Abstract
Response surface methodology based on the 24 factorial central composite design (CCD) was applied to optimize the liquefaction and saccharification process for the enhancement of sugar production from raw corn starch. Optimum level of the pre and post-cooking α-amylase and glucoamylase dose were found to be 3.0 and 1.5 U/ml, respectively, while saccharification temperature for the maximum sugar production was found to be 50 °C. The quadratic model predicted that maximum conversion efficiency for the enhanced sugar production from raw corn starch was 93.65 % under the above mentioned optimum level of variables. Additional experiments performed in triplicate with the optimized level of variables showed maximum conversion efficiency of 94.12 %, in close agreement between the predicted and experimental results. The generated sugar was subjected for the bioethanol production in separate fermentation system using yeast while simultaneous saccharification and fermentation system was also evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conventional method of starch hydrolysis using acid has been replaced by processes using starch saccharifying enzymes, accounting for approximately 15 % share in the world enzyme market. There are numerous bacteria and fungi from which amylases can be isolated but the most thoroughly studied are Bacillus and Aspergillus spp. [1]. Industrially important enzymes including amylases have traditionally been obtained from submerged cultures because of ease of handling and greater control of environmental factors such as temperature and pH. Solid state fermentation (SSF) is an interesting alternative since the metabolites produced are concentrated and purification procedures are less costly [2, 3]. A low-cost SSF process can supply enzyme extracts with a high potential for application in the cold hydrolysis of raw starch from agroindustrial cakes [4].
Increasing price of crude oil and other fossil fuels have raised interest in alternative fuel sources around the world. The most common bio-fuel is bio-ethanol, accounting for more than 90 % of total bio-fuel usage. Fuel alcohol production from starch needs constant process improvement in the biomass conversion to fuel alcohol for making it economically viable. Around 60 % of the total ethanol is produced by fermentation [5]. Research and development efforts aimed at commercial production of ethanol by fermentation from renewable resources such as crop residues and biomass waste [6–11], municipal solid wastes (MSW) [12–16], municipal sludge [17], and dairy/cattle manures [18] have increased.
Several methods reported for the fermentative production of ethanol from starch include (1) simultaneous saccharification and fermentation with a mixed culture of amylolytic and an ethanol-producing microorganism [19], (2) use of amylolytic enzymes from bacteria and fermenting yeast both together for the saccharification and fermentation of starch [20], (3) addition of glucoamylase to the raw starchy materials prior to fermentation, which is a common practice in industry [21].
We have already optimized the production and purification process of glucoamylase and α-amylase from Colletotrichum sp. KCP1 and Bacillus amyloliquefaciens KCP2, respectively [22–24]. In the present investigation we have used a low cost SSF system for production of thermo-stable liquefying and saccharifying amylases from isolates for the hydrolysis of the raw corn starch as well as mess food waste. Response surface methodology (RSM) using central composite design was employed for the optimization of liquefaction and saccharification for the enhanced sugar production. The generated sugar slurry was again subjected for the alcohol production using yeast strain.
Materials and Methods
Microorganisms and Maintenance
A bacterial isolate identified as Bacillus amyloliquefaciens KCP2 using 16s rDNA (Accession No: KF112071) and the fungal isolate identified as Colletotrichum sp. KCP1 using 18S rDNA (Accession No: GU353321) were studied previously for the alpha-amylase and glucoamylase production, respectively under solid state fermentation [22, 24] have been selected in the present investigation based on the suitability of the organisms to grow on solid state base such as wheat bran, the property of thermostable alpha-amylase and fungal glucoamylase and the levels of enzyme yields.
Bacillus amyloliquefaciens KCP2 was stored at 4 °C on Bushnell Hass Agar (BHA) slant containing 1 % starch. Bushnell Hass mineral salts solution has the following composition (gm/lit): MgSO4 0.2, KH2PO4 1.00, CaCl2 0.02, FeCl3 0.05, NH4NO3 1.00, K2HPO4 1.00 while Colletotrichum sp. KCP1 was stored at 4 °C on potato dextrose agar (PDA). These cultures were subculture every 2–3 weeks of interval.
Glucoamylase Production by Colletotrichum sp. KCP1 Under SSF
Previously optimized medium having starch 1.5 g, whey 0.1 ml and casein acid hydrolysate 0.1 g, per 5 g of wheat bran was used for production of glucoamylase under SSF. Production flasks were inoculated with 3 agar plugs of 8 mm diameter from 7 days grown fungal culture on Czapek Dox agar plate and incubated at 30 °C for 5 days. Subsequently the enzyme was extracted with 50 ml of 0.05 M sodium acetate buffer (pH 5.0) on rotary shaker at 150 rpm for 30 min at 25 °C. The content was filtered through muslin cloth, centrifuged at 8,000 rpm for 15 min and clear supernatant was used for determining glucoamylase activity expressed as U/gds (Units/gram dry substrate) and liberated reducing sugars (glucose equivalents) were estimated by dinitrosalicylic acid (DNS) method [25]. One unit of glucoamylase is defined as the amount of enzyme releasing 1 µmol of glucose equivalent per minute under the assay conditions.
Alpha Amylase Production by B. amyloliquefaciens KCP2 Under SSF
Alpha amylase production was carried out in 250 ml Erlenmeyer flasks containing starch 0.01 g, ammonium sulphate 0.2 g and 5 mM calcium chloride per 5 g wheat bran as solid substrate and 10 ml of the salt solution (gm/lit: MgSO4 0.2, KH2PO4 1.00, FeCl3 0.05, NH4NO3 1.00, K2HPO4 1.00) to provide the adequate moisture content. After inoculation, all the experimental flasks were incubated under static condition at 37 ± 2 °C and were harvested after 72 h interval followed by the enzyme extraction with 40 ml of 0.05 M phosphate buffer (pH 8.0) on a rotary shaker at 150 rpm for 30 min at 25 °C. The content was filtered through muslin cloth, centrifuged at 8,000 rpm for 25 min and the clear supernatant was used for determining alpha-amylase activity, which is expressed as U/gds (Units/gram dry substrate). The reaction mixture consisted of 1.0 ml of 1 % starch, 0.9 ml 0.05 M phosphate buffer (pH 8.0), and 0.1 ml of enzyme extract. After 10 min of incubation at 65 °C, the liberated reducing sugars were estimated by the dinitrosalicylic acid (DNS) method [25]. The colour developed was read at 560 nm using a spectrophotometer (Shimadzu UV-160).
Substrates Used for the Saccharification Study
Raw Corn Starch
Fresh corns were procured from the market and corn seed separated from the corn cobs. Corn seed were crushed and soaked in water for separation of starch which settled at the base of container. At a regular time interval the water was changed and deposited starch was collected. The process continued till the release of the starch stopped. The starch thus collected was subjected to drying at 60 °C, till fine powder was formed.
Food Waste
Food waste was collected from the mess of Vallabh Vidyanagar, Gujarat where around 500 students take their meals daily. It was mixed with water at ratio of 1:2 and crushed into small particles. The characteristic of the food waste mixture used in this study were pH 5.7, total solid 11.2 %, and total chemical oxygen demand 82.4 g/l. The average elemental composition of food waste mixture was carbon 52.8 %, nitrogen 5.9 %, hydrogen 6.8 % and oxygen 34.5 %. These characteristic of the food mixture were found to be similar to other reported values [26, 27].
Application of Microbial Enzymes in the Hydrolysis of Raw Corn Starch Using Response Surface Methodology
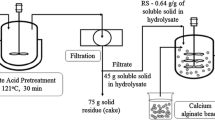
Raw corn starch (10 %) slurry was prepared in 250 ml Erlenmeyer flask with distilled water followed by addition of bacterial alpha amylase and the temperature was maintained at 65–70 °C during pre-cooking in water bath for 30 min. The slurry of raw corn starch was then cooked under pressure in an autoclave at 121 °C for 30 min. After gelatinization, the contents of the flasks were allowed to cool and another dose of alpha amylase was added while the temperature was maintained at 65 °C for another 30 min for starch liquefaction. After cooling the liquefied mash fungal glucoamylase was added for saccharification. The flasks was then incubated for 12 h and the liberated reducing sugars were determined according to [25] (Fig. 1). All the results represent the mean of at least three independent experiments. Conversion efficiency in mashing was calculated as follows:
Sugars obtainable from starch hydrolysis as determined from stoichiometrical calculation were 1.01 g/g [28].
The interactive effect of four variables (pre-cooking alpha amylase, post-cooking alpha amylase, glucoamylase and saccharification temperature) which influence the raw corn starch saccharification significantly were analysed and optimized by response surface Central Composite Design (CCD). RSM is useful for small number of variables (up to five) but is impractical for large number of variables, due to high number of experimental runs required. According to the design, the total number of treatment combinations is 2k + 2k + no, where k is the number of independent variables and no is the number of repetition of experiments at the central point. Each factor in the design was studied at five different levels (−α, −1, 0, +1, +α) as shown in Table 1. All variables were set at a central coded value of zero. The minimum and maximum ranges of variables were determined on the basis of our previous experiments. The full experimental plan with respect to their values in actual and coded form is presented in Table 2. The % conversion efficiency of microbial enzymes for raw corn starch saccharification was measured in triplicate for all 30 different experimental runs. The raw corn starch saccharification was analyzed using a second order polynomial equation, and the data were fitted into the equation by multiple regression procedure. The model equation for analysis is given as:
where βo, βi, βii and βij represent the constant process effect in total, the linear, quadratic effect of Xi and the interaction effect between Xi and Xj, respectively for the raw corn starch saccharification. Later, an experiment was run using the optimum values for variables given by response optimization to confirm the predicted value and experimental value of raw corn starch saccharification.
Software and Data Analysis
The results of the experimental design were analyzed and interpreted using Design Expert Version 8.0 (Stat-Ease Inc., Minneapolis, Minnesota, USA) statistical software.
Fermentation of Mess Food Waste and Raw Corn Starch Slurry
The experiment was carried out in 250 ml of Erlenmeyer flasks with working volume of 100 ml. The collected mess food sample was diluted with water at a ratio of 1:2 and was treated with the optimized condition and level of post and pre-cooking alpha amylase and glucoamylase for maximum sugar production. The liquid phase of the food waste hydrolysate obtained by enzymatic saccharification was stored in the −20 °C freezer before ethanol fermentation. Ethanol fermentation was carried out using Saccharomyces cerevisiae under anaerobic condition using food waste hydrolysate without addition of any nutrient components while 0.5 g % of yeast extract was added to the raw corn starch slurry. To ensure anaerobic condition, the flasks were sealed by the rubber stopper after inoculation of 2 % (v/v) pre-cultured inocula and were kept at 30 ± 2 °C for 48 h.
Simultaneous Saccharification and Fermentation (ssf)
At the end of liquefaction process, the raw corn starch slurry and food waste were cooled to 30 ± 2 °C from 65 ± 2 °C and subjected to simultaneous saccharification fermentation with yeast culture, after supplementation of fungal glucoamylase and with 0.5 g % of yeast extract in case of raw corn starch slurry while food waste slurry was used without addition of any nutrient components. The flasks were covered with (a sterile plug or) rubber stopper and incubated at 30 ± 2 °C in rotary shaker at 200 rpm. Samples of the fermentation slurry were collected after 48 h of fermentation period for estimating ethanol production.
Ethanol Extraction and Estimation
The fermentation slurry was subjected to distillation at 79 °C using Soxhlet’s apparatus. The ethanol content from distillate was measured using dichromate method after qualitative estimation by gas chromatography.
Results and Discussion
Response surface methodology is generally used to investigate a combined effect of several variables and to find optimum conditions for a multi-variable system. The most common experimental design used in RSM is CCD which has equal predictability in all directions from the center. In addition, CCDs are optimized designs for fitting quadratic models [29].
In the present work, experiments were planned to obtain a quadratic model consisting of 24 trails. The plan includes thirty experiments and two levels of concentration for each variable. In order to study the combined effect of these variables to obtained maximum saccharification, experiments were performed at different combinations. Table 2 summarizes the central composite experimental plan along with the predicted and observed response for each individual experiment. It shows the conversion efficiency (%) of the microbial enzyme corresponding to combined effect of all four variables in the specified ranges. RSM was used for the optimization of enzymatic hydrolysis of maize starch for the higher glucose production and the maximum conversion efficiency of 96.25 % was achieved [28].
The optimum levels of the selected variables were obtained by solving the regression equation and by analyzing the response surface contour and surface plots [30]. The regression equation obtained after the analysis of variance (ANOVA) provides an estimate of the level of %conversion efficiency of the microbial enzyme as a function of combine effect of pre-cooking alpha amylase, post-cooking alpha amylase, glucoamylase and saccharification temperature.
The % conversion efficiency of the enzymes may be best predicted by the following model:
where Y is conversion efficiency (%), A is pre-cooking alpha amylase dose (U/ml), B is post cooking alpha amylase dose (U/ml), C is glucoamylase dose (U/ml) and D is saccharification temperature (°C).
Statistical significance of respective model equation was checked using F-test analysis of variance (ANOVA) (Table 3). The model F-value of 5.41 implies the model is significant. There is only a 0.12 % chance that a model F-value could be large due to noise. Values of Prob > F less than 0.0500 indicate model terms are significant. In this case A, B, D, A2, B2 and D2 are found to be significant model terms. The lack of fit F-value of 1.00 implies the lack of fit is not significant relative to the pure error. Non-significant lack of fit indicates that the experimental data obtained are in good agreement with the model. Adequate Precision measures the signal to noise ratio. A ratio greater than 4 is desirable. In our case we found the ratio of 7.654 indicates an adequate signal for the model. The R2 value (multiple correlation coefficient) closer to 1 denotes better correlation between observed and predicted values. The coefficient of variation (CV) indicates the degree of precision with which the experiments are compared. The lower reliability of the experiment is usually indicated by high value of CV. In the present case a low CV (5.44 %) denotes that the experiments performed are reliable.
The effect of interaction of variables to generate more sugars and consequently increase the overall % conversion efficiency was studied against any two independent variables while keeping the other independent variables at their constant level. These response surface plots or contour plots can be used to predict the optimal values for different test variables. Therefore, six response surfaces were obtained by considering all the possible combinations. Three-dimensional response plot shown in Fig. 2a describes the behaviour of microbial enzyme for maximum conversion efficiency (%), main effect, interaction effect and squared effect (nonlinear) of pre-cooking alpha amylase and post cooking alpha amylase at different dose level. Both the components at their lower dose did not show any significant effect on the conversion efficiency. The shape of the response surface curves showed a good interaction between these tested variables and increase in the dose level of both variables leads to maximum sugar production and ultimately high conversion efficiency. Figure 2b depicts three dimensional curve and contour plot of the calculated response surface from the interaction between glucoamylase and pre-cooking alpha amylase dose while keeping fixed level of other two variables. Interaction of both glucoamylase and alpha amylase at more than middle level showed positive effect and leads to maximum sugar production while both variables at their lower dose did not show any significant effect. Figure 2c shows the interaction between the saccharification temperature and pre-cooking alpha amylase dose on sugar production from raw corn starch. Higher level of both the variables did not show any significant rise in the sugar production while middle level of temperature and higher middle level of pre-cooking alpha amylase dose leads to maximum sugar production. Glucoamylase and post cooking alpha amylase at their lower level did not show any significant effect on the sugar production (Fig. 2d). The interaction of both the variables showed positive effect at their higher level and leads to maximum sugar production. As shown in Fig. 2e, the interaction between the temperature and post-cooking alpha amylase showed prominent effect on the sugar production. Simultaneous increase in the both variables leads to gradual increase in the % conversion efficiency up to certain level. Maximum conversion efficiency (%) was observed when both variables were kept at their middle level while lower level showed negative effect on sugar generation. Increase in the saccharification temperature leads to gradual increase in the %conversion efficiency up to higher middle level (Fig. 2f). The lower level of glucoamylase and saccharification temperature did not show any significant effect on the sugar production but the interaction of both the variables were found to be positive for enhanced sugar production from raw corn starch. Saccharification temperature between 50 and 60 °C was found to be effective for enhanced activity of glucoamylase and ultimately leads to higher conversion efficiency (%).
Response surface graph showing the interaction effect of a pre-cooking α-amylase and post-cooking α-amylase keeping fixed level of glucoamylase (1.52 U) and temperature (50 °C), b pre-cooking α-amylase and glucoamylase keeping fixed level of post-cooking alpha-amylase (3.00 U) and temperature (50 °C), c pre-cooking α-amylase and saccharification temperature keeping fixed level of post-cooking alpha-amylase (3.00 U) and glucoamylase (1.52 U), d post-cooking α-amylase and glucoamylase keeping fixed level of pre-cooking alpha-amylase (3.00 U) and temperature (50 °C), e post-cooking α-amylase and saccharification temperature keeping fixed level of pre-cooking alpha-amylase (3.00 U) and glucoamylase (1.52 U), f glucoamylase and saccharification temperature keeping fixed level of pre-cooking alpha-amylase (3.00 U) and post-cooking alpha-amylase (3.00 U) on the conversion efficiency (%) for the enhanced sugar production
Validation of the Quadratic Model
Validation was carried out under conditions predicted by the response surface model. The optimal level estimated for each variable in enzyme system for higher sugar production from raw corn starch were 3 U/ml dose of both pre and post cooking alpha amylase, 1.5 U/ml dose of glucoamylase and 50 °C as saccharification temperature. The predicted conversion efficiency (%) obtained from the model using the above optimum level of variables was 93.65 %. To validate the prediction of the model, additional experiments in triplicate were performed with the optimized enzyme system. These experiments yielded the maximum conversion efficiency of 94.12 %. Good agreements between the predicted and experimental results verified the validity of the model and the existence of the optimal points.
Fermentation
Liquefaction process involves a partial hydrolysis of starch to maltodextrins at high temperature and the reduction of starch-paste viscosity by the action of thermo-stable α-amylase produced by the Bacillus amyloliquefaciens KCP2. The efficiency of the fermentation process depends on the glucose concentration produced by the saccharification process [31]. During separate saccharification and fermentation process, the ethanol production was found to be 16.20 and 21.2 g/l using mess food waste and corn starch as substrate, respectively (Fig. 3). Food waste is a complex mixture containing sugar, starch, cellulose, protein and fat, it was expected that the optimum saccharification conditions might be changed. Ethanol fermentation was performed by simultaneous saccharification and fermentation (ssf) process, where glucoamylase produced by the Colletotrichum sp. KCP1 and yeast were added simultaneously. The ssf decreases the product inhibition on enzyme as well as the osmotic stress on yeast cells. In this study, ssf was operated at 30 °C according to a high ethanol production rate reported in literature [31–33]. In spite of the economical advantage of ssf over separate hydrolysis and fermentation, the critical problem of ssf is the difference in the temperature optima of the enzyme and the fermenting microorganisms. It was noted that this temperature was lower than the optimum temperature of saccharifying enzymes used in this study. The ethanol concentration of 19 and 25 g/l was reported during simultaneous saccharification and fermentation of Arundo donax—a perennial herbaceous crop and steam pre-treated corn stover, respectively after 72 h of fermentation period [34]. During simultaneous saccharification and fermentation process, the ethanol production was found to be 11.21 and 15.02 g/l using mess food waste and corn starch as substrate, respectively.
Conclusion
Bacillus amyloliquefaciens KCP2 α-amylase and glucoamylase from Colletotrichum sp. KCP1 have been employed for the liquefaction and saccharification of the raw corn starch for the maximum sugar generation. The sugar slurry was then subjected for ethanol fermentation for 48 h using yeast culture. Statistical optimization of enzymatic hydrolysis of raw corn starch has been successfully carried out using RSM based on the 24 factorial CCD. The optimum values for the tested variables for the maximum conversion efficiency were: pre- and post-cooking α-amylase dose 3.0 U/ml, glucoamylase dose 1.5 U/ml at a saccharification temperature of 50 °C. The maximum conversion efficiency of 94.12 % was achieved. This design proved to be useful in the optimization of enzymatic liquefaction and saccharification process. Maximum alcohol production was found to be 16.20 and 21.2 g/l from mess food waste and corn starch, respectively after 48 h of fermentation period.
References
Soni, S.K., Kaur, A., Gupta, J.K.: A solid state fermentation based bacterial α-amylase and fungal glucoamylase system and its suitability for the hydrolysis of wheat starch. Process Biochem. 39, 185–192 (2003)
Nigam, P., Singh, D.: Enzyme and microbial systems involved in starch processing. Enzyme Microb. Technol. 17, 770–778 (1995)
Pandey, A., Soccol, C.R., Mitchell, D.: New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 35, 1153–1169 (2000)
de Castro, A., de Andréa, T., Carvalho, D., Teixeira, M., dos Reis Castilho, L., Freire, D.: Valorization of residual agroindustrial cakes by fungal production of multienzyme complexes and their use in cold hydrolysis of raw starch. Waste Biomass Valoriz. 2, 291–302 (2011)
Mojović, L., Nikolić, S., Rakin, M., Vukasinović, M.: Production of bioethanol from corn meal hydrolyzates. Fuel 85, 1750–1755 (2006)
Asada, C., Nakamura, Y.: Chemical characteristics and ethanol fermentation of the cellulose component in autohydrolyzed bagasse. Biotechnol. Bioprocess Eng. 10, 346–352 (2005)
Kádár, Z., Szengyel, Z., Réczey, K.: Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind. Crops Prod. 20, 103–110 (2004)
Kim, S., Dale, B.E.: Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 26, 361–375 (2004)
Lissens, G., Klinke, H., Verstraete, W., Ahring, B., Thomsen, A.B.: Wet oxidation pre-treatment of woody yard waste: parameter optimization and enzymatic digestibility for ethanol production. J. Chem. Technol. Biotechnol. 79, 889–895 (2004)
Fan, Z., South, C., Lyford, K., Munsie, J., van Walsum, P., Lynd, L.R.: Conversion of paper sludge to ethanol in a semicontinuous solids-fed reactor. Bioprocess Biosyst. Eng. 26, 93–101 (2003)
Nakamura, Y., Sawada, T.: Ethanol production from artificial domestic household waste solubilized by steam explosion. Biotechnol. Bioprocess Eng. 8, 205–209 (2003)
Mtui, G., Nakamura, Y.: Bioconversion of lignocellulosic waste from selected dumping sites in Dar es Salaam, Tanzania. Biodegradation 16, 493–499 (2005)
Lark, N., Xia, Y., Qin, C.-G., Gong, C., Tsao, G.: Production of ethanol from recycled paper sludge using cellulase and yeast Kluveromyces marxianus. Biomass Bioenergy 12, 135–143 (1997)
Green, M., Shelef, G.: Ethanol fermentation of acid hydrolysate of municipal solid waste. Chem. Eng. J. 40, B25–B28 (1989)
Green, M., Kimchie, S., Malester, A., Rugg, B., Shelef, G.: Utilization of municipal solid wastes (MSW) for alcohol production. Biol. Wastes 26, 285–295 (1988)
Yücel, Y., Göycıncık, S.: Optimization of ethanol production from spent tea waste by Saccharomyces cerevisiae using statistical experimental designs. Biomass Convers. Bioref. (2014). doi:10.1007/s13399-014-0138-2
Cheung, S.W., Anderson, B.C.: Laboratory investigation of ethanol production from municipal primary wastewater solids. Bioresour. Technol. 59, 81–96 (1997)
Wen, Z., Liao, W., Chen, S.: Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour. Technol. 91, 31–39 (2004)
Verma, G., Nigam, P., Singh, D., Chaudhary, K.: Bioconversion of starch to ethanol in a single-step process by coculture of amylolytic yeasts and Saccharomyces cerevisiae 21. Bioresour. Technol. 72, 261–266 (2000)
Hoshino, K., Taniguchi, M., Marumoto, H., Fujii, M.: Continuous ethanol production from raw starch using a reversibly soluble-autoprecipitating amylase and flocculating yeast cells. J. Ferment. Bioeng. 69, 228–233 (1990)
Yamade, K., Fukushima, S.: Continuous alcohol production from starchy materials with a novel immobilized cell/enzyme bioreactor. J. Ferment. Bioeng. 67, 97–101 (1989)
Prajapati, V.S., Trivedi, U.B., Patel, K.C.: Optimization of glucoamylase production by Colletotrichum sp. KCP1 using statistical methodology. Food Sci. Biotechnol. 22, 31–38 (2013)
Prajapati, V.S., Trivedi, U.B., Patel, K.C.: Kinetic and thermodynamic characterization of glucoamylase from Colletotrichum sp. KCP1. Indian J. Microbiol. 54, 87–93 (2014)
Prajapati, V.S., Trivedi, U.B., Patel, K.C.: A statistical approach for the production of thermostable and alklophilic alpha-amylase from Bacillus amyloliquefaciens KCP2 under solid-state fermentation. 3 Biotech. (2014). doi:10.1007/s13205-014-0213-1
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Han, S.-K., Shin, H.-S.: Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrog. Energy 29, 569–577 (2004)
Rao, M., Singh, S.: Bioenergy conversion studies of organic fraction of MSW: kinetic studies and gas yield–organic loading relationships for process optimisation. Bioresour. Technol. 95, 173–185 (2004)
Kunamneni, A., Singh, S.: Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem. Eng. J. 27, 179–190 (2005)
Gao, Y.-L., Ju, X.-R., Jiang, H.-H.: Use of response surface methodology to investigate the effect of food constituents on Staphylococcus aureus inactivation by high pressure and mild heat. Process Biochem. 41, 362–369 (2006)
Abdelhay, A., Magnin, J.-P., Gondrexon, N., Baup, S., Willison, J.: Optimization and modeling of phenanthrene degradation by Mycobacterium sp. 6PY1 in a biphasic medium using response-surface methodology. Appl. Microbiol. Biotechnol. 78, 881–888 (2008)
Kim, J.K., Oh, B.R., Shin, H.-J., Eom, C.-Y., Kim, S.W.: Statistical optimization of enzymatic saccharification and ethanol fermentation using food waste. Process Biochem. 43, 1308–1312 (2008)
Reddy, L., Reddy, O.: Rapid and enhanced production of ethanol in very high gravity (VHG) sugar fermentation by Saccharomyces cerevisiae: role of finger millet (Eleusine coracana L.) flour. Process Biochem. 41, 726–729 (2006)
Wang, S., Ingledew, W., Thomas, K., Sosulski, K., Sosulski, F.: Optimization of fermentation temperature and mash specific gravity for fuel alcohol production. Cereal Chem. 76, 82–86 (1999)
Öhgren, K., Rudolf, A., Galbe, M., Zacchi, G.: Fuel ethanol production from steam-pretreated corn stover using SSF at higher dry matter content. Biomass Bioenergy 30, 863–869 (2006)
Acknowledgments
The authors are grateful to the Department of Biotechnology, Ministry of Sciences and Technology, Government of India, for providing the financial assistance during the course of this investigation.
Conflict of interest
We all authors declare that this manuscript does not have any financial/commercial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prajapati, V., Trivedi, U. & Patel, K.C. Bioethanol Production from the Raw Corn Starch and Food Waste Employing Simultaneous Saccharification and Fermentation Approach. Waste Biomass Valor 6, 191–200 (2015). https://doi.org/10.1007/s12649-014-9338-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-014-9338-z