.: Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 8, 60 (2008)
Willats, W.G., Mccartney, L., Mackie, W., Knox, J.P.: Pectin: cell biology and prospects for functional analysis. Plant Mol. Biol. 47, 9–27 (2001)
Pedrolli, D.B., Monteiro, A.C., Gomes, E., Carmona, E.C.: Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 3, 9–18 (2009)
Chen, J., Liu, W., Liu, C.M., Li, T., Liang, R.H., Luo, S.J.: Pectin modifications: a review. Critical reviews in food sci. nutri. 55, 1684–1698 (2015)
Sharma, H.K., Xu, C., Qin, W.: Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valoriz. 10, 235–251 (2019)
Chaturvedi, V., Verma, P.: An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value-added products. 3 Biotech. 3, 415–431 (2013)
Puligundla, P., Oh, S.E., Mok, C.: Microwave-assisted pretreatment technologies for the conversion of lignocellulosic biomass to sugars and ethanol: a review. Carbon Letters (Carbon Lett.). 17, 1–10 (2016)
Tu, W.C., Hallett, J.P.: Recent advances in the pretreatment of lignocellulosic biomass. Current opinion Green Sustainable Chem. 20, 11–17 (2019)
Kuhad, R.C., Singh, A.: Lignocellulose Biotechnology: Future Prospects, Pp 400. I.K. International Ltd, New Delhi (2007)
Kuhad, R.C., Gupta, R., Singh, A.: Microbial celluloses and their industrial applications. Enzymes Res. 2011, 1–10 (2011)
Mtui, G.Y.S.: Lignocellulolytic enzymes from tropical fungi: types, substrates and applications- review. Scientific Res. Essays. 15, 1544–1555 (2012)
Agrawal, R., Semwal, S., Kumar, R., Mathur, A., Gupta, R.P., Tuli, D.K., Satlewal, A.: Synergistic enzyme cocktail to enhance hydrolysis of steam exploded wheat straw at pilot scale. Front. Energy Res. 6, 122 (2018)
Henrissat, B., Davies, G.: Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7, 637–644 (1997)
Beguin, P., Aubert, J.P.: The biological degradation of cellulose. FEMS Microbiol. Rev. 13, 25–58 (1994)
Lynd, L.R., Weimer, P.J., van Zyl, W.H., Pretorius, I.S.: Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 (2002)
Bhat, M.K., Bhat, S.: Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 15, 583–620 (1997)
Maki, M., Leung, K.T., Qin, W.: The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 5, 500–516 (2009)
Adsul, M., Sandhu, S.K., Singhania, R.R., Gupta, R., Puri, S.K., Mathur, A.: Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzyme Microbial Technol. 133, 109442 (2020)
Sajith, S., Priji, P., Sreedevi, S., Benjamin, S.: An overview on fungal cellulases with an industrial perspective. J. Nutr. Food Sci. 6, 1–13 (2016)
Sweeney, M.D., Xu, F.: Biomass converting enzymes as industrial biocatalysts for fuels and chemicals: recent developments. Catalysts. 2, 244–263 (2012)
Walia, A., Guleria, S., Mehta, P., Chauhan, A., Parkash, J.: Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech. 7, 11 (2017)
Chauhan, P.S., Puri, N., Sharma, P., Gupta, N.: Mannanases: microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 93, 1817–1830 (2012)
Dekker, R.F.H.: Biodegradation of the hemicelluloses. In: Higuchi, T. (ed.) Biosynthesis and Biodegradation of Wood Components, pp. 505–533. Academic Press, New York (1985)
Niladevi, K.N.: Ligninolytic Enzymes, in: Biotechnology for Agro-Industrial Residues Utilization, pp. 397–414. Springer, Dordrecht (2009)
Sokan-Adeaga, A.A., Ana, G.R.E.E., Sokan-Adeaga, M.A., Sokan-Adeaga, E.D.: Lignocelluloses: an economical and ecological resource for bio-ethanol production – a review. Intl. J. Natural Resour. Ecology and Management. 1, 128–144 (2016)
Hofrichter, M.: Lignin conversion by manganese peroxidase (MnP). Enzyme Microbio. Technol. 30, 454–466 (2002)
Tian, J.H., Pourcher, A.M., Bouchez, T., Gelhaye, E., Peu, P.: Occurrence of lignin degradation genotypes and phenotypes among prokaryotes- review. Appl. Microbiol. Biotechnol. 98, 9527–9544 (2014)
Heinzkill, M., Bech, L., Halkier, T., Schneider, P., Anke, T.: Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl. Environ. Microbio. 64, 1601–1606 (1998)
Huang, X.F., Santhanam, N., Badri, D.V., Hunter, W.J., Manter, D.K., Decker, S.R., Vivanco, J.M., Reardon, K.F.: Isolation and characterization of lignin-degrading bacteria from rainforest soils. Biotechnol. Bioeng. 110, 1616–1626 (2013)
Fisher, A.B., Fong, S.S.: Lignin biodegradation and industrial implications. AIMS Bioeng. 1, 92–112 (2014)
Singh, R., Kumar, M., Mittal, A., Mehta, P.K.: Microbial enzymes: industrial progress in 21st century. 3 Biotech. 6, 1–15 (2016)
Maciel, M.J., Silva, A.C., Ribeiro, H.C.T.: Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. Electron. J. Biotechnol. 13, 14–15 (2010)
Placido, J., Capareda, S.: Ligninolytic enzymes: a biotechnological alternative for bioethanol production. Biores. Bioprocess. 2, 1–12 (2015)
Lobos, S., Larram, J., Salas, L., Cullen, D., Vicuna, R.: Isoenzymes of manganese dependent peroxidase and laccase produced by the lignin degrading basidiomycete Ceriporiopsis subvermispora. Microbio. 14, 2691–2698 (1994)
Hammel, K.E., Cullen, D.: Role of fungal peroxidases in biological ligninolysis. Curr. Opin. Plant Biol. 11, 349–355 (2008)
Kashyap, D.R., Vohra, P.K., Chopra, S., Tewari, R.: Applications of pectinases in the commercial sector: a review. Bioresour. Technol. 77, 215–227 (2001)
Jayani, R.S., Saxena, S., Gupta, R.: Microbial pectinolytic enzymes: a review. Process Biochem. 40, 2931–2944 (2005)
Garg, G., Singh, A., Kaur, A., Singh, R., Kaur, J., Mahajan, R.: Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech. 6, 47 (2016)
Geetha, M., Saranraj, P., Mahalakshmi, S., Reetha, D.: Screening of pectinase producing bacteria and fungi for its pectinolytic activity using fruit waste. Int. J. Biochem. Biotechnol. Sci. 1, 30–42 (2012)
Pandey, A., Selvakumar, P., Soccol, C.R., Nigam, P.: Solid-state fermentation for the production of industrial enzymes. Curr. Sci. 77, 149–162 (2009)
Grethlein, H.E., Converse, A.O.: Common aspects of acid prehydrolysis and steam explosion for pretreating wood. Bioresour. Technol. 36, 77–82 (1991)
Howard, R.L., Abotsi, E., Jansen-van-Rensburg, E.L., Howard, S.R.: Lignocellulose biotechnology: issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2, 602–619 (2003)
Cho, E.J., Trinh, L.T.P., Song, Y., Lee, Y.G., Bae, H.J.: Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 298, 122386 (2020)
Garcia, A., Gandini, A., Labidi, J., Belgacem, N., Bras, J.: Industrial and crop wastes: a new source for nanocellulose biorefinery. Ind. Crop. Prod. 93, 26–38 (2016)
Liu, X., Duan, X., Wei, W., Wang, S., Ni, B.J.: Photocatalytic conversion of lignocellulosic biomass to valuable products. Green Chem. 21, 4266–4289 (2019)
Kobayashi, F.A.: Synthesis and utilization of sugar compounds derived from lignocellulosic biomass. Green Chem. 15, 1740–1763 (2013)
Werpy, T.A., Petersen, G.: Top value-added chemicals from biomass: I. Results of screening for potential candidates from sugars and synthesis gas. National Renewable Energy Lab United States (2004). https://doi.org/10.2172/15008859
Bozell, J.J., Petersen, G.R.: Technology development for the production of biobased products from biorefinery carbohydrates-the U.S. Department of Energy’s "top 10" revisited. Green Chem. 12, 539–554 (2010)
Luque, R., Clark, J.H., Yoshida, K.: Gai, PL: efficient aqueous hydrogenation of biomass platform molecules using supported metal nanoparticles on Starbons. Chem. Comm. 45, 5305–5307 (2009)
Delhomme, C., Weuster-Botz, D., Kuhn, F.E.: Succinic acid from renewable resources as a C4 building-block chemical-a review of the catalytic possibilities in aqueous media. Green Chem. 11, 13–26 (2009)
Nhuan, P.N., Kleff, S., Schwegmann, S.: Review succinic acid: technology development and commercialization. Fermentation. 3, 1–14 (2017)
Mäki-Arvela, P., Simakova, L.I., Salmi, T., Murzin, D.Y.: Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 114, 1909–1971 (2014)
Kahlich, D., Wiechern, U., Lindner, J.: Propylene Oxide, In Ullmann’s Encyclopedia of Industrial Chemistry, (Ed.). Wiley-VCH (2011). https://doi.org/10.1002/14356007.a22_239.pub2
Alsaheb, R.A.A., Aladdin, A., Othman, N.Z., Malek, R.A., Leng, O.M., Aziz, R., El Enshasy, H.A.: Lactic acid applications in pharmaceutical and cosmeceutical industries. J. Chem. Pharm. Res. 7, 729–735 (2015)
Nee; Nigam, P.S.: Chapter 3: Production of Organic Acids from Agro-Industrial Residues, Book Biotechnology for Agro Industries, pp. 38–58. Springer, New Delhi (2009)
Fernandes, D.R., Rocha, A.S., Mai, E.F., Mota, C.J.A., Teixeira, D.S.A.: Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A: General. 425-426, 199–204 (2012)
Bozell, J.J., Moens, L., Elliott, D.C., Wang, Y., Neuenscwander, G.G., Fitzpatrick, S.W., Bilski, R.J., Jarnefeld, J.L.: Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recy. 28, 227–239 (2000)
Dutta, S., De, S., Saha, B.: A brief summary of the synthesis of polyester building-block chemicals and biofuels from 5-hydroxymethylfurfural. ChemPlusChem. 77, 259–272 (2012)
Yan, K., Wu, G., Lafleur, T., Jarvis, C.: Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sust. Energ. Rev. 38, 663–676 (2014)
Mariscal, R., Maireles-Torres, P., Ojeda, M., Sadaba, I., Lopez, G.M.: Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Env. Sci. 9, 1144–1189 (2016)
Takkellapati, S., Li, T., Gonzalez, M.A.: An overview of biorefinery derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Techn. Environ. Policy. 20, 1615–1630 (2018)
Van-Putten, R.J., van der Waal, J.C., De Jong, E., Rasrendra, C.B., Heeres, H.J., De Vries, J.G.: Hydroxymethylfurfural: a versatile platform chemical made from renewable resources. Chem. Rev. 113, 1499–1597 (2013)
Bai, R., Zhang, H., Mei, F., Wang, S., Li, T., Gu, Y., Li, G.: One-pot synthesis of glycidol from glycerol and dimethyl carbonate over a highly efficient and easily available solid catalyst NaAlO2. Green Chem. 15, 2929–2934 (2013)
Almeida, J.R., Fávaro, L.C., Quirino, B.F.: Biodiesel biorefinery: opportunities and challenges for microbial production of fuels and chemicals from glycerol waste. Biotechnol. Biofuels. 5, 48 (2012)
Santacesaria, E., Tesser, R., Di Serio, M., Casale, L., Verde, D.: New process for producing Epichlorohydrin via glycerol chlorination. Ind. Eng. Chem. Res. 49, 964–970 (2010)
Pappenberger, G., Hohmann, H.P.: Industrial production of L-ascorbic acid (vitamin C) and D-isoascorbic acid. Adv. Biochem. Eng. Biotechnol. 143, 143–188 (2014)
Parker, J.D., Parker, J.O.: Nitrate therapy for stable angina pectoris. N. Engl. J. Med. 338, 520–531 (1998)
Zhang, J., Li, J., Wu, S.B., Liu, Y.: Advances in catalytic production and utilization of sorbitol. Ind. Eng. Chem. Res. 52, 11799–11815 (2013)
Star-Colibri: Strategic targets for 2020-collaboration initiative on biorefineries: Background information and biorefinery status, potential and Sustainability. https://edepot.wur.nl/158542 (2010). Accessed 18 Mar 2010
Lugani, Y., Oberoi, S., Sooch, B.S.: Xylitol: a sugar substitute for patients of diabetes mellitus. World J. Pharm. Pharm. Sci. 6, 741–749 (2017)
Guo, X., Zhang, R., Li, Z., Dai, D., Li, C., Zhou, X.: A novel pathway construction in Candida tropicalis for direct xylitol conversion from corncob xylan. Bioresour. Technol. 128, 547–552 (2013)
Kim, S.: Evaluation of alkali-pretreated soybean straw for lignocellulosic bioethanol production. Int. J. Polym. Sci. 2018, 1–7 (2018)
Subramani, V., Gangwal, S.K.: A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol. Energy Fuel. 22, 814–839 (2008)
Wyman, C.E., Kumar, R., Cai, C.M.: Bioethanol from Lignocellulosic Biomass. U.C. Riverside (2017). https://doi.org/10.1007/978-1-4939-2493-6_521-3
Abubackar, H.N., Veiga, M.C., Kennes, C.: Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuels Bioprod. Biorefin. 5, 93–114 (2011)
Choi, D.W., Chipman, D.C., Bents, S.C., Brown, R.C.: A techno-economic analysis of polyhydroxyalkanoate and hydrogen production from syngas fermentation of gasified biomass. Appl. Biochem. Biotechnol. 160, 1032–1046 (2010)
Misawa, N.: Pathway engineering of plants toward astaxanthin production. Plant Biotechnol. 26, 93–99 (2009)
Jaswir, I., Noviendri, D., Harini, R.F., Octavianti, F.: Carotenoids: sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 5, 7119–7131 (2011)
Lou, S.N., Hsu, Y.S., Ho, C.T.: Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. J. Food and drug Analysis. 22, 290–295 (2014)
Bradshaw, H.D., Schemske, D.W.: Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 426, 176–178 (2003)
Saini, A., Panesar, P.S., Bera, M.B.: Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions based delivery system. Biores. Bioprocess. 6, 1–12 (2019)
Minatel, O., Borges, C.V., Ferreira, M.I., Gomez, H.A.G., Chen, C.Y.O., Lima, G.P.P.: Phenolics compounds: Functional properties, impact of processing and bioavailability- In Phenolic compounds- biological activity (2017). https://doi.org/10.5772/66368
Wang, C., Kelley, S.S., Venditti, R.A.: Lignin-based thermoplastic materials. ChemSusChem. 9, 770–783 (2016)
Potočnik, J.: Renewable energy sources and the realities of setting an energy agenda. Science. 315, 810–811 (2007)
Scacchi, C.C.O., Gonzalez-Garcia, S., Caserini, S., Rigamonti, L.: Greenhouse gases emissions and energy use of wheat grain-based bioethanol fuel blends. Sci. Total Environ. 21, 5010–5018 (2010)
Wang, S., Dai, G., Yang, H., Luo, Z.: Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog. Energy Combust. Sci. 62, 33–86 (2017)
Rosenbaum, M., He, Z., Angenent, L.T.: Light energy to bioelectricity: photosynthetic microbial fuel cells. Current Opinion in Biotechnol. 21, 259–264 (2010)
Velasquez-Orta, S.B., Curtis, T.P., Logan, B.E.: Energy from algae using microbial fuel cells. Biotechnol. Bioeng. 103, 1068–1076 (2009)
Rashid, N., Cui, Y.F., Rehman, M.S.U., Han, J.I.: Enhanced electricity generation by using algae biomass and activated sludge in microbial fuel cell. Sci. Total Environ. 456-457, 91–97 (2013)
Ren, Z., Ward, T.E., Regan, J.M.: Electricity production from cellulose in a microbial fuel cell using a defined binary culture. Environ. Sci. Technol. 41, 4781–4786 (2007)
Gujjala, L.K., Kumar, S.J., Talukdar, B., Dash, A., Kumar, S., Sherpa, K.C., Banerjee, R.: Biodiesel from oleaginous microbes: opportunities and challenges. Biofuels. 10, 45–59 (2019)
Yousuf, A.: Biodiesel from lignocellulosic biomass–prospects and challenges. Waste Manag. 32, 2061–2067 (2012)
Thushari, I., Babel, S.: Biodiesel production from waste palm cooking oil using solid acid catalyst derived from coconut meal residue. Waste Biomass Valor. 11, 4941–4956 (2020)
Vanarasi, P., Singh, P., Auer, M., Adams, P.D., Simmons, B.A., Singh, S.: Survey of renewable chemicals produced from lignocellulosic biomass during ionic liquid pretreatment. Biotechnol. Biofuels. 6, 1–9 (2013)
Agrawal, A., Kaushik, N., Biswas, S.: Derivatives and applications of lignin – an insight – review article. The Scitech. 1, 2348–2311 (2014)
Bozell, J.J., Holladay, J.E., Johnson, D., White, D.F.: Top Value-Added Candidates from Biomass, Volume II: Results of Screening for Potential Candidates from Biorefinery Lignin, pp. 48–77. Pacific Northwest National Laboratory, Springfield (2007)
Ritala, A., Häkkinen, S.T., Toivari, M., Wiebe, M.G.: Single cell protein—state-of-the-art, industrial landscape and patents 2001-2016. Front. Microbiol. 8, 2009 (2017)
Mondal, A.K., Sengupta, S., Bhowal, J., Bhattacharya, D.K.: Utilization of fruit wastes in producing single cell protein. Int. J. Environ. Sci. Technol. 1, 430–438 (2012)
Anupama, R.P.: Value-added food: single cell protein. Biotechnol. Adv. 18, 445–479 (2000)
Turnbull, W.H., Leeds, A.R., Edwards, G.D.: Mycoprotein reduces blood lipids in free-living subjects. Am. J. Clin. Nutr. 55, 415–419 (1992)
NSF.: Breaking the chemical and engineering barriers to lignocellulosic biofuels: Next generation hydrocarbon biorefineries. Ed. George W. Huber, University of Massachusetts Amherst, pp 180. National Science Foundation, Chemical, Bioengineering, Environmental, and Transport Systems Division, Washington DC (2008)
Meyer, S., Glaser, B., Quicker, P.: Technical, economical, and climate-related aspects of biochar production technologies: a literature review. Environ. Sci. Technol. 45, 9473–9483 (2011)
Srinivasan, P., Sarmah, K.A., Smernik, R., Das, O., Farid, M., Gao, W.: A feasibility study of agricultural and sewage biomass as biochar, bioenergy and biocomposites feedstock: production, characterization and potential applications. Sci. Total Environ. 512-513, 495–505 (2015)
Xiao, X., Chen, B., Chen, Z., Zhu, L., Schnoor, L.J.: Insight into multiple and multilevel structures of biochars and their potential environmental applications: a critical review. Environ. Sci. Technol. 52, 5027–5047 (2018)
Yang, D.J., Li, H., Qin, Y.L., Zhong, R.S., Bai, M.X., Qiu, X.Q.: Structure and properties of sodium lignosulfonate with different molecular weight used as dye dispersant. J. Dispers. Sci. Technol. 36, 532–539 (2015)
Qin, Y., Lin, X., Lu, Y., Wu, S., Yang, D., Qiu, X., Fang, Y., Wang, T.: Preparation of a low reducing effect sulfonatd alkali lignin and application as dye dispersant. Polymers. 10, 982 (2018)
Qiu, X., Yu, J., Yang, J., Mo, W., Qian, Y.: Whitening sulfonated alkali lignin via H2O2/UV radiation and its application as dye dispersant. ACS Sustain. Chem. Eng. 6, 1055–1060 (2018)
Bacakova, L., Pajorova, J., Bacakova, M., Skogberg, A., Kallio, P., Kolarova, K., Svorcik, V.: Versatile application of nanocellulose: from industry to skin tissue engineering and wound healing. Nanomaterials. 9, 1–39 (2019)
de Souza, A.P., Grandis, A., Leite, D.C., Buckeridge, M.S.: Sugarcane as a bioenergy source: history, performance, and perspectives for second-generation bioethanol. Bioenergy Res. 7, 24–35 (2014)
Emaga, T.H., Robert, C., Ronkart, S.N., Wathelet, B., Paquot, M.: Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 99, 4346–4354 (2008)
Rabemanolontsoa, H., Saka, S.: Comparative study on chemical composition of various biomass species. RSC Adv. 3, 3946–3956 (2013)
Bhushan, S., Kalia, K., Sharma, M., Singh, B., Ahuja, P.S.: Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 28, 285–296 (2008)
Banerjee, J., Singh, R., Vijayaraghavan, R., MacFarlane, D., Patti, A.F., Arora, A.: Bioactives from fruit processing wastes: green approaches to valuable chemicals. Food Chem. 225, 10–22 (2017)
Zheng, Y.X., Wang, Y.L., Pan, J., Zhang, J.R., Dai, Y., Chen, K.Y.: Semi-continuous production of high-activity pectinases by immobilized Rhizopus oryzae using tobacco wastewater as substrate and their utilization in the hydrolysis of pectin-containing lignocellulosic biomass at high solid content. Biores.Technol. 241, 1138–1144 (2017)
Wang, J., Chio, C., Chen, X., Su, E., Cao, F., Jin, Y., Qin, W.: Efficient saccharification of agave biomass using Aspergillus niger produced low-cost enzyme cocktail with hyperactive pectinase activity. Bioresour. Technol. 272, 26–33 (2019)
Di-Blasi, C., Branca, C., Galgano, A.: Biomass screening for the production of furfural via thermal decomposition. Ind. Eng. Chem. Res. 49, 2658–2671 (2010)
Thite, V.S., Nerurkar, A.S.: Physicochemical characterization of pectinase activity from Bacillus spp. and their accessory role in synergism with crude xylanase and commercial cellulase in enzyme cocktail mediated saccharification of agrowaste biomass. J. applied microbio. 124, 1147–1163 (2018)
Rahimi, S., Hasanloo, T.: The effect of temperature and pH on biomass and bioactive compound production in Silybum marianum hairy root cultures. Res. J. Pharmacognosy. 3, 53–59 (2016)
Lee, M.J., Son, K.H., Oh, M.M.: Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Horticul. Environ. Biotechnol. 57, 139–147 (2016)
Nitsos, C., Matsakas, L., Triantafyllidis, K., Rova, U., Christakopoulos, P.: Investigation of different pretreatment methods of Mediterranean-type ecosystem agricultural residues: characterisation of pretreatment products, high-solids enzymatic hydrolysis and bioethanol production. Biofuels. 9, 545–558 (2018)
Schmidt, L.M., Mthembu, L.D., Reddy, P., Deenadayalu, N., Kaltschmitt, M., Smirnova, I.: Levulinic acid production integrated into a sugarcane bagasse based biorefinery using thermal-enzymatic pretreatment. Indus. crops products. 99, 172–178 (2017)
Tao, P., Zhang, Y., Wu, Z., Liao, X., Nie, S.: Enzymatic pretreatment for cellulose nanofibrils isolation from bagasse pulp: transition of cellulose crystal structure. Carbohydr. Polym. 214, 1–7 (2019)
Funding
No funding was provided for this review.
Author information
Authors and Affiliations
Contributions
SS did the major contribution in writing the manuscript, A.L.M.K. did proofread, and all authors have helped in writing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrestha, S., Kognou, A.L.M., Zhang, J. et al. Different Facets of Lignocellulosic Biomass Including Pectin and Its Perspectives. Waste Biomass Valor 12, 4805–4823 (2021). https://doi.org/10.1007/s12649-020-01305-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01305-w