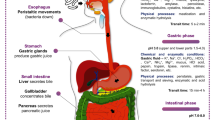
Abstract
Probiotic bacteria are known to have ability to tolerate inhospitable conditions experienced during food preparation, food storage, and gastrointestinal tract of consumer. As probiotics are living cells, they are adversely affected by the harsh environment of the carrier matrix as well as low pH, bile salts, oxidative stress, osmotic pressure, and commensal microflora of the host. To overcome the unfavorable environments, many probiotics switch on the cell-mediated protection mechanisms, which helps them to survive, acclimatize and remain operational in the harsh circumstances. In this review, we provide comprehensive understanding on the different stresses experienced by the probiotic when added in carrier food as well as during human gastrointestinal tract transit. Under such situation how these health beneficial bacteria protect themselves by activation of several defense systems and get adapted to the lethal environments.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elie Metchnikoff, a Nobel Award winner, is credited with developing the initial notion of probiotics. In 1908, he postulated that the bacteria in fermented milk might be reason for the Balkan population’s high life expectancy. Metchnikoff’s theory, however, was ignored for almost a century. The potential health advantages of probiotic lactic acid bacteria have revived interest in them during the past two decades, and as a result, probiotics are now considered essential to human [1]. Probiotics are projected to have a USD 57.8 billion market value in 2022. With an expected CAGR of 8.1% by 2027, it will likely reach USD 85.4 billion [2]. Live microorganisms known as “probiotics” are said to have health advantage when taken in sufficient amounts [3], mainly includes lactic acid bacteria (LAB), Propionibacteria and Bifidobacteria. Many probiotics which belong to genera such as Enterococcus, Bacillus, Escherichia, etc., do not have GRAS status and not included in qualified presumption of safety (QPS), in addition to that they are comparatively less popular probiotic than LAB, Bifidobacteria and Propionibacteria. Hence, most of the studies discussed in this review are related to these three genera. LAB are rod or spherical, gram-positive, acid-tolerant, typically nonsporulating, low-GC bacteria that have similar physiological and metabolic traits [4]. Propionibacterium is a gram-positive, mesophilic, aerotolerant, non-motile, and non-spore-forming bacterium that has a high GC content. It has low nutritional needs and can persist in unfavorable conditions [5]. Bifidobacterium is a genus of gram-positive, nonmotile, often branched rod, anaerobic bacteria [6]. While Lactobacilli are widely regarded as helpful microbes and some strains, such as probiotics, are even thought to promote wellness, their broad historical use has helped them gain approval as generally recognized as safe (GRAS) for human [7].
The human digestive tract has been found to benefit greatly from probiotic microbes and milk-based foods recognized as an effective delivery system since long time. Probiotic products are also growing in popularity using other non-dairy alternatives, such as plant-based foods including grains, fruits and vegetables that have low allergic response rates [8]. Probiotics or products containing probiotics can be helpful in intestinal illnesses, inflammatory bowel disease, diabetes, allergy, inability to digest milk sugar [9], vitamin production [10, 11], tightening of gut barrier [12,13,14], boosts immunity [15, 16], intestinal motility [17, 18], gut microbiome [19,20,21], and lifestyle-related diseases by reducing their clinical symptoms [22,23,24,25]. To obtain the claimed health benefits from the commercial probiotic strain, they should be in the active physiological state because under stressful environment they utilize its energy toward the self-survival and do not provide mentioned health benefit to the host [26, 27].
Nowadays, commercially available probiotics are taken orally as nutritional supplements, such as sachets, pills, capsules, or as one of the ingredients of foods like milk-based products and/or plant-based products. Primary target site of probiotic bacteria is the human intestine; thus, they must reach there in active physiological state to obtain claimed therapeutic benefits. Probiotic bacteria differ in their ability to remain alive and multiply in various carrier matrices as a result of stressors associated with various food processing methods, storage environments and gastrointestinal tract (GIT) [22]. Any alteration to the bacterial DNA, protein, or vital component that reduces viability of cell is known as stress. A cell will strive to re-establish its metabolic pattern in an effort to either survive or grow more quickly as a result of such modifications [28]. As probiotics are live entity, they would face various stresses like presence of organic acids, heating injury, ice crystals piercing, high osmotic pressure in surrounding, antimicrobial compounds, dissolved oxygen in food, and low water activity, either in the carrier matrix or GIT [29]. The metabolic pathways of LAB may be greatly affected by certain stressful situations, hence, limiting their therapeutic efficacy in terms of inhibiting pathogen through secretion of antimicrobial compounds, competing with pathogens for adherence to the intestinal epithelium, boosts immunity by interacting with the intestinal cell line, enhance gut barrier function, produces bioactive compounds, etc. [12, 15, 22, 30]. In fact, a stressed carrier matrix alters the physiology of microbial cell, which may impair the effectiveness of probiotic bacteria [26, 27]. Probiotics may find it difficult to survive in the food, which could hinder their delivery to the target site. On the contrary, many LAB are equipped with adaptation mechanisms and counteract these stresses by multiple responses, e.g., acid neutralization under acid stress, secretion of heat shock proteins during heat stress, rerouting metabolic pathways during bile and acid stress, and modification of cell envelope under osmotic stress. They are safeguarded from severe environmental shocks by these adaption techniques.
It is crucial to have a strong knowledge of how probiotic bacteria respond to external factors to choose strains that will work better as starter cultures and probiotics [31, 32]. It is also crucial to fully comprehend the parameters that enable bacterial viability and the mechanism that enables them to endure inhospitable environments while maintaining a normal physiological state [33, 34]. The objectives of this review were to discuss the various response strategies used by the bacteria to combat the stressful conditions encountered during food preparation as well as the GIT transit and to give an insight to application of stress-adapted probiotics in a customized food preparation such as low-pH food (fermented milk or fruit juices), food stored at very low temperature (ice cream and frozen dessert), and freeze-dried probiotic cells.
Factors Affecting the Viability of Probiotics
Probiotics experiences the different stresses at different stages of food manufacturing, i.e., probiotic preservation (freeze dried, DVS), fermentation in food matrix, refrigerated storage, and human GIT (digestive enzymes, stomach pH, bile, osmotic constraints, commensal microbes, etc.). Following are the stress factors which hampers the growth of probiotic microorganisms [35] such as moisture/water activity, low pH, high bile concentration, oxygen permeability through packaging, osmotic stress (due to sugar and salt addition), metabolites of other bacteria, nutrient depletion/competition for nutrients, post-acidification, presence of harmful microbes, storage condition (temperature, humidity), mechanical stress (pressing, vigorous shaking/centrifuge), heat stress, and chilling injuries. For probiotics to remain alive, become widely distributed, and provide their health advantages, they must adapt to this challenging environment and be protected. When probiotic bacteria are subjected to different stressful environments, they are known to activate number of defense mechanisms in order to overcome the stress and remain viable.
General and Common Stress Responses
When probiotics are under stress, they try to safeguard themselves by expressing numerous coping strategies in the matrix. These coping strategies are more or less same and common for environmental stresses like acid stress, bile stress, heat stress, cold stress, osmotic stress, oxidative stress, etc. The most common defence mechanisms exhibited by probiotics in the stressful conditions are discussed below [36]. General and common stress responses exhibited by probiotic bacteria are shown in Fig. 1.
General stress responses exhibited by probiotic bacteria [36]
Accumulation of Compatible Solutes and Energy Storage Compounds
A smaller organic molecule which is polar, water soluble, and having neutral isoelectric point is known as a compatible solute. It acts as an osmolyte and helps bacterial cells to acclimatize to osmotic stress [37]. To increase the proliferation of cells and re-establish original pressure during osmotic stress, bacteria increase the concentration of compatible solutes [37], which the cell either transports from the surrounding or produces itself [38]. There are two groups of compatible solutes: the first group includes sugars and polyols (trehalose, glycerol), while the second group includes amino acids [39].
Trehalose assists in refolding of protein, inhibits protein aggregation, and safeguards cellular proteins from reactive oxygen species (ROS) damage [40]. Trehalose may also help keep plasma membranes intact [41]. After the external carbon supply has been used up, trehalose can serve as an internal source of carbon [40]. Bacteria were known to accumulate trehalose in their surrounding medium when exposed to high sugar content. It was reported that trehalose concentration is increased by Propionibacterium freudenreichii and Lactobacillus casei under osmotic stress [40, 42]. In contrast to a chemically defined medium, the solutes accumulate more readily. Some challenging conditions may also cause P. freudenreichii to increase the concentration of trehalose such as very low temperature [43], presence of oxygen, and low pH [40, 41]. Moreover, trehalose also helps in reducing viability loss of freeze-dried cells during storage [44].
Glutamate and lysin accumulations help the lactobacilli cell under many adverse environment [34]. Propionibacterium acidopropionicii concentrated arginine and aspartate following acid stress [45]. Under acidic environments, L. plantarum activates the lysine degradation pathway [46]. Phosphates and glycogen are found to be used as energy storage compound by several probiotic bacteria.
Regulation of Energy Production
During stressful conditions, a cell regulates the various metabolic pathways and substrate conversion to counteract the stress and maintain the homeostasis. Under normal condition, ATPase synthesize ATP by proton motive force and stores energy. But under stressful conditions like acid and bile stress, this protein works in reversible fashion, it expulses the proton from the cytoplasm by hydrolysing ATP [47, 48]. Adenosine triphosphatase (ATPase) activity was regulated at transcriptional level [49] and ATPase expression was correlated with bile stress and acid tolerance [45]. When grown in MRS medium at 37 °C with constant pH 4.8 for 14 h, L. rhamnosus GG overexpressed F0F1-ATP synthase genes while proteins taking part in DNA and RNA synthesis were significantly reduced [50]. Similar results were observed in the following study. Increased production of F0F1-ATPase was reported at pH 4.8 than pH 7 by a probiotic strain, B. longum NCIMB 8809 [51].
Even substrate conversion is also redirected under stresses. For example, during acid stress Lactobacilli decreases lactic acid production by rerouting the glucose metabolic pathway [34]. Under cold stress, Propionibacteria restrict the production of propionate and acetate from lactate by diverting pyruvate toward other pathways [43]. The arginine deaminase (ADI) pathway is about five times more active in P. acidopropionici and Lactobacilli to prevent pHi from dropping too much. By this way, arginine can be broken down to produce ATP, ammonia, and carbon dioxide [45, 52, 53]. The pH homeostasis is achieved by the release of ammonia and carbon dioxide [45] and energy generated used by the ATPase to get rid of protons from the cell.
Impact on Bacterial Envelope
Probiotic bacteria experience cell envelope injury during harsh environment [34]. The cell membrane acts as a first line of protection shield against any hurdle. The stressors, such as presence of organic acids, freezing temperature, high temperature, and bile salts, can have an impact on the cell membrane. Moreover, cell envelop plays an extremely crucial role in maintaining cell intact and reducing the osmotic pressure under osmotic stress [34]. Different adaptive strategies are employed by bacteria to restore the integrity of the membrane and cell wall.
Variations in fluidity tend to be countered by modification of membrane constituents as a defense strategy that take place in stressful situations to maintain intact bilayer structure. As a result of acid stress, L. casei is found to produce more cyclopropane fatty acids and less unsaturated to saturated fat. It helps the cell to prevent inflow of proton by making cytoplasmic membrane stiffer and more compact [49]. It was also reported that unsaturated fatty acids in cytoplasmic membranes of L. helveticus increased when it was under heat stress, which reduced membrane fluidity [54]. P. freudenreichii produces branched chain fatty acids through degradation of branched amino acids using a variety of enzymes [55], helping to keep the membrane fluid to fend off cold stress.
Increase in hydrophobicity of cell wall is one of the response mechanisms adopted by many probiotics against different stresses. Many Lactobacilli were found to overproduce S-layer proteins during bile, acid, heat, and osmotic stress [56, 57]. Such S-layer proteins function as a coating of insulation, thereby, protecting the cell from any injury and lethal effect. It was also observed that exopolysaccharides (EPS) defend bacteria from stress like heat, bile, low pH, and osmotic. Such EPS can be either tightly or weakly linked to the cell surface [58,59,60].
Production of Chaperones and Stress Responsive Proteases
Under extreme stress, the expression of chaperones and proteases is rapidly accelerated by the bacteria. They either refold the denatured protein to correct configuration or degrade them. When damage is irreparable, proteases serve as the ultimate line of defense by promoting recycling of amino acids of denatured protein. By destroying proteins whose activities are no longer necessary as a result of changes to environment, the process of proteolysis of cellular proteins can significantly contribute to homeostasis [34]. Proteins that have been damaged were correctly folded by chaperones and DnaK (heat shock protein) is a widely recognized cellular chaperone which bring damaged proteins to correct configuration [34].
When L. rhamnosus is exposed to pH 4.8, the ClpE chaperone concentration was two times greater in the late lag phase of growth, demonstrating its defensive function in acidic stress [50]. In a study, S. thermophilus produced higher amount of elongation factor G (EF-G) and Tuf proteins after 3 h of exposure to acidic pH 5 than at normal physiological pH 6.8 [61]. EF-G promotes the translocation step in bacterial protein synthesis and Tuf involved in molecular chaperone activity. Various stress responsive proteins such as small heat shock proteins (HSP), cold shock proteins, antifreeze proteins, mRNA binding proteins, moonlight protein (possess adhesive property), etc., are overproduced during heat, cold, osmotic, bile, and acid stress. Main stress response mechanisms of bacteria under various stresses like osmotic, acid, oxidative, heat, cold and bile are shown in Fig. 2.
Main stress response mechanisms of bacteria under various stresses like osmotic, acid, oxidative, heat, cold, and bile [36]. Peptidoglycan is represented in blue. Membrane lipids under normal growth are represented in gray. Amounts of saturated (blue), unsaturated (red), and cyclic (yellow) fatty acids are modulated by treatments. S-layer proteins, which may be involved in adaptation, are represented in yellow and red outside the peptidoglycan. Lipoteichoic acids, whose length is modulated, are presented in green. Inducible transmembrane ATPase and Osmoprotectant uptake systems are represented in pink and blue, respectively. In the cytoplasm, general stress proteins are represented by different colors. Colored circles represent different osmoprotectant and energy storage compounds. Crosses on circles mean the conversion of the molecule. The chromosome is represented in black
Individual Stress: Impact and Response by Probiotics
Acid Stress
Lactic acid production during fermentation by lactic acid bacteria causes acidification of carrier matrix. This undissociated organic acid enters the cell through simple diffusion and dissociate inside the cell due to high pH. This leads to cause acidic cytoplasm and damages DNA, proteins, and many vital biomolecules which are necessary for the cell’s viability [62]. Low pH in gastric conditions also prevents the microbial colonization in GIT [63]. Many bacteria activated the defense mechanism during acid stress; such studies are discussed below.
Neutralization of cytoplasm by metabolism of amino acid is one of the mechanisms activated during acid stress by the probiotic bacteria. Amino acid decarboxylation produces ATP as well as neutralize acid by producing alkaline metabolites. Overexpression of glutamic acid decarboxylase (GAD) genes of L. reuteri strain 100–23 in mouse stomach shows the activation of protection system against acid stress [64]. In a study, histidine decarboxylation pathway from Str. thermophilus CHCC1524 introduced to L. lactis NZ9000 showed tenfold higher acid stress survival at acid stress, 2.5 pH for 2 h compared to wild type [65]. Such Lactobacilli have potential to be utilized in low-pH carrier matrix like fermented foods.
Malate decarboxylation is called malolactic fermentation (MLF). It releases CO2 which neutralizes the protons [66]. When 30 mM malate added to the carrier matrix, it improved the low pH 2.5 survival of the L. casei ATCC 334 [49]. Many probiotic bacteria are found to produce EPS, which shield themselves against low pH. The L. helveticus ATCC 15807 secreted greater EPS at pH 4.5 than normal physiological pH. EPS prevent the penetration of free H+ into the cell cytoplasm [67].
Probiotics can boost the functioning of the F0F1 ATPase that utilizes ATP to propel the evacuation of H+ from the cell and so maintain pHi homeostasis [63]. L. rhamnosus GG increased F0F1-ATP synthase production, when grown in whey broth (5% hydrolyzed whey, 0.6% casein hydrolysate and 0.0015% MnSO4, and water, pH 5.8). Various other acid stress responses such as cell signaling by LuxS (involved in quorum sensing), ClpE (degradation of misfolded proteins during stress responses), and peptidoglycan biosynthesis along with F0F1-ATP synthase were upregulated in whey broth having pH 4.8 compared to pH 5.8 [50].
Probiotics fight against low pH stress through over production of common stress proteins and chaperones such as GroEL, GroES, DnaK, and Clp [68]. It was reported that the HSP and chaperones (DnaK, GrpE, GroEL, and GroES) were abundant in L. plantarum 423 under acid stress, i.e., in MRS at 2.5 pH for 2 h [46]. Alteration in cell membrane composition is another protection strategy in probiotics during acid stress. The composition of fatty acids in cell membrane of previously acid exposed cells of L. casei ATCC 334 was checked in a study. The higher contents of saturated fatty acid (SFA) and cyclopropane fatty acid (CFA) were reported [49]. These SFA and CFA play functional role in modulation of membrane features like fluidity, hydrophobicity, and proton permeability under adverse environment.
In one study, L. plantarum 423 was added to MRS broth with 2.5 pH for 2 h. By analyzing proteomic profile, it was found that proteins involved in transcription, translation, and cell division were decreased under stress conditions. These proteins are not vital for cell’s viability but they are involved in cell growth and divisions. Further they studied glucose consumed and lactic acid produced in MRS broth under low pH and normal physiological environment. Under control condition 12.9 g/L glucose was consumed and 2.7 g/L lactic acid was produced, whereas in stress condition 19.0 g/L glucose was consumed and 0.7 g/L lactic acid was produced [46]. Increased glucose consumption and reduced lactic acid production during acid stress mean that cell is utilizing glucose as a source of energy to cope with stressful condition. It channelizes the metabolic pathway of glucose in such a way that end product is not acid but some other neutral and /or alkaline substances under acid stress. Figure 3 presents an overview of the many modes of acid resistance in lactic acid bacteria. Acid stress response in various beneficial bacteria is given in Table 1.
Mechanisms of acid tolerance in lactic acid bacteria [adopted from 141]. ADP adenosine diphosphate, AI-2 auto-inducer 2, ATP adenosine triphosphate, CFA cyclopropane fatty acids, Dnak molecular chaperone protein, GABA γ-aminobutyrate, GAD glutamate decarboxylase, LuxS S-ribosylhomocysteinelyase, Nth endonuclease, RecA DNA repair protein, RecO DNA repair protein, Shsp small heat shock protein, SmnA AP endonuclease, TCS two-component signal system, and UvrA ultraviolet excinuclease
Bile Stress
In order to move through the host’ GI system, probiotic bacteria must overcome many key obstacles and one such obstacle is bile in the human intestine. The natural level of bile in the human gut is around 0.05 to 2%. Bile acid serves as a biological detergent and exhibits variety of negative consequences, such as DNA damage, protein misfolding or denaturation, formation of secondary structure of RNA, reducing pH of cell cytoplasm, and dissolving cellular lipids [69, 70]. Research findings have shown that probiotic bacteria respond to bile hurdle by increasing the level of chaperones, proteases, proteins involved in bile detoxification and export, redox enzymes, and cell wall– and membrane-bound constituents, which consistently alters cell envelope characteristics [71,72,73].
Many probiotics possess enzyme, bile salt hydrolase which provides protection against bile stress. Moreover, probiotic LAB cleans the cellular environment from bile employing export mechanisms. In both Lactobacilli and Bifidobacteria, many transporter proteins and potential bile efflux systems were characterized as well as located using transcriptome method [68, 71, 74,75,76]. When L. fermentum NCDC 605 was exposed to 1.2% bile in MRS broth for 6 h, the following changes were observed: alterations in the energy metabolism, such as a rise in ATP synthesis; alterations in glycolytic end product concentrations; alteration in shape and size of L. fermentum NCDC 605 cells; molecular chaperones and proteases genes upregulated. All these alterations in L. fermentum NCDC 605 tend to help the cell to survive under bile stress [77]. The morphological changes may be due to alteration in cell surface by (a) excretion of EPS, (b) modifications to the cell membrane’s fatty acid makeup, and (c) changes in surface-associated proteins.
One of the enzymes called bile salt hydrolase (BSH) is involved in deconjugation of primary bile salt and releases free insoluble cholic acid (unconjugated acid) and a residue of taurine or glycine. Other commensal microbes could then disintegrate the unconjugated acids or excreted out by host [78]. When L. plantarum Lp91 was exposed to 2% bile for 3 h in MRS broth, the expression of bsh gene increased six times compared to control [79]. The multidrug transporters and bile efflux pumps are the primary mechanisms in Bifidobacterium breve UCC2003 for bile acid detoxification during gastrointestinal transit. When bile efflux pump Bbr_0838 in B. breve UCC2003 has been inactivated, through insertional mutation, the capacity of the genetically modified cells to grow in the presence of cholic acid (0.05%) has been reduced significantly (p < 0.05) than the unmodified, original cells [76].
In another study, B. breve UCC2003 was allowed to form biofilms in reinforced clostridial medium for 24 h during many stressful conditions like acidic environment at pH 4, pH 4.5, pH 5, and pH 5.8; bile stress at porcine 0.05%, 0.1%, 0.5%, 1%, and 2%; salt stress at NaCl 94.5 mM, 103 mM, 171 mM, 256 mM, and 426 mM; osmotic stress at sucrose 1.46 mM, 2.92 mM, 14.6 mM, 29.2 mM, and 58 mM. They found that at 2% porcine concentration significantly (p < 0.05) increased the higher biofilm formation as compared to other. Also, among all the stress conditions, bile stress at various concentration produced highest biofilm [80]. That shows that the biofilm formation is one of the important mechanisms of B. breve UCC2003 activated against bile stress in the GIT transit. In another study on bile stress, when probiotic L. salivarius Ren exposed to MRS containing 0.75 g/L bile for 14 h, various changes such as maltose and glycerol were utilized in carbohydrate metabolism to produce additional energy, overproduction of the enzymes involved in cell surface charge modification, secretion of cell envelope bound haemolysin-like protein (hinder bile penetration), overexpression of ATP-binding cassette (ABC) transporters (for expulsion of toxic intracellular bile), and overexpression of proteolytic system (to give additional amino acids to repair damaged proteins) were recorded [81]. Figure 4 shows various bile response mechanisms discovered and defined in Lactobacilli. Bile stress responses in various beneficial bacteria are given in Table 2.
Bile response mechanisms identified and characterized in Lactobacilli [69]
Cold Stress
Probiotic may be exposed to low temperatures during the storage of bacterial formulations before they are utilized in food production and during refrigerated storage of food products. Moreover, freezing and freeze drying are general techniques to preserve and concentrated probiotics lead to cold stress to the cells.
Low storage temperatures of probiotics can cause stiffening of the cell membranes, reducing vital enzyme functionality and lower down RNA transcription and protein translation rate, which may lead to growth arrest in a cell. Moreover, the ice crystals generated during freezing can permanently injure the bacterial cell envelope by punching and piercing. Furthermore, solutes begin to accumulate inside the cell during refrigerated storage due to conversion of liquid water into solid ice. Hence, cells experience desiccation and osmotic pressure gradient under cold environment. Since most probiotics are sold in freeze-dried form, the ability of probiotic to remain alive in cold environments is extremely important. It was reported that P. freudenreichii elevate the concentration of branched chain fatty acids in cytoplasmic membrane by synthesizing from branched amino acids, eventually maintaining required fluidity under the cold stress [55]. In probiotic Lactobacilli, cold stress stimulates many antifreeze and cold shock proteins (CSP) that bind to RNA, which prevent secondary structure generation and bolster transcription, translation, and ribosomal activity to keep the cell active under stress [82, 83]. Freezing resistant enzymes secreted by lactic bacteria are also capable of supporting both RNA and protein synthesis at extremely low temperatures [83].
The damage and piercing caused by the ice crystal formation during freezing were prevented by expression of antifreeze proteins by the probiotic Lactobacilli [84]. Osmotic pressure gradient formed during low temperature storage was found to balance by bacteria through secretion and accumulation of compatible solutes such as glycerol, trehalose, and amino acids like glycine, glutamate, lysine, arginine, betaine, and proline [85]. In a study, L. delbrueckii subsp. bulgaricus LBB.B5 were exposed to milk at 4 °C for 5 days and 37 °C for 16 h. Increased levels of many stress tolerance proteins like AddB, UvrC, RecA, and DnaJ were observed at lower temperature of exposure [86]. The cold-stress response of probiotic L. plantarum K25 was measured by comparing differentially expressed (DE) protein profiles after incubation at 10 °C for 72 h and 37 °C for 14 h. Various proteins upregulated after exposure to 10 °C compared to 37 °C were DNA repair, transcription, translation, quorum sensing, and ABC transporters [87]. Cold stress response in various beneficial bacteria is given in Table 3.
Heat Stress
Heat is a common technological stress because probiotics frequently have to deal with it at different phases of food production. Probiotics may encounter high temperatures like 60 °C during various stages of food preparation. Even immensely high temperature during spray drying can result in brief heat shocks of up to 200 °C. When biomolecules like DNA, RNA, and protein are subjected to elevated temperatures, they denature and loses their native property, which hampers metabolic activity [88]. Additionally, heat stress increases cell membrane flexibility, affecting the vital activity of cell, and may irreversibly damage cells and causes cell death [89]. Bacterial cells can tolerate milder heat challenges up to 65 °C, but it may reduce the stability of non-covalent bonds, causes cell envelope disruption, affecting function of ribosome, and leads to proteins denaturation [34].
Promoting the synthesis of particular proteins is one of the adaptive methods found in L. kefiranofaciens M1 to prevent cell damage [90]. These proteins were HSP, phosphoenolpyruvate-protein phosphotransferase, chaperone, chaperonins, and cofactors. They are crucial for facilitating proper packing and eventual transport of nascent polypeptides [91]. The GroEL/GroES chaperonin is one of the chaperone proteins that probiotic Lactobacilli use to deal with heat stress [92]. It was observed that B. longum synthesize HSP in heat stress and also adapt to higher temperature [93]. Interestingly, several HSP from lactic bacteria have capacity to attach and stabilize cell membranes. Such HSP is also known as lipochaperone [94, 95]. Saturated and straight-chain fatty acids that contribute to optimal liquidity necessary for normal membrane function were found in LAB that grew under heat stress [96].
The effect of heat adaptation (pre-exposure to sub-sublethal stress) on production of EPS by B. bifidum was checked in a study. The cells were exposed to 42 °C for 5 min and then grown in MRS containing 0.5 g/L of L-cysteine at 37 °C for 24 h anaerobically. L-cystein acts as oxygen remover and addition of this creates anaerobic conditions in the media, thus, enhancing the growth of anaerobic bacteria, B. bifidum. The EPS production was significantly higher in pre-exposed cells than the non-heat-exposed cells [97]. This finding shows the crucial role of EPS in protecting B. bifidum during heat stress. The SDS PAGE analysis of intracellular proteins of L. casei, heat stressed at 45 °C, 50 °C, 55 °C, and 60 °C for 60 min, was carried out. In all heat-stressed cells, variations in protein content at 40–55 kDa, 60 kDa, and 70 kDa were linked to overexpression of DnaJ, GroEL, and DnaK [98].
Adherence to HeLa cells and fatty acid composition of L. casei introduce to two heat treatments such as 37 °C for 10 min and 45 °C for 10 min were measured. HeLa cells adherence and ratio of unsaturated fatty acids (USFA) to SFA in 45 °C for 10 min treatment were 31.33% and 0.36, respectively, whereas in 37 °C for 10 min treatment were 28.66% and 0.40, respectively [96]. In case of 45 °C for 10 min treatment, the significant (p < 0.05) rise in adherence ability indicates the L. casei cell attaches to other cells to get away from the stressful condition, whereas significant reduction in the ratio of USFA to SFA means there is increase in concentration of SFA which prevents melting of cell membrane at high temperature and maintains the proper fluidity required for the normal functioning of the cell.
In one of the studies, cells of probiotic Enterococcus faecium HL7 were kept at 52 °C and 47 °C for 15 min for heat adaptation. Then % survival was calculated for the control and heat-adapted cells at 60 °C after every 10 min up to 40 min. Complete death of all the cells occurred in control, while heat-adapted cell survival was found to reduce at the end of 60 min. Cells adapted to 52 °C exhibited greater survival than 47 °C. Cells heat adapted to 52 °C had comparatively higher SFA and lower USFA than the remaining treatments. Cells with a reduced level of USF or with increased level of SFA have a reduced cytoplasmic membrane flexibility which is correlated to greater heat resistance [99]. Viability of heat-treated (52 °C/15 min) and non-heat-treated E. faecium HL7 cells after subjected to different stress, such as 0.01% hydrogen peroxide (oxidative stress), 20% ethanol, 3 pH (acid stress), and 12 pH, was evaluated. It was discovered that the heat-adapted cells managed to survive at significantly (p < 0.05) higher number than the non-adapted cells [99]. When cells are exposed to a particular stress, it activated various general stress response mechanisms that help the cell to survive and fight against other stressful conditions. This mechanism is also known as cross protection. In the above study, when E. faecium HL7 cells were previously treated with sublethal heat stress, it improved the survival rate under various stresses. It indicated the cross protection in the heat-adapted cells. Heat stress response in various beneficial bacteria is given in Table 4.
Osmotic Stress
Osmotic stress is experienced by probiotic bacteria in the growth medium, in the course of food production and in the GIT. Probiotic bacteria undergo osmotic stress when solute contents in food preparation were changed like salt in cheese, high sugary foods, pickle, etc. [100]. Water moves out of the cell as osmotic pressure rise, triggering cell contraction, loss of cell turgor pressure, and altering cytoplasmic solute content. All of which have a negative impact on bacterial survival [101]. Compatible solutes either from the surrounding medium or secreted by the cell were extremely helpful to combat the osmotic stress. Most of the compatible solutes do not have any charge at pH 7; hence, without interfering with the metabolism, uncharged compatible solutes can be collected in large concentrations. Compatible solutes were observed to preserve proteins in their correct conformation during osmotic pressure.
Osmotic stress could stimulate the secretion of trehalose in P. freudenreichii and L. casei [42]. Lactobacilli were reported to modulate the cytoplasmic level of amino acids such as proline and glutamate during osmotic adaptation [34]. The osmotically induced OpuABC (or Bus ABC) transporter accumulates glycine betaine in P. freudenreichii during osmotic stress [42]. The high levels of sugar in the carrier matrix of LAB allow formation of metabolites such as mannitol (non-fermentable carbohydrate). The non-fermentable carbohydrates were found to boost cell survival during spray drying by raising osmotic pressure and causing cells to osmotically adapt [102]. The LAB cells activated several defence mechanisms when pre-exposed to sub-lethal osmotic stress, could have increased survival after spray drying [42]. The LAB produced EPS in the surrounding medium, thereby prevented cell damage due to dehydration in osmotic stress. The hydroxyl group present on the polysaccharides of EPS was responsible for the water binding ability [103]. The Leu. mesenteroides 406 produced 25.83 g/L EPS in MRS broth containing 5% NaCl compared to 16.02 g/L EPS in control after 48 h at 28 °C [104].
Osmotic stress triggers production or import of K+ or compatible solute in probiotics, which protect cell against lethal damage. L. acidophilus and L. casei secrete protective molecules like DnaK and HtrS operon proteins that safeguard the bacteria against damage caused by high salt concentration in medium [105]. The impact of osmoadaptation of B. bifidum CCFM16 cells on multiplication rate during osmotic stress was determined in a study. Medium added with 0.3% NaCl gives osmotic stress of 100 mOsm/kg. In MRS containing 1 g/L L-cysteine, B. bifidum CCFM16 was exposed to an osmotic environment that increased steadily over the course of 1000 generations. Then the hyperosmotic-tolerance mutant and parent strains were exposed to 1300 mOsm/kg osmotic stress. The generation time of extremely high osmotic pressure–resistant mutant B. bifidum CCFM16m is 1/3 of its parent strain B. bifidum CCFM16 [106]. The finding of this study suggests that the B. bifidum CCFM16 develops various defense mechanisms to cope against harsh osmotic stress when gradually grown in increasing level of osmotic pressure. Such mutant strain has potential to survive in higher number during freeze dying and refrigerated storage in food matrix.
Lactobacillus rhamnosus GG was previously exposed to 4% NaCl and 4.5 pH in MRS for 24 h for stress adaptation. The cell count reduction of stress-adapted L. rhamnosus GG (0.2 log reduction) was comparatively lesser than non-stress-adapted L. rhamnosus GG (0.5 log reduction) in yoghurt matrix during refrigerated storage [107]. This indicates the adaptive response of L. rhamnosus GG to sublethal osmotic and pH stress had improved the survival in the carrier matrix during low-temperature storage.
Osmotic stress response in various beneficial bacteria is given in Table 5.
Oxidative Stress
Aerobic conditions experienced by probiotics during food processing as well as GIT transit lead to oxidative stress to the sensitive bacterial strain. The oxygen acts as toxic compound by reacting with iron of heme-dependent cytochrome oxidase in electron transport chain to create ROS [108]. Metabolic conversion of oxygen generates ROS like superoxide (O2−), hydroxyl radicals (HO•), and hydrogen peroxide (H2O2). These ROS are highly unstable entities and are responsible for the oxidative chain reaction which damages several critical biomolecules like proteins, DNA, RNA, and lipids, which affects cells viability. ROS can freely pass through the semipermeable membrane and greater amount of ROS ceases LAB cell multiplication. Since many LAB and bifidobacteria lack catalase and superoxide dismutase (SOD) enzyme activity, they were unable to neutralize hydrogen peroxide and ROS, which made them vulnerable to oxygen [109].
Certain LAB like L. plantarum does not have ROS-neutralizing enzymes such as SOD. They defend themselves by other strategies, i.e., concentration of manganese inside the cell and utilizing Mn-dependent mechanisms of superoxide neutralization [110]. Aerotolerant anaerobe LAB Leu. mesenteroides, which lacks SOD, neutralized ROS by encouraging EPS production and cellular aggregation. EPS expel dissolved oxygen from medium to relieve oxidative stress [111]; EPS also neutralizes the ROS by binding with them and cellular aggregation protects the inner cells from the ROS. The fatty acid constituents in bifidobacterial cell membrane were found to alter in oxidative stress [112], suggesting that such modifications might strengthen tolerance to ROS.
The redox mechanisms of Lactobacillus spp. may contribute to the downregulation of ROS-forming enzymes. In addition, nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf-2) and nuclear factor kappa B (NF-κB) were two common transcription factors, through which Lactobacillus spp. modulated oxidative stress [113]. The active cells of L. plantarum CAUH2 were suspended in MRS supplemented with 3 mM, 4 mM, 5 mM, 6 mM, and 7 mM H2O2 to study the oxidative stress response. The concentrations 6 mM and 7 mM H2O2 inhibited L. plantarum CAUH2 growth, but cells were able to survive up to 5 mM H2O2 stress. It was also observed that L. plantarum CAUH2 changed its carbon source utilizing profile and modified glycolytic pathway to produce more ATP under 5 mM H2O2 stress. At transcription level, the antioxidant enzymes like NADH peroxidase, thioredoxin reductase, and glutathione peroxidase were 6.11, 36.76, and 6.23 times upregulated under 5 mM H2O2 stress [114]. The surviving cell numbers of L. rhamnosus hsryfm 1301 increased from 3.7 log CFU in non-adapted cells to 7.8 log CFU in pre-adapted (5 mM H2O2 for 1 h) cells in the presence of 0.5 mM H2O2 [115]. Since oxidative stress improved the survival rate of adapted cells, pre-adaptation to oxidative stress has potential to ameliorate the aerotolerance in probiotic bacteria during food processing.
Oxidative stress response in various beneficial bacteria is given in Table 6.
Conclusion
Various environmental stresses are encountered by probiotics during food preparation and passage through the GIT. All the response mechanisms used by probiotics to various stresses are species and strain dependent. While under stress, probiotics respond by triggering a number of regulatory functions, such as control gene expression, modification in cell membrane composition, and alteration in metabolic pathways. Exposure of probiotic bacteria to sublethal stress activates the multiple stress tolerance mechanisms in the stress-adapted cell. This review will be helpful to the people involved in development of a new/novel probiotic food, i.e., selection of the carrier matrix with most effective probiotic strain in active physiological state giving maximum therapeutic benefit during GIT transit. Furthermore, this information lays the path for the development of biological and technology strategies to enhance probiotic robustness. It is required to conduct more genomic studies for better understanding of bacterial cell’s behavior under stress. Additionally, there is also requirement to perform animal studies for the stressed cells to check for any pathogenicity, efficacy, and effectiveness. Trials can be conducted to prepare customized foods by utilizing stress-adapted probiotics.
Availability of Data and Materials
Raw data not published in supplementary materials and data are not available for this review paper.
References
Kitazawa H, Alvarez S, Suvorov A, Melnikov V, Villena J, Sánchez B (2015) Recent advances and future perspective in microbiota and probiotics. BioMed Res Inter https://doi.org/10.1155/2015/275631
Neutraceutical products market (2023) Top market reports https://www.marketsandmarkets.com/Market-Reports/nutraceutical-product-market-145393943Accessed on 01 February 2023
Ganguly NK, Bhattacharya SK, Sesikeran B, Nair GB, Ramakrishna BS, Sachdev HPS, Hemalatha R (2011) ICMR-DBT guidelines for evaluation of probiotics in food. Indian J Med Res 134:22–25
Żukiewicz-Sobczak W, Wróblewska P, Adamczuk P, Silny W (2014) Probiotic lactic acid bacteria and their potential in the prevention and treatment of allergic diseases. Cent Eur J Immunol 39:104–108
Rabah H, Carmo FLRD, Jan G (2017) Dairy propionibacteria: versatile probiotics. Microorganisms. https://doi.org/10.3390/microorganisms5020024
Gaspar P, Carvalho AL, Vinga S, Santos H, Neves AR (2013) From physiology to systems metabolic engineering for the production of biochemicals by lactic acid bacteria. Biotechnol Adv 31:764–788. https://doi.org/10.1016/j.biotechadv.2013.03.011
Marcial-Coba MS, Cieplak T, Cahú TB, Blennow A, Knøchel S, Nielsen DS (2018) Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct 9:5868–5879. https://doi.org/10.1039/C8FO01331D
Gupta S, Abu-Ghannam N (2012) Probiotic fermentation of plant based products: possibilities and opportunities. Crit Rev Food Sci Nutr 52:183–199. https://doi.org/10.1080/10408398.2010.499779
Pakdaman MN, Udani JK, Molina JP, Shahani M (2015) The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance-a randomized, double-blind, placebo-controlled, crossover clinical trial. Nutri J. https://doi.org/10.1186/s12937-016-0172-y
Strozzi GP, Mogna L (2008) Quantification of folic acid in human feces after administration of Bifidobacterium probiotic strains. J Clin Gastroenterol 42:S179–S184. https://doi.org/10.1097/mcg.0b013e31818087d8
LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P (2017) Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Factories 16:1–10. https://doi.org/10.1186/s12934-017-0691-z
Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC (2010) Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol 10:1–11. https://doi.org/10.1186/1471-2180-10-316
Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM (2010) Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol-Gastroint Liver Physiol 298:G851–G859. https://doi.org/10.1152/ajpgi.00327.2009
Du W, Xu H, Mei X, Cao X, Gong L, Wu Y, Li Y, Yu D, Liu S, Wang Y, Li W (2018) Probiotic Bacillus enhance the intestinal epithelial cell barrier and immune function of piglets. Benef Microbes 9:743–754. https://doi.org/10.3920/BM2017.0142
Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M (2005) Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-β-bearing regulatory cells. J Immunol 174:3237–3246. https://doi.org/10.4049/jimmunol.174.6.3237
Huebner C, Ding Y, Petermann I, Knapp C, Ferguson LR (2011) The probiotic Escherichia coli Nissle 1917 reduces pathogen invasion and modulates cytokine expression in Caco-2 cells infected with Crohn’s disease-associated E. coli LF82. Appl Environ Microbiol 77:2541–2544. https://doi.org/10.1128/AEM.01601-10
Koebnick C, Wagner I, Leitzmann P, Stern U, Zunft HJ (2003) Probiotic beverage containing Lactobacillus casei Shirota improves gastrointestinal symptoms in patients with chronic constipation. Can J Gastroenteol Hepatol 17:655–659. https://doi.org/10.1155/2003/654907
Bekkali NL, Bongers ME, Van den Berg MM, Liem O, Benninga MA (2007) The role of a probiotics mixture in the treatment of childhood constipation: a pilot study. Nutri J. https://doi.org/10.1186/1475-2891-6-17
Simone M, Gozzoli C, Quartieri A, Mazzola G, Di Gioia D, Amaretti A, Raimondi S, Rossi M (2014) The probiotic Bifidobacterium breve B632 inhibited the growth of Enterobacteriaceae within colicky infant microbiota cultures. BioMed Res Int. https://doi.org/10.1155/2014/301053
Aloisio I, Prodam F, Giglione E, Bozzi Cionci N, Solito A, Bellone S, Baffoni L, Mogna L, Pane M, Bona G, Di Gioia D (2018) Three-month feeding integration with bifidobacterium strains prevents gastrointestinal symptoms in healthy newborns. Front Nutr. https://doi.org/10.3389/fnut.2018.00039
Roychowdhury S, Cadnum J, Glueck B, Obrenovich M, Donskey C, Cresci GA (2018) Faecalibacterium prausnitzii and a prebiotic protect intestinal health in a mouse model of antibiotic and Clostridium difficile exposure. J Parenter Enteral Nutr 42:1156–1167. https://doi.org/10.1002/jpen.1053
Xiao J, Zhang Y, Yang Z (2014) Lactic acid bacteria in health and disease. In: Zhang H, Cai Y (eds) Lactic acid bacteria: fundamentals and practice. Springer, Dordrecht. pp 303–374. https://doi.org/10.1007/978-94-017-8841-0_2
Ambalam P, Raman M, Purama RK, Doble M (2016) Probiotics, prebiotics and colorectal cancer prevention. Best Pract Res Clin Obstet Gynaecol 30:119–131. https://doi.org/10.1016/j.bpg.2016.02.009
Tarrah A, Castilhos JD, Rossi RC, Duarte VDS, Ziegler DR, Corich V, Giacomini A (2018) In vitro probiotic potential and anti-cancer activity of newly isolated folate-producing Streptococcus thermophilus strains. Front Microbiol. https://doi.org/10.3389/fmicb.2018.02214
Bautista-Gallego J, Arroyo-López FN, Bordons A, Jiménez-Díaz R (2019) New trends in table olive fermentation. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01880
Muller JA, Ross RP, Sybesma WFH, Fitzgerald GF, Stanton C (2011) Modification of the technical properties of Lactobacillus johnsonii NCC 533 by supplementing the growth medium with unsaturated fatty acids. Appl Environ Microbiol 77:6889–6898. https://doi.org/10.1128/AEM.05213-11
Du Toit E, Vesterlund S, Gueimonde M, Salminen S (2013) Assessment of the effect of stress-tolerance acquisition on some basic characteristics of specific probiotics. Int J Food Microbiol 165:51–56. https://doi.org/10.1016/j.ijfoodmicro.2013.04.022
Booth IR (2002) Stress and the single cell: intrapopulation diversity is a mechanism to ensure survival upon exposure to stress. Int J Food Microbiol 78:19–30. https://doi.org/10.1016/S0168-1605(02)00239-8
Giraffa G (2012) Selection and design of lactic acid bacteria probiotic cultures. Eng Life Sci 12:391–398. https://doi.org/10.1002/elsc.201100118
Kathiriya MR, Hati S, Prajapati JB, Vekariya YV (2016) Assessment of in vitro probiotic potential of lactic acid bacteria. Res Rev J Dairy Sci Tech 5:17–30
Abhisingha M, Dumnil J, Pitaksutheepong C (2018) Selection of potential probiotic Lactobacillus with inhibitory activity against Salmonella and fecal coliform bacteria. Probiotics Antimicrob Proteins 10:218–227. https://doi.org/10.1007/s12602-017-9304-8
Dowarah R, Verma AK, Agarwal N, Singh P, Singh BR (2018) Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE. https://doi.org/10.1371/journal.pone.0192978
Grujović MŽ, Mladenović KG, Nikodijević DD, Čomić LR (2019) Autochthonous lactic acid bacteria—presentation of potential probiotics application. Biotechnol Lett 41:1319–1331. https://doi.org/10.1007/s10529-019-02729-8
Papadimitriou K, Alegria A, Bron PA, de Angelis M, Gobbetti M, Kleerebezem M, Kok J (2016) Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev 80:837–890. https://doi.org/10.1128/MMBR.00076-15
de Melo Pereira GV, de Oliveira CB, Júnior AIM, Thomaz-Soccol V, Soccol CR (2018) How to select a probiotic? A review and update of methods and criteria. Biotechnol Adv 36:2060–2076. https://doi.org/10.1016/j.biotechadv.2018.09.003
Gaucher F, Bonnassie S, Rabah H, Marchand P, Blanc P, Jeantet R, Jan G (2019) Adaptation of beneficial Propionibacteria, lactobacilli, and Bifidobacteria improves tolerance toward technological and digestive stresses. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00841
Csonka LN (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147. https://doi.org/10.1128/mr.53.1.121-147.1989
Csonka LN, Hanson AD (1991) Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol 45:569–606. https://doi.org/10.1146/annurev.mi.45.100191.003033
Roesser M, Müller V (2001) Osmoadaptation in bacteria and archaea: common principles and differences. Environ Microbiol 3:743–754. https://doi.org/10.1046/j.1462-2920.2001.00252.x
Cardoso FS, Gaspar P, Hugenholtz J, Ramos A, Santos H (2004) Enhancement of trehalose production in dairy propionibacteria through manipulation of environmental conditions. Int J Food Microbiol 91:195–204. https://doi.org/10.1016/S0168-1605(03)00387-8
Cardoso FS, Castro RF, Borges N, Santos H (2007) Biochemical and genetic characterization of the pathways for trehalose metabolism in Propionibacterium freudenreichii, and their role in stress response. Microbiology 153:270–280. https://doi.org/10.1099/mic.0.29262-0
Huang S, Cauty C, Dolivet A, Le Loir Y, Chen XD, Schuck P, Jeantet R (2016) Double use of highly concentrated sweet whey to improve the biomass production and viability of spray-dried probiotic bacteria. J Funct Foods 23:453–463. https://doi.org/10.1016/j.jff.2016.02.050
Dalmasso M, Aubert J, Even S, Falentin H, Maillard MB, Parayre S, Thierry A (2012) Accumulation of intracellular glycogen and trehalose by Propionibacterium freudenreichii under conditions mimicking cheese ripening in the cold. Appl Environ Microbiol 78:6357–6364. https://doi.org/10.1128/AEM.00561-12
Giulio BD, Orlando P, Barba G, Coppola R, Rosa MD, Sada A, Nazzaro F (2005) Use of alginate and cryo-protective sugars to improve the viability of lactic acid bacteria after freezing and freeze-drying. World J Microbiol Biotechnol 21:739–746. https://doi.org/10.1007/s11274-004-4735-2
Guan N, Liu L, Shin H, Chen RR, Zhang J, Li J et al (2013) Systems-level understanding of how Propionibacterium acidipropionici respond to propionic acid stress at the microenvironment levels: mechanism and application. J Biotechnol 167:56–63. https://doi.org/10.1016/j.jbiotec.2013.06.008
Heunis T, Deane S, Smit S, Dicks LM (2014) Proteomic profiling of the acid stress response in Lactobacillus plantarum 423. J Proteome Res 13:4028–4039. https://doi.org/10.1021/pr500353x
van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E (2002) Stress responses in lactic acid bacteria. In: Siezen RJ, Kok J, Abee T, Schasfsma G (eds) Lactic acid bacteria: genetics, metabolism and applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-2029-8_12
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453. https://doi.org/10.1128/MMBR.67.3.429-453.2003
Broadbent JR, Larsen RL, Deibel V, Steele JL (2010) Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J Bacteriol 192:2445–2458. https://doi.org/10.1128/JB.01618-09
Koponen J, Laakso K, Koskenniemi K, Kankainen M, Savijoki K, Nyman TA, Varmanen P (2012) Effect of acid stress on protein expression and phosphorylation in Lactobacillus rhamnosus GG. J Proteom 75:1357–1374. https://doi.org/10.1016/j.jprot.2011.11.009
Sánchez B, Champomier-Vergès M-C, Collado MC, Anglade P, Baraige F, Sanz Y et al (2007) Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl Environ Microbiol 73:6450–6459. https://doi.org/10.1128/AEM.00886-07
Rollan G, Lorca GL, Font de Valdez G (2003) Arginine catabolism and acid tolerance response in Lactobacillus reuteri isolated from sourdough. Food Microbiol 20:313–319. https://doi.org/10.1016/S0740-0020(02)00139-9
Teixeira JS, Seeras A, Sanchez-Maldonado AF, Zhang C, Su MSW, Gänzle MG (2014) Glutamine, glutamate, and arginine-based acid resistance in Lactobacillus reuteri. Food Microbiol 42:172–180. https://doi.org/10.1016/j.fm.2014.03.015
Guerzoni ME, Lanciotti R, Cocconcelli PS (2001) Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiol 147:2255–2264. https://doi.org/10.1099/00221287-147-8-2255
Gagnaire V, Jardin J, Rabah H, Briard-Bion V, Jan G (2015) Emmental cheese environment enhances Propionibacterium freudenreichii stress tolerance. PLoS One. https://doi.org/10.1371/2Fjournal.pone.0135780
Khaleghi M, Kermanshahi RK (2012) Effect of environmental stresses on S-layer production in Lactobacillus acidophilus ATCC 4356. In: Petre M (ed) Advances in applied biotechnology. InTech, pp 209–224. https://doi.org/10.5772/1096
Grosu-Tudor SS, Brown L, Hebert EM, Brezeanu A, Brinzan A, Fadda S et al (2016) S-layer production by Lactobacillus acidophilus IBB 801 under environmental stress conditions. Appl Microbiol Biotechnol 100:4573–4583. https://doi.org/10.1007/s00253-016-7355-5
Alp G, Aslim B (2010) Relationship between the resistance to bile salts and low pH with exopolysaccharide (EPS) production of Bifidobacterium spp. isolated from infants feces and breast milk. Anaerobe 16:101–105. https://doi.org/10.1016/j.anaerobe.2009.06.006
Stack HM, Kearney N, Stanton C, Fitzgerald GF, Ross RP (2010) Association of beta-glucan endogenous production with increased stress tolerance of intestinal lactobacilli. Appl Environ Microbiol 76:500–507. https://doi.org/10.1128/AEM.01524-09
Caggianiello G, Kleerebezem M, Spano G (2016) Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Applied Microbiol Biotechnol 100:3877–3886. https://doi.org/10.1007/00253-016-7471-2
Arena S, D’Ambrosio C, Renzone G, Rullo R, Ledda L, Vitale F et al (2006) A study of Streptococcus thermophilus proteome by integrated analytical procedures and differential expression investigations. Proteomics 6:181–192. https://doi.org/10.1002/pmic.200402109
Wu C, Zhang J, Wang M, Du G, Chen J (2012) Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J Ind Microbiol Biotechnol 39:1031–1039. https://doi.org/10.1007/s10295-012-1104-2
Montoro BP, Benomar N, Gómez NC, Ennahar S, Horvatovich P, Knapp CW, Abriouel H (2018) Proteomic analysis of Lactobacillus pentosus for the identification of potential markers involved in acid resistance and their influence on other probiotic features. Food Microbiol 72:31–38. https://doi.org/10.1016/j.fm.2017.11.006
Wilson CM, Loach D, Lawley B, Bell T, Sims IM, O’Toole PW, Tannock GW (2014) Lactobacillus reuteri 100–23 modulates urea hydrolysis in the murine stomach. App Environ Microbiol 80:6104–6113. https://doi.org/10.1128/AEM.01876-14
Trip H, Mulder NL, Lolkema JS (2012) Improved acid stress survival of Lactococcus lactis expressing the histidine decarboxylation pathway of Streptococcus thermophilus CHCC1524. Journal Biol Chem 287:11195–11204. https://doi.org/10.1074/jbc.m111.330704
Sumby KM, Grbin PR, Jiranek V (2014) Implications of new research and technologies for malolactic fermentation in wine. Appl Microbiol Biotechnol 98:8111–8132. https://doi.org/10.1007/s00253-014-5976-0
Torino MI, Font de Valdez G, Mozzi F (2015) Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00834
Ferreira AB, De Oliveira MV, Freitas FS, Alfenas-Zerbini P, Da Silva DF, De Queiroz MV, Borges AC, De Moraes CA (2013) Increased expression of clp genes in Lactobacillus delbrueckii UFV H2b20 exposed to acid stress and bile salts. Benef Microbes 4:367–374. https://doi.org/10.3920/BM2013.0022
Ruiz L, Margolles A, Sánchez B (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00396
He J, Wang W, Wu Z, Pan D, Guo Y, Cai Z, Lian L (2019) Effect of Lactobacillus reuteri on intestinal microbiota and immune parameters: Involvement of sex differences. J Funct Foods 53:36–43. https://doi.org/10.1016/j.jff.2018.12.010
Zaidi AH, Bakkes PJ, Krom BP, van der Mei HC, Driessen AJ (2011) Cholate-stimulated biofilm formation by Lactococcus lactis cells. Appl Environ Microbiol 77:2602–2610. https://doi.org/10.1128/AEM.01709-10
Alcantara C, Zuniga M (2012) Proteomic and transcriptomic analysis of the response to bile stress of Lactobacillus casei BL23. Microbiol 158:1206–1218. https://doi.org/10.1099/mic.0.055657-0
Ambalam P, Kondepudi KK, Nilsson I, Wadström T, Ljungh Å (2012) Bile stimulates cell surface hydrophobicity, Congo red binding and biofilm formation of Lactobacillus strains. FEMS Microbiol Lett 333:10–19. https://doi.org/10.1111/j.1574-6968.2012.02590.x
An H, Zhai Z, Yin S, Luo Y, Han B, Hao Y (2011) Coexpression of the superoxide dismutase and the catalase provides remarkable oxidative stress resistance in Lactobacillus rhamnosus. J Agric Food Chem 59:3851–3856. https://doi.org/10.1021/jf200251k
Hamon E, Horvatovich P, Izquierdo E, Bringel F, Marchioni E, Aoudé-Werner D, Ennahar S (2011) Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiol 11:1–11. https://doi.org/10.1186/1471-2180-11-63
Ruiz L, Zomer A, O’Connell-Motherway M, van Sinderen D, Margolles A (2012) Discovering novel bile protection systems in Bifidobacterium breve UCC2003 through functional genomics. App Environ Microbiol 78:1123–1131. https://doi.org/10.1128/AEM.06060-11
Ali SA, Singh P, Tomar SK, Mohanty AK, Behare P (2020) Proteomics fingerprints of systemic mechanisms of adaptation to bile in Lactobacillus fermentum. J Proteomics. https://doi.org/10.1016/j.jprot.2019.103600
Lambert JM, Bongers RS, de Vos WM, Kleerebezem M (2008) Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. App Environ Microbiol 74:4719–4726. https://doi.org/10.1128/AEM.00137-08
Duary RK, Batish VK, Grover S (2012) Relative gene expression of bile salt hydrolase and surface proteins in two putative indigenous Lactobacillus plantarum strains under in vitro gut conditions. Mol Biol Rep 39:2541–2552. https://doi.org/10.1007/s11033-011-1006-9
Kelly SM, Lanigan N, O’Neill IJ, Bottacini F, Lugli GA, Viappiani A, van Sinderen D (2020) Bifidobacterial biofilm formation is a multifactorial adaptive phenomenon in response to bile exposure. Sci Rep. https://doi.org/10.1038/s41598-020-68179-9
Wang G, Zhai Z, Ren F, Li Z, Zhang B, Hao Y (2020) Combined transcriptomic and proteomic analysis of the response to bile stress in a centenarian-originated probiotic Lactobacillus salivarius Ren. Food Res Int. https://doi.org/10.1016/j.foodres.2020.109331
Song S, Bae DW, Lim K, Griffiths MW, Oh S (2014) Cold stress improves the ability of Lactobacillus plantarum L67 to survive freezing. Int J Food Microbiol 191:135–143. https://doi.org/10.1016/j.ijfoodmicro.2014.09.017
Mangiagalli M, Sarusi G, Kaleda A, Bar Dolev M, Nardone V, Nardini VVF, M, (2018) Structure of a bacterial ice binding protein with two faces of interaction with ice. FEBS J 285:1653–1666. https://doi.org/10.1111/febs.14434
Polo L, Manes-Lazaro R, Olmeda I, Cruz-Pio LE, Medina A, Ferrer S, Pardo I (2017) Influence of freezing temperatures prior to freeze-drying on viability of yeasts and lactic acid bacteria isolated from wine. J Appl Microbiol 122(6):1603–1614. https://doi.org/10.1111/jam.13465
Keto-Timonen R, Hietala N, Palonen E, Hakakorpi A, Lindström M, Korkeala H (2016) Cold shock proteins: a minireview with special emphasis on Csp-family of enteropathogenic Yersinia. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01151
Yin X, Salemi MR, Phinney BS, Gotcheva V, Angelov A, Marco ML (2017) Proteomes of Lactobacillus delbrueckii subsp. bulgaricus LBB. B5 incubated in milk at optimal and low temperatures. mSystems. https://doi.org/10.1128/mSystems.00027-17
Liu S, Ma Y, Zheng Y, Zhao W, Zhao X, Luo T, Yang Z (2020) Cold-stress response of probiotic Lactobacillus plantarum K25 by iTRAQ proteomic analysis. J Microbiol Biotechnol 30:187–195. https://doi.org/10.4014/jmb.1909.09021
Bove P, Russo P, Capozzi V, Gallone A, Spano G, Fiocco D (2013) Lactobacillus plantarum passage through an oro-gastrointestinal tract simulator: Carrier matrix effect and transcriptional analysis of genes associated to stress and probiosis. Microbiol Res 168:351–359. https://doi.org/10.1016/j.micres.2013.01.004
Ferrando V, Quiberoni A, Reinheimer J, Suárez V (2016) Functional properties of Lactobacillus plantarum strains: a study in vitro of heat stress influence. Food Microbiol 54:154–161. https://doi.org/10.1016/j.fm.2015.10.003
Chen MJ, Tang HY, Chiang ML (2017) Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol 66:20–27. https://doi.org/10.1016/j.fm.2017.03.020
Hernández-Alcántara AM, Wacher C, Llamas MG, López P, Pérez-Chabela ML (2018) Probiotic properties and stress response of thermotolerant lactic acid bacteria isolated from cooked meat products. LWT 91:249–257. https://doi.org/10.1016/j.lwt.2017.12.063
Russo P, De La Luz MM, Capozzi V, De Palencia PF, López P, Spano G, Fiocco D (2012) Comparative proteomic analysis of Lactobacillus plantarum WCFS1 and DctsR mutant strains under physiological and heat stress conditions. Int J Mol Sci. https://doi.org/10.3390/ijms130910680
Khaskheli GB, Zuo F, Yu R, Chen S (2015) Overexpression of small heat shock protein enhances heat-and salt-stress tolerance of Bifidobacterium longum NCC2705. Curr Microbiol 71:8–15. https://doi.org/10.1007/s00284-015-0811-0
Capozzi V, Weidmann S, Fiocco D, Rieu A, Hols P, Guzzo J, Spano G (2011) Inactivation of a small heat shock protein affects cell morphology and membrane fluidity in Lactobacillus plantarum WCFS1. Res Microbiol 162:419–425. https://doi.org/10.1016/j.resmic.2011.02.010
Maitre M, Weidmann S, Rieu A, Fenel D, Schoehn G, Ebel C, Coves J, Guzzo J (2012) The oligomer plasticity of the small heat-shock protein Lo18 from Oenococcus oeni influences its role in both membrane stabilization and protein protection. Biochem J 444:97–104. https://doi.org/10.1042/BJ20120066
Haddaji N, Mahdhi AK, Krifi B, Ismail MB, Bakhrouf A (2015) Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnv047
Nguyen HT, Razafindralambo H, Blecker C, N’Yapo C, Thonart P, Delvigne F (2014) Stochastic exposure to sub-lethal high temperature enhances exopolysaccharides (EPS) excretion and improves Bifidobacterium bifidum cell survival to freeze–drying. Biochem Eng J 88:85–94. https://doi.org/10.1016/j.bej.2014.04.005
Bommasamudram J, Muthu A, Devappa S (2022) Effect of sub-lethal heat stress on viability of Lacticaseibacillus casei N in spray-dried powders. LWT. https://doi.org/10.1016/j.lwt.2021.112904
Álvarez-Ordóñez A, Fernández A, López M, Arenas R, Bernardo A (2008) Modifications in membrane fatty acid composition of Salmonella typhimurium in response to growth conditions and their effect on heat resistance. Int J Food Microbiol 123:212–219. https://doi.org/10.1016/j.ijfoodmicro.2008.01.015
Waśko A, Polak-Berecka M, Gustaw W (2013) Increased viability of probiotic Lactobacillus rhamnosus after osmotic stress. Acta Aliment 42:520–528. https://doi.org/10.1556/aalim.42.2013.4.7
Metris A, George SM, Mulholland F, Carter AT, Baranyi J (2014) Metabolic shift of Escherichia coli under salt stress in the presence of glycine betaine. Appl Environ Microbiol 80:4745–4756. https://doi.org/10.1128/AEM.00599-14
Peighambardoust SH, Golshan Tafti A, Hesari J (2011) Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food Sci Technol 22:215–224. https://doi.org/10.1016/j.tifs.2011.01.009
Nguyen PT, Nguyen TT, Bui DC, Hong PT, Hoang QK, Nguyen HT (2020) Exopolysaccharide production by lactic acid bacteria: the manipulation of environmental stresses for industrial applications. AIMS Microbiol 6:451–469. https://doi.org/10.3934/2Fmicrobiol.2020027
Grosu-Tudor SS, Zamfir M (2014) Exopolysaccharide production by selected lactic acid bacteria isolated from fermented vegetables. Sci Bull Ser F Biotechnol 18:107–114
Palomino MM, Waehner PM, Fina Martin J, Ojeda P, Malone L, Sánchez Rivas C, Ruzal SM (2016) Influence of osmotic stress on the profile and gene expression of surface layer proteins in Lactobacillus acidophilus ATCC 4356. App Microbiol Biotechnol 100:8475–8484. https://doi.org/10.1007/s00253-016-7698-y
Zhang Y, Mao B, Tang X, Liu X, Zhao J, Zhang H, Chen W (2022) Integrative genome and metabolome analysis reveal the potential mechanism of osmotic stress tolerance in Bifidobacterium bifidum. LWT. https://doi.org/10.1016/j.lwt.2022.113199
Settachaimongkon S, van Valenberg HJ, Winata V, Wang X, Nout MR, van Hooijdonk TC, Zwietering MH, Smid EJ (2015) Effect of sublethal preculturing on the survival of probiotics and metabolite formation in set-yoghurt. Food Microbiol 49:104–115. https://doi.org/10.1016/j.fm.2015.01.011
Mitra S, Nguyen LN, Akter M, Park G, Choi EH, Kaushik NK (2019) Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers. https://doi.org/10.3390/cancers11071030
Cesselin B, Derré-Bobillo A, Fernandez A, Lamberet G, Lechardeur D, Yamamoto Y, Gaudu P (2011) Responses of lactic acid bacteria to oxidative stress. In: Tsakalidou E, Papadimitriou K (eds) Stress responses of lactic acid bacteria, Springer Boston, MA, pp 111–127
Barnese K, Gralla EB, Valentine JS, Cabelli DE (2012) Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc Natl Acad Sci USA 109:6892–6897. https://doi.org/10.1073/pnas.1203051109
Yan M, Wang BH, Xu X, der Meister JT, Tabγač HT, Hwang FF, Liu Z (2018) Extrusion of dissolved oxygen by exopolysaccharide from Leuconostoc mesenteroides and its implications in relief of the oxygen stress. Front Microbiol. https://doi.org/10.3389/fmicb.2018.02467
Oberg TS, Ward RE, Steele JL, Broadbent JR (2013) Genetic and physiological responses of Bifidobacterium animalis subsp. lactis to hydrogen peroxide stress. J Bacteriol 195:3743–3751. https://doi.org/10.1128/JB.00279-13
Kong Y, Olejar KJ, On SL, Chelikani V (2020) The potential of Lactobacillus spp. for modulating oxidative stress in the gastrointestinal tract. Antioxidants. https://doi.org/10.3390/antiox9070610
Zhai Z, Yang Y, Wang H, Wang G, Ren F, Li Z, Hao Y (2020) Global transcriptomic analysis of Lactobacillus plantarum CAUH2 in response to hydrogen peroxide stress. Food Microbiol. https://doi.org/10.1016/j.fm.2019.103389
Zhang C, Lu J, Yang D, Chen X, Huang Y, Gu R (2018) Stress influenced the aerotolerance of Lactobacillus rhamnosus hsryfm 1301. Biotechnol Lett 40:729–735. https://doi.org/10.1007/s10529-018-2523-6
Jin J, Qin Q, Guo H, Liu S, Ge S, Zhang H, Ren F (2015) Effect of pre-stressing on the acid-stress response in Bifidobacterium revealed using proteomic and physiological approaches. PLoS ONE. https://doi.org/10.1371/journal.pone.0117702
Bi J, Liu S, Du G, Chen J (2016) Bile salt tolerance of Lactococcus lactis is enhanced by expression of bile salt hydrolase thereby producing less bile acid in the cells. Biotechnol Lett 38:659–665. https://doi.org/10.1007/s10529-015-2018-7
Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR (2008) Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA 105:13580–13585. https://doi.org/10.1073/pnas.0804437105
Fanning S, Hall LJ, van Sinderen D (2012) Bifidobacterium breve UCC2003 surface exopolysaccharide production is a beneficial trait mediating commensal-host interaction through immune modulation and pathogen protection. Gut Microbes 3:420–425. https://doi.org/10.4161/gmic.20630
Pfeiler EA, Klaenhammer TR (2009) Role of transporter proteins in bile tolerance of Lactobacillus acidophilus. Appl Environ Microbiol 75:6013–6016. https://doi.org/10.1128/AEM.00495-09
Whitehead K, Versalovic J, Roos S, Britton RA (2008) Genomic and genetic characterization of the bile stress response of probiotic Lactobacillus reuteri ATCC 55730. Appl Environ Microbiol 74:1812–1819. https://doi.org/10.1128/AEM.02259-07
Ruiz L, Sánchez B, Ruas-Madiedo P, De Los Reyes-Gavilán CG, Margolles A (2007) Cell envelope changes in Bifidobacterium animalis ssp. lactis as a response to bile. FEMS Microbiol Lett 274:316–322. https://doi.org/10.1111/j.1574-6968.2007.00854.x
Jia FF, Zheng HQ, Sun SR, Pang XH, Liang Y, Shang JC, Meng XC (2018) Role of luxS in stress tolerance and adhesion ability in Lactobacillus plantarum KLDS1. 0391. BioMed Res Int. https://doi.org/10.1155/2018/4506829
Aoudia N, Rieu A, Briandet R, Deschamps J, Chluba J, Jego G, Guzzo J (2016) Biofilms of Lactobacillus plantarum and Lactobacillus fermentum: effect on stress responses, antagonistic effects on pathogen growth and immunomodulatory properties. Food Microbiol 53:51–59. https://doi.org/10.1016/j.fm.2015.04.009
Khaleghi M, Kermanshahi RK, Yaghoobi MM, Zarkesh-Esfahani SH, Baghizadeh A (2010) Assessment of bile salt effects on s-layer production, slp gene expression and some physicochemical properties of Lactobacillus acidophilus ATCC 4356. J Microbiol Biotechnol 20:749–756. https://doi.org/10.4014/jmb.0906.06050
Fiocco D, Capozzi V, Goffin P, Hols P, Spano G (2007) Improved adaptation to heat, cold, and solvent tolerance in Lactobacillus plantarum. Appl Microbiol Biotechnol 77:909–915. https://doi.org/10.1007/s00253-007-1228-x
Varcamonti M, Arsenijevic S, Martirani L, Fusco D, Naclerio G, De Felice M (2006) Expression of the heat shock gene clpL of Streptococcus thermophilus is induced by both heat and cold shock. Microb Cell Fact. https://doi.org/10.1186/1475-2859-5-6
Dalmasso M, Aubert J, Briard-Bion V, Chuat V, Deutsch SM, Even S, Thierry A (2012) A temporal-omic study of Propionibacterium freudenreichii CIRM-BIA1T adaptation strategies in conditions mimicking cheese ripening in the cold. PLoS One. https://doi.org/10.1371/2Fjournal.pone.0029083
Meneghel J, Passot S, Cenard S, Réfrégiers M, Jamme F, Fonseca F (2017) Subcellular membrane fluidity of Lactobacillus delbrueckii subsp. bulgaricus under cold and osmotic stress. Appl Microbiol Biotechnol 101:6907–6917. https://doi.org/10.1007/s00253-017-8444-9
Pandi S, Basheer S (2016) Adaptation of Lactobacillus sp. and Saccharomyces sp. to heat stress. Int J Microbiol Allied Sci 283:7–16
Palomino MM, Allievi MC, Gründling A, Sanchez-Rivas C, Ruzal SM (2013) Osmotic stress adaptation in Lactobacillus casei BL23 leads to structural changes in the cell wall polymer lipoteichoic acid. Microbiol 159:2416–2426. https://doi.org/10.1099/mic.0.070607-0
Calderini E, Celebioglu HU, Villarroel J, Jacobsen S, Svensson B, Pessione E (2017) Comparative proteomics of oxidative stress response of Lactobacillus acidophilus NCFM reveals effects on DNA repair and cysteine de novo synthesis. Proteomics. https://doi.org/10.1002/pmic.201600178
Guo Q, Li S, Xie Y, Zhang Q, Liu M, Xu Z, Yang Y (2017) The NAD+-dependent deacetylase, Bifidobacterium longum Sir2 in response to oxidative stress by deacetylating SigH (σH) and FOXO3a in Bifidobacterium longum and HEK293T cell respectively. Free Radic Biol Med 108:929–939. https://doi.org/10.1016/j.freeradbiomed.2017.05.012
Ge Q, Ge P, Jiang D, Du N, Chen J, Yuan L, Zhou G (2018) A novel and simple cell-based electrochemical biosensor for evaluating the antioxidant capacity of Lactobacillus plantarum strains isolated from Chinese dry-cured ham. Biosens Bioelectron 99:555–563. https://doi.org/10.1016/j.bios.2017.08.037
Maresca D, Zotta T, Mauriello G (2018) Adaptation to aerobic environment of Lactobacillus johnsonii/gasseri strains. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00157
Li Y, Hugenholtz J, Abee T, Molenaar D (2003) Glutathione protects Lactococcus lactis against oxidative stress. Appl Environ Microbiol 69:5739–5745. https://doi.org/10.1128/AEM.69.10.5739-5745.2003
Rochat T, Gratadoux JJ, Gruss A, Corthier G, Maguin E, Langella P, Van De Guchte M (2006) Production of a heterologous nonheme catalase by Lactobacillus casei: an efficient tool for removal of H2O2 and protection of Lactobacillus bulgaricus from oxidative stress in milk. Appl Environ Microbiol 72:5143–5149. https://doi.org/10.1128/AEM.00482-06
Miyoshi A, Rochat T, Gratadoux JJ, Le Loir Y, Oliveira SC, Langella P, Azevedo V (2003) Oxidative stress in Lactococcus lactis. Genet Mol Res 2:348–359
Hols P, Hancy F, Fontaine L, Grossiord B, Prozzi D, Leblond-Bourget N, Kleerebezem M (2005) New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol Rev 29:435–463. https://doi.org/10.1016/j.fmrre.2005.04.008
Goffin P, Muscariello L, Lorquet F, Stukkens A, Prozzi D, Sacco M, Hols P (2006) Involvement of pyruvate oxidase activity and acetate production in the survival of Lactobacillus plantarum during the stationary phase of aerobic growth. Appl Environ Microbiol 72:7933–7940. https://doi.org/10.1128/AEM.00659-06
Wang C, Cui Y, Qu X (2018) Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch Microbiol 200:195–201. https://doi.org/10.1007/s00203-017-1446-2
Author information
Authors and Affiliations
Contributions
Mital R. Kathiriya and Subrota Hati designed the concept, compiled the information, wrote of the manuscript, and edited of the manuscript. Yogesh V. Vekariya contributed in writing and editing the original manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kathiriya, M.R., Vekariya, Y.V. & Hati, S. Understanding the Probiotic Bacterial Responses Against Various Stresses in Food Matrix and Gastrointestinal Tract: A Review. Probiotics & Antimicro. Prot. 15, 1032–1048 (2023). https://doi.org/10.1007/s12602-023-10104-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10104-3