Abstract
Extracellular glucoamylase of Colletotrichum sp. KCP1 produced through solid state fermentation was purified by two steps purification process comprising ammonium sulphate precipitation followed by gel permeation chromatography (GPC). The Recovery of glucoamylase after GPC was 50.40 % with 19.3-fold increase in specific activity. The molecular weight of enzyme was found to be 162.18 kDa by native-PAGE and was dimeric protein of two sub-units with molecular weight of 94.62 and 67.60 kDa as determined by SDS-PAGE. Activation energy for starch hydrolysis was 26.45 kJ mol−1 while temperature quotient (Q 10 ) was found to be 1.9. The enzyme was found to be stable over wide pH range and thermally stable at 40–50 °C up to 120 min while exhibited maximum activity at 50 °C with pH 5.0. The pKa1 and pKa2 of ionisable groups of active site controlling V max were 3.5 and 6.8, respectively. V max , K m and K cat for starch hydrolysis were found to be 58.82 U ml−1, 1.17 mg (starch) ml−1 and 449 s−1, respectively. Activation energy for irreversible inactivation (E a(d)) of glucoamylase was 74.85 kJ mol−1. Thermodynamic parameters of irreversible inactivation of glucoamylase and starch hydrolysis were also determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glucoamylases (1,4-d-glucan glucanohydrolase; EC 3.2.1.3), also referred to as amyloglucosidases, are exo-acting amylases releasing glucose from non-reducing end of starch and related oligosaccharides. Glucoamylase is second to proteases in worldwide distribution and sales among industrial enzymes. Glucoamylases are widely distributed among many species of animals, plants and microorganisms [1]. Among microbes bacteria, yeast and fungi are capable of producing these enzymes. Filamentous fungi however constitute major source among all microorganisms. Microbial strains of genus Aspergillus and Rhizopus are mainly used for commercial production of glucoamylase [2]. The preferences for glucoamylase from these sources in starch processing industries are due to their good thermostability and high activity at neutral pH [2, 3]. This enzyme is generally regarded as safe (GRAS) by the Food and Drug Administration (FDA), USA.
The primary commercial application of glucoamylase is for production of glucose syrup from starch. This syrup can be used for fermentation, production of crystalline glucose, or as starting material for fructose syrup [4, 5]. Moreover, the enzyme is extensively used in brewing, textile, food, paper and pharmaceutical industries [5].
Each industrial application demands enzymes with specific kinetic properties, making it important to exploit new microbial sources of enzyme. The operating range of glucoamylase from various microbial sources, in terms of pH, temperature and compatibility with other enzymes, must be investigated to exploit its efficiency of action in variety of food processing operations. Industrial enzymatic hydrolysis of starch is not only influenced by variables related to chemical and physical nature of starch and its suspensions but also by those variables associated with catalytic process (pH, temperature, enzyme/substrate ratio or enzymatic deactivation and inhibition phenomenon) [6]. Studies on the thermodynamic stability of enzymes have provided some essential insights into factors that determine enzyme stability. Thermodynamic data for an enzyme and its catalysed reaction are essential in prediction of the extent of reaction and the position of any process in which these reaction were occur. Enzyme thermostability encompasses thermodynamic and kinetic stabilities [7].
Present work reports purification and characterization of glucoamylase from Colletotrichum sp. KCP1 with respect to its ability to convert soluble starch into glucose as it is new isolate and there are no reports on characterization of glucoamylase produced by Colletotrichum spp. Thermodynamic parameters are investigated for soluble starch hydrolysis and irreversible inactivation of this enzyme. Investigations on kinetic and thermostable properties of glucoamylase from Colletotrichum sp. KCP1 will help to determine suitability of this enzyme for further application.
Materials and Methods
Fungal Strain and Enzyme Production
A fungal isolate from soil identified as Colletotrichum sp. KCP1 using 18S rDNA partial genome sequencing and studied previously for optimization of glucoamylase production under solid state fermentation [8] has been used in the present investigation. Previously optimized medium having starch concentration 1.5 g, whey 0.1 ml and casein acid hydrolysate 0.1 g, per 5 g of wheat bran was used for production of glucoamylase under SSF. Production flasks were inoculated with 3 agar plugs of 8 mm diameter from 7 days old Czapek Dox agar plate and incubated at 30 °C for 5 days. Subsequently the enzyme was extracted with 50 ml of 0.05 M sodium acetate buffer (pH 5.0) on rotary shaker at 150 rpm for 30 min at 25 °C. The content was filtered through muslin cloth, centrifuged at 8,000 rpm for 15 min and clear supernatant was used for determining glucoamylase activity expressed as U gds−1 (Units gram−1 dry substrate) and liberated reducing sugars (glucose equivalents) were estimated by dinitrosalicylic acid (DNS) method [9]. One unit of glucoamylase is defined as the amount of enzyme releasing 1 μmol of glucose equivalent per minute under the assay conditions.
Purification of Glucoamylase
The crude enzyme was subjected to two steps purification process comprising fractional precipitation by ammonium sulphate followed by the gel permeation chromatography. The crude enzyme was precipitated to 70 % saturation at 4 °C with ammonium sulphate and left overnight at 4 °C. The precipitated enzyme was collected by centrifugation, dissolved in 0.05 M sodium acetate buffer (pH 5.5) and dialysed against the same buffer up to 24 h with three changes of equal intervals. For column chromatography 2 mg of ammonium sulphate concentrated protein after dialysis was applied to 23 × 1 cm column containing Sephadex G-100 equilibrated with 0.05 M sodium acetate buffer (pH 5.5). Protein was eluted by applying same buffer and fractions of 1 ml each were collected at flow rate of 0.75 ml min−1. These fractions were checked for protein concentration at 280 nm and glucoamylase activity [1].
Characterization of Glucoamylase
Polyacrylamide Gel Electrophoresis
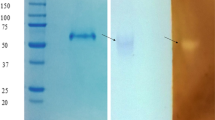
The purity of enzyme and its molecular mass was determined by native-polyacrylamide gel electrophoresis (native-PAGE) and sodium dodecyl sulphate polyacylamide gel electrophoresis (SDS-PAGE), respectively, using Laemmli system [10]. Gel was stained with the silver staining and standard proteins with molecular weight ranging from 14.3 to 205 kDa were used for SDS PAGE calibration, while catalase (240 kDa), bovine albumin (67 kDa), egg albumin (43 kDa), trypsin soybean inhibitor (20.1 kDa) and lactoglobulin (18.4 kDa) were used for native-PAGE calibration. Calibration graph was obtained by plotting logarithum of molecular weight versus migration distance which yield linear relationship. Total Lab Quant software was used for confirmation of molecular weight. Activity staining was performed by incubating gel in sodium acetate buffer (pH 5.5) containing 2 % starch for 30 min at 50 °C and visualized with 0.1 % I2 + 1.5 % KI solution.
Optimum Temperature, Activation Energy and Temperature Quotient (Q 10 )
Glucoamylase was assayed at different temperatures ranging from 30 to 70 °C and activation energy (E a ) was determined by applying Arrhenius plot. The effect of temperature on rate of reaction was expressed in terms of Q 10 which is the factor by which rate increases due to rise in temperature by 10 °C [11].
Effect of pH on Enzyme Activity and Stability
The optimum pH was determined by measuring activity at 55 °C using various buffers: sodium acetate (pH 4–5), sodium phosphate (pH 6–8) and glycine–NaOH (pH 9.0) while stability was tested after 48 h at 4 °C and residual activity was determined. Dixon plot was applied to determine pKa of ionizable groups of active site residues [11].
Kinetics and Thermodynamics of Starch Hydrolysis
The kinetic constants (V max, K m, K cat and K cat/K m) were determined by Michaelis–Menten kinetics. The thermodynamic parameters for substrate hydrolysis were calculated by rearranging Eyring’s absolute rate equation derived from transition state theory [12].
where k b Boltzmann’s constant (R/N) 1.38 × 10−23 J K−1, T absolute temperature (K), h Planck’s constant 6.626 × 10−34 J s, N Avogadro’s number 6.02 × 1023 mol−1, R gas constant 8.314 J K−1 mol−1, ∆H* enthalpy of activation, ∆S* entropy of activation.
Thermal Inactivation and Thermodynamics of Enzyme Activity
Isothermal inactivation treatment was performed in water bath at temperatures ranging from 40 to 70 °C at pH 5.5, using 0.05 M sodium acetate buffer. Enzyme inactivation often follows first-order kinetics [13].
Where k d is inactivation rate constant; A o and A are initial enzyme activity and remaining activity after heating for time t, respectively. The deactivation energy (E a(d) ) was determined based on Arrhenius equation.
It is common to express first-order reactions in terms of D values and z value. Decimal reduction time, is defined as the time needed for reduction of 90 % of initial activity while z value represents the increase of temperature required to reduce 90 % of decimal reduction time [12, 13].
where T and T ref are temperature of treatment and reference temperature (both in °C), respectively, D and D ref are decimal reduction time at temperature of treatment and at reference temperature, respectively.
Thermodynamics of irreversible inactivation of glucoamylase was determined by rearranging the Eyring’s absolute rate equation. [12].
Statistical Analysis
All experiments in the present investigation were performed in replicates of three (n = 3) and data represented as average values ± standard deviation.
Results and Discussion
Purification of Glucoamylase
The extracellular glucoamylase was purified using two steps purification process, ammonium sulphate precipitation and column chromatography (Table 1). The complete precipitation of glucoamylase was observed at 70 % of ammonium sulphate at 4 °C. The overall purification after gel permeation chromatography was found to be 19.30-fold with 50.40 % recovery. The specific activity was enhanced to 36.68 U mg−1 protein. Glucoamylases from Humicola sp. and Fusarium solani were purified to 39-fold with 33 % recovery and 26.2-fold with 31.8 % recovery, respectively [1, 14] while 25.3-fold purification was reported in case of Monascus purpureus [15]. In the present study, after purification the purified protein showed a single band on native-PAGE with amylolytic activity on solubilised starch-PAGE (Fig. 1a). Two bands were observed when purified protein was subjected to SDS-PAGE (Fig. 1b). The molecular masses of subunits estimated from relative mobility of the standard proteins on SDS-PAGE were 94.62 and 67.60 kDa, respectively. The protein band on native-PAGE showed molecular weight of 162.18 kDa when compared with molecular weight standards. The presence of one activity band on native-PAGE with two subunit bands on SDS-PAGE has been reported by Koc and Metin [16] and Marlida et al. [5].
Determination of Temperature Optima, Activation Energy and Temperature Quotient (Q 10 ) of the Glucoamylase
Optimum temperature of purified glucoamylase for hydrolysis of starch was found to be 50 °C which showed good similarity with other reported enzymes [14, 17]. Many glucoamylases function at thermophilic temperatures, usually 50–60 °C. The enzymes from Aspergillus niger, Aspergillus awamori var. kawachi and Arthrobotrys amerospora were optimally active at 50, 60 and 55 °C, respectively [18, 19]. Figure 2 shows that the activity increased until 55 °C, decreasing for temperatures above this value indicating inactivation of enzyme at higher temperature. The activation energy of glucoamylase was found to be 26.45 kJ mol−1 (Fig. 3) which is significantly lower than other reported amylases [20, 21]. Temperature quotient for enzyme was found to be 1.9, which is comparatively higher than that of Humicola sp.; 1.0 [14] and Thermomucor indicae-sedudaticae; 1.35 [22].
Effect of pH on Enzyme Activity and Stability
The hydrolysis of soluble starch by purified glucoamylase at various pH (4–9) at 50 °C was determined. The enzyme exhibited high activity in range of 4.5–6.5 with optimum activity at pH 5.0 (Fig. 4 a) which showed good similarity with glucoamylase form Acremonium sp. [5]. Most of fungal glucoamylases have pH optima in range of 4–6 [5, 23]. The pKa1 and pKa2 values of acidic and basic limbs of the active site residues determined by the Dixon plot were 3.5 and 6.8, respectively. The purified enzyme was stable and retained more than 80 % residual activity in pH range from 4 to 8 after 48 h while 57.89 % residual activity was observed at pH 9 (Fig. 4b). The glucoamylase showed high stability over wide pH range for relatively long time indicates its suitability for industrial purpose [3, 16].
Kinetics and Thermodynamics of Starch Hydrolysis
The K m and V max values of glucoamylase determined through Lineweaver–Burk plot for hydrolysis of soluble starch at 55 °C were 1.17 mg ml−1 and 58.82 U ml−1, respectively (Fig. 5), where as K cat value was 449 s−1. The efficiency constant (K cat /K m ) was 383.76, indicating high catalytic power of enzyme. The K m values of 1.9, 3.5 and 10 mg ml−1 for starch have been reported for glucoamylases from F. solani [1], A. niger [24] and Acremonium sp. [5]. A comparison with above results shows that glucoamylase from Colletotrichum sp. has approximately 1.7, 3.1 and 9-fold lower K m values, respectively which indicates higher affinity of enzyme towards starch. The efficiency constant of glucoamylase was found to be about 1.4 and 2.8 times higher than other reported glucoamylase [1, 14] which confirm higher affinity of enzyme towards starch hydrolysis.
The enthalpy of activation (∆H*), Gibbs free energy (∆G*) and entropy of activation (∆S*) for starch hydrolysis by glucoamylase were calculated as 23.973, 57.851 and −113.68 J mol−1, respectively. The ∆H*, ∆G* and ∆S* for starch hydrolysis by glucoamylase from Arachniotus citrinus and Humicola sp. previously reported were 35.64 and 18.36 kJ mol−1, 75 and 69.06 kJ mol−1, −124 and −154.56 J mol−1, respectively [14, 23]. The lower enthalpy (∆H*) value of glucoamylase showed that the formation of transition state or activated complex between enzyme-substrate was more efficient. Moreover, lower ∆G* value suggested that the conversion of its transition complex into products was more spontaneous than that of A. citrinus and Humicola sp. The feasibility and extent of a chemical reaction is best determined by measuring change in Gibbs free energy (∆G*) for substrate hydrolysis, i.e. the conversion of ES complex into products. The lower is the free energy change the more feasible is the reaction, i.e. the conversion of the reactant to product will be spontaneous. The entropy was slightly lower, which explained that the transition complex had lesser disorder. The free energy for activation of substrate binding (∆G* E–S ) and formation of activation complex (∆G* E–T ) were 0.388 and −14.741 kJ mol−1, respectively. This again confirmed high affinity of enzyme towards starch for hydrolysis and its spontaneous conversion into glucose.
Thermostability and Thermodynamics of the Glucoamylase
The pseudo-first-order plot is shown in Fig. 6 for irreversible thermal denaturation of glucoamylase. The enzyme was thermally stable at 40 and 50 °C and showed >95 % residual activity after 120 min of incubation period. However, at 60 °C, 63 % of residual activity was observed after 30 min of incubation period while above this temperature rapid loss in enzyme activity was observed and displayed half life of 44 min at 70 °C. There was an average reduction of around 14 % in enzyme activity after 120 h of incubation at temperature above 50 °C, while total loss of activity was observed after 120 min of incubation at 60 and 70 °C. Many fungal glucoamylases have been reported to be stable at considerably higher temperature for short time periods while glucoamylase produced by Lactobacillus amylovorus was thermolabile and its activity decreased after 10 min exposure at 60 °C [25]. The glucoamylase from F. solani was thermally unstable at 60 °C and displayed half life of about 26 min [1].
The inactivation constant increases with the temperature, indicating that high temperatures increase the rate of inactivation. Moreover, decimal reduction time decreases with increasing temperature, as expected (Table 2). The enzyme showed good stability at low temperatures (30–50 °C), presenting D value higher than 1,000 min and did not show much difference between D values in these temperatures. However, for temperatures higher than 50 °C, enzyme was found to be less stable, since D values were found to be lower. Deactivation energy E a(d) for glucoamylase calculated using an Arrhenius plot (Fig. 7) was found to be 74.85 kJ mol−1, which was considerably lower than other reported enzymes [13]. The glucoamylase presented z value of 6.19, which indicates that to reduce 90 % of decimal reduction time, it is necessary to increase temperature by 6.19 °C [26].
The ∆H*, ∆G* and ∆S* of irreversible thermal inactivation of glucoamylase were equal to 72.25, 70.69 and 5 × 10−3 kJ mol−1, respectively (Table 2). Out of which ∆H* and ∆G* were decreased to 72.091 and 70.532 kJ mol−1, respectively at 50 °C, while ∆S* increased to 5.2 × 10−3 kJ mol−1 K−1. An increase in temperature leads to decrease in free energy while there was slight increase in entropy, but increase or decrease in entropy was not significant. It is observed that low entropy values are unique in biological system. Solvent and structural effects are the two major factors which influence the numerical values of ∆H* and ∆S*. The possible reason for low entropy is compaction of the enzyme molecule and such changes can arise from the formation of charged particles around the enzyme molecule and the ordering of solvent molecule.
The lower values of K m and ∆G* of the glucoamylase from Colletotrichum sp. indicates its higher affinity to catalyze soluble starch hydrolysis while lower (E a ) activation energy leads to its higher reactivity. Kinetic and thermodynamic parameters of the enzyme suggested that it may be used commercially for the production of glucose in starch processing industry and will help to determine the suitability of this enzyme for application in food processing industries. Present information has novelty as it depict for the first time kinetics and thermodynamics of soluble starch hydrolysis and irreversible inactivation of glucoamylase from Colletotrichum sp.
References
Bhatti HN, Rashid MH, Nawaz R, Asgher M, Perveen R, Jabbar A (2007) Purification and characterization of novel glucoamylase from Fusarium solani. Food Chem 103:338–343
Pandey A (1995) Glucoamylase research: an overview. Starch 47:439–445
Norouzian D, Azim A, Jeno MS, Murrauy MY (2006) Fungal glucoamylases. Biotechnol Adv 44:80–85
Fogarty WM (1983) Microbial amylases. In: Fogarty WM (ed) Microbial enzyme and biotechnology. Applied Science Publishers, London, pp 1–90
Marlida Y, Hassan SN, Radu SZ, Baker J (2000) Purification and characterization of sago starch degrading glucoamylase from Acremonium sp. endophytic fungus. Food Chem 71:221–227
Sanroman A, Murado MA, Lema JM (1996) The influence of substrate structure on kinetics of hydrolysis of starch by glucoamylase. Appl Biochem Biotechnol 59:127–130
Gohel V, Naseby DC (2007) Thermostabilization of chitinolytic enzymes of Pantoea dispersa. Biochem Eng J 35:150–157
Prajapati VS, Trivedi UB, Patel KC (2012) Optimization of glucoamylase production by Colletotrichum sp. KCP1 using statistical methodology. Food Sci Biotechnol 22:31–38
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Laemmli UK (1970) Cleavage of structural proteins during the assembly of head of bacteriophage T-4. Nature 227:680–685
Dixon M, Webb EC (1979) Enzymes: enzyme kinetics. Academic Press, New York, pp 47–206
Eyring H, Stearn AE (1939) The application of theory of absolute reaction rates to protein. Chem Rev 24:253–270
Treichel H, Mazutti MA, Maugeri F, Rodrigues MI (2009) Technical viability of production, partial characterization of inulinase using pretreated agroindustrial residues. Bioprocess Biosys Eng 32:425–433
Muhammad R, Raheela P, Muhammad RJ, Habibullah N, Muhammad HR (2007) Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzyme Microb Technol 41:558–564
El-Sayed SM, El-Aassar SA, Abdel-Meguid DI (2000) Leaching, purification and some properties of glucoamylase from solid state cultures of Monascus purpureus ATCC 16437. Afr J Mycol Biotechnol 8:1–18
Koc O, Metin K (2010) Purification and characterization of thermostable glucoamylase produced by Aspergillus flavus HBF34. Afr J Biotechnol 23:3414–3424
Tosi LRO, Terenzi HF, Jorge JA (1993) Purification and characterization of an extracellular glucoamylase from thermophilic fungus Humicola grisea var. thermoidea. Can J Microbiol 39:846–852
Venkatatramu K, Manjunath P, Rao MRR (1975) Glucoamylase of Aspergillus niger NRRL 330. Ind J Biochem Biophys 12:107–114
Norouzian D, Rostami K, Nouri ID, Saleh M (2000) Subsite mapping of purified glucoamylases I, II, III produced by Arthrobotrys amerospora ATCC 34468. World J Microbiol Biotechnol 16:155–161
Mishra R, Maheshwari R (1995) Amylases of thermophilic fungus Thermomyces lanuginosus: their purification, properties, action on starch and response to heat. J Biosci 21:653–672
Zafar I, Rashid MH, Jabbar A, Malana MA, Khalid AM, Rajoka MI (2003) Kinetics of enhanced thermostability of an extracellular glucoamylase from Arachniotus sp. Biotechnol Lett 25:1667–1670
Kumar S, Satyanarayana T (2003) Purification and kinetics of raw starch hydrolyzing, thermostable and neutral glucoamylase produced by thermophilic mould Thermomucor indicae-seudaticae. Biotechnol Prog 19:936–944
Niaz M, Ghafoor MY, Jabbar A, Wahid A, Rasul E, Ahmed R (2004) Properties of glucoamylase from mesophilic fungus Arachniotus citrinus produced under solid-state growth conditions. Int J Biotechnol 1:223–231
Selvakumar P, Ashakumary L, Helen A, Pandey A (1996) Purification and characterization of glucoamylase produced by Aspergillus niger in solid state fermentation. Lett Appl Microbiol 23:403–406
James JA, Lee BH (1995) Characterization of glucoamylase from Lactobacillus amylovorus ATCC 33621. Curr Microbiol 34:186–191
Raviyan P, Tang J, Rasco BA (2003) Thermal stability of alpha-amylase from Aspergillus oryzae entrapped in polyacrylamide. J Agric Food Chem 51:5462–5466
Acknowledgments
The authors are grateful to Department of Biotechnology, Ministry of Sciences and Technology, India for providing financial assistance during the course of this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prajapati, V.S., Trivedi, U.B. & Patel, K.C. Kinetic and Thermodynamic Characterization of Glucoamylase from Colletotrichum sp. KCP1. Indian J Microbiol 54, 87–93 (2014). https://doi.org/10.1007/s12088-013-0413-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-013-0413-0