Abstract
The advocacy of producing biofuels from wastes would answer the call for energy and environmental sustainability. This call is very timely considering the issues of global warming, increasing greenhouse gas emissions, diminishing natural resources, and enlarging human population. For one, the increasing generation of waste pulps from the growing numbers of starch-producing industries using cassava (Manihot esculenta Crantz) has become alarming because the improper disposal of these causes putrefaction odor, leachate contamination on water bodies, illnesses/diseases of community residents, and so on. In this work, the potential of cassava waste pulps (CWP) from starch industry was assessed with regard to the extraction of bioethanol via aerobic fermentation. The effect of yeast loading (0–4 tsp) and mashing duration (3–11 min) was evaluated on their influence on the bioethanol yield in CWP fermentation through central composite design of the response surface methodology. The result showed that 5.93 ± 0.03 mL of bioethanol could be extracted from a kilogram of fresh CWP after 7-day aerobic fermentation at conditions of 7 min mashing duration (42 °C) and 1 tsp yeast loading. Yeast loading and mashing duration are both significant with regard to bioethanol production. The gas chromatography analysis revealed 0.08% v/v bioethanol in the fermentation broth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A more environment-conscious society today urges many to find environmentally benign technologies that would minimize environmental burden of heavy pollutant emissions to the atmosphere, high load effluents to water bodies, and fast depletion of natural resources. One of the current challenges is the development of alternative liquid-phase energy resources considering not only the finite and fast depleting fossil fuel reserves but also the high greenhouse gas emissions that cause increasing global climate temperature or the so-called greenhouse effect (Höök and Tang 2013; Barreto 2018). A new, sustainable, and eco-friendly liquid biofuel source to substitute the crude oil is now gaining increasing importance and even becoming an urgent necessity. Among the bioresources that are readily available, relatively low-cost, and do not pose a threat to food security is the organic wastes for the production of biofuels as value-added products. One of the industries that produce huge heaps of organic wastes is the starch-producing industry.
The prominent voluminous wastes from starch-producing businesses are the waste pulps of cassava or Manihot esculenta Crantz as residuals of the production with approximately 72–85% moisture content (Hermiati et al. 2012). For instance, the PhilAgro Industrial Corporation, one of the starch-producing industries in the Philippines, generates 100 tons of cassava waste pulps (CWP) daily. Most of the cassava used in this corporation is derived from the harvest of Northern Mindanao, the top producer of cassava in the Philippines, with a 33.5% share to the national production (PSA 2018). In 2017, the Philippines had approximately 590,000 metric tons of cassava production (Lacson 2017), and around 15–20% of the original processed root in dry weight basis ends up as solid wastes (Charmongkolpradit and Luampon 2017). In the global scale, over 270 million tons of cassava were produced in the year 2016 (Chuasuwan 2017). This huge production entails enormous volumes of CWP that are usually dumped indiscriminately due to the perception that it cannot be further utilized (Archibong et al. 2016). Leaving the CWP to rot in an open area contributes some environmental problems such as the production of offensive and strong putrefaction odor due to rapid spoilage and the leachate contamination of water bodies (Bacusmo 2000). Disposal challenges of CWP may be aggravated in the future due to the consistent demand for cassava-based products like starch.
Heightened with concerns on environmental and energy sustainability, it is timely for academicians/scientists and industry experts to closely investigate the possible utilization of CWP as an alternative resource. These pulps have been explored with limited success in the production of briquettes, surfactants, and fertilizers (Ubalua 2007). For animal husbandry, the CWP as an ingredient in the feed formulation has been opposed due to considerable contents of anti-nutrients like hydrocyanic acid, tannic acid, and oxalate (Oluremi and Nwosu 2002). Bioethanol extraction is one of the applications that can be explored with CWP due to its high residual starch level, low ash content and small particle size of lignocellulosic fiber (Archibong et al. 2016). Since the waste material is abundantly available and readily collectible like in the Philippines, its potential for the production of bio-products like bioethanol is huge. It could help in relieving the current CWP disposal problems while creating significant possibilities in the landscape of renewable energy resources with minimal environmental management costs (Panichnumsin et al. 2012). In fact, a case study in Brazil revealed up to 86% reduction of greenhouse gases emissions as one benefit of bioethanol production and usage (Adekunle et al. 2016).
In 2017, the production of bioethanol worldwide reached 105.5 million m3 (REN21 2018). Though the global community has acknowledged the role of bioethanol for energy security; environmental and economic drawbacks of producing bioethanol from first-generation feedstocks (sugar or starch from sugarcane, corn, and wheat) have switched attentions of using second-generation feedstocks (municipal solid waste, crop residues, sludge, livestock manure, and others) (Nair et al. 2017).
Related studies on the extraction of bioethanol from second-generation materials like wastes have been reported with limited success. One of the considerations that conclusively demonstrated higher bioethanol production is the pre-treatment of the substrate. A study of bioethanol extraction from sugarcane bagasse demonstrated that hydrodynamic cavitation coupled with alkaline-hydrogen peroxide pre-treatment resulted in high hydrolysis yield (> 95%) (Terán Hilares et al. 2018). The same trend was found in the production of ethanol using hydrodynamic cavitation alone in sugarcane bagasse (Terán Hilares et al. 2017a, b). Likewise, a dry corn mill employing the hydrodynamic cavitation pre-treatment resulted in high ethanol yield (Ramirez-Cadavid et al. 2016).
At the moment, however, there are only limited works that optimized the extraction of bioethanol from CWP. Additionally, the literature shows that there is no study that tried mashing duration as thermal pre-treatment before extraction of bioethanol from CWP. This low-cost pre-treatment process is hypothesized to help extend the contact between the CWP cell wall and the enzyme so that sugar recovery would be improved. The primary objective of this study, therefore, was optimizing the possible extraction of bioethanol from thermally treated (mashed) CWP via aerobic fermentation through the central composite design of the response surface methodology. The effects of mashing duration and yeast loading were investigated, and the optimum conditions in the extraction of bioethanol were determined. The bioethanol extraction was modeled and statistically analyzed through 3D plots. The developed predictive model equation was verified via experimentations to compare and validate actual and predicted bioethanol extract volume.
2 Materials and methods
2.1 Chemicals
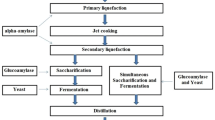
The distiller’s active dry yeast (Saccharomyces cerevisiae) used in this study was purchased from Redstar, USA. While the alpha-amylase, produced from Aspergillus oryzae, used in fermentation was purchased from the University of the Philippines Los Baños.
2.2 Collection and preparation of cassava waste pulps
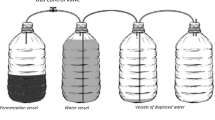
The cassava waste pulps (CWP) were collected from PhilAgro Industrial Corporation located at Maasin, Nicdao, Baungon, Bukidnon, Philippines. The collected samples were kept cold in the ice box during transport to avoid contamination. Very shortly, the samples were held in the refrigerator at 20 °C before use to prevent degradation and possible wild fermentation. The pH of the CWP sample was determined using a pH meter (Yieryi, TPH01608, China).
2.3 Parametric study of reducing sugar concentration during fermentation
The mashing duration and yeast loading were varied with the aim of maximizing the percent decrease of reducing sugar in the CWP substrate. High percent decrease of reducing sugar content that is used up during fermentation would suggest a potential of high bioethanol yield. The sugar concentration, expressed in oBrix, was measured using refractometer (YH Equipment, RHB0-80, China).
The initial ranges of the values of the chosen variables for the parametric study were based on the previous work (Periyasamy et al. 2009) with the same yeast strain (Saccharomyces cerevisiae) as follows: mashing duration (5, 7, 9, 11 min), and yeast loading (1, 2, 3, 4 tsp). Taking the center of one variable as constant while varying the other variable, the peak with high reducing sugar percent decrease in the graph was identified. The known peak value was used as the level 0 of the design of the experiment. In each run, CWP mass was held constant at 200 g with alpha-amylase of 30 mL.
2.4 Experimental design of bioethanol extraction
Central composite design (CCD) of the response surface methodology (RSM) was employed using Design Expert 7.0 software (stat-Ease, Inc, USA). The range and level of variables viz. mashing duration and yeast loading were based on the results of parametric studies with alpha-amylase fixed at 30 mL.
The combinations of all the runs generated by the CCD were followed. During mashing pre-treatment at a predetermined time according to CCD, mash tuns filled with CWP (the ratio was 250 mL H2O to 200 g pulp) with alpha-amylase were cooked at a monitored temperature of 42 ± 2 °C. After that, a predetermined amount of yeast was added to the cooled-down mixture and consequently underwent fermentation for 7 days (Wahyuono et al. 2015).
2.5 Determination of bioethanol yield from cassava waste pulps
The initial sugar concentration (So) of the CWP at 40 °C before mashing was measured using a refractometer and recorded. After 7-day fermentation, the fermented samples were strained, and the juice was separated from the substrate. Then, the juice was centrifuged and filtered further, and a clear liquid was obtained as filtrate. The final sugar concentration (Sf) of the filtrate was measured and recorded. The concentration of sugar that was used up in the fermentation broth was calculated as reducing sugar using Eq. 1.
Assuming that glucose was produced during fermentation and all converted into bioethanol, the yield was computed using the difference of So and Sf and following the procedures and computations published elsewhere (Ernest and Stefan 2018).
2.6 Optimization and product analysis
Optimization of bioethanol extraction was completed using the numerical optimization feature of Design Expert 7.0 software, selected based on desirability. The bioethanol yields achieved according to experimental runs were analyzed, the 3D graph was produced, and the suggested predictive model equation was known. After analysis, the generated predictive model equation that could calculate bioethanol yield according to examined variables was validated by experimental runs.
The bioethanol produced from CWP underwent gas chromatography analysis. The HP-GC 7820 gas chromatogram determined the compositions of the produced bioethanol, and the analysis was done at Pilipinas Kao, Incorporated in Jasaan, Misamis Oriental, Philippines.
3 Results and discussion
3.1 pH of the cassava waste pulps
The pH of the substrate before it underwent fermentation was near-neutral (6.4 ± 0.1). The pH obtained was close to the optimum pH determined in the production of bioethanol from damaged corn grains of 5.8 (Gawande and Patil 2018). This result is desirable because yeast cells thrive more in acidic than in basic environment (Dequin 2001), thereby promoting bioethanol production. Also, the a-amylase works well with pH of 5–6 (Uncu and Cekmecelioglu 2011). Moreover, since the S. cerevisiae tolerates in a wide range of pH which makes it commonly employed yeast in industrial application (Lin et al. 2012), the slight difference of pH of CWP for bioethanol production is not an issue for the current work. This result is supported by another work whereby it is emphasized that for fermentation using S. cerevisiae microbes, a pH of 6.5–7.5 is essential to sustain the bacteria’s growth (Aditiya et al. 2016).
3.2 Results of the parametric study
The first set of experiments was conducted to investigate the individual effects of mashing duration and yeast loading onto the reduction of reducing sugar concentration in the fermentation broth. Figure 1 shows the trend of the reduction of the concentration of reducing sugar from a cassava waste pulps (CWP) produced at variable mashing durations with 3 tsp yeast loading. The parametric study result showed that the reduction of sugar content of the CWP as substrate peaks at 7 min mashing duration with 36.4%. Changing yeast loading at a constant mashing duration of 7 min, the reducing sugar percentage peaked at 2 tsp with 35.7% (Fig. 2). Previous work revealed variable reducing sugar yields from cassava pulps such as 9.0% (Hanh et al. 2011), 16.5% (Jaleel et al. 1988), and up to 60% (Elemike et al. 2015). These variabilities of reducing sugar, as revealed in the result of parametric analysis and the reported values, made the subsequent study in optimization more interesting. The peak values of reducing sugar identified after parametric examination were the basis in assigning center values (level 0) of the design of experiment (DOE) in the optimization study. In this work, the center values of the DOE were 7 min mashing duration and 2 tsp yeast loading. Table 1 shows the levels and range of variables in the investigation for bioethanol extraction.
3.3 Bioethanol yield from cassava waste pulps
The aim of this work to explore the possible use of CWP for bioethanol production has been achieved with percent yields of bioethanol range between 3.24 and 9.71 mL per kg of fresh CWP (Table 2). Using bioethanol density of 0.789 g/mL, this result is equivalent to 0.0026 to 0.0077 g bioethanol per g of fresh CWP which is significantly lower when compared to other results using agri-wastes (Table 3). The low yield can be attributed to a high moisture content of the CWP as compared to other feedstocks. In dry basis, the bioethanol yield from CWP is equivalent to 0.013 to 0.049 g/g which is comparable to those derived from olive mill solid wastes of 0.03 g/g (Abu et al. 2014), from wood chips of up to 0.04 g/g (Horisawa et al. 2015), and from sugarcane bagasse of 0.03–0.08 g/g (Nasirpour and Mousavi 2018). Another likely reason for a slightly lower yield of bioethanol from CWP is the temperature employed during pre-treatment. The 42 °C might not be able to completely break down the lignin structure of the CWP. The mashing temperature was chosen to reduce energy consumption during pre-treatment. A hot water pre-treatment in a previous study (Muktham et al. 2016) at 200–230 °C resulted in much higher bioethanol yield. This means that the temperature has to be considered in improving the bioethanol yield during pre-treatment. Though the yield is a little lower than those derived from other feedstocks, this upshot is remarkable considering the environmental gains deriving from the production of bioethanol from CWP. The conversion of CWP into bioethanol is a manifestation of cleaner production, waste minimization, and value-adding of a new product. The use of the produced bioethanol would likewise bring a cleaner environment considering that the engines burning bioethanol emits less pollution into the atmosphere. Further investigation of the quantification of the comparative advantages such as energy and environment benefits shall be done in the future to make this work more meaningful.
3.4 Modeling analysis results
The ANOVA determined the two-factor interval (2FI) as the model to predict the yield of bioethanol from CWP with a p value of 0.0344 (Table 4). This implied that the predictive model equation generated could reliably calculate bioethanol yield with only 3.44% chance that error in computation could occur due to the unpredictable data swings. The F value of 0.31 implied that the lack of fit was not significant relative to pure error and that the model has 82.13% confidence that lack of fit could not occur due to the data swings and variations.
The 2FI model equation in terms of an actual factor is given in Eq. 2 where A is the yeast loading (tsp), and B is the mashing duration (min).
The bioethanol yield, as affected by the examined variables, could be predicted or computed using the generated 2FI equation with acceptable error. The linear term B (mashing duration), and term A (yeast loading) when both taken singly, could significantly affect the increase of bioethanol yield as revealed in the equation due to positive numerical coefficients. This means that increasing the yeast loading and mashing duration would lead to the increase in bioethanol yield. On the other hand, the interaction of yeast loading and mashing duration, as described by AB, would result in the significant decrease in bioethanol yield. This trend means that there are interaction effects between the yeast loading and mashing duration that would result in bioethanol reduction.
With the use of Eq. 2, the predicted/theoretical values were computed which are almost equal to the actual values that were determined during experimentation (Table 5). This data strengthened the claim that the model equation fitted the data well with a high assurance of exactness and reliability. It could mean that the fitness is robust, reliable and could support the claim that 2FI model could predict bioethanol yield from CWP as affected by the chosen variables.
3.5 Effects of the operating variables on bioethanol yield
The examined variables such as yeast loading and mashing duration were further evaluated according to their effects on bioethanol yield from CWP fermentation through a visual 3D plot (Fig. 3). As the amount of yeast increased, the bioethanol yield also increased as observed in another study (Periyasamy et al. 2009). Particularly, the increase in mashing duration as pre-treatment resulted in a sharp increment increase in bioethanol yield at the lower yeast loading. This result can be explained by the rapid conversion of cellulosic content to sugar like glucose that can be used by the sufficient amount of yeast to achieve fermentation (Nasirpour and Mousavi 2018). At longer mashing duration, the yield decreases with an increase in yeast loading. The decrease is probably due to the decomposition and derivation of the bioethanol to other compounds (Nasirpour and Mousavi 2018) that were produced by the increased amount of yeast.
Additionally, the figure showed maximum bioethanol yield at 9 min mashing time and 1 tsp of yeast. Future research may consider exploring longer mashing duration because the current work cannot give a hint to an inversion of the trend if mashing duration is further increased beyond 9 min with yeast loading of 1 tsp. Also, the current work cannot rule out that higher yield could be achieved at higher mashing duration at yeast loading of less than 1 tsp.
3.6 Numerical optimization and analysis of the bioethanol yield
The combination of chosen variables suggested by the Design Expert 7.0 software with the highest desirability level was chosen for experimental validation. The criteria in numerical optimization using the software were: maximize bioethanol yield while minimizing the values of chosen variables. After criteria setting, the analysis generated optimal conditions of 7 min mashing duration and 1 tsp yeast loading. Verification runs using optimal conditions were conducted, and the average bioethanol actual yield was compared to the theoretical yield (Table 6). The actual bioethanol yield of 5.93 ± 0.03 mL/kg falls a little above from the theoretical yield of 5.87 mL of bioethanol in each kilogram of fresh CWP. One reason is the model whereby 2FI emerged as the most appropriate and not quadratic. The 2FI as a significant model, instead of the quadratic, means that the levels of the chosen variables were not in the optimal range. They might be a little lower or higher than the value of optimum bioethanol yield. Nonetheless, the bioethanol yield can still be determined using the model equation generated by simply plugging in the values of yeast loading and mashing duration with a less considerable error. The 5.93 mL bioethanol of every fresh CWP is equivalent to 39.53 mL bioethanol for every kg of dried CWP, assuming 85% MC of fresh CWP (Hermiati et al. 2012). The analysis of bioethanol via gas chromatography showed 0.08% v/v in the fermentation broth.
4 Conclusion
This study evaluated the possibility of bioethanol production from cassava waste pulps (CWP), a by-product of starch-producing industry, via mashing pre-treatment followed by the fermentation process. The result showed that alpha-amylase from Aspergillus oryzae successfully fermented the CWP at unaltered pH of 6.4. The bioethanol yield of 5.93 ± 0.03 mL per kg fresh CWP (equivalent to 39.53 mL per kg of dry CWP) was obtained at 7 min mashing duration and 1 tsp yeast loading. The predictive model equation that could reliably calculate bioethanol yield is the two-factor interval (2FI) with a p value of 0.0344. It was found out that yeast loading and mashing duration significantly affect the bioethanol yield. Furthermore, the gas chromatography analysis showed that the fermented juice derived from CWP is composed of 0.08% v/v bioethanol. This study brings an original contribution to the development of sustainable bioethanol production, toward a cleaner energy mix. However, further research is needed, along with the paths suggested in this study, as well as focused on the life-cycle analysis of net energy contribution.
References
Abu H, Najami N, Dosoretz C et al (2014) Potential of bioethanol production from olive mill solid wastes. Bioresour Technol 152:24–30. https://doi.org/10.1016/j.biortech.2013.10.102
Adekunle A, Orsat V, Raghavan V (2016) Lignocellulosic bioethanol: a review and design conceptualization study of production from cassava peels. Renew Sustain Energy Rev 64:518–530. https://doi.org/10.1016/j.rser.2016.06.064
Aditiya HB, Mahlia TMI, Chong WT et al (2016) Second generation bioethanol production: a critical review. Renew Sustain Energy Rev 66:631–653. https://doi.org/10.1016/j.rser.2016.07.015
Archibong EJ, Obika IE, Okafor OI et al (2016) Ethanol production from cassava wastes (pulp and peel) using alcohol tolerant yeast isolated from palm wine. Am J Life Sci Res 4:92–97. https://doi.org/10.21859/ajlsr-040305
Bacusmo J (2000) Eighty-four status and potentials of the Philippines cassava industry. Bioresour Technol 12:84–101
Barreto RA (2018) Fossil fuels, alternative energy and economic growth. Econ Model. https://doi.org/10.1016/j.econmod.2018.06.019
Charmongkolpradit S, Luampon R (2017) Study of thin layer drying model for cassava pulp. Energy Procedia 138:354–359. https://doi.org/10.1016/j.egypro.2017.10.138
Chuasuwan C (2017) Cassava Industry. Thailand Industry Outlook
Dequin S (2001) The potential of genetic engineering for improving brewing, wine-making and baking yeasts. Appl Microbiol Biotechnol 56:577–588. https://doi.org/10.1007/s002530100700
Elemike EE, Oseghale OC, Okoye AC (2015) Utilization of cellulosic cassava waste for bio-ethanol production. J Environ Chem Eng 3:2797–2800. https://doi.org/10.1016/j.jece.2015.10.021
Ernest Z, Stefan V (2018) Theoretical yield of ethyl alcohol computation. In: Socratic Chemistry. https://socratic.org/questions/if-250g-of-sugar-is-completely-fermented-to-ethanol-what-is-the-theoretical-yiel. Accessed 12 July 2018
Gawande SB, Patil ID (2018) Experimental investigation and optimization for production of bioethanol from damaged corn grains. Mater Today Proc 5:1509–1517. https://doi.org/10.1016/j.matpr.2017.11.240
Hanh VT, Phuong LB, Phong TT et al (2011) Biomass as sustainable energy and environmental technology. In: 8th Biomass Asia workshop. Vietnam Academy of Science and Technology, Vietnam
Hermiati E, Mangunwidjaja D, Sunarti TC et al (2012) Potential utilization of cassava pulp for ethanol production in Indonesia. Sci Res Essays 7:100–106. https://doi.org/10.5897/SREX11.022
Höök M, Tang X (2013) Depletion of fossil fuels and anthropogenic climate change—a review. Energy Policy 52:797–809. https://doi.org/10.1016/j.enpol.2012.10.046
Horisawa S, Ando H, Ariga O, Sakuma Y (2015) Direct ethanol production from cellulosic materials by consolidated biological processing using the wood rot fungus Schizophyllum commune. Bioresour Technol 197:37–41. https://doi.org/10.1016/j.biortech.2015.08.031
Jaleel SA, Srikanta S, Ghildyal NP, Lonsane BK (1988) Simultaneous solid phase fermentation and saccharification of cassava fibrous residue for production of ethanol. Starch-Stärke 40:55–58. https://doi.org/10.1002/star.19880400205
Lacson NE (2017) Cassava production. Manila, Philippines
Lin Y, Zhang W, Li C et al (2012) Factors affecting ethanol fermentation using Saccharomyces cerevisiae BY4742. Biomass Bioenergy 47:395–401. https://doi.org/10.1016/j.biombioe.2012.09.019
Muktham R, Bhargava SK, Bankupalli S, Ball AS (2016) A review on 1st and 2nd generation bioethanol production-recent progress. J Sustain Bioenergy Syst 6:72–92. https://doi.org/10.4236/jsbs.2016.63008
Nair RB, Lennartsson PR, Taherzadeh MJ (2017) Bioethanol production from agricultural and municipal wastes. In: Wong JW-C, Tyagi RD, Pandey A (eds) Current developments in biotechnology and bioengineering: solid waste management. Elsevier, Amsterdam, pp 157–190
Nasirpour N, Mousavi SM (2018) RSM based optimization of PEG assisted ionic liquid pretreatment of sugarcane bagasse for enhanced bioethanol production: effect of process parameters. Biomass Bioenergy 116:89–98. https://doi.org/10.1016/j.biombioe.2018.06.008
Oluremi OIA, Nwosu A (2002) The effect of soaked cassava peels on weanling rabbits. J Food Technol Afr 17:12–15. https://doi.org/10.1108/10650750910961929
Panichnumsin P, Nopharatana A, Ahring B, Chaiprasert P (2012) Enhanced biomethanation in co-digestion of cassava pulp and pig manure using a two-phase anaerobic system. J Sustain Energy Environ 3:73–79
Periyasamy S, Venkatachalam S, Ramasamy S, Srinivasan V (2009) Production of bio-ethanol from sugar molasses using Saccharomyces cerevisiae. Mod Appl Sci 3:32–37. https://doi.org/10.5539/mas.v3n8p32
PSA (2018) Major vegetables and root crops quarterly bulletin. Philippine Statistics Authority, Manila
Ramirez-Cadavid DA, Kozyuk O, Lyle P, Michel FC (2016) Effects of hydrodynamic cavitation on dry mill corn ethanol production. Process Biochem 51:500–508. https://doi.org/10.1016/j.procbio.2016.01.001
REN21 (2018) Renewables 2018 Global Status Report. Paris
Terán Hilares R, de Almeida GF, Ahmed MA et al (2017a) Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass—a parametric study. Bioresour Technol 235:301–308. https://doi.org/10.1016/j.biortech.2017.03.125
Terán Hilares R, Ienny JV, Marcelino PF et al (2017b) Ethanol production in a simultaneous saccharification and fermentation process with interconnected reactors employing hydrodynamic cavitation-pretreated sugarcane bagasse as raw material. Bioresour Technol 243:652–659. https://doi.org/10.1016/j.biortech.2017.06.159
Terán Hilares R, Kamoei DV, Ahmed MA et al (2018) A new approach for bioethanol production from sugarcane bagasse using hydrodynamic cavitation assisted-pretreatment and column reactors. Ultrason Sonochem 43:219–226. https://doi.org/10.1016/j.ultsonch.2018.01.016
Ubalua AO (2007) Cassava wastes: treatment options and value addition alternatives. Afr J Biotechnol 6:2065–2073
Uncu ON, Cekmecelioglu D (2011) Cost-effective approach to ethanol production and optimization by response surface methodology. Waste Manag 31:636–643. https://doi.org/10.1016/j.wasman.2010.12.007
Wahyuono RA, Hakim MN, Santoso SA (2015) Feasibility study on the production of bioethanol from tapioca solid waste to meet the national demand of biofuel. Energy Procedia 65:324–330. https://doi.org/10.1016/j.egypro.2015.01.060
Acknowledgements
The authors would like to thank the faculty and staff of the College of Engineering and Technology of the University of Science and Technology of Southern Philippines—Claveria for the support in the conduct of this study. Special thanks to PhilAgro Industrial Corporation for the biomass, and Pilipinas Kao Inc. for the gas chromatography analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Icalina, M.A.A., Jaro, R.H., Potato, D.N.C. et al. Assessing bioethanol extraction potential from waste pulps of cassava (Manihot esculenta Crantz) via aerobic fermentation. Energ. Ecol. Environ. 3, 279–287 (2018). https://doi.org/10.1007/s40974-018-0100-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40974-018-0100-4