Abstract
Probiotics prove useful in correcting and preventing numerous health conditions, including those having severe impact on society, e.g., obesity and cancer. Notably, these capabilities of probiotics appear to be associated with their antioxidant properties. The mechanisms of antioxidant action of probiotics range from immediate biochemical scavenging of reactive substances to induction of signaling events leading to increased capacity of the host’s cytoprotective systems. Since the antioxidant effects of probiotics significantly vary in types and details, a broad selection of methods of assessment of these properties is required in order to identify, characterize, and develop novel probiotics for medical purposes, as well as to explain the mechanisms of action of probiotics already in use in healthcare. This review revises the versatile toolbox, which can be used to assess the antioxidant properties of probiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are numerous reports on the ability of probiotic bacteria to correct negative effects of various non-infectious pathologies such as allergies, toxicoses of different etiology, obesity, etc.; in addition, some probiotics appear to be capable of preventing cancer [1,2,3,4,5,6,7]. The broad spectrum of health-promoting activity of probiotics can be attributed to their metabolic products protecting eukaryotic host’s cells from negative influence of various factors, including oxidative stress [6].
Besides displaying a plethora of health-promoting functions which are often strain specific [8, 9], some probiotic bacteria demonstrate strong antioxidative potentials [10]. Specifically, Lactobacillus fermentum (Lb. fermentum) strains were shown to have resistance to several reactive oxygen species (ROS) such as hydrogen peroxide, superoxide, and hydroxyl radicals [11]. In addition, some metabolites, such as exopolysaccharides, synthesized, and excreted by probiotic bacteria, were shown to have antioxidant activity [12]. Extracts of Bifidobacterium animalis 01 were found to scavenge ROS in vitro and in vivo [13]. Also, oxidative stress associated with type 2 diabetes was shown to be decreased by multispecies probiotic preparations, and Lactobacillus rhamnosus demonstrated strong antioxidant activity in situations of elevated physical stress in humans [14].
One of the widely investigated topics in dietary-based biomedicine is probiotics for amelioration of oxidative stress-related diseases by direct sequestration of ROS and augmentation of antioxidant defense systems operating in the human body [15,16,17]. The production of free radicals at high levels in the gut can exert cytotoxic effects on the membrane phospholipids of the intestinal epithelial cells, resulting in the formation of toxic products such as malondialdehyde (MDA). Similarly, the occurrence of severe peroxidative changes in the gut due to lipids and free radicals reaction resulting in enhanced lipid peroxidation has been found to be commonly associated with the onset of numerous diseases. Thus, probiotics are an important factor affecting oxidative status of the gut by exhibiting direct antioxidant properties and by inducing the intrinsic human signaling antioxidant defense [15, 18].
Studying the mechanisms underlying health-promoting functions of probiotic bacteria will enhance our knowledge of symbiotic microbe-host interactions [19,20,21,22,23]. As a result, we expect to find new approaches in using nature-derived biologically active substances in gastro-intestinal health care, immunomodulation, prophylaxis of cancer, stress (UV and radiation)-protection, and growth/regeneration promotion.
An important part of a “tool kit” for these studies is the methods to assess the antioxidative properties of probiotics. This paper provides an overview of such methods. These methods can be divided into two distinct groups: those assessing the effects on the oxidative status systems signaling and those testing the biochemical antioxidant properties of probiotics.
Oxidative Status and Inflammation Systems Signaling-Based Techniques
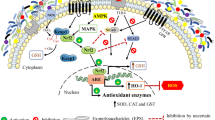
As far as humans and animals have evolved genetic programs through intervention of antioxidative enzymes for protection against oxidative stresses, the level of expression of some eukaryotic genes could be used to indirectly assess the antioxidant capacity of probiotics administered to the objects. Therefore, nuclear factor erythroid 2-related factor 2 (NFE2L2, also known as NRF2) has been recognized as one of the key transcriptional factors that can play a significant protective role by controlling the antioxidant response element-dependent gene regulation in response to oxidative stress [15].
Generally, the basic interactomic approach [24] is used in this type of studies: probiotics are analyzed with respect to their ability to induce a set of genes (or protein products) regulated by a single transcription factor or being a part of a signaling pathway—e.g. NFE2L2, AP-1, NF-κB, etc. For example, Chauhan et al. tested antioxidant properties of Lb. fermentum Lf1 through assessing the NFE2L2/AP-1 and PPARGC1A pathways activation in the HT-29 cells [15]. Endo et al. tested the effects of MIYAIRI 588 probiotic on rats using similar approach; however, they only assessed NFE2L2/AP-1 targets (NQO1, HMPOX1, TXN) on the protein level [25]. Gao et al. used the NFE2L2 protein expression assessment-based variation of the method, together with numerous biochemical tests, to study the signaling antioxidant activity of the Lactobacillus plantarum FC225 strain [26]. The effects of probiotic Lactobacillus reuteri ATCC PTA 6475 on pro-inflammatory cytokines regulated by the AP-1 component JUN were revealed by Lin et al. [27].
The same approach is sometimes employed using prooxidant/pro-inflammatory signaling systems as the reporters. For example, the effects of the combined Lactobacillus delbruekii and Lb. fermentum probiotic on the NF-κB signaling pathway at the protein level were studied by Hegazy and El-Bedewy [28]. A similar investigation focused at probiotic Lb. rhamnosus GR-1 was performed by Karlsson et al. [29]. Being involved in cytokine signaling, NF-κB is often in the focus of the studies dedicated to testing of probiotics effects on the human gut microbiota interactions [30].
A study involving a combined analysis of activation of NF-κB and AP-1 was undertaken by Wehkamp and co-authors; these investigators tested the signaling effects of Escherichia coli Nissle 1917 probiotic on the human intestinalepithelial cells [31, 32]. Schlee et al. investigated several oxidative status related and non-related pathways to assess the signaling effects of several probiotics and of a probiotic cocktail [33].
Biochemical Approaches to Probiotics Antioxidant Properties Testing
There are several group of methods, and several variances within these methods, which are routinely used for the antioxidant properties of probiotics. These methods range from those analyzing total pro- or antioxidant capacities of the reporter system, to those quantifying separate indices of oxidative status of the reporter system. Several methods rely on detection of changes in free radical production modulated by probiotics introduced into the radical-generating systems. Other methodological options are also available (varying in reporter substance type, e.g., fluorescent probes and primary exogenous ROS) that are not routinely used in probiotics screening and research but are of potential interest for the field.
Reviewing of the biochemical methods starts with the total prooxidant/antioxidant assays. Please note, brief ready-to-use protocols are given in the papers cited.
Total Prooxidant/Antioxidant Assays
Several generalized indices of oxidative status of biological systems are used to date. These are, for example, oxygen radical antioxidant capacity (ORAC), total oxidant capacity (TOC), also known as total oxidant status (TOS); total antioxidant capacity (TAC), also known as total antioxidant status (TAS), total antioxidant response (TAR), antioxidant potential (AOP), or non-enzymatic antioxidant capacity (NEAC) [34,35,36,37]. Biochemical principles and mechanisms underlying these assays are described in detail elsewhere in numerous experimental reports and reviews.
Antioxidant activity can be monitored by a variety of methods based on different mechanisms such as hydrogen atom transfer (HAT), single electron transfer (SET), reducing power, etc.
Oxygen radical antioxidant capacity (ORAC) is the most used HAT method. Other HAT-based methods share the same principle, with the examples being total radical trapping antioxidant parameter (TRAP) and crocin bleaching assays. In these methods, peroxyl radicals produced by a generating system react with a probe resulting in the loss of fluorescence or absorbance that is registered as decay curves. Commonly used peroxyl radical generators are a group of azo-compounds, e.g., 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) (hydrophilic) and 2,2′-azobis(2,4-dimethylnaleronitrile (AMVN) (hydrophobic). A model antioxidant, Trolox (a vitamin E analog) is usually used as reference, and ORAC values of the tested antioxidants/probiotics are reported as Trolox equivalents [36]. A commonly used reporter fluorescent probe is fluorescein (Fig. 1) [38]. The ORAC method was used to test the antioxidant properties of the Lb. fermentum LF31 [38].
SET methods typically use Trolox as standard antioxidant. Among SET-based methods, the Trolox equivalent antioxidant capacity (TEAC) assay is one of the most frequently used to date. The assay measures the ability of antioxidants to scavenge the stable radical cation 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), a chromophore with maximum absorption at 734 nm (Fig. 2). Its absorbance at this wavelength decreases in the presence of antioxidants [36]. Another example of TEAC chromophores is 2,2-diphenyl-1-picrylhydrazyl with maximum absorption at 520 nm(Fig. 3) [36, 39]. In a large-scale study by Amaretti et al., TEAC assay was used to test several probiotics, including 7 Bifidobacterium, 11 Lactobacillus, 6 Lactococcus, and 10 Streptococcus thermophilus strains [40].
The reducing power of antioxidants can be measured through their redox reactions with transition metal ions—iron (ferric reducing antioxidant potential, FRAP) and copper (cupric reducing antioxidant capacity, CUPRAC). The TAS and TOS/TOC methods by Erel et al. [41] employ oxido-reduction of iron ions [36].
The TOS/TOC assay is based on the oxidation of ferrous ion to ferric ion in the presence of oxidants in acidic medium [36]. The resulting complex ferric ion-xylenol orange is colored [36], and can be measured at 560 nm [42].
The TAS method is based on the generation of hydroxyl radical via Fenton reaction, and the rate of the reaction is monitored via the analysis of absorbance of colored dianisidyl radicals (absorbance is registered at ~440 nm) [41]. The mixture of ortho-dianisidine, ferrous ammonium sulfate, and hydrogen peroxide solution produces oxidized o-dianisidine molecules into dianisidyl radicals, leading to a bright yellow-brown color development (Fig. 4). Antioxidants suppress the color formation [36]. The TAS method has been used to study several probiotics, including Protexin [43].
The TOS/TOC assay is calibrated with hydrogen peroxide and results are expressed in terms of hydrogen peroxide micromolar equivalents per liter (μmol H2O2 Eq/L), whereas the TAS assay is calibrated with a stable antioxidant standard solution, which is traditionally the Trolox, and results are expressed as mmol Trolox Eq/L [36]. Total oxidant status assay is relatively rarely used for studying the probiotics properties. An example of such a study is the one performed by Anwar et al. on the Protexin probiotic [43].
Unfortunately, total oxidative/antioxidant indices reflect too complex events, and thus they are not readily reproducible. Different TOC/TAC assays sometimes do not correlate with each other, and even considering the same method or methods with similar mechanisms, the results are often conflicting [36].
Thus, more specific methods are often used together or apart from the total prooxidant/antioxidant assays. These methods are subdivided into two categories: those assessing dynamics of isolated redox processes, and those analyzing endpoint products of such processes. One of the most frequently used assays of the first category is the lipid peroxidation detection.
Lipid Peroxidation Detection Using TBA
Several variations of the basic principle of the assay [44] are used today. An acidified (with 1% phosphoric acid) homogenate is treated with TBA (0.6%), and the mixture is then heated on a boiling water bath for 45 min. At this stage, the reaction occurs. 2-thiobarbituric acid reacts with MDA or other chemically similar molecules (TBA-reactive substances) at 25 °C. One molecule of MDA or other chemically related substance reacts with 2 molecules of 2-thiobarbituric acid via a Knoevenagel-type condensation to yield chromophores with absorbance maximum at 532 nm. These chromophores require extraction, thus subsequently, an equal volume of n-butanol is added to the sample, and the solution is thoroughly mixed to allow for extraction of the products of the reaction. The butanol phase is then separated by centrifugation, and absorbance is measured at 520 and 535 nm [15]. For preparation of the standard curve, overnight digestion of various concentrations of 1,1,3,3-tetraethoxypropane (0.1 mM) in presence of 0.2 N HCl can be used [15]. The method was used, for example, to study the antioxidant properties of Lactobacillus brevis CD2, Lb. salivarius FV2, and Lb. plantarum FV9 [45].
Endpoint Products of Redox Processes
Over the years, MDA and TBARS were the most often analyzed markers of oxidative stress. In line with these markers, oxidized LDLs, antibodies to oxidized LDLs, 4-hydroxynonenal (4-HNE), acrolein, advanced lipid oxidation products, advanced protein oxidation products, advanced glycation end products, disulfides, carbonyls, 3-nitrotyrosine, reactive aldehydes, reduced sugars, 8-oxy-2-deoxyguanosine were also used [25, 36, 44, 46]. These factors are easily detected using respective specific techniques, from ELISA to HPLC with UV detection (HPLC-UV), ultra-performance liquid chromatography with tandem mass spectrometry (HPLC/UPLC-MS/MS), and gas chromatography–mass spectrometry (GC-MS) [46]. These markers are also supplemented with more accurate ones, such as isoprostanes and their metabolites, and allantoin [46].
Isoprostanes
Isoprostanes are prostaglandin (PG) isomers that are generated from polyunsaturated fatty acids, mainly from arachidonic acid (and additionally from docosahexaenoic and eicosapentaenoic acids) by a non-enzymatic process that involves in situ peroxidation of membrane phospholipids by free radicals and ROS [46]. Isoprostanes are reliable markers of oxidative damage in vivo and in vitro [46]. Isoprostanes are suitable oxidative stress markers: they are stable specific products of ROS-induced lipid peroxidation, and they have been found in detectable quantities as a free form in all biological fluids and as esterified form in normal tissues and they are unaffected by lipid content in diet. Current methods for determination of isoprostanes are ELISA, LC-MS, and GC-MS [46]. Isoprostanes served as a marker of oxidative status in human subjects that were assigned with a diet containing the Lb. fermentum ME-3 probiotic [47].
Allantoin
In humans, allantoin is the end product of non-enzymatic oxidation of uric acid. Allantoin is a promising biomarker of systemic oxidative status in humans because concentration of allantoin does not depend on variations of uric acid level, it is stable regardless of the storage or sample preparation, and additionally it is easily detected in biological material of human samples [46]. Allantoin is an extremely polar compound; therefore, quantitative determination in plasma, serum, or urine is difficult. It requires the use of sensitive and specific analytical techniques: capillary zone electrophoresis (CZE), enzymatic assay and enzyme cycling method, capillary electrophoresis with UV detection (CE-UV), HPLC-UV, HPLC/UPLC-MS/MS, and GC-MS [46]. Allantoin was among compounds tested in infant rhesus monkeys fed with diet containing the Bif. animalis subsp. lactis HN019 probiotic [48].
Radical-Generating Systems Used for the Antioxidant Assays
Several radical-generating systems are routinely used in antioxidants and probiotics testing.
Pyrogallol Autoxidation
The method utilizes the iron ions or luminol-enhanced autoxidation of pyrogallol accompanied by release of superoxide anion [26, 49]. In this method, the test compound or a probiotic affect the rate of release of chromophoric products of the reaction (detection at 320 or 420 nm) [26, 49]. This method was used, for example, in a study by Gao et al. where the antioxidant activity of the Lb. plantarum FC225 strain was elucidated [26].
DPPH Radical-Generating/Reporting System
The DPPH (1,1-diphenyl-2-picrylhydrazyl) solution is a stable radical-generating system [50]. Usually, the 0.1 mM DPPH solution in methanol [26] or ethanol [50] is mixed with the test compound or a probiotic. The decrease in absorbance at 517 nm is measured at 0 and 5 min and then every 15 min until the reaction reaches its plateau. The percentage of DPPH remaining at the steady-state is calculated as a function of the molar ratio of antioxidant to DPPH [26]. Lb. plantarum FC225 strain antioxidant effect was studied using this method [26].
1,10-Phenanthroline/Ferrous Sulfate Radical-Generating System
In this assay, the hydroxyl radical scavenging activity of the test compound is analyzed using the mixture of 1,10-phenanthroline (0.75 mM), FeSO4 (0.75 mM), and H2O2 (0.01%) producing a colored product registered at 536 nm [26]. This method was used to study Lb. plantarum FC225 strain’s antioxidant properties [26].
Anti-lipid Peroxidation Activity Test—the Egg Yolk/Ferrous Sulfate System
According to this approach, anti-lipid peroxidation activity is determined following a simple procedure. Equal volumes of PBS and fresh egg yolk are mixed and stirred for 10 min, and then the mix is diluted 1:25 with PBS. One milliliter of the resulting solution, 0.5 mL of the sample, 1 mL PBS, and 1 mL FeSO4 (0.01 mM) are mixed; the mixture is shaken at 37 °C for 15 min, and then 1 mL of 2.5% trichloroacetic acid is added. The solution is thoroughly mixed, centrifuged at 4000 g for 20 min, then 3 mL of the supernatant is mixed with 2 mL 0.8% 2-thiobarbituric acid and heated to 100 °C for 10 min. The absorbance of the mixture is measured at 532 nm [26].
Although effects of probiotics are often analyzed with respect to chemical content of egg yolk [51, 52], the method is rarely used to test for anti-lipid peroxidation activity.
Superoxide Anion Detection Methods
Numerous substances react with superoxide and allow for its detection via calorimetric or fluorescent methods [25]. The most frequently used ones are: redox-sensitive fluorescent dye dihydroethidium (compatible with tissues samples) [25]; ferricytochrome C (when reduced, it can be measured spectrophotometrically at 550 nm) [53]; nitroblue tetrazolium (the reaction product absorbance is measured at 550 nm) [54, 55]; 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole (NBD-Cl) (upon reacting with superoxide anion, it produces a product with absorbance measured at 470 nm; the same product is also fluorescent, with ex./em. maxima of 470/550 nm) [55]; 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) (the reaction product absorbance is measured at 470 nm) [55].
These and other fluorescent/chromophor probes can and are readily used for biochemical assessment of antioxidant properties of probiotics in cell-free and cellular assays [56,57,58].
Biosensors in Assessment of Antioxidative and Related Properties of Probiotics
In vivo studies on animal objects are usually rather laborious and time-consuming. Тo identify potential protectors among probiotics, much simpler model systems are required [59].
The considerable universality of the antioxidant defense mechanisms, a consequence of evolutionary antiquity of its mechanisms, allows using simple unicellular system, including prokaryotes, as model objects to test antioxidant properties of bioactive factors. An approach implying using of biosensors is an example of such solution [60].
A biosensor is defined as an analytical device, which integrates a biological recognition element with a physical transducer to generate a measurable signal proportional to the concentration of the analyzed compound [61].
The biosensor approach is not too common; however, there were some attempts to utilize it in probiotics studies. A typical approach for using cellular biosensors was proposed by Grimoud et al. [62]. Briefly, for the screening of potential protective (anti-inflammatory and anti-proliferative) properties, the authors used a two-stage screening system based on a modified eukaryotic cell line. The first step of screening was based on the HT-29 cells with modified expression pattern. The pattern of inflammation was characterized by analyzing the secreted interleukins. Secreted substances were quantified using classical chemiluminescent ELISA test. Then, further testing was carried out using the inflammatory cell culture model consisting of inflammatory-activated transgenic Caco-2 cells transfected with a reporter gene under the control of the NF-κB inducible promoter. This method is attributed to biosensor type because the detecting system consists of the biological part (cells) and the technical component (a luminometer). In this study, the following microorganisms were screened: Bifidobacterium bifidum, Bif. breve, Bif. longum, B if. pseudocatenulatum, Lactobacillus acidophilus, Lb. buchneri, Lb. farciminis, Lb. helveticus, Lb. plantarum, Lb. rhamnosus, Lb. lactis, Pediococcus acidilactici, and Streptococcus thermophilus. As a result, anti-inflammatory properties of 11 strains were tested. It was also found that B if. breve and Lactococcus lactis (Lc. lactis), in a composition of symbiotic preparations, significantly decreased proliferation of cancer cells.
It is worth considering the approach of in vitro screening of probiotic properties proposed in [38]. The antioxidant performance of Lb. fermentum LF31 with prebiotic supplement was shown in human colon cultured cells using oxygen radical absorbance capacity (ORAC) method and the potency of the strain was compared with that of the positive control, Trolox. Authors observed a statistically significant free radical scavenging capacity of Lb. fermentum LF31.
Speaking of single-cell systems, it should be noted that bacteria grow faster and are easier to operate with when compared to eukaryotic cell culture. Signal system based on luminescence is a tool of choice in bacterial biosensor studies, since luminescent signal is one of the most easily detected. If methods under review are to be applied in large-scale pharmacological research, the speed of screening will be a crucial factor.
In one of our own studies [63] bacterial biosensors based on E. coli MG 1655 (pSoxS-lux), E. coli MG1655 (pRecA-lux), and E.coli MG1655 pColD-lux were used as a single-cell model systems. These biosensors are the genetically modified strains of E. coli, containing the plasmid carrying lux CDABE operon from Photorhabdus luminescens under the control of appropriate promoters, SoxS, RecA, etc. This operon is responsible for bioluminescence and provides luciferase used in this test as a reporter [64].
A biosensor strain with the PsoxS promoter detects the presence of oxidants forming a cell superoxide anion radical in the medium. A characteristic feature of oxidative stress in E. coli is the induction of genes of the antioxidant system and increasing the activity of antioxidant enzymes encoded by these genes [65]. Therefore, in the genetic constructs that constitute the basis of biosensors responsive to oxidative stress, the promoters of these genes were used. The PsoxS promoter specifically reacts to the superoxide anion radicals. Biosensors with pRecA and pColD plasmids report on the presence of factors that cause DNA damage in the cell. The sensitivity of these biosensors is about 10−8 M of the inductor [64]. To activate SoxS promoter, paraquat (1,1′-dimethyl-4,4′-bipyridylium dichloride) was used. This compound triggers oxidative stress, switching the cell bioenergetics to generation of superoxide anion, instead of ATP synthesis [66, 67].
In addition, an activity of Bacillus amyloliquefaciens B-1895 (soil isolate) and Bacillus subtilis KATMIRA1933 (isolated from the fermented dairy product YoguFarm™) was studied. Probiotic properties of B. amyloliquefaciens В-1895 manifest in stimulation of growth and tolerance to pathogens of fish and birds [68, 69]. The subtilosin preparation obtained from B. subtilis KATMIRA1933 was confirmed as being safe for human tissues, having spermicidal activity [70], and active against foodborne [71] and vaginal [72, 73] pathogens. Preparations of both fermentates demonstrated antioxidant activity [68,69,70,71,72,73].
In another work [74], a similar approach for use of biosensors was proposed with some modifications: genetically engineered constructs were made in Bif. longum. Authors constructed a bifidobacteria-based biosensor that could be used to analyze the metabolic state of the cells. In this case, the probiotic strain itself was a biosensor. An insect (Pyrophorus plagiophthalamus) luciferase gene was introduced into the genome of the bacteria to construct a bifidobacterial luminescent biosensor that could be used for a quick screening. Light emission is the signal of the metabolic state changes of cells. Experiments with luminescent Bif. longum indicate that, under acidic stress condition, bifidogenic prebiotics such as FOS or lactulose can considerably improve the cell physiology.
Applying this approach makes it possible to study the metabolic activity of the probiotic preparation under different conditions, which allows choosing the optimum combination of additional compounds in synbiotic preparations, for example, in order to help bifidobacteria to survive gastric transit, or to increase its beneficial properties. This approach seems promising and, with minor modification (e.g., introduction of stress-inducible promoters to the construct) can be applied to problems discussed above.
In general, we can conclude that there is some trend in applying biosensors in probiotic screening. Most of authors use microplate tests, with luminescent of fluorescent signal as detection tool.
A Brief Summary of Methods Used to Assess the Antioxidative Properties of Probiotics
Table 1 summarizes the brief results of the studies that employed the reviewed methods of assessment of the oxidative status-modulating properties of probiotics.
A Comparative Analysis of Methods under Description
As seen from current review, the methods utilized for measuring of antioxidant activity of probiotics can be subdivided into biochemical and signaling-based techniques. The most straightforward methods employ chemical systems for generation of ROS, and one can even choose a system producing specific radicals. In these methods, reporting substances are external just as the radical-generating systems, and no eukaryotic cells are required to run the test.
The second group of methods relies on external or cellular eukaryotic sources of ROS, while detected are cell-derived substances only. Remarkably, there are sub-groups of such techniques, and these reflect an important biological fact: ROS are produced all over the eukaryotic cell, in all of its compartments. Although ROS are generally universal, the consequences of their generation are dramatically different: most impact may fall on lipids, proteins, small molecules, DNA, and RNA. Consequently, this initial impact affects the secondary events. To list a few examples, lipid oxidation may lead to chain reactions of lipid peroxidation: oxidation of calcium channels of endoplasmic reticulum leads to cytoplasmic calcium flux further leading to endoplasmic reticulum stress and oxidation of cytoplasmic signaling proteins leads to induction of redox-activated transcription factors controlling ROS-generating enzymes. Thus, different analytical methods are required and employed to study specific roles of probiotics in development of the primary and secondary events and their consequences. In addition, just as it is true for the first group of methods, specific methods of the second group are used to test particular properties in a given probiotic. The rationale is standard for biomedicine: therapeutical intervention should be as targeted as possible. This is especially true for modulation of redox process, because there are many sources of ROS inside the cell, and these ROS have numerous physiological functions that are spatiotemporally specific.
The third group of methods resembles the second one, with one essential difference: the detection is based on cellular signals deriving from cellular sensing of and reactions towards redox processes. As redox regulation is vast and diverse, specific signaling systems and levels of these systems (pre-mRNAs, mRNA, proteins and their modifications) to be analyzed are chosen based on research needs. For example, pre-mRNAs, mRNAs, and signaling proteins modifications are used to address changes in cellular signaling pattern, while proteins quantities and enzymatic activities are analyzed to assess the cellular response.
The fourth group of methods is somewhat similar to the third group in being based on assessment of cellular reactions towards ROS, rather than on assessment of direct chemical consequences of generation of ROS, but it is distinct, as it utilizes prokaryotic and eukaryotic biosensors.
In summary, antioxidant activity testing methods used in probiotics research are extremely versatile. They range from cell-free radical generation testing (for assessing the direct inherent antioxidant activity) and to RNA/protein expression analysis in eukaryotic cells co-culture and animal models. Firstly, this vast variety allows one to choose the most appropriate method for assessing a specific property of the probiotic strain being under consideration or development. Secondly, in probiotics development, these methods are conveniently combined into a panel of tests of increasing complexity thus making positive and negative selection of strains both fast and cheap. Moreover, each type (or step in the multi-step approach) of testing methods is represented by generally interchangeable techniques varying in details, such as radical generation system used, mode of detection, positive control or reference substance, pathway targets analyzed, etc. Consequently, these groups of tests are flexible enough to meet the needs and capabilities of every given lab. At the same time, utilization of several techniques within the same group of methods allows to account non-intended interactions of the agent (probiotic) being tested and the assay system components. Thus, the abovementioned wide variety of methods, which can be used to assess antioxidant properties of probiotics, lays a solid basis for reliable data interpretation.
Moreover, probiotics, as a group of antioxidants, are inherently much more “customizable” than any other group of antioxidants, and their potential redox roles are far more complex and wide-ranged that these of the latter. On the other hand, it is evident that cellular antioxidants such as probiotics, have much more complex effects when used as treatment, when compared to molecular antioxidants. Thus, much more complex testing is required for probiotics than for molecular antioxidants. As a consequence, the antioxidant properties testing “toolbox” used in probiotics research should be more diverse than that used in molecular antioxidants research, and concomitant use of tests from different groups is required to comprehensively characterize the intrinsic complexity of effects of probiotics in modulation of redox processes and oxidative status of the host cells.
References
Prescott S, Nowak-Węgrzyn A (2011) Strategies to prevent or reduce allergic disease. Ann Nutr Metab 59(Suppl 1):28–42. https://doi.org/10.1159/000334150
Priebe MG, Vonk RJ, Sun X et al (2002) The physiology of colonic metabolism. Possibilities for interventions with pre-and probiotics. Eur J Nutr 41(Suppl 1):I2–I10
Khansari N, Shakiba Y, Mahmoudi M (2009) Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents Inflamm 3:73–80
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T (2010) Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64(6):636–643. https://doi.org/10.1038/ejcn.2010.19
Vanderhoof JA, Mitmesser SH (2010) Probiotics in the management of children with allergy and other disorders of intestinal inflammation. Benefic Microbes 1(4):351–356. https://doi.org/10.3920/BM2010.0034
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63(14):3615–3626. https://doi.org/10.1021/jf506326t
Cenci G, Caldini G, Trotta F, Bosi P (2008) In vitro inhibitory activity of probiotic spore-forming bacilli against genotoxins. Lett Appl Microbiol 46(3):331–337. https://doi.org/10.1111/j.1472-765X.2007.02314.x
Cox CM, Dalloul RA (2014) Immunomodulatory role of probiotics in poultry and potential in ovo application. Benefic Microbes 6(1):45–52. https://doi.org/10.3920/BM2014.0062
Floch MH (2014) Recommendations for probiotic use in humans - a 2014 update. Pharmaceuticals (Basel) 7(10):999–1007. https://doi.org/10.3390/ph7100999
Achuthan AA, Duary RK, Madathil A, Panwar H, Kumar H, Batish VK, Grover S (2012) Antioxidative potential of lactobacilli isolated from the gut of Indian people. Mol Biol Rep 39(8):7887–7897. https://doi.org/10.1007/s11033-012-1633-9
Kullisaar T, Zilmer M, Mikelsaar M (2002) Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72(3):215–224. https://doi.org/10.1016/S0168-1605(01)00674-2
Mahdhi A, Leban N, Chakroun A et al (2017) Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb Pathog 109:214–220. https://doi.org/10.1016/j.micpath.2017.05.046
Shen Q, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of Bifidobacterium animalis 01 isolated from centenarians. Curr Microbiol 62(4):1097–1103. https://doi.org/10.1007/s00284-010-9827-7
Wang Y, Wu Y, Wang Y et al (2017) Antioxidant properties of probiotic bacteria. Nutrients 9:E521. https://doi.org/10.3390/nu9050521
Chauhan R, Vasanthakumari AS, Panwar H et al (2014) Amelioration of colitis in mouse model by exploring antioxidative potentials of an indigenous probiotic strain of Lactobacillus fermentum Lf 1. Biomed Res Int 2014(206732):1–12. https://doi.org/10.1155/2014/206732
Isolauri E, Kirjavainen PV, Salminen S (2002) Probiotics: a role in the treatment of intestinal infection and inflammation? Gut 50(suppl 3):iii54–iii59
Matsuu M, Shichijo K, Okaichi K et al (2003) The protective effect of fermented milk kefir on radiation-induced apoptosis in colonic crypt cells of rats. J Radiat Res 44(2):111–115. https://doi.org/10.1269/jrr.44.111
Stilling RM, Dinan TG, Cryan JF (2014) Microbial genes, brain and behaviour–epigenetic regulation of the gut-brain axis. Genes Brain Behav 13(1):69–86. https://doi.org/10.1111/gbb.12109
Renner HW, Münzner R (1991) The possible role of probiotics as dietary antimutagen. Mutat Res 262(4):239–245. https://doi.org/10.1016/0165-7992(91)90090-Q
Lo PR, RC Y, Chou CC et al (2004) Determinations of the antimutagenic activities of several probiotic bifidobacteria under acidic and bile conditions against benzo [a] pyrene by a modified Ames test. Int J Food Microbiol 93(2):249–257. https://doi.org/10.1016/j.ijfoodmicro.2003.11.008
Lopitz-Otsoa F, Rementeria A, Elguezabal N, Garaizar J (2006) Kefir: a symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev Iberoam Micol 23(2):67–74. https://doi.org/10.1016/S1130-1406(06)70016-X
Gotteland M, Brunser O, Cruchet S (2006) Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther 23(8):1077–1086. https://doi.org/10.1111/j.1365-2036.2006.02868.x
Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B (2014) The human gut microbiota: a dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res 76(1):2–10. https://doi.org/10.1038/pr.2014.49
Zolotukhin P, Kozlova Y, Dovzhik A, Kovalenko K, Kutsyn K, Aleksandrova A, Shkurat T (2013) Oxidative status interactome map: towards novel approaches in experiment planning, data analysis, diagnostics and therapy. Mol BioSyst 9(8):2085–2096. https://doi.org/10.1039/c3mb70096h
Endo H, Niioka M, Kobayashi N, Tanaka M, Watanabe T (2013) Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: new insight into the probiotics for the gut-liver axis. PLoS One 8(5):e63388. https://doi.org/10.1371/journal.pone.0063388
Gao D, Gao Z, Zhu G (2013) Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct 4(6):982–989. https://doi.org/10.1039/c3fo30316k
Lin YP, Thibodeaux CH, Peña JA, Ferry GD, Versalovic J (2008) Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis 14(8):1068–1083. https://doi.org/10.1002/ibd.20448
Hegazy SK, El-Bedewy MM (2010) Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J Gastroenterol 16(33):4145–4151. https://doi.org/10.3748/wjg.v16.i33.4145
Karlsson M, Scherbak N, Khalaf H, Olsson PE, Jass J (2012) Substances released from probiotic Lactobacillus rhamnosus GR–1 potentiate NF-κB activity in Escherichia coli-stimulated urinary bladder cells. FEMS Immunol Med Microbiol 66(2):147–156. https://doi.org/10.1111/j.1574-695X.2012.00994.x
Wagner RD, Johnson SJ (2012) Probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J Biomed Sci 19:58. https://doi.org/10.1186/1423-0127-19-58
Wehkamp J, Harder J, Wehkamp K, Meissner BW, Schlee M, Enders C, Sonnenborn U, Nuding S, Bengmark S, Fellermann K, Schroder JM, Stange EF (2004) NF-kappaB- and AP-1-mediated induction of human beta defensin–2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 72(10):5750–5758. https://doi.org/10.1128/IAI.72.10.5750-5758.2004
Schlee M, Wehkamp J, Altenhoefer A (2007) Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun 75(5):2399–2407. https://doi.org/10.1128/IAI.01563-06
Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K (2008) Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol 151(3):528–535. https://doi.org/10.1111/j.1365-2249.2007.03587.x
Balcerczyk A, Grzelak A, Janaszewska A, Jakubowski W, Koziol S, Marszalek M, Rychlik B, Soszynski M, Bilinski T, Bartosz G (2003) Thiols as major determinants of the total antioxidant capacity. Biofactors 17(1-4):75–82. https://doi.org/10.1002/biof.5520170108
Pinchuk I, Shoval H, Dotan Y, Lichtenberg D (2012) Evaluation of antioxidants: scope, limitations and relevance of assays. Chem Phys Lipids 165(6):638–647. https://doi.org/10.1016/j.chemphyslip.2012.05.003
Peluso I, Cavaliere A, Palmery M (2016) Plasma total antioxidant capacity and peroxidation biomarkers in psoriasis. J Biomed Sci 23(52):52. https://doi.org/10.1186/s12929-016-0268-x
Fraga CG, Oteiza PI, Galleano M (2014) In vitro measurements and interpretation of total antioxidant capacity. Biochim Biophys Acta 1840(2):931–934. https://doi.org/10.1016/j.bbagen.2013.06.030
Persichetti E, De Michele A, Codini M, Traina G (2014) Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition 30(7-8):936–938. https://doi.org/10.1016/j.nut.2013.12.009
Kavitha Rani PR, Fernandez A, George A, Remadevi VK, Sudarsanakumar MR, Laila SP, Arif M (2015) Synthesis, spectral characterization, molecular structure and pharmacological studies of N'-(1, 4-naphtho-quinone–2yl) isonicotinohydrazide. Spectrochim Acta A Mol Biomol Spectrosc 135:1156–1161. https://doi.org/10.1016/j.saa.2014.07.092
Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97(2):809–817. https://doi.org/10.1007/s00253-012-4241-7
Erel O (2004) A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 37(2):112–119. https://doi.org/10.1016/j.clinbiochem.2003.10.014
Gay C, Collins J, Gebicki JM (1999) Determination of iron in solutions with the ferric-xylenol orange complex. Anal Biochem 273(2):143–148. https://doi.org/10.1006/abio.1999.4207
Anwar H, Rahman ZU, Javed I, Muhammad F (2012) Effect of protein, probiotic, and symbiotic supplementation on serum biological health markers of molted layers. Poult Sci 91(10):2606–2613. https://doi.org/10.3382/ps.2012-02172
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituricacid test. Anal Biochem 86(1):271–278
Barbonetti A, Cinque B, Vassallo MR et al (2011) Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil Steril 95(8):2485–2488. https://doi.org/10.1016/j.fertnstert.2011.03.066
Czerska M, Mikołajewska K, Zieliński M, Gromadzińska J, Wąsowicz W (2015) Today’s oxidative stress markers. Med Pr 66(3):393–405. https://doi.org/10.13075/mp.5893.00137
Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M (2003) Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr 90(02):449–456. https://doi.org/10.1079/BJN2003896
He X, Slupsky CM, Dekker JW et al (2016) Integrated role of Bifidobacterium animalis subsp. lactis supplementation in gut microbiota, immunity, and metabolism of infant rhesus monkeys. mSystems 1
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I (1998) Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl–2 picrylhydrazyl radical. Biochem Pharmacol 56(2):213–222. https://doi.org/10.1016/S0006-2952(98)00128-2
Salma U, Miah AG, Tsujii H, Schellander K, Südekum KH (2012) Effect of dietary Rhodobacter capsulatus on lipid fractions and egg-yolk fatty acid composition in laying hens. J Anim Physiol Anim Nutr (Berl) 96(6):1091–1100. https://doi.org/10.1111/j.1439-0396.2011.01224.x
Tang SG, Sieo CC, Kalavathy R et al (2015) Chemical compositions of egg yolks and egg quality of laying hens fed prebiotic, probiotic, and synbiotic diets. J Food Sci 80(8):C1686–C1695. https://doi.org/10.1111/1750-3841.12947
Nauseef WM (2014) Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim Biophys Acta 1840(2):757–767. https://doi.org/10.1016/j.bbagen.2013.04.040
Wang L, Liu S, Zheng Z, Pi Z, Song F, Liu Z (2015) Rapid assay for testing superoxide anion radical scavenging activities to natural pigments by ultra-high performance liquid chromatography-diode-array detection method. Anal Methods 7(4):1535–1542. https://doi.org/10.1039/C4AY02690J
Olojo RO, Xia RH, Abramson JJ (2005) Spectrophotometric and fluorometric assay of superoxide ion using 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole. Anal Biochem 339(2):338–344. https://doi.org/10.1016/j.ab.2005.01.032
Hosoki K, Nakamura A, Nagao M, Hiraguchi Y, Tokuda R, Wada H, Nobori T, Fujisawa T (2010) Differential activation of eosinophils by 'probiotic' Bifidobacterium bifidum and ‘pathogenic’ Clostridium difficile. Int Arch Allergy Immunol 152(Suppl 1):83–89. https://doi.org/10.1159/000312131
Harikrishnan R, Balasundaram C, Heo MS (2010) Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol 29(5):868–874. https://doi.org/10.1016/j.fsi.2010.07.031
Kodali VP, Sen R (2008) Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol J 3(2):245–251. https://doi.org/10.1002/biot.200700208
McBain AJ, Macfarlane GT (2001) Modulation of genotoxic enzyme activities by non-digestible oligosaccharide metabolism in in-vitro human gut bacterial ecosystems. J Med Microbiol 50(9):833–842. https://doi.org/10.1099/0022-1317-50-9-833
Kotova VY, Manukhov IV, Zavilgelskii GB (2010) Lux-biosensors for detection of SOS-response, heat shock, and oxidative stress. Appl Biochem Microbiol 46(8):781–788. https://doi.org/10.1134/S0003683810080089
Su L, Jia W, Hou C, Lei Y (2011) Microbial biosensors: a review. Biosens Bioelectron 26(5):1788–1799. https://doi.org/10.1016/j.bios.2010.09.005
Grimoud J, Durand H, De Souza S et al (2010) In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int J Food Microbiol 144(1):42–50. https://doi.org/10.1016/j.ijfoodmicro.2010.09.007
Prazdnova EV, Chistyakov VA, Churilov MN, Mazanko MS, Bren AB, Volski A, Chikindas ML (2015) DNA-protection and antioxidant properties of fermentates from Bacillus amyloliquefaciens B–1895 and Bacillus subtilis KATMIRA1933. Lett Appl Microbiol 61(6):549–554. https://doi.org/10.1111/lam.12491
Zavilgelsky GB, Kotova VY, Manukhov IV (2007) Action of 1,1-dimethylhydrazine on bacterial cells is determined by hydrogen peroxide. Mutat Res 634(1-2):172–176. https://doi.org/10.1016/j.mrgentox.2007.07.012
Farr SB, Kogoma T (1991) Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev 55(4):561–585
Liochev SI, Hausladen A, Beyer WF, Fridovich I (1994) NADPH: ferredoxin oxidoreductase acts as a paraquatdiaphorase and is a member of the soxRS regulon. Proc Natl Acad Sci U S A 91(4):1328–1331. https://doi.org/10.1073/pnas.91.4.1328
Miller RL, Sun GY, Sun AY et al (2007) Cytotoxicity of paraquat in microglial cells: involvement of PKCδ-and ERK1/2-dependent NADPH oxidase. Brain Res 1167:129–139. https://doi.org/10.1016/j.brainres.2007.06.046
Chistyakov V, Melnikov V, Chikindas ML et al (2015) Poultry-beneficial solid-state Bacillus amyloliquefaciens B-1895 fermented soybean formulation. Biosci Microbiota Food Health 34(1):25–28. https://doi.org/10.12938/bmfh.2014-012
Karlyshev AV, Melnikov VG, Chistyakov VA (2014) Draft genome sequence of Bacillus amyloliquefaciens B-1895. Genome Announc 2(3):e00633–e00614. https://doi.org/10.1128/genomeA.00633-14
Sutyak KE, Anderson RA, Dover SE, Feathergill KA, Aroutcheva AA, Faro S, Chikindas ML (2008) Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect Dis Obstet Gynecol 2008:ID540758. https://doi.org/10.1155/2008/540758
Amrouche T, Sutyak Noll K, Wang Y, Huang Q, Chikindas ML (2010) Antibacterial activity of subtilosin alone and combined with curcumin, poly-lysine and zinclactate against Listeria monocytogenes strains. Probiotics Antimicrob Proteins 2(4):250–257. https://doi.org/10.1007/s12602-010-9042-7
Sutyak KE, Wirawan RE, Aroutcheva AA, Chikindas ML (2008) Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J Appl Microbiol 104(4):1067–1074. https://doi.org/10.1111/j.1365-2672.2007.03626.x
Noll KS, Sinko PJ, Chikindas ML (2011) Elucidation of the molecular mechanisms of action of the natural antimicrobial peptide subtilosin against the bacterial vaginosis-associated pathogen Gardnerella vaginalis. Probiotics Antimicrob Proteins 3(1):41–47. https://doi.org/10.1007/s12602-010-9061-4
Guglielmetti S, Ciranna A, Mora D, Parini C, Karp M (2008) Construction, characterization and exemplificative application of bioluminescent Bifidobacterium longum biovar longum. Int J Food Microbiol 124(3):285–290. https://doi.org/10.1016/j.ijfoodmicro.2008.03.033
Abdhul K, Ganesh M, Shanmughapriya S, Kanagavel M, Anbarasu K, Natarajaseenivasan K (2014) Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int J BiolMacromol 70:450–454. https://doi.org/10.1016/j.ijbiomac.2014.07.026
Balcázar JL, de Blas I, Ruiz-Zarzuela I et al (2007) Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunol Med Microbiol 51(1):185–193. https://doi.org/10.1111/j.1574-695X.2007.00294.x
Acknowledgements
The authors would like to thank Dr. Vijendra Mishra for critical reading and extremely helpful comments.
Funding
Preparation of this paper was supported by the grant of the Russian Science Foundation (Project No. 16-16-04032).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zolotukhin, P.V., Prazdnova, E.V. & Chistyakov, V.A. Methods to Assess the Antioxidative Properties of Probiotics. Probiotics & Antimicro. Prot. 10, 589–599 (2018). https://doi.org/10.1007/s12602-017-9375-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9375-6