Abstract
Objective
The objective of this study was to evaluate the probiotic potential as well as the ability of adhesion and aggregation of natural and autochthonous lactic acid bacteria, isolated from traditionally made cheese.
Results
Lactic acid bacteria from natural food sources can be promising probiotic candidates and they can be used in natural food preservation or like starter cultures. Tested autochthonous isolates showed tolerance to the simulated gastrointestinal condition as well as the sensitivity to clinically relevant antibiotics, especially to ampicillin (MIC at 0.195 μg mL−1 for lactobacilli and from 0.195 to 3.125 μg mL−1 for lactococci). Among isolates, the highest percentage of adhesion was detected with chloroform, while the adhesion ability of selected isolates to pig intestinal epithelium was in the correlation with the results of adhesion ability with solvents. The auto-aggregation ability of isolates was demonstrated, while co-aggregation with Escherichia coli was strain specific.
Conclusion
The results indicated the potential probiotic properties of the isolates and give evidence for further investigation and potential application in the dairy industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are defined as viable microorganisms which have a positive impact on medical condition of their host (FAO/WHO 2006). Lactic acid bacteria (LAB) are the most commonly used microorganisms as probiotics, because they have “Generally Recognized as Safe” (GRAS) status and because they are desirable members of the intestinal microflora (Shokryazdan et al. 2014). The tolerance to gastrointestinal conditions (Hernandez-Hernandez et al. 2012) as well as the safety aspect of LABs, which include antimicrobial resistance (Vesković-Moračanin et al. 2017) and haemolysis on blood agar (Kaktcham et al. 2012), are the major criteria for selection of probiotics bacteria.
A high number of in vitro models have been used for studying the adhesion of probiotic to epithelial cells. Many difficulties were found, which have led to the development of in vitro model systems for the preliminary selection of potentially adherent strains (Kos et al. 2003; Carasi et al. 2014; Garriga et al. 2014; Sitepu et al. 2016). It is known that Lactobacillus reuteri could inhibit the adherence of enteropathogenic E. coli to human intestinal epithelium (Walsham et al. 2016). Some authors indicated that auto-aggregation ability of LAB was necessary for adhesion to intestinal epithelial cells. They also indicated that LAB, with co-aggregation ability, could form a barrier that prevent colonization by pathogenic microorganisms (Kos et al. 2003; Younes et al. 2012).
The increase of interest regarding the commercial utilization of Lactobacillus strains isolated from traditionally and naturally fermented dairy products, was noticed (Magdoub et al. 2015). But, Solieri et al. (2014) indicated that there is no universal strain that would provide all probiotic benefits, not even strain of the same species.
The aims of this study were to evaluate the tolerance of selected LABs to different gastrointestinal conditions; their sensitivity to clinically relevant antibiotics; their ability to synthesize biogenic amines and ability to grow on media with phenol as well as detection of haemolysis on blood agar. Also, the aims were to evaluate the ability of auto-aggregation, co-aggregation and adhesion of LABs, as well as the investigation of the adhesive properties of selected LAB to pig ileal epithelial cells, by using the in vitro model.
Material and methods
Bacteria used in study
Four isolates of Lactobacillus genera (Lb. fermentum KGPMF28, Lb. fermentum KGPMF29, Lb. brevis KGPMF35, Lb. plantarum KGPMF62) and six isolates of Lactococcus genera (L. lactis subsp. lactis KGPMF23, L. lactis subsp. lactis biovar. diacetylactis KGPMF50, L. lactis subsp. lactis biovar. diacetylactis KGPMF54, L. lactis subsp. lactis biovar. diacetylactis KGPMF55, L. lactis subsp. lactis biovar. diacetylactis KGPMF57 and L. lactis subsp. lactis biovar. diacetylactis KGPMF59) were used in this study. All tested bacteria were isolated from Sokobanja’s cheese (Southeastern Serbia) and provided by the Microbiology Laboratory, Faculty of Science, University of Kragujevac, Serbia. The cheese was made on traditional way, without adding any bacterial starter culture, so the isolates tested in this study presents the natural and autochthonous microflora. These isolates were chosen according to the previously investigated biochemical characteristics and antagonistic potential against enterobacteria isolated from the same cheese (Muruzović et al. 2018a, b). The bacterial strains were kept in glycerol stock at − 80 °C. Lactobacillus plantarum LP299v was used as reference strain. Escherichia coli, a clinical isolate, was a generous gift from the Institute of Public Health, Kragujevac, Serbia. Before experimental use, working cultures were revitalized by two consecutives in MRS broth (for LAB) and Nutrient broth (for E. coli).
In vitro gastrointestinal transit tolerance assay
The acid tolerance of LAB was studied in different pH solutions, which were prepared by adjusting the hydrochloric acid (HCl) (Zorka Šabac, Šabac, Serbia) to pH levels of 3, 4 and 5. Sterile MRS broth (pH 6.5) served as a control. Inoculum was prepared from overnight culture of LABs (turbidity of initial suspension 108 CFU mL−1). Inoculum of each bacterial strain (10 μL) was added to the standard or modified MRS broth. The incubation was carried out at 37 °C/48 h. Optical densities of bacterial growth were determined with an ELISA plate reader at 600 nm. Results were performed in triplicate.
Simulated gastric and small intestinal juice tolerance assays were performed according to the method described in Huang and Adams (2004), with some modifications. Overnight cultures were inoculated in ratio 1:10. Simulated gastric juice were prepared by suspending 0.22% (w/v) pepsin (Merck, New Jersey, USA) in sterile filtered 0.5% (w/v) NaCl solution with the pH adjusted to 2. 96-well microtiter plates were incubated at 37 °C/3 h, because this is the time of staying food in the stomach (Zoumpopoulou et al. 2008). The results were determined with ELISA plate reader at 600 nm, in triplicate (Bassyouni et al. 2012).
Simulated small intestinal juice were prepared by suspending 0.2% (w/v) of pancreatin (Sigma-Aldrich, St. Louis, USA) in filter sterile 0.5% NaCl (w/v) solution with 0.4% of bile salts (Sigma-Aldrich, St. Louis, USA) and adjusting pH to 8 with adding sterile 0.1 M NaOH. 96-well microtiter plates were incubated at 37 °C/4 h, because this is the time of staying food in small intestine (Kumar and Murugalatha 2012). Results were performed in triplicate. The number of viable LABs was determined by transferring the appropriate samples onto the MRS agar plates. The percentage of survival was calculated using the following formula:
α—CFU mL−1 of the assayed strain (uninoculated MRS (pH 6.5), at 37 °C/48 h); β—CFU mL−1 of the same strain after incubation with the different gastrointestinal conditions.
Antibiotic sensitivity
The antibiotics sensitivity of LABs was investigated by using the microdilution method with resazurin and the minimum inhibitory concentration (MIC) was determined (Sarker et al. 2007). Tetracycline, ampicillin, gentamicin, vancomycin and polymyxin B (Sigma Chemicals Co., USA), in concentration range from 0.05–4000 μg mL−1, were used for this study. The method was described in detail in Muruzović et al. (2016).
Synthesis of biogenic amines and haemolysis on blood agar
The ability of isolates to synthesize biogenic amines (histamine and tyramine) from histidine and tyrosine, as well as haemolysis on blood agar plates, were analysed by method described in Jeong and Lee (2015).
Growth in the presence of phenol
Growth of isolates in the presence of phenol was determined by method described in Šušković et al. (2001).
Investigation of auto-aggregation and co-aggregation ability
The auto-aggregation ability of LABs isolates, as well as co-aggregation ability with E. coli was monitored by a modified method described in Ocaña and Nader-Macías (2002) and Tuo et al. (2013).
The auto-aggregation ability of examined isolates was monitored in PBS buffer (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, pH 7.5). The cells of overnight culture were settled down by centrifugation at 5000 rpm for 15 min, after which they were twice washed in PBS buffer, and then re-suspended in 4 mL of the same buffer, so the number of cells was approximately 108 CFU mL−1. The suspension was well mixed in Vortex; 200 µL from the suspension surface was transferred into the micro tube with 1800 µL of PBS and the optical density was monitored at 600 nm (A0). The same procedure was repeated after 1, 2, 3, 4, 5 and 24 h (At). The percent of auto-aggregation was calculated by following formula:
where At represents the absorbance of supernatant after 1, 2, 3, 4, 5 and 24 h.
For the investigation of co-aggregation ability, the cells of examined LABs and E. coli were prepared identically as in the previous method, and re-suspended in PBS buffer, per 2 mL of each suspension of both types of bacteria for which the co-aggregation ability was monitored. Suspensions were mixed well in Vortex, 200 µL from the suspension surface was transferred into the micro tube with 1800 µL of PBS buffer and optical density was monitored at 600 nm (A0). The same procedure was repeated after 2 h (At). The percent of co-aggregation was calculated by following formula:
where At represents the absorbance of supernatant after 2 h.
Microbial adhesion to solvents
Microbial adhesion to solvents (MATS) was measured according to the method described in Rosenberg et al. (1980), with some modifications (Kos et al. 2003; Palomares et al. 2007; Collado et al. 2008). After 24 h of incubation, the bacteria, which grew in the MRS broth, were harvested in the stationary phase by centrifugation at 5000 rpm for 15 min, washed twice, and resuspended in 0.1 M KNO3 (pH 6.2), so the number of cells was approximately 108 CFU mL−1. The optical density of the cell suspension was measured at 600 nm (A0). 1 mL of the solvent was added to 3 mL of cell suspension. After 10 min of incubation at room temperature, the two-phase system was mixed in Vortex, for 2 min. The aqueous phase was removed after 20 min of incubation at room temperature, and its optical density was measured at 600 nm (A1). The percentage of bacterial adhesion to solvent was calculated as:
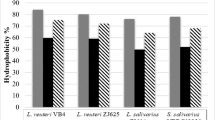
Three different solvents were tested in this study: xylene (Sineks, Belgrade, Serbia), which is an apolar solvent; chloroform (Alkaloid, Skoplje, Macedonia), a monopolar and acidic solvent; and ethyl acetate (Zorka Šabac, Šabac, Serbia) a monopolar and basic solvent. Only bacterial adhesion to xylene reflects the cell surface hydrophobicity or hydrophilicity. The values of MATS obtained with the two other solvents, chloroform and ethyl acetate, were regarded as a measure of electron donor (basic) and electron acceptor (acidic) characteristics of bacteria, respectively. According to Ocaña and Nader-Macías (2002), the percent of hydrophobicity was expressed as: 0–35%—low hydrophobicity; 36–70%—middle hydrophobicity; 71–100%—high hydrophobicity.
In vitro test for determination of adhesion ability of LAB to pig intestinal epithelium
The adhesion ability of Lb. brevis KGPMF35, Lb. fermentum KGPMF29 and L. lactis subsp. lactis biovar. diacetylactis KGPMF57 to the pig intestinal epithelium was tested according to the method described in Kos et al. (2003), with modifications. Pig ileal intestinal epithelium was used because of the similarity of porcine and human intestinal tracts. Ileal samples were collected from 9-month-old pig. Immediately after sacrificing the animal, the intestinal epithelium was stored at 4 °C in a refrigerator. Before the experiment, the intestinal epithelium, was cut to an appropriate length (1 cm2) and held for 30 min in PBS buffer (Alfa Aesar GmbH & Co, Karlsruhe, Germany) at 4 °C in a refrigerator, in order to loosen surface mucus. Furthermore, epithelium was washed three times in PBS buffer, mixing on a rotary shaker, in order to remove surplus of fat. Prepared samples were aseptically transferred to the Erlenmeyer flasks, which contained 20 mL of MRS broth (Torlak, Belgrade, Serbia), previously inoculated with 200 μL of with overnight culture of bacteria. Flacks were incubated for 24 h at 37 °C. After incubation, the ileal samples were washed with sterile saline, in order to remove free-floating bacteria, and fixed with methanol. After drying, the samples were staining with a florescent color, acridine orange, for 2 min. Excess color was removed by washing with distilled water. The samples were examined and photographed by florescent microscope (Nikon, Ti-Eclipse, 400x, Austria). Intestinal epithelium in uninoculated MRS broth and in PBS buffer served as sterility control.
Statistical analysis
All data were presented as means ± standard deviations, by using Microsoft Excel (Redmond, Washington, DC, USA). Differences between bacterial tolerance to gastrointestinal conditions, as well as sensitivity on tested antibiotic were tested by using one-way ANOVA and Paired-T test. Paired T-test was used for statistical processing of the results of adhesion to different solvents. Spearman's correlation coefficient was used for determination of correlation between auto-aggregation and microbial adhesion to solvents. All statistical analyses were performed with SPSS (IBM SPSS Statistics 20).
Results
In vitro gastrointestinal transit tolerance assay
Probiotic strains need to be resistant to low pH values, since they need to pass through from the conditions of stomach. Although the optical densities of bacterial growth were reduced, all the isolates showed the growth ability at low pH (Table 1).
Among lactobacilli, only KGPMF35 isolate showed more than 100% of growth after 1 h of incubation in simulated gastric juice tolerance. The percentage of survival, after 3 h of incubation, obtained from 79.5 to 88.9%. Among lactococci, only KGPMF23 showed more than 100% of growth after 1 h of incubation, while KGPMF55 isolate showed decrease of growth. The percentage of survival was in range from 58.2 to 86.6% (Table 2).
The growth of all isolates after 4 h of incubation in stimulated small intestinal juice lead to further decrease. The percentage of survival of lactobacilli was in range from 26.2 to 63% and for lactococci was from 43.9 to 52.8% (Table 3).
Antibiotic sensitivity
The results of sensitivity of isolates to clinically relevant antibiotics was presented in Table 4. The results were checked according to LAB resistance criteria proposed for antibiotics of human and veterinary importance by the European Food Safety Authority (EFSA).
Lactobacillus spp. showed significant sensitivity to ampicillin and tetracycline, compared to the other tested antibiotics (p < 0.05). MIC values for lactobacilli obtained from 0.125 to 3 μg mL−1 for tetracycline and from 0.195 to 64 μg mL−1 for ampicillin. Lactococcus spp. showed significant sensitivity to ampicillin, tetracycline and vancomycin (p < 0.05). MIC values obtained from 1.56–3.125 μg mL−1 for tetracycline, from 0.195–3.125 μg mL−1 for ampicillin and from 1.56–3.125 μg mL−1 for vancomycin.
Synthesis of biogenic amines, growth on media with phenol and haemolysis on blood agar
Tested LAB isolates showed no ability to synthesize histamine and tyramine (biogenic amines), which is desirable characteristic when selecting possible probiotics. In the medium with 0.1, 0.2 and 0.3% of phenol, the most of examined LABs grew well. The exceptions were KGPMF50 and KGPMF55 isolates, which showed no growth in medium with 0.3% of phenol. Lactobacilli and lactococci showed no α or β haemolysis.
The auto-aggregation and co-aggregation ability of LABs
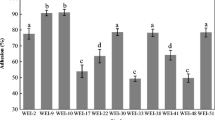
The auto-aggregation ability of LAB was measured over a period of 5 h. Results indicated that the tested strains exhibited a strong auto-aggregating phenotype. The observed auto-aggregation was not lost after washing and suspending the bacterial cells in PBS, which is probably related to cell surface component. When compare the auto-aggregation between groups of bacteria, it could be concluded that it was slightly better in group of Lactobacillus spp. (Fig. 1), then in Lactococcus spp. (Fig. 2). The percentage of auto-aggregation of lactobacilli ranged from 41.89 to 53.74%. The best auto-aggregation ability was showed by Lb. brevis KGPMF35 isolate. The tested lactococci showed auto-aggregation ability in a percentage ranged from 35.82 to 50.2% (Fig. 2).
Co-aggregation ability of isolated LAB with E. coli also was examined (Table 5). Results indicated that all tested Lactobacillus strains demonstrated co-aggregation with E. coli, while co-aggregation of Lactococcus spp. was a strain specific.
The ability of adhesion to different solvents
The MATS method was used to evaluate the hydrophobic/hydrophilic of the cell surface properties of LABs. Lb. plantarum LP 299v was used for comparative purposes. In order to assess the Lewis acid–base characteristics of the bacterial cell surfaces, bacterial adhesion with chloroform and ethyl acetate was tested. The results indicated that tested bacteria had a stronger affinity with chloroform (10.79–21.57%), which is an acidic solvent and electron acceptor, than with ethyl acetate (4.95–12.84%), which is a basic solvent and electron donor. Affinity for xylene was not observed (Table 6).
In vitro adhesion to pig intestinal epithelium
The ability of LABs isolated from cheese to adhere to pig epithelium, was investigated, using the florescence microscope. The adhesion was noticed for all selected bacteria (Fig. 3).
Discussion
In this paper, for the first time, it was investigated the probiotic potential, as well as the adhesion and aggregation ability of ten strains of LAB, isolated from traditionally made cheese from Southeastern Serbia. All isolates showed a tolerance to simulated gastrointestinal conditions and potential for further investigation. Similar results have been previously reported by other studies, where authors analyzed LABs isolated from different fermented products (Ramos et al. 2013; Leite et al. 2015; Pavli et al. 2016).
Tetracycline, ampicillin, gentamicin, vancomycin and polymyxin B (a part of mixed therapy of bacterial vaginosis) are chosen in order to detect a wide range of determinants for resistance. According to EFSA (2012), “any bacterial strain carrying an acquired resistance to antimicrobial that is shown to be due to the acquisition of genetic determinant presents the greatest potential for horizontal spread and should not be used as a feed additive”. However, the use of probiotics is clinically relevant for patients using antimicrobial drugs, thus, being susceptible to antimicrobials, makes the strains unsuitable to this purpose. But, Pereira et al. (2015) indicated that there is a lack of agreement on the interpretive breakpoints for probiotic bacteria. Also, they indicated that data that confirms antimicrobial susceptibility of Lactobacillus strains are scarce. Uroić et al. (2014) investigated antibiotics sensitivity of LAB isolated from Serbian and Croatian cheeses and showed that all isolates were susceptible to the antibiotics, which was confirmed in our study. Leite et al. (2015) indicated that lactococci, isolated from Brazilian kefir, were susceptible to the tetracycline, erythromycin, clindamycin, ampicillin, and aminoglycosides. The susceptible to the vancomycin was the exception. In our study, lactococci were sensitive to vancomycin. The results observed in this paper conformed that tested LABs showed no resistance to tested antibiotics, according to EFSA (2012) breakpoints, so they are safe for clinical use. But, non-parameterized MICs and breakpoints for probiotic bacteria are still a technical problem, because we do not have all needed parameters for antimicrobial susceptibility of LAB, given their clinical relevance as probiotics (Pereira et al. 2015).
There are many studies which indicated that the biofilm formation of LAB is associated with adhesion properties (Elhadidy and Zahran 2014; Živković et al. 2016; Popović et al. 2018). Previously studies of LAB, isolated from Sokobanja cheese, indicated that they showed the ability to produce moderate biofilm (Muruzović et al. 2018a, c). In the present study, their ability of adhesion with different solvents was demonstrated. Tested Lactobacillus isolates showed a significant correlation between auto-aggregation ability and affinity with chloroform (p < 0.05). In general, tested isolates showed a better affinity with chloroform (acidic solvent) than with ethyl acetate (basic solvent) (p < 0.05). Kaewnopparat et al. (2013) indicated that LAB processed the ability to colonize the human gut and to increase the concentration of excreted antimicrobial substances in the process of co-aggregation. This could be one more mechanism for the control of development of pathogen strains. Lactobacillus spp., isolated from Serbian cheese, showed a high percent of co-aggregation with E. coli clinical isolate, after 2 h of incubation. Li et al. (2015) indicated that selecting LABs with higher adhering ability, according to the aggregation ability, is not a desirable method, because, so far, some authors showed that these characteristics were strain specific (Lee and Salminen 2009; Li et al. 2015), which was confirmed in our research, too. Janković et al. (2012) indicated that Lb. plantarum, isolated from home-made cow and sheep cheeses, showed a co-aggregation ability with some food–borne pathogens. Idoui (2014) demonstrated the ability of Lb. fermentum to co-aggregate with Salmonella spp., Klebsiella spp. and E. coli.
Some studies indicated that selection of probiotic LAB primarily was done based on their hydrophobicity against xylene (Palomares et al. 2007), hexadecane (Pringsulaka et al. 2015) and toluene (Dowarah et al. 2018). The ability of adherence and colonization of intestinal epithelium cells of the host are important characteristic which have a role in the inhibition of the colonization by pathogens strains (Pringsulaka et al. 2015). Dowarah et al. (2018) reported the ability of in vitro adhesion by Lb. acidophilus PF01, Lb. acidophilus CF07 and Pediococcus acidilactici FT28 to duodenal epithelium cells of pig. The adhesion of LABs, isolated from Serbian cheese, was confirmed through the in vitro investigation of the adhesion ability to pig intestinal epithelium cells.
Conclusions
The tested LABs, isolated from Sokobanja’s cheese, showed tolerance to extremes of gastrointestinal conditions, sensitivity to selected antibiotics, as well as the ability to survive in the presence of different concentrations of phenol. They showed no ability to synthesize histamine and tyramine, which is desirable characteristic when selecting possible probiotics or starter cultures. All isolates showed the ability of auto-aggregation and the co-aggregation with E. coli clinical isolate. The adhesion ability of bacteria is an important characteristic, because LAB, with process this ability, can be used as a mechanical barrier for adhesion of other bacteria, like enterobacteria or potential pathogenic bacteria, to epithelium. Based on the results of tolerance to extremes of gastrointestinal conditions, adhesion and aggregation ability, it could be concluded that KGPMF28, KGPMF29, KGPMF35 and KGPMF57 isolates showed the potential for future investigation and usage.
References
Bassyouni RH, Abdel-all WS, Fadl MG, Abdel-all S, Kamel Z (2012) Characterization of lactic acid bacteria isolated from dairy products in Egypt as a probiotic. Life Sci J 9:2924–2933
Carasi P, Ambrosis NM, De Antoni GL, Bressollier P, Urdaci MC, de los Angeles Serradell M (2014) Adhesion properties of potentially probiotic Lactobacillus kefiri to gastrointestinal mucus. J Dairy Res 81(1):16–23
Collado MC, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226:1065–1073
Dowarah R, Verma AK, Agarwal N, Singh P, Singh BR (2018) Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 13(3):e0192978. https://doi.org/10.1371/journal.pone.0192978
Elhadidy M, Zahran E (2014) Biofilm mediates Enterococcus faecalis adhesion, invasion and survival into bovine mammary epithelial cells. Lett Appl Microbiol 58:248–254
European Food Safety Authority-EFSA (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human or veterinary importance. EFSA J 10:1–10
FAO/WHO (World Health Organization) (2006) Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food Nutr Pap 85:2.
Garriga M, Rubio R, Aymerich T, Ruas-Madiedo P (2014) Potentially probiotic and bioprotective lactic acid bacteria starter cultures antagonise the Listeria monocytogenes adhesion to HT29 colonocyte-like cells. Benef Microbes 6(3):337–343
Hernandez-Hernandez O, Muthaiyan A, Moreno FJ, Montilla A, Sanz ML, Rickeet SC (2012) Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol 30:355–361
Huang Y, Adams MC (2004) In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol 91:253–260
Idou T (2014) Probiotic properties of Lactobacillus strains isolated from gizzard of local poultry. Iran J Microbiol 6(2):120–126
Janković T, Frece J, Abram M, Gobin I (2012) Aggregation ability of potential probiotic Lactobacillus plantarum strains. Int J Sanit Eng Res 6:19–24
Jeong DW, Lee JH (2015) Antibiotic resistance, hemolysis and biogenic amine production assessments of Leuconostoc and Weissella isolates for kimchi starter development. LWT Food Sci Technol 64:1078–1084
Kaewnopparat S, Dangmanee N, Kaewnopparat N, Srichana T, Chulasiri M, Settharaksa S (2013) In vitro probiotic properties of Lactobacillus fermentum SK5 isolated from vagina of a healthy woman. Anaerobe 22:6–13
Kaktcham PM, Zambou NF, Tchouanguep FM, El-Soda M, Choudhary MI (2012) Antimicrobial and safety properties of lactobacilli isolated from two Cameroonian traditional fermented foods. Sci Pharm 80:189–203
Kos B, Šušković J, Vuković S, Šimpraga M, Frece J, Matošić S (2003) Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J Appl Microbiol 94:981–987
Kumar AM, Murugalatha N (2012) Isolation of Lactobacillus plantarum from cow milk and screening for the presence of sugar alcohol producing gene. J Clin Microbiol Antimicrob 4:16–22
Lee YK, Salminen S (2009) Handbook of probiotics and prebiotics. Wiley, pp 386
Leite AMO, Miguel MAL, Peixoto RS, Ruas-Madiedo P, Paschoalin VMF, Mayo B, Delgado S (2015) Probiotic potential of selected lactic acid bacteria strains isolated from Brazilian kefir grains. J Dairy Sci 98:3622–3632
Li Q, Liu X, Dong M, Zhou J, Wang Y (2015) Aggregation and adhesion abilities of 18 lactic acid bacteria strains isolated from traditional fermented food. Int J Agric Policy Res 3(2):84–92
Magdoub MNI, Hassan ZMR, Effat BAM, Sadek ZIM, Tawfik NF, Mabrouk AMM (2015) Probiotic properties of some lactic acid bacteria isolated from Egyptian dairy products. Int J Curr Microbiol Appl Sci 4:758–766
Muruzović MŽ, Mladenović KG, Stefanović OD, Vasić SM, Čomić LR (2016) Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial, and antibiofilm activities. J Food Drug Anal 24:539–547
Muruzović MŽ, Mladenović KG, Žugić Petrović TD, Čomić LR (2018a) Characterization of lactic acid bacteria isolated from traditionally made Serbian cheese and evaluation of their antagonistic potential against Enterobacteriaceae. J Food Process Preserv 42(4):e13577. https://doi.org/10.1111/jfpp.13577
Muruzović MŽ, Mladenović KG, Đilas MD, Stefanović OD, Čomić LR (2018b) In vitro evaluation of antimicrobial potential and ability of biofilm formation of autochthonous Lactobacillus spp. and Lactococcus spp. isolated from traditionally made cheese from Southeastern Serbia. J Food Process Preserv 42(11):13776
Muruzović MŽ, Mladenović KG, Čomić LR (2018) In vitro evaluation of resistance to environmental stress by planktonic and biofilm form of lactic acid bacteria isolated from traditionally made cheese from Serbia. Food Biosci 23:54–59
Ocaña V, Nader-Macías ME (2002) Vaginal lactobacilli: self- and co-aggregation ability. Br J Biomed Sci 59:183–190
Palomares IC, Morales PR, Felix AE (2007) Evaluation of probiotic properties in Lactobacillus isolated from small intestine of piglets. Rev Latinoameric Microbiol 49(3–4):46–54
Pavli PG, Argyri AA, Papadopoulou OS, Nychas GE, Chorianopoulos NG, Tassou CC (2016) Probiotic potential of lactic acid bacteria from traditional fermented dairy and meat products: assessment by in vitro tests and molecular characterization. J Probiotics Health 4:157–165
Pereira NG, Figueiredo FJB, Dias-Souza MV (2015) Antimicrobial susceptibility of commercial probiotic Lactobacillus strains. J Appl Pharm Sci 2(2):14–17
Popović N, Dinić M, Tolinački M, Mihajlović S, Terzić-Vidojević A, Bojić S, Djokić J, Golić N, Veljović K (2018) New insight into biofilm formation ability, the presence of virulence genes and probiotic potential of Enterococcus sp. dairy isolates. Front Microbiol 9:78
Pringsulaka O, Rueangyotchanthana K, Suwannasai N, Watanapokasin N, Amnueysit P, Sunthornthummas S, Sukkhum A, Sarawaneeyaruk S, Rangsiruji A (2015) In vitro screening of lactic acid bacteria for multi-strain probiotics. Livestock Sci 174:66–73
Ramos CL, Thorsen L, Schwan RF, Jespersen L (2013) Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol 36:22–29
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324
Shokryazdan P, Sieo CC, Kalavathy R, Liang JB, Alithee NB, Jahromi MF, Ho YW (2014) Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res Int 2014:1–16
Sitepu GR, Nursyirwani N, Efriyeldi E (2016) Adhesion of lactic acid bacteria (LAB) to intestinal epithelial cells of red snapper (Lutjanus argentimaculatus) in inhibiting Vibrio alginolyticus. J Online Mahasiswa 3(2):1–10
Solieri L, Bianchi A, Mottolese G, Lemmetti F, Giudici P (2014) Tailoring the probiotic potential of non-starter Lactobacillus strains from ripened Parmigiano Reggiano cheese by in vitro screening and principal component analysis. Food Microbiol 38:240–249
Šušković J, Kos B, Goreta J, Matosić S (2001) Role of lactic acid bacteria and bifidobacteria in symbiotic effect. Food Technol Biotechnol 39:227–235
Tuo Y, Yu H, Ai L, Wu Z, Guo B, Chen W (2013) Aggregation and adhesion properties of 22 Lactobacillus strains. J Dairy Sci 96:4252–4257
Uroić K, Nikolić M, Koslć B, Pavunc L, Beganović J, Lukić J, Jovčić B, Filipić B, Miljković M, Golić N, Topisirović L, Čadež N, Raspor P, Šušković J (2014) Probiotic properties of lactic acid bacteria isolated from Croatian fresh soft cheese and Serbian white pickled cheese. Food Technol Biotechnol 52:232–241
Vesković Moračanin S, Djukić D, Zdolec N, Milijašević M, Mašković P (2017) Antimicrobial resistance of lactic acid bacteria in fermented food. J Hyg Eng Des 18:25–35
Walsham ADS, MacKenzie DA, Cook V, Wemyss-Holden S, Hews CL, Juge N, Schüller S (2016) Lactobacillus reuteri inhibition of enteropathogenic Escherichia coli adherence to human intestinal epithelium. Front Microbiol 7:244
Younes JA, van der Mei HC, van den Heuvel E, Busscher HJ, Reid G (2012) Adhesion forces and coaggregation between vaginal staphylococci and lactobacilli. PLoS ONE 7(5):1–8
Živković M, Miljković MS, Ruas-Madiedo P, Markelić MB, Veljović K, Tolinački M, Sokocić S, Korać A, Golić N (2016) EPS-SJ exopolisaccharide produced by the strain Lactobacillus paracasei subsp. paracasei BGSJ2-8 is involved in adhesion to epithelial intestinal cells and decrease on E. coli association to Caco-2 cells. Front Microbiol 7:286
Zoumpopoulou G, Foligne B, Christodoulou K, Grangette C, Pot B, Tsakalidou E (2008) Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int J Food Microbiol 121:18–26
Acknowledgements
This investigation was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 41010).
Author information
Authors and Affiliations
Contributions
MŽG conceived and designed the experiments; DDN designed and photographed samples by florescent microscope and interpreted the results of adhesion; MŽG and KGM processed the results; KGM and LRČ take charge of the preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest with the current work or its publication.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grujović, M.Ž., Mladenović, K.G., Nikodijević, D.D. et al. Autochthonous lactic acid bacteria—presentation of potential probiotics application. Biotechnol Lett 41, 1319–1331 (2019). https://doi.org/10.1007/s10529-019-02729-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02729-8