Abstract
In this paper, the antibacterial effects of the Bacillus amyloliquefaciens-produced bacteriocin subtilosin, both alone and in combination with curcumin, ε-poly-l-lysine (poly-lysine), or zinc lactate, were examined against Listeria monocytogenes. Results indicated that subtilosin inhibits both of the studied bacterial strains, Scott A (wild-type, nisin sensitive) and NR30 (nisin resistant). However, L. monocytogenes Scott A was more sensitive to subtilosin and pure curcumin. In addition, subtilosin was more active at an acidic pH. Subtilosin in combination with encapsulated curcumin displayed partial synergy against L. monocytogenes ScottA. It also had synergistic activity against both L. monocytogenes Scott A and L. monocytogenes NR30 when combined with zinc lactate. Only an additive effect was observed for subtilosin when combined with non-encapsulated curcumin or poly-lysine against the mentioned strains. Thus, using the combination of subtilosin with curcumin, poly-lysine, or zinc lactate, a lower effective dose can be used to control L. monocytogenes infection. Our findings suggest that subtilosin could be used as alternative bacteriocin to nisin, providing an opportunity to use a novel natural and efficacious biopreservative against L. monocytogenes in food preservation. This is the first report on the effects of the combination of subtilosin with natural antimicrobials on L. monocytogenes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last few decades, natural antimicrobial substances have been widely explored and investigated for their potential use as food preservatives and pharmaceuticals. Indeed, bacteriocins such as nisin and weak organic acids such as lactic acid are produced by lactic acid bacteria and are commercially used to control pathogens in some foods [5]. Additionally, many compounds derived from plant extracts, such as curcumin, are employed in food preparations and have been shown to possess antimicrobial activity against foodborne pathogens [19, 29, 35]. Curcumin, a yellow-pigmented compound, is widely used to color many foods (fruit and vegetable products, cereal products, meat and meat products, etc.) [16]. This phytochemical was shown to have antibacterial activity [28]. Poly-lysine is a natural cationic polypeptide produced by Streptomyces albulus ssp. lysinololymerus and has GRAS status in certain food applications [1]. Recently, poly-lysine has been shown to act synergistically with nisin against L. monocytogenes [3]. Salts of lactic acid such as sodium and potassium are used as preservatives in some refrigerated ready-to-eat cooked meats [2]. Among nature-derived antimicrobials, bacteriocins from lactic acid bacteria are attractive in terms of their wide range of antibacterial activities, as well as because of their potential use as safe and natural food preservatives [7]. However, many natural antimicrobials have a limited spectrum of activity and are effective only at very high concentrations that may alter the organoleptic properties of treated foods. A possible solution may be using combinations of antimicrobials at low concentrations [33]. Indeed, mixing antimicrobials with different inhibitory activities might lead to compositions with apparent synergistic or additive effects which may themselves be very useful in food preservation. A mixture of preservatives with different mechanisms of action can be used efficiently in microbial control, creating a multiple hurdle against the targeted bacteria. For example, nisin has been used in combination with sodium lactate in hurdle technology [26]. Naghmouchi et al. [25] also demonstrated that nisin, when synergistically combined with polymyxin, was able to effectively control the growth of both Gram-negative and Gram-positive pathogens. This promising approach has become more interesting for the food industry because the use of a combination of antimicrobials may increase the rates of microbial killing, help avoid the emergence of resistant variants, expand the spectrum of activity, and reduce antimicrobial concentrations employed in food preservation [4, 18, 39].
Listeria monocytogenes is a Gram-positive bacterium occurring naturally in the environment as well as in a variety of commodities, such as uncooked meats and vegetables and processed foods such as soft cheeses [9]. It causes listeriosis, which is recognized as a serious public health problem primarily affecting pregnant women, newborns, and adults with a weakened immune system [10, 15, 27]. To control this pathogen in foods, different strategies are applied, and particular attention has been focused on the use of bacteriocins, both alone and in combination with natural antimicrobial substances, as a safe and effective alternative to chemical preservatives [24]. Subtilosin A is a bacteriocin produced by the Gram-positive, spore forming bacterium Bacillus subtilis, which is often found in Oriental fermented foods [43]. Shelburne et al. [30] reported that subtilosin A demonstrated an antimicrobial activity against a wide range of bacteria including Gram-positive and Gram-negative organisms, both aerobes and anaerobes. Recently, Sutyak et al. [34] isolated subtilosin from the dairy product-derived Bacillus amyloliquefaciens, a phylogenetically close relative of B. subtilis. This bacteriocin was shown to be active against the pathogens L. monocytogenes, Gardnerella vaginalis, and Streptococcus agalactiae. However, no studies were reported on the effects of subtilosin, in combination with other antimicrobials, on L. monocytogenes. The aim of this study was to assess the effects of subtilosin both alone and in combination with several natural antimicrobials against the broadly studied foodborne pathogen L. monocytogenes Scott A and its nisin-resistant mutant, L. monocytogenes NR30. The latter selection is justified by emerging listerial resistance to nisin and, consequently, the need for alternative bacteriocins active against nisin-resistant mutants, both alone and in combination with other natural antimicrobials. Thus, the susceptibility of L. monocytogenes strains to the combination of subtilosin with curcumin (plant extract-derived compound) versus encapsulated curcumin, poly-lysine (peptide), and zinc lactate was assayed using the checkerboard method.
Materials and Methods
Bacterial Strains and Growth Conditions
The subtilosin-producing organism, B. amyloliquefaciens, was isolated by Sutyak et al. [34] from the yogurt-flavored cultured beverage Yogu Farm™ (JSL Foods, Los Angeles, CA, USA). After inoculation from the stock culture, the subcultures were grown aerobically overnight in MRS broth at 37 °C without agitation. Micrococcus luteus ATCC 10420 was grown aerobically in Trypticase Soy Broth supplemented with 0.6% yeast extract (Difco, MI) and 0.25% glucose at 30 °C. M. luteus is commonly used by researchers in academia and industry as a reference microorganism due to its extreme sensitivity to nisin and many other bacteriocins. According to the previously published recommendation by McEntire et al. [24], L. monocytogenes ScottA was grown at 37 °C and L. monocytogenes NR30 was grown at 30 °C (both strains were obtained from the laboratory culture collection). After inoculation from the stock, the cultures were grown overnight to exponential phase at their respective temperatures in Brain Heart Infusion Broth (BHI, OXOID, Basingstoke, UK).
Production and Purification of Subtilosin
Subtilosin production was carried out using 500 mL of MRS broth (Difco™, Detroit, MI, USA) inoculated with Bacillus amyloliquefaciens (1% inoculum from an overnight culture) and incubated for 24 h at 37 °C without agitation. Cell-free supernatant from this culture was used for bacteriocin purification by ammonium sulfate precipitation and column chromatography. All these steps were followed according to protocols described by Sutyak et al. [34]. The crude bacteriocin was tested in a well diffusion assay against the indicator organism M. luteus [c. 106 CFU/mL].
Antimicrobial Solutions Preparation
Curcumin was a gift from Sabinsa Corporation (Piscataway, NJ, USA), which contains 85% curcumin, 11% demethoxycurcumin, and 4% bisdemethoxycurcumin [41]. It was used without further purification. Encapsulated curcumin powders were prepared as follows: the solution containing maltodextrin (Maltrin M100, Grain Processing Corp., Muscatine, IA, USA), medium chain triglycerides (Neobee 1053, Stepan Co., Northfield, IL, USA) with dissolved curcumin, Tween 40 (SigmaUltra, Sigma–Aldrich Company, St. Louis, MO, USA), and Span 20 (Sigma–Aldrich) were homogenized in a high pressure homogenizer (EmulsiFlex-C3, Avestin, Ottawa, Canada) for 3 cycles at 1,000 bar to generate an oil-in-water (O/W) emulsion. The emulsion was fed into a spray dryer (Model GB 22, Yamato Scientific, Tokyo, Japan). The inlet temperature, air flow, rate of feeding, and atomization pressure were 160 °C, 0.45 m3/min, 2 mL/min, and 0.45 MPa, respectively. The powders obtained were mixtures containing 0.73% (w/w) curcumin and matrix of encapsulation (3.92% emulsifier, 14.6% medium chain triglyceride, 73% maltodextrin, and 8.75% water). Matrix powders without curcumin were made to be used as a control in experiments. Stock solutions of pure curcumin (2 mg/mL), encapsulated curcumin (0.5 mg/mL), and matrix (0.2 mg/mL) were prepared by dissolving the powder in pure ethanol and dilution in BHI broth. The poly-lysine stock solution containing 25% poly-lysine was a gift from Chisso America Inc. (Hayward, CA, USA, Lot# 1031005). Zinc lactate (PURAMEX, PURAC Biochem, Gorinchem, The Netherlands) was dissolved in sterile water to produce solutions with final concentrations of 1–10% (w/v). Then, diluted solutions were prepared from the stock solution using BHI broth.
Determination of Minimal Inhibitory Concentrations
Antimicrobial substances were investigated individually for their antimicrobial activity using the broth microdilution method. L. monocytogenes cells were subcultured from stock agar plates and grown overnight in BHI broth at 30 °C. Serial dilutions of antimicrobials (subtilosin: 400–20 μg/mL; pure curcumin: 2,000–100 μg/mL; encapsulated curcumin: 800–20 μg/mL; matrix: 500–100 μg/mL; poly-lysine: 300–10 μg/mL; zinc lactate: 20,000–1,000 μg/mL) were prepared from stock solutions. Fifty microliters of each dilution was aseptically added to the wells of a sterile 96-well plate (Corning Inc., Corning, NY, USA). The antimicrobial alone, cells alone, and medium alone were used as controls. An overnight culture of L. monocytogenes was diluted in fresh BHI broth at a 1:100 ratio, yielding an approximate inoculum size of 1 × 107 colony forming units (CFU)/mL, of which 100 μL was added to all wells. The inoculated 96-well plate was placed in a computer-controlled and temperature-controlled MRX Dynex Revelation Microplate Reader (Dynex Technologies, Chantilly, VA, USA) to measure the absorbance (turbidity) of the wells. The absorbance was recorded at 630 nm every 30 min over a 24-h incubation period at 30 °C. The lowest antimicrobial concentration not exhibiting bacterial growth (no OD increase) in the wells was determined to be the minimal inhibitory concentration (MIC). Assays were undertaken at least two times in triplicate.
Checkerboard Assay
The interaction between antimicrobials against L. monocytogenes strains was performed using a “checkerboard” assay. This method allows for investigation into the interaction of two antimicrobial substances used at different concentrations at the same time. In this experiment, a two-dimensional array of serial concentrations of test compounds was used as the basis for calculation of a fractional inhibitory concentration (FIC) index to demonstrate that paired combinations of antimicrobials can exert inhibitory effects that are more than the sum of their effects alone (synergy), equal to the sum of their effects alone (additive), or less than the sum of their effects alone (antagonism) [21]. The checkerboard assay was conducted according to the protocol given by Badaoui Najjar et al. [3]. Aliquots (50 μL) of antibacterial dilution were added to the wells of a 96-well plate (Corning) in a vertical orientation (first antimicrobial) and horizontal orientation (second antimicrobial) so that the plate would contain various concentration combinations of the two compounds. Wells with cells alone (no antimicrobial added) were used as a control. The wells containing antimicrobial alone were used as an additional control for the possible precipitation of antimicrobials and/or contamination of the media itself. In order to verify whether the matrix used in curcumin encapsulation had an additional inhibitory effect on bacterial growth, we added diluted matrix solution to three wells. The wells were inoculated with 100 μL of L. monocytogenes diluted a 100-fold, and the plate was incubated at 30 °C. To prevent spills and mixing of neighboring wells, the plate was covered with a hydrophobic film (Fisher). The assay was performed for 24 h at 30 °C in a MRX Dynex Revelation Microplate Reader (Dynex). Optical density indicating turbidity in the wells was measured every 30 min at a wavelength of 630 nm. An automated 5-s shake preceded each reading to assure even distribution of the microorganisms in each well. Each experiment was conducted three times in duplicate. The fractional inhibitory concentration (FIC) was calculated by dividing the MIC of the antibacterial in the mixture by the MIC of the same antibacterial alone. The FIC index was calculated according to the formula:
Respectively, [subtilosin] and [antimic.] indicate the concentrations of subtilosin and antimicrobial used for partial inhibition. The FIC index defines the nature of the interaction. According to the FIC index, the interaction was considered as a synergy at <0.5, partial synergy at 0.5–0.75, additive at 0.75–1.0, indifference at >1.0, and antagonism at >4.0 [27].
Isobologram
Alternately, the results of the checkerboard assay can be represented graphically by plotting the points formed by pairs of concentrations from different combination experiments on a graph known as an isobologram [12]. MIC values of the two antimicrobials used alone were determined and plotted on the x- and y-axis and joined by a line. Then, the mixed concentrations of the two compounds are plotted and compared with the previous line. The isobologram can be interpreted by examining the position of the ratio points and extrapolating synergy (below the line), antagonism (above the line), or additive effect (on the line). Each experiment was performed three times in duplicates.
Graphical Presentation of the Data
Each experiment was performed twice in triplicates and presented in Figs. 1 and 2 as an average of these values.
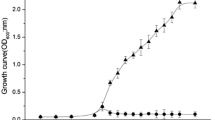
Effect of combination of subtilosin (15 μg/mL) with encapsulated curcumin (60 μg/mL) on L. monocytogenes Scott A growth in BHI broth (pH 7.4). Filled diamond cells alone, filled triangle cells with matrix of encapsulation, open triangle subtilosin alone, open circle encapsulated curcumin alone, open diamond subtilosin with encapsulated curcumin
Effect of combination of subtilosin (57.5 μg/mL) with encapsulated curcumin (20 μg/mL) L. monocytogenes NR30 growth in BHI broth (pH 7.4). Filled diamond cells alone, filled triangle cells with matrix of encapsulation, open triangle subtilosin alone, open circle encapsulated curcumin alone, open diamond subtilosin with encapsulated curcumin. The negative values of the data corresponding to the mixture encapsulated curcumin + subtilosin (open diamond) are the result of subtracting the control OD values (encapsulated curcumin + subtilosin + cells) from the experimental OD values (encapsulated curcumin + subtilosin)
Results
MIC Determination
To compare the antibacterial activity of subtilosin, curcumin, poly-lysine, and zinc lactate against both L. monocytogenes ScottA and L. monocytogenes NR30, we evaluated their MIC in BHI broth. Encapsulated curcumin, an antibacterial substance progressively released in culture media, was tested in comparison with curcumin itself, which is well known for its low solubility at higher concentrations in aqueous media like BHI broth where precipitation occurred. The results presented in Table 1 show that each of the tested antimicrobial agents inhibits the growth of both bacterial strains, although subtilosin was more active, especially when the medium pH is lower. Indeed, the MICs of subtilosin were eightfolds (L. monocytogenes NR30) or 12-folds (L. monocytogenes ScottA) lower at pH 5. However, L. monocytogenes ScottA was found to be more sensitive to subtilosin and pure curcumin than L. monocytogenes NR 30. In contrast, the latter was very sensitive to encapsulated curcumin and zinc lactate, while both bacterial strains shown the same sensitivity to poly-lysine (Table 1).
Interactions Between Antimicrobials Against L. monocytogenes Strains
As the study of antimicrobial combinations is so important, we wanted to expand the application of the antimicrobial activity method from individual antimicrobials to mixtures. Once the independent MIC was determined, the fractional inhibitory concentration (FIC) and FIC indices were calculated for the different combinations.
Interaction Between Subtilosin and Curcumin
We sought to determine the interaction between subtilosin and encapsulated curcumin or pure curcumin in BHI broth (pH 7.4) against the aforementioned pathogens. Thus, we compared the inhibitory effect of combinations using different concentrations of pure and encapsulated curcumin. Only bacterial growth curves obtained with the combination of subtilosin and encapsulated curcumin are presented in this paper. The growth data reported in Figs. 1 and 2 illustrate the effects of combining subtilosin with encapsulated curcumin against L. monocytogenes ScottA and L. monocytogenes NR30. In general, both of the growth curves (Figs. 1, 2) showed the same trend, with partial inhibition caused by subtilosin alone and encapsulated curcumin alone and with full inhibition induced by the combination of subtilosin with encapsulated curcumin. The complete inhibition of L. monocytogenes ScottA and L. monocytogenes NR 30, respectively, was observed at sub-inhibitory concentrations of subtilosin and encapsulated curcumin corresponding to 15 and 60 μg/mL versus 57.5 and 20 μg/mL, leading to FIC indices of 0.74 (partial synergy) and 0.95 (additive effect). With respect to the control cultures, there was no significant difference between the growth curves of cells alone and cells treated with the encapsulation matrix, indicating the matrix did not have any intrinsic inhibitory activity (Figs. 1, 2).
According to the isobologram (Fig. 3), the interaction between subtilosin and encapsulated curcumin (15 and 60 μg/mL, respectively) was synergistic.
Interaction Between Subtilosin and Poly-Lysine and Zinc Lactate
In order to further investigate the antimicrobial interaction of subtilosin with other natural antimicrobials, we experimented using several different concentrations of poly-lysine and zinc lactate. Based on previous reports that bacteriocins and zinc lactate would optimally interact against L. monocytogenes strains at an acidic pH [24], we conducted assays with the combination of subtilosin and zinc lactate in BHI broth at pH 5.0. A synergistic interaction was observed between subtilosin and zinc lactate (2.5 and 25 μg/mL, respectively) against L. monocytogenes NR30 when tested at an acidic pH (data not shown). The FIC indices for all combinations of antimicrobials are shown in Table 2.
With reference to the FIC scale, synergistic effects were found for subtilosin combined with zinc lactate against L. monocytogenes ScottA (FIC index = 0.67) and L. monocytogenes NR30 (FIC index = 0.36), while an additive effect occurred when subtilosin was combined with pure curcumin or poly-lysine. These results indicate that the combination of subtilosin with zinc lactate was more effective against both strains of the pathogen at pH 5.0.
Discussion
The antimicrobial activity of subtilosin alone and its interaction with the natural antimicrobials curcumin, poly-lysine, and zinc lactate was investigated against two strains of the major foodborne pathogen L. monocytogenes (wild-type ScottA and nisin-resistant NR30). The antimicrobial action of subtilosin is posited to be dependent on its composition/molecular structure. Kawulka et al. [17] demonstrated that subtilosin A has only one lysine and a total of three aspartate and glutamate residues, suggesting that it may not interact solely with the cell membrane at physiological pH and may first bind to a surface receptor. It has been proposed that subtilosin A may bind to lipid bilayers of bacterial cells, resulting in leakage of the aqueous contents of small unilamellar vesicles. The interaction of subtilosin A with the cell membrane was shown to be modulated by the lipid composition of the target, with the concentration used in the assay being an important factor [36]. In addition, the antimicrobial activity of subtilosin may be a function of its interaction with membrane-associated receptors, similar to that seen with the antibiotic nisin [42].
Curcumin, a yellow polyphenol extracted from the rhizome of turmeric [31], has been reported to be insoluble at high concentrations (>25 μM) in buffer solution at pH 7.0 [14], but this phytochemical was found to have antimicrobial activity [8, 20, 23]. Given that the water insolubility and instability of curcumin could reduce its biological activity, we were interested in exploring the activity of encapsulated curcumin both alone and combined with subtilosin as a means of improving its relative antimicrobial activity. Pronounced inhibition of listerial growth (shown by low MICs) was observed when using low concentrations of encapsulated versus non-encapsulated curcumin. These results indicate that the antibacterial activity of curcumin was improved by entrapment of the bioactive compound, allowing for a progressive release. Although the encapsulation matrix contains surfactants, it did not carry any innate antimicrobial activity. There is an extended lag in listerial growth in the presence of the matrix alone when compared to the controls. However, as is obvious from both Figs. 1 and 2, the foodborne pathogen reaches the same cell density in the stationary phase both in the presence and in the absence of the matrix, indicating the matrix does not inhibit microbial growth. Furthermore, the solubility of encapsulated curcumin in the media was likely increased due to the chemical composition of the encapsulation matrix. The water solubility of curcumin has reportedly been improved by incorporation into various surfactant micellar systems such as cyclodextrins [13, 37]. In addition, nano-sized curcumin particles can and may facilitate the permeation of curcumin through the cellular membrane, which would further improve the uptake of curcumin by listerial cells. Curcumin, previously described as a poly-phenolic compound, may be active against bacterial cell membranes since cyclic hydrocarbons, which are well known for their toxicity to microorganisms, were reported to target in vitro cytoplasmic membranes where the lipophilic compounds preferentially reside [32]. Toxic effects on membrane structure and function have been generally used to explain the antimicrobial action of some phytochemicals [6, 11, 38]; however, the specific mechanisms involved in the antimicrobial action of curcumin remain poorly characterized. Indeed, it has been reported that some phytochemicals, such as those essential oils possessing the strongest antibacterial properties, are usually composed of phenolic compounds [22, 40]. Thus, it seems reasonable to assume that the mechanism of action and antimicrobial efficacy of curcumin and the phenolic compounds in essential oils would be similar.
Our findings agree well with those from the experimental work of McEntire et al. [24], who argued in favor of the synergy between nisin and zinc lactate against L. monocytogenes ScottA and L. monocytogenes NR 30. These authors demonstrated that treatment with zinc lactate increased the nisin-induced loss of intracellular ATP for both organisms. However, our observations are slightly different from those reported by Badaoui Najjar et al. [3], who demonstrated synergy between nisin and ε-poly-l-lysine against L. monocytogenes ScottA. Regardless, the results obtained in our experiments allow us to suggest that the inhibitory action of subtilosin is potentially increased by its interaction with curcumin, poly-lysine, or zinc lactate.
In conclusion, subtilosin inhibits both nisin-sensitive and nisin-resistant strains of L. monocytogenes. Subtilosin partially synergizes with encapsulated curcumin and zinc lactate against L. monocytogenes ScottA but acts additively with pure curcumin and poly-lysine against L. monocytogenes NR30. Encapsulated curcumin has more antibacterial activity than non-encapsulated, especially when combined with subtilosin, and could be a suitable candidate for further investigations in a food matrix. It should still be experimentally confirmed whether the higher activity of encapsulated curcumin is entirely due to the improved solubility and deliverability of the antimicrobial or if the matrix significantly contributes to the enhanced antimicrobial effect. Further studies should be focused on the molecular mechanisms of interaction between two antimicrobials in order to clarify how one antimicrobial favors the action of another on a bacterial cell. The proper use of these antimicrobial combinations could prevent the emergence of bacteriocin-resistant bacteria, generated by the continuous use of only a single antimicrobial substance. Our observations lead us to suggest that subtilosin could be used as alternative bacteriocin to nisin, providing an opportunity to use a novel and efficacious biopreservative against L. monocytogenes strains in food products. This is the first report concerning the synergistic effects of subtilosin in combination with a natural antimicrobial against L. monocytogenes, particularly the nisin-resistant mutant. Future biopreservative applications of this novel bacteriocin strongly depend on an assessment of its antibacterial activity when combined with the previously mentioned antimicrobials in food matrices.
References
Anonymous, CFSAN/Office of Food Additive Safety (2004) GRAS. Notice No. GRN 000135
Anonymous, FDA. Federal Register (1988) Nisin preparation: affirmation of GRAS status as a direct human food ingredient. 21 CFR Part 184, Fed Regist 53:11247–11251
Badaoui Najjar M, Kashtanov D, Chikindas ML (2007) ε-Poly-l-lysine and nisin A act synergistically against Gram-positive food-borne pathogens Bacillus cereus and Listeria monocytogenes. Lett Appl Microbiol 45:13–18
Branen JK, Davidson PM (2004) Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int J Food Microbiol 90:63–74
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Cristani M, D’arrigo M, Mandalari G, Castelli F, Sarpietro MG, Micieli D, Venuti V, Bisignano G, Saija A, Trombetta D (2007) Interaction of four monoterpenes contained in essential oils with model membranes: implications for their antibacterial activity. J Agric Food Chem 55:6300–6308
Deegan LH, Cotter PD, Hill C, Ross P (2006) Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int Dairy J 16:1058–1071
Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Lori V, Maino M, Comparato G, Fanigliulo L, Morana E, Pilotto A, Martelli L, Martelli M, Leandro G, Franze A (2007) A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter 12:238–243
Farber JM, Peterkin PI (1991) Listeria monocytogenes, a foodborne pathogen. Microbiol Rev 55:476–511
Gandhi M, Chikindas ML (2007) Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol 113:1–15
Gill AO, Holley RA (2006) Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol 108:1–9
Hall MJ, Middleton RF, Westmacott D (1983) The fractional inhibitory concentration (FIC) index as a measure of synergy. J Antimicrob Chemother 11:427–433
Humphrey AM (1980) Chlorophyll. Food Chem 5:57
Hung WC, Chen FY, Lee CC, Sun Y, Lee MT, Huang HW (2008) Membrane-thinning effect of curcumin. Biophys J 94:4331–4338
ICMFS (2002) Listeria monocytogenes in cooked sausage (frankfurters). In: ICMFS (ed) Microorganisms in foods, microbiological testing in food safety management. Kluwer, New York, pp 285–312
JECFA (2004) Curcumin chemical and technical assessment (CTA). Sixty-first report of the Joint FAO/WHO Expert Committee on Food Additives, Technical Report Series, WHO, Geneva, Switzerland. Available at: ftp://ftp.fao.org/es/esn/jecfa/cta/CTA_61_Curcumin.pdf
Kawulka KE, Sprules T, Diaper CM, Whittal RM, McKay RT, Mercier P, Zuber P, Vederas JC (2004) Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to α-carbon cross-links: formation and reduction of α-thio-α-amino acid derivatives. Biochemistry 43:3385–3395
Koga T, Inoue H, Ishii C, Okazaki Y, Domon H, Utsui Y (2000) Effect of plaunotol in combination with clarithromycin or amoxycillin on Helicobacter pylori in vitro and in vivo. J Antimicrob Chemother 50:133–136
Kotzekidou P, Giannakidis P, Boulamatsis A (2008) Antimicrobial activity of some plant extracts and essential oils against foodborne pathogens in vitro and on the fate of inoculated pathogens in chocolate. Food Sci Technol 41:119–127
Kumar S, Narain U, Tripathi S, Misra K (2001) Synthesis of curcumin bioconjugates and study of their antibacterial activities against beta-lactamase-producing microorganisms. Bioconjug Chem 12:464–469
Kumar KA, Mazumdar K, Dutta NK, Karak P, Dastidar SG, Ray R (2004) Evaluation of synergism between the aminoglycoside antibiotic streptomycin and the cardiovascular agent amlodipine. Biol Pharm Bull 27:1116–1120
Lin YT, Labbe RG, Shetty K (2004) Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl Environ Microbiol 70:5672–5678
Mahady GB, Pendland SL, Yun G, Lu ZZ (2002) Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res 22:4179–4181
McEntire JC, Montville TJ, Chikindas ML (2003) Synergy between nisin and select lactates against Listeria monocytogenes is due to the metal cations. J Food Prot 66:1631–1636
Naghmouchi K, Drider D, Baah J, Teather R (2010) Nisin A and polymyxin B as synergistic inhibitors of Gram-positive and Gram-negative bacteria. Probiotics Antimicrob Prot. doi: 10.1007/s12602-009-9033-8
Nykanen A, Weckman K, Lapvetelainen A (2000) Synergistic inhibition of Listeria monocytogenes on cold-smoked rainbow trout by nisin and sodium lactate. Int J Food Microbiol 61:63–72
Odds FC (2003) Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1
Rai D, Singh JK, Roy N, Panda D (2008) Curcumin inhibits FtsZ assembly: an attractive mechanism for its antibacterial activity. Biochem J 410:147–155
Sandasi M, Leonard CM, Viljoen AM (2008) The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 19:1070–1075
Shelburne CE, An FY, Dholpe V, Ramamoorthy A, Lopatin DE, Lantz MS (2007) The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J Antimicrob Chemother 9:1–4
Shishodia S, Sethi G, Aggarwal BB (2005) Curcumin: getting back to the roots. Ann N Y Acad Sci 1056:206–217
Sikkema J, de Bont JA, Poolmann B (1994) Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269:8022–8028
Sofos JN, Beuchat LR, Davidson PM, Johnson EA (1998) Naturally occurring antimicrobials in food. Regul Toxicol Pharmacol 28:71–72
Sutyak KE, Wirawan RE, Aroutcheva AA, Chikindas ML (2007) Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J Appl Microbiol 104:1067–1074
Tajbakhsh S, Mohammadi K, Deilami I, Zandi K, Fouladvand M, Ramedani E, Asayesh G (2008) Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr J Biotechnol 7:3832–3835
Thennarasu S, Lee DK, Poon A, Kawulka KE, Vederas JC, Ramamoorthy A (2005) Membrane permeabilization, orientation, and antimicrobial mechanism of subtilosin A. Chem Phys Lipids 137:38–51
Tønnesen HH (2002) Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Pharmazie 57:820–824
Turina AV, Nolan MV, Zygadlo JA, Perillo MA (2006) Natural terpenes: self-assembly and membrane partitioning. Biophys Chem 122:101–113
van Vuuren SF, Viljoen AM (2007) Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Frag J 22:540–544
Veldhuizen EJA, Tjeerdsma-van Bokhoven JL, Zweijtzer C, Burt SA, Haagsman HP (2006) Structural requirements for the antimicrobial activity of carvacrol. J Agric Food Chem 54:1874–1879
Wang XY, Jiang Y, Wang YW, Huang MT, Ho CT, Huang QR (2008) Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem 108:419–424
Wiedemann I, Breukink E, van Kraaij C, Kuipers OP, Bierbaum G, de Kruijff B, Sahl HG (2001) Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J Biol Chem 276:1772–1779
Zheng G, Slavik MF (1999) Isolation, partial purification and characterization of a bacteriocin produced by a newly isolated Bacillus subtilis strain. Lett Appl Microbiol 28:363–367
Acknowledgments
The authors thank Dr. T. M. Montville (Food Science Department, Rutgers University, NJ) for his contributions to the discussed research. T. Amrouche is a recipient of a grant from the Fulbright Fellowships program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tahar Amrouche and Katia Sutyak Noll contributed equally to the work within this manuscript.
Rights and permissions
About this article
Cite this article
Amrouche, T., Sutyak Noll, K., Wang, Y. et al. Antibacterial Activity of Subtilosin Alone and Combined with Curcumin, Poly-Lysine and Zinc Lactate Against Listeria monocytogenes Strains. Probiotics & Antimicro. Prot. 2, 250–257 (2010). https://doi.org/10.1007/s12602-010-9042-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-010-9042-7