Abstract
Oxidative stress is one of the major causes of degenerative conditions occurring at cellular level with serious health implications. This study was aimed at investigating the antioxidative potentials of probiotic lactobacilli of Indian gut origin and their ability to augment antioxidant defense enzyme systems in the host cells under oxidative stress conditions. A total of 39 Lactobacillus cultures were assessed for their resistance against reactive oxygen species. Most of the cultures were moderately to strongly resistant towards 0.4 mM H2O2. The Lactobacillus isolate CH4 was the most H2O2 resistant culture with only 0.06 log cycle reduction. Majority of the cultures demonstrated high resistance towards hydroxyl ions and Lp21 was the most resistant with log count reduction of 0.20 fold only. Almost all the cultures were also quite resistant to superoxide anions. Lp21 also showed the highest superoxide dismutase content (0.8971 U). Amongst the 39 cultures, Lactobacillus spp. S3 showed the highest total antioxidative activity of 77.85 ± 0.13 % followed by Lp55 (56.1 ± 1.2 %) in terms of per cent inhibition of linolenic acid oxidation. Lp9 up-regulated the expression of superoxide dismutase 2 gene in HT-29 cells both at 0.1 mM (1.997 folds) and 1.0 mM H2O2 (2.058 folds) concentrations. In case of glutathione peroxidase-1, Lp9, Lp91 and Lp55 showed significant (P < 0.001) up-regulation in the gene expression to the level of 5.451, 8.706 and 10.083 folds, respectively when HT-29 was challenged with 0.1 mM H2O2. The expression of catalase gene was also significantly up-regulated by all the cultures at 0.1 mM H2O2 conditions. It can be concluded that the antioxidative efficacy of the putative probiotic lactobacilli varied considerably between species and strains and the potential strains can be explored as prospective antioxidants to manage oxidative stress induced diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological oxidation is a natural and controlled process in our body essential to many biological processes. One of the paradoxes of life is that the oxygen that sustains aerobic life is not only fundamentally essential molecule indispensable for energy metabolism and respiration, but has also been implicated in many diseases and degenerative conditions through excessive generation of free radicals [1]. These radicals are highly unstable and react rapidly with other groups or substances in the body, virtually with all the vital cell components leading to cell or tissue injury. Excessive free radical production can damage lipids, proteins and DNA besides compromising cell function leading to cell death by necrosis or apoptosis. Oxidative stress (OxS) is caused by an imbalance between the production of reactive oxygen species (ROS), or free radicals and antioxidant defenses, which through a series of events alter the normal cellular functions. High grade OxS has been implicated in the pathogenesis of several inflammatory gastro-intestinal metabolic disorders (inflammatory bowel disease), the vascular system (atherosclerosis, myocardial infarction, stroke, and vascular dysfunctionality), the nervous system (Alzheimer’s disease, Parkinson’s disease), the liver (ethanol damage, cirrhosis), the skin (several dermatoses), the pancreas (diabetes mellitus) and the eyes (age-related macular degeneration and retinopathy). All living organisms have inbuilt antioxidative defense systems to counter the injurious effects of excessive amounts of ROS and other kinds of free radicals produced in our body by different mechanisms. However, under certain situations, the antioxidant systems operating in our body may not be sufficient to prevent/suppress the damage attributed to excessive levels of oxidants (free radicals). In such situations, antioxidants from external sources can be used through oral route to protect affected human subjects against oxidative stress related diseases [2–5].
It has now been well recognized that some probiotic lactobacilli possess strong antioxidative activity, and are able to decrease the risk of accumulation of ROS during the ingestion of food [6, 7]. Since, currently there has been enormous interest in the antioxidative agents because of their purportive positive effects on human health, the quest for finding novel antioxidants continues. This attribute is now considered as a valuable functional property for selection of probiotic strains for application in health foods. Although, antioxidative potentials of probiotics are currently the focus of attention, the benefits of this important property have not been fully explored as yet. A few studies have been conducted with some established and proven strains of probiotic lactobacilli and bifidobacteria to harness their antioxidative potentials [4–7]. However, similar information on probiotic strains isolated from gut of Indian people is lacking. Hence, this study was primarily undertaken with the objective of assessing the antioxidative efficacy of putative probiotic strains of lactobacilli of Indian gut origin in HT-29 cells an established in vitro cell culture model so that they could eventually be explored as biotherapeutics in the management of oxidative stress induced diseases such as Inflammatory Bowel Disease (IBD), Crohn’s disease (CD) and Ulcerative colitis (UC).
Materials and methods
Bacterial cultures
A total of 39 cultures were used in this study, which included 30 putative probiotic Lactobacillus isolates from Indian gut maintained at Molecular Biology Unit, Dairy Microbiology Division, National Dairy Research Institute, Karnal and nine standard probiotic Lactobacillus reference strains procured from different sources as listed in Table 1. The identity and probiotic attributes of these cultures were established previously in our laboratory [8–10]. The cultures were propagated and maintained in de Mann Rogosa Sharpe (MRS, HiMedia, India) medium. Stock cultures were maintained as glycerol stocks at −70 °C. Prior to use, lactobacilli were sub-cultured three times in MRS medium at 37 °C for overnight (16–18 h). The cultures were examined periodically under the microscope after Gram staining to ensure their purity.
Resistance of lactobacilli towards reactive oxygen species
The effect of different reactive oxygen species namely hydrogen peroxide, hydroxyl ions and superoxide radicals on the viability of all the probiotic lactobacilli was determined as per the methods used by Kullisaar et al. [11].
Resistance to hydrogen peroxide
The test Lactobacillus cultures were grown overnight (16–18 h) in MRS broth at 37 °C and harvested by centrifugation at 10,000 rpm for 10 min at 5 °C. The cell pellet was washed twice in isotonic saline. The washed cells were resuspended in isotonic saline at the level of 109 cfu/mL and incubated with 0.4 mM hydrogen peroxide (Sigma, USA) at 37 °C. The aliquots were drawn at 60 min intervals and plated onto MRS Agar (HiMedia, India) followed by incubation at 37 °C for 48 h in 5 % CO2 incubator.
Resistance to hydroxyl radicals
The cell suspension of the test cultures was prepared in isotonic saline at the level of 109 cfu/mL as described previously. The respective cell suspensions were exposed to hydroxyl radicals which were generated via the Fenton reaction [12]. The hydroxyl radical generation was performed in the solution which contained terephthalic acid (1,4-benzenedicarboxylic acid, Sigma, USA) in phosphate buffer. This step was followed by addition of Lactobacillus cultures at the level of 109 cfu/mL along with CuSO4·5H2O. The reaction was initiated by the addition of 1 mM H2O2. Aliquots were drawn at 15 min time intervals and the number of viable cells was estimated in each sample as cfu on MRS agar plates after incubation at 37 °C for 48 h in 5 % CO2 incubator.
Resistance to superoxide anions
Resistance of lactobacilli to superoxide anions induced by paraquat (Sigma, USA) was determined using the diffusion assay procedure [13]. An aliquot of 0.1 mL of the standardized cell suspension containing 109 cfu/mL was spread onto MRS agar plate containing 0.5 % glucose and allowed to dry. Paper disk spotted with an aliquot of 10 μL each of 5, 10, 20, 50 and 100 mM paraquat (Sigma, USA) was placed in the centre of the agar plate and incubated overnight at 37 °C. The zone of growth inhibition (mm) was measured.
Superoxide dismutase activity
The superoxide dismutase (SOD) activity in the test cultures was measured by using commercially available reagent kit (Fluka, Sigma Aldrich, Switzerland). SOD Assay Kit-WST allows very convenient SOD assaying by utilizing Dojindo’s highly water soluble tetrazolium salt, WST-1(2-(4-iodophenyl)-3(4-nitrophenyl)-5-(2,4-disulfophenyl)2H-tetrazolium,monosodium salt) that produces a water soluble formazan dye upon reaction with a superoxide anion. Inhibition of this reaction by sample indicates its superoxide dismutase activity and can be determined by a colorimetric method. Lactobacillus cultures were inoculated at 1 % in MRS broth and incubated at 37 °C in carbon dioxide incubator (5 % CO2) for 16–18 h. The bacterial cells were harvested by centrifugation at 10,000 rpm for 10 min at 4 °C. The harvested cells were washed twice in phosphate buffered saline (PBS) and resuspended in the same buffer. The cell suspension was then sonicated (Vibracell; Sonics and Materials, Danbury, Conn) for 5 min by using 40 % amplitude with constant cooling. The bacterial lysates were centrifuged at 10,000 rpm for 10 min at 4 °C and phenylmethane sulphonyl fluoride (PMSF) at 1 mM was added to the supernatant and stored at −20 °C until further use. After the incubation of reaction mixture with the sample (lactobacilli lysates) at 37 °C for 20 min, the absorbance was measured at 450 nm. The SOD activity was quantified by measuring the decrease in colour development at 450 nm. One SOD enzyme unit is defined as the amount of protein that produces a 50 % inhibition of the nitrite formation under the assay conditions.
Total antioxidative activity (TAA)
The total antioxidative activity (TAA) of the test cultures was determined using linolenic acid test (LA test) as per Kullisaar et al. [11]. This test evaluates the ability of the sample to inhibit linolenic acid (Sigma, USA) oxidation [14, 15]. Initially, the cell lysates were prepared by sonicating the standardized cell suspension of 109 cfu/mL (Vibracell; Sonics and Materials, Danbury, Conn) of each test culture for 5 min by using 40 % amplitude with constant cooling. The standard solution of linolenic acid was prepared in 96 % ethanol (1:100). The working solution was prepared by appropriately diluting the linolenic acid standard. For setting up the reaction, an aliquot of 0.01 % sodium dodecyl sulphate (lauryl sulfate, HiMedia, India) and the sample (0.045 mL of lactobacilli cell lysates) was added to 0.4 mL working solution of linolenic acid. The reaction was started by adding FeSO4·7H2O (Himedia, India) and the mixture was incubated at 37 °C for 60 min. The reaction was then stopped by addition of Butylated hydroxyl toluene (BHT, Sigma) followed by treatment with 0.5 mL of acetate buffer (pH 3.5) consisting of glacial acetic acid and sodium acetate trihydrate (HiMedia, India) and subsequently heated with freshly prepared thiobarbituric acid solution (TBA) (Sigma, USA) at 80 °C for 40 min. After cooling, the mixture was acidified by adding 5 M HCl, extracted with 1-butanol and centrifuged at 3,000×g for 10 min followed by measurement of absorbance of butanol fraction. The TAA of the sample was expressed as the inhibition by sample of LA-standard peroxidation as follows: [1 − (A534 (sample)/A534 (LA as control)] × 100. The higher numerical value (%) of TAA indicates the higher TAA of the sample. Peroxidation of LA-standard in the isotonic saline (without samples) served as a control.
Propagation and maintenance of cell lines
The human adenocarcinoma epithelial cell line HT-29 (mucus secreting) was cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM; Sigma, USA) supplemented with 10 % (v/v) heat-inactivated (30 min, 56 °C) fetal bovine serum (Sigma, USA), 25 mM HEPES (Sigma, USA), 100 U/mL penicillin (Sigma, USA), and 100 μg/mL streptomycin (Sigma, USA) in 25 cm2 culture flask (Falcon, Beckton Dickinson, Sparks, MD) at 37 °C in an atmosphere of 5 % CO2/95 % air. The cultures were fed with fresh medium every alternate day and trypsinized using 0.25 % Trypsin–EDTA solution (Sigma, USA) at 37 °C. The cell suspension with 1 × 105 cells prepared in 4 mL complete DMEM medium was transferred to each well of six-well tissue culture plates (Falcon 6-well, Beckton Dickinson, Sparks, MD, USA). The medium was changed every alternate day. When cells reached 80 % confluency, the spent medium was completely removed twenty-four hours prior to challenge study and cells were fed with DMEM medium lacking antibiotics.
Challenge of HT-29 cells with probiotic and hydrogen peroxide
The cell line was incubated with probiotic culture (1 × 109 cfu/mL) at 37 °C, 5 % CO2 for 4 h. After incubation period, it was challenged with H2O2 (Sigma) either at 0.1 mM or 1.0 mM H2O2 and incubated for 1 h/30 min, respectively.
Extraction of RNA
After the incubation of both treated and untreated samples, the growth medium was removed and trypsinized with 0.25 % Trypsin–EDTA solution (Sigma, USA) and cells were pelleted by centrifugation at 1,700 rpm for 4 min. The total RNA was isolated by TRI Reagent (Sigma, USA). RNA was suspended in 50 μL of DEPC treated/RNase free water and stored at −80 °C until further use. Purity of the total RNA extracted was determined spectrophotometrically based on 260/280 nm ratio and the integrity of RNA was checked by electrophoresing on 1 % agarose gel. Residual DNA was removed by treating RNA with RNase free DNaseI as per the instructions furnished by the manufacturer (Promega, USA). RNA was stored at −80 °C until further use. Nucleic acid concentration was measured at 260 nm and 1 μg of the DNase treated RNA was transcribed into cDNA, using ImPromII reverse transcriptase kit (Promega, USA) and random primers following the protocol as per the manufacturer’s instructions. Untreated HT-29 cells grown in DMEM served as control.
LightCycler quantitative real-time PCR
Real-time PCR reaction was performed in a LightCycler 480 instrument (Roche), with Relative Quantification Software (version LCS480 1.5.0.39, Roche) using fluorescence signal detection (SYBR Green) after each amplification cycle. Each reaction was performed in a 10 μL reaction volume containing 5 μL of 2× SYBR Green PCR Master Mix (Roche), 2.5 μL of properly diluted cDNA (typically 30 ng/μL of cDNA for all the genes targeted for RT-qPCR), 0.5 μL of each primer at 10 μM and 1.5 μL of nuclease-free water. Negative controls (with no DNA template, only primer pair, water and 2× SYBR Green PCR Master Mix) for each primer set were included in each run. The primers used for the target genes along with the size of respective amplicons were SOD2 F: 5′-gcc ctg gaa cct cac atc aac-3′ and SOD2 R: 5′-caa cgc ctc ctg gta ctt ctc-3′, 115 bp [16]; GPx-1 F: 5′-ccg acc cca agc tca tca cc-3′ and GPx-1 R: 5′-gat gtc agg ctc gat gtc aat gg-3′, 150 bp [17]; CAT F: 5′-tgg aca agt aca atg ctg ag-3′ and CAT R: 5′-tta cac gga tga acg cta ag-3′, 155 bp [16] and for the reference gene B-ACTIN used as internal control for normalization were B-ACTIN F: 5′-tgg ctg ggg tgt tga agg tct-3′ and B-ACTIN R: 5′-agc acg gca tcg tca cca act-3′, 238 bp [18].
The thermal cycling conditions for SOD2 and CAT included: initial denaturation (95 °C for 5 min), followed by amplification and quantification program of 35 cycles (30 s at 95 °C, 45 s at 57 °C and 45 s at 72 °C with a single fluorescence measurement), melt curve program (60 to 95 °C with a heating rate of 0.11 °C s−1 and a continuous fluorescence measurement) and finally a cooling step to 40 °C. While for GPx-1, amplification was 40 cycles (30 s at 95 °C, 1 min at 60 °C and 1 min at 72 °C with a single fluorescence measurement). Measurement of gene expression with regard to each RNA extractions were obtained in triplicate, and the mean of these values were used for further analysis.
Data analysis of relative target gene expression
Generation of quantitative data by RT-qPCR is based on the number of cycles required for optimal amplification generated fluorescence to reach a specific threshold of detection (the quantification cycle; Cq value) [19]. Real-time PCR amplification efficiencies were determined for each set of primers with the slope of a linear regression model [20]. The cDNA samples were diluted in a range of 100, 50, 25, 5, 1, and 0.25 ng and used as RT-qPCR templates. The standard curves were generated by plotting the log cDNA values against Cq values obtained over the range of dilutions. The slope of the curves was used to determine the reaction efficiency (E) as E = 10[−1/slope] [21]. Randomization test method, as a part of the REST software, was used to assess statistical significance of up- or down-regulation of the target genes after normalization to the reference gene. For up-regulation, the factor of regulation is equal to the given value in the Randomization Data Output Box. While for down-regulation, the regulation factor is illustrated as a reciprocal values (1/expression ratio) [22]. Randomization test randomly reallocates the observed values in different sample groups to the values in the control group. Relative expression is then estimated for each of the new groups and the effect of randomization in each group is evaluated. If this effect is not significant, it provides sufficient evidence that the observed treatment effect was not due to the random allocation. Statistical analysis was considered significant at P ≤ 0.05. Data that emerged from this study has been presented in the form of whisker-box plots. The top and bottom of each box represent the 25th- to 75th-percentile values while the 50th percentile (median) is the horizontal dotted line in the rectangle. The vertical bars represent the minimum and maximum values that are not considered outliers.
Results and discussion
Resistance to reactive oxygen species (ROS)
Hydrogen peroxide
Most of the Lactobacillus cultures under investigation in this study exhibited a moderate to strong resistance towards H2O2 at 0.4 mM concentration. The reduction in log cycles of the Lactobacillus cultures exposed to 0.4 mM H2O2 ranged from 0.06 to 5.09 (data not shown). Amongst the indigenous Lactobacillus isolates, CH4 was the most resistant since it survived maximally at the physiological concentration of H2O2 as can be reflected from the lowest log cycle reduction (0.06 fold). It was followed by Lp43, Lp58 and Lp77 with corresponding log cycle reduction values of 0.24, 0.33 and 0.59 only (Fig. 1). However, the least resistant culture was S1 showing the highest reduction in log counts (5.09 folds). Based on per cent survival, the most H2O2 resistant reference probiotic Lactobacillus strain was LA1-2407 (0.13 fold reduction in log counts) followed by Lj 5273 (0.46 fold reduction) and L. casei Shirota (0.89 fold reduction). Almost all the reference probiotic strains used in the study demonstrated high H2O2 resistance. The comparative appraisal of the results clearly suggest that in terms of H2O2 resistance, the indigenous Lactobacillus isolates performed either equally or even better than the reference strains. Our results with regard to H2O2 resistance of Lactobacillus isolates are consistent with those of Kullisaar et al. and Lee et al. [11, 23] who also demonstrated high H2O2 resistance in their respective probiotic Lactobacillus strains both at 0.4 and 1 mM concentrations, respectively.
Resistance to hydroxyl radicals
Free hydroxyl radicals constitute another type of reactive oxygen species implicated in several pathological conditions. These free hydroxyl radicals are extremely reactive by either abstracting hydrogen from or adding hydroxyl radicals to biological molecules. Hydroxyl radicals can thus damage virtually all types of macro molecules viz. carbohydrates, nucleic acids, proteins, lipids and amino acids. All the Lactobacillus cultures included in the study were also subjected to 1 mM hydroxyl radicals generated via the Fenton reaction [12] for assessing their resistance towards these reactive oxygen species. There was a wide variation in the resistance of different Lactobacillus strains. The most resistant Lactobacillus culture towards hydroxyl ions was Lp21 and Lp52 was the most susceptible as can be evidenced from their corresponding log cycle reductions in viable counts i.e. 0.20 (Lp21) and 2.99 (Lp52), respectively (Fig. 2). Similarly, amongst the reference probiotic Lactobacillus strains, L. casei Shirota and La14962 were the most resistant towards hydroxyl ions with respective log cycle decline at 0.45 and 0.456 folds only, closely followed by LGG and LaNCFM with log cycle reduction values of 0.51 and 0.70, respectively. On an average, 80–90 % of the test Lactobacillus cultures remained viable even after 1 h of treatment. Our results in this regard are slightly at variance from those recorded by Kullisaar et al. [11] who reported that two of the highly antioxidative strains of L. fermentum E-3 and E-18 used in their study could survive for only 34 min at 1 mM hydroxyl radicals in comparison to 60 min survival recorded for some of the indigenous probiotic strains in our study at the same concentration. However, our findings are in agreement with those of Kim et al. [24], who also demonstrated high resistance of their test probiotic strains towards hydroxyl radicals. Lee et al. [23], on the other hand, had reported that L. casei KCTC3260 remained viable in presence of 0.4 mM hydroxyl radicals even after 7 h which was more than that of L. rhamnosus GG. However, 50 % of L. casei KCTC3108 and L. casei 01 strain had no viability after 4 h treatment, thereby, suggesting that the viability or survival of lactobacilli may be strain dependent and upon the dose of the hydroxyl radicals. Apart from the antioxidative enzymes, the only non enzymatic mechanism to protect important cellular structures from the damaging effect of these radicals is the glutathione system [11, 24].
Resistance to superoxide anions
Since, superoxide anions are the other key ROS which can also cause damage to biological molecules such as DNA, lipids and proteins through oxidative stress [25], we also assessed the resistance of our probiotic Lactobacillus strains against superoxide anions. Superoxide dependent growth inhibition was not discernible with any of the cultures tested in the study indicating their resistance towards the superoxide anions at 10 mM paraquat concentration. However, when the concentration of paraquat was increased to 20 and 50 mM, distinct zone of inhibition was recorded with only one isolate i.e. S3 suggesting that the same was sensitive to superoxide anions at higher paraquat concentration. Our results are in accordance with those of Kullisaar et al. [11], who too could not establish superoxide dependent growth inhibition with any of the four cultures namely L. fermentum E-3, E-18, E 338-1 thereby, suggesting the resistance of these strains to superoxide anions. Lee et al. [23], on the other hand, found mixed results as two of their Lactobacillus cultures viz. Lactobacillus rhamnosus GG and L. casei KCTC 3260 were resistant to 10 mM paraquat while the others viz. L. casei 01 and L. casei KCTC 3109 sensitive to the same concentration which further suggest resistance towards superoxide anions was highly strain specific. The possible mechanism of resistance of Lactobacillus strains to superoxide anions reported in our study and the aforesaid reports could be attributed to their endogenous superoxide dismutase activities which can protect these cells from the damaging effects of free superoxide anions by scavenging them.
Superoxide dismutase activity (SOD)
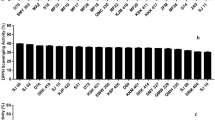
The data on SOD activity of all the probiotic lactobacilli under investigation recorded in Table 2 indicate that the level of SOD in the test cultures ranged from 0.0242 to 0.8971 U. Out of all the 39 cultures examined, Lp21 produced the highest superoxide dismutase content (0.8971 U) closely followed by Lp43 (0.8945 U). In comparison to indigenous Lactobacillus isolates, SOD activity of the reference probiotic strains was almost negligible and ranged from 0.1259 to 0.5981 U with L. plantarum CSCC5276 showing as much as 0.5981 U of the enzyme activity.
Nearly all the cells and cellular organisms use SOD to eliminate superoxide. However, one of the notable exceptions, is L. plantarum and other related lactobacilli. These bacteria use a different mechanism for eliminating superoxide. But nevertheless, a few previous reports do show that L. plantarum and related lactobacilli also contain SOD [26, 27]. The lack of endogenous SODs and catalase may account for the high sensitivity of most species of Lactobacillus to oxidative stress. However, L. plantarum has developed an alternative non-enzymatic defense system that involves the accumulation of high intracellular concentrations of manganese ions, which can scavenge O2 −. In our study, a positive correlation between the concentration of SOD in an organism and its tolerance to oxygen free radicals was demonstrated as reported previously [28]. However, Gregory and Fridovich [29] reported that L. plantarum lacked SOD although it was further reported that when grown aerobically on glucose-containing medium, both the catalase-positive (T-1403-5) and a catalase negative strain (8014) of L. plantarum possessed low but readily detectable levels of superoxide dismutase. The results obtained in our study with regard to SOD activity of the test probiotic lactobacilli are consistent with similar findings on SOD activity in the cell free extracts of lactobacilli as reported previously [11, 30]. On comparative evaluation, it is quite evident that some of our indigenous Lactobacillus isolates namely Lp21, Lp43 and Lp57 expressed relatively higher superoxide dismutase activity than that recorded with the reference cultures, thereby, clearly indicating their versatility as possible biotherapeutics in the management of oxidative stress induced diseases.
Total antioxidative activity (TAA)
Inhibition of lipid peroxidation is used as a biomarker for assessing the total antioxidative activity of bioactive ingredients including probiotics [31]. In our study, we used TBA assay exploring the linolenic acid induced inhibition of lipid peroxidation as the basis for determining the per cent TAA activity of our test cultures. The total antioxidative activity of the Lactobacillus cultures in their respective cell lysates was determined in terms of per cent inhibition of the peroxidation of linolenic acid. The high numerical per cent value (%) indicates the high total antioxidative activity of the sample. Amongst the 39 cultures, Lactobacillus spp. S3 showed the highest per cent TAA value i.e. 77.85 ± 0.13 followed by Lp55 (56.1 ± 1.2), Lp91 (48.39 ± 1.42) and Lp78 (42.02 ± 1.1) as compared to the reference cultures which showed relatively lower TAA values ranging from 4.5 ± 0.28 (La1 2407) to 43.8 ± 1.97 (LaNCFM), thereby, indicating that indigenous isolates showed better antioxidative potential (Table 3). On comparative evaluation, Lp10 was the least antioxidative culture as it could express only 1.3 ± 1.3 % TAA. However, TAA of most of the cultures in the present study ranged from 20 to 40 %. Our results in this regard are in agreement with the earlier findings of Kullisaar et al. [11] and Ou et al. [32] as well as by several other investigators [23, 33, 34]. However, our results are slightly at variance with those of Hutt et al. [35], who recorded relatively much lower per cent TAA values (11–24 %) associated with some of the common reference strains of probiotics. However, the variation in TAA of probiotic cultures may also be strain specific as is the case with many of other probiotic properties.
Expression of antioxidative enzyme systems by reverse transcription-qPCR (RT-qPCR)
Five potential putative probiotic indigenous lactobacilli namely Lp9, Lp91, Lp55, CH4 and S3 demonstrating moderately to high resistance to reactive oxygen species, SOD activity and TAA along with three standard probiotic strains CSCC5276, LGG and LaNCFM, were selected for further studies to establish their antioxidative efficacy in HT-29 cells under oxidative stress conditions. The expression of genes encoding antioxidative enzymes in the cell line model pre-challenged with probiotic strains before subjecting to oxidative stress with H2O2 (0.1 and 1 mM) was analysed by reverse transcription-qPCR (RT-qPCR). Specificity of the primers for the target genes along with their RT-qPCR reaction efficiencies were calculated (Figs. 1S, 2S) with higher linearity (Pearson correlation coefficient r > 0.998).
Relative expression of superoxide dismutase (SOD2)
When HT-29 cells were subjected to H2O2 alone at 0.1 mM, there was a significant (P < 0.001) up-regulation in the expression of SOD2 by 1.540 fold (Table 4; Fig. 3S). However, the level of expression (−1.194 fold) was non significant at 1.0 mM H2O2 concentration. Amongst the probiotic strains used in the study, Lp9, CSCC5276 and LaNCFM up regulated the SOD2 significantly (P < 0.001) by 1.997, 1.556 and 1.545 folds, respectively at 0.1 mM H2O2 for 1 h. The level of expression was further increased in the host cells at 1 mM H2O2 by 2.058 and 2.181 folds with Lp9 and CSCC5276, respectively. Apart from this, CH4, LGG, Lp91 and S3 also up regulated the gene expression at significant level by 2.273, 1.949, 1.826 and 1.746 folds, respectively under the same set of conditions. Lp55 on the other hand did not evoke any significant effect on SOD2 expression under both the conditions of oxidative stress indicating the high variability between the probiotic strains with regard to SOD expression. Although, our findings in this regard can not be substantiated because of lack of any published reports on similar lines under in vitro conditions, similar observations made by some investigators under in vivo conditions using animal models by supplementation of probiotics as such or through oral feeding of probiotic formulations [36] and dahi [37] could be adequate to substantiate our results. However, the outcome of these studies could not be replicated in two clinical studies involving young healthy women as the subject [38, 39], thereby, necessitating the need for pursuing more in vitro studies with proven cell line models for direct validation of our results.
Relative expression of glutathione peroxidase-1 (GPx-1)
The expression of GPx-1 in HT-29 cells subjected to 0.1 mM H2O2 treatment alone for 1 h was significantly (P < 0.001) up regulated by 1.632 fold whereas at 1.0 mM H2O2 treatment for 30 min, there was 2.088 folds increase in the expression (Table 4; Fig. 3S) which however was not significant. It was interesting to note that virtually all the probiotic strains used in the study significantly (P < 0.001) up regulated GPx-1 expression in HT-29 cells at 0.1 mM H2O2. The relative expression of GPx-1 in HT-29 cells was up regulated significantly by LaNCFM, Lp55, Lp91 and LGG under this condition by 11.560, 10.083, 8.706 and 6.336 folds, respectively. However, the corresponding figures obtained at 1.0 mM H2O2 decreased to 4.489, 3.969, 4.856 and 4.353 folds, respectively and were still significant. A comparative appraisal of our results indicate that both the indigenous probiotic lactobacilli and the reference strains demonstrated up regulation of GPx-1 expression in HT-29 cells at both the concentrations of H2O2, although this ability was highly strain specific. Since, there are no direct published reports on the role of probiotics in inducing expression of antioxidant enzymes in vitro cell culture models under oxidative stress, the findings of our study in this regard can not be substantiated, However, the outcome of in vivo studies conducted in this regard clearly demonstrated the efficacy of probiotics in triggering the expression of antioxidant enzymes including GPx-1 under oxidative stress. In one such study, D’Souza et al. [36] reported that Glutathione peroxidase was up-regulated in children suffering from Nectrotizing Enterocolitis (NEC) when the patients were fed with probiotic Lactobacillus casei. Similarly, Yadav et al. [37], reported that feeding of probiotic dahi significantly increased the concentration of GSH and GPx in different tissues in streptozotocin (STZ) and high fructose diet (HFD) induced diabetes rats. However, our findings on GPx-1 expression in HT-29 cells under oxidative stress conditions are at variance with those of Fabian and Elmadfa [38] who reported that the mean activities of GSH-Px decreased significantly (P < 0.001) in the probiotic group after consumption of yoghurt daily for four weeks in healthy women.
Relative expression of catalase (CAT)
The antioxidative effect of probiotic lactobacilli with regard to induction of CAT gene expression in HT-29 cells under oxidative stress with H2O2 was also investigated in this study (Table 4; Fig. 3S). Treatment of HT-29 cells with H2O2 led to significant (P < 0.001) up-regulation of CAT gene at both 0.1 mM (1.511 fold) and 1.0 mM (1.597 fold). All the probiotic strains, the subject of this study were able to up regulate the expression of CAT in HT-29 cells at significant levels at 0.1 mM H2O2. The fold increase in the level of expression brought about with Lp55, Lp9, Lp91, CSCC5276 and LaNCFM were 3.785, 3.717, 3.660, 3.694 and 3.391, respectively. However, the up regulation of gene expression was considerably decreased at 1 mM H2O2. Higher levels of catalase indicate that oxidative stress can be overcome as catalase improves detoxification of hydrogen peroxide. Such changes could reduce damaging effects by oxidants and protect cells from oxidative damage. Therefore, a high level of expression could be associated with less genetic damage due to the exposures that involve colorectal cancer risk. However, the results on the expression of CAT gene cannot be substantiated due to lack of literature on in vitro expression of CAT gene modulated by probiotic strains under oxidative stress conditions.
Hence, it can be concluded from this study that many of the indigenous probiotic Lactobacillus cultures demonstrated strong antioxidative properties by resisting the toxic effects of different reactive oxygen species and expressed higher per cent TAA values (inhibition of lipid peroxidation) and SOD activity. However, the antioxidative effects with regard to scavenging different free radicals varied considerably between different species and strains. The individual antioxidative activities exhibited may not be expressed at the same level in all the probiotic strains. Nevertheless, some of the indigenous putative probiotic Lactobacillus isolates particularly Lp21, Lp43, Lp55, Lp57, Lp9, Lp91 and CH4 were superior in their antioxidative functions in comparison to reference strains. It can be further inferred from our study that probiotics do enhance the level of antioxidant enzymes viz. SOD2, GPx-1 and CAT in HT-29 cells and the possible mechanism of the host resistance towards H2O2, hydroxyl ions and superoxide anions could be through mediation of the above endogenous/exogenous antioxidative enzymes which can confer resistance and protection to host cells towards these free oxygen radicals by scavenging them. The exogenously produced antioxidant enzymes produced by probiotic strains along with their lipid peroxidation inhibition activity (TAA) can further complement the protection of gut epithelial cells against oxidative stress. However, effects of dose response also need to be worked out critically under different levels of oxidative stress to optimally express the antioxidative efficacy of each probiotic strain. The shortlisted novel indigenous probiotic Lactobacillus isolates exhibiting high antioxidative efficacy could be the prospective candidate probiotics for pursuing more intensive in vivo studies to validate their antioxidative potentials in appropriate animal models and clinical studies. After establishing their in vivo efficacy, these novel probiotic strains can be explored in the management of chronic oxidative stress induced inflammatory gut diseases like IBD, CD and other metabolic disorders.
References
Marx JL (1985) Oxygen free radicals linked to many diseases. Science 235:529–531
Zommara M, Tachibana N, Sakono M et al (1996) Whey from cultured skim milk decrease serum cholesterol and increases antioxidant enzymes in liver and red blood cells in rats. Nutr Res 16:293–302
Oxman T, Shapira M, Diver A et al (2000) A new method of long-term preventive cardioprotection using Lactobacillus. Am J Physiol Heart Circ Physiol 278:H1717–H1724
Terahara M, Nishide S, Kaneko T (2000) Preventive effect of Lactobacillus delbrueckii subsp. bulgaricus on the oxidation of LDL. Biosci Biotechnol Biochem 64:1868–1873
Kullisaar T, Songisepp E, Mikelsaar M et al (2003) Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr 90:449–456
Kaizu H, Sasaki M, Nakajima H et al (1993) Effect of antioxidative lactic acid bacteria on rats fed a diet deficient in vitamin E. J Dairy Sci 76:2493–2499
Peuhkuri K, Lahteenmaki T, Sievi E et al (1996) Antioxidative properties of Lactobacillus GG measured as prostacyclin and nitric oxide production in endothelial cell culture. Nutr Today (Suppl) 31:53S–54S
Kaushik JK, Kumar A, Duary RK et al (2009) Functional and probiotic attributes of an indigenous isolate of Lactobacillus plantarum. PLoS One 4(12):e8099. doi:10.1371/journal.pone.0008099
Kumar R, Grover S, Batish VK (2011) Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague–Dawley rats. Br J Nutr 105:561–573. doi:10.1017/S0007114510003740
Kumar R, Grover S, Batish VK (2011) Molecular identification and typing of putative probiotic indigenous Lactobacillus plantarum strain Lp91 of human origin by specific primed-PCR assays. Probiotics Antimicrob Prot 3:186–193. doi:10.1007/s12602-011-9083-6
Kullisaar T, Zilmer M, Mikelsaar M et al (2002) Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol 72:215–224
Barreto JC, Smith GS, Strobel NH et al (1995) Terephthalic acid: a dosimeter for the detection of hydroxyl radicals in vitro. Life Sci 56(4):PL89–PL96
Bauer AW, Kirby WMM, Sherris JC et al (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Pahhkla R, Zilmer M, Kullisaar T et al (1998) Comparison of the antioxidant activity of melatonin and pinoline in vitro. J Pineal Res 24:96–101
Starkopf J, Zilmer K, Vihalemm T et al (1995) Time course study of oxidative stress during open heart surgery. Scand J Thorac Cardiovasc Surg 29:181–186
Sauer J, Richter KK, Pool-Zobel BL (2007) Physiological concentrations of butyrate favorably modulate genes of oxidative and metabolic stress in primary human colon cells. J Nutr Biochem 18:736–745
Vanhoutvin SALW, Troost FJ, Hamer HM et al (2009) Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One 4:e6759. doi:10.1371/journal.pone.0006759
Lallemant B, Evrard A, Combescure C et al (2009) Reference gene selection for head and neck squamous cell carcinoma gene expression studies. BMC Mol Biol 10:78. doi:10.1186/1471-2199-10-78
Bustin SA, Benes V, Garson JA et al (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. doi:10.1373/clinchem.2008.112797
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RTPCR. Nucleic Acids Res 29:e45
Rasmussen R (2001) Quantification on the LightCycler. In: Meuer S, Wittwer C, Nakagawara K (eds) Rapid cycle real-time PCR, methods and applications. Springer Press, Heidelberg, pp 21–34
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Lee J, Hwang KT, Chung MY et al (2005) Resistance of Lactobacillus casei KCTC 3260 to reactive oxygen species (ROS): role for a metal ion chelating effect. J Food Sci 70:M388–M391. doi:10.1111/j.1365-2621.2005.tb11524.x
Kim HS, Jeong SG, Ham JS et al (2006) Antioxidative and probiotic properties of Lactobacillus gasseri NLRI-312 isolated from Korean infant feces. Asian Aust J Anim Sci 19(9):1335–1341
De Freitas JM, Meneghini R (2001) Iron and its sensitive balance in the cell. Mutat Res 475:153–159
Gonzalez SN, Nadra-Chaud CA, Apella MC et al (1991) Evidence of superoxide dismutase in Lactobacillus acidophilus. Chem Pharm Bull 39:1065–1067
Yousten AA, Johnson JL, Salin M (1975) Oxygen metabolism of catalase-negative and catalase-positive strains of Lactobacillus plantarum. J Bacteriol 123:242–247
Tally FP, Goldin BR, Jacovus NV et al (1977) Superoxide dismutase in anaerobic bacteria of clinical significance. Infect Immun 16:20–25
Gregory EM, Fridovich L (1974) Oxygen metabolism in Lactobacillus plantarum. J Bacteriol 117:166–169
Gonzalez SN, Apella MC, Romero N et al (1989) SOD activity in some strains of lactobacilli: induction by manganese. Chem Pharm Bull 37:3026–3028
Rael LT, Thomas GW, Craun ML (2004) Lipid peroxidation and the thiobarbituric acid assay: standardization of the assay when using saturated and unsaturated fatty acids. J Biochem Mol Bio 37:749–752
Ou CC, Ko JL, Lin MY (2006) Antioxidative effects of intracellular extracts of yogurt bacteria on lipid peroxidation and intestine 407 cells. J Food Drug Anal 14(3):304–310
Lin MY, Yen CL (1999) Inhibition of lipid peroxidation by Lactobacillus acidophilus and Bifidobacterium longum. J Agric Food Chem 47:3661–3664
Zhang C, Li XY, Zhao L et al (2007) Lipopolysaccharide (LPS) upregulates the expression of heme oxygenase-1 in mouse placenta. Placenta 28:951–957
Hutt P, Shchepetova J, Loivukene K et al (2006) Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero and uropathogens. J Appl Microbiol 100:1324–1332
D’Souza A, Fordjour L, Ahmad A et al (2010) Effects of probiotics, prebiotics and synbiotics on messenger RNA expression of Caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula-fed neonatal rats. Ped Res 67:526–531
Yadav H, Jain S, Sinha PR (2008) Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res 75:189–195
Fabian E, Elmadfa I (2007) The effect of daily consumption of probiotic and conventional yoghurt on oxidant and anti-oxidant parameters in plasma of young healthy women. Int J Vitam Nutr Res 77:79–88
Chamari M, Djazayery A, Jalali M et al (2008) The effect of daily consumption of probiotic and conventional yogurt on some oxidative stress factors in plasma of young healthy women. ARYA Atheroscler J 4(4):175–179
Acknowledgments
We gratefully acknowledge the Director, National Dairy Research Institute (NDRI, Karnal, India) for providing facilities to carry out the study. The financial support received from National Dairy Research Institute (NDRI, India) and Indian Council of Agricultural Research (ICAR, India) in terms of providing fellowship to the first author of the paper to carry out her Master’s programme is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Achuthan, A.A., Duary, R.K., Madathil, A. et al. Antioxidative potential of lactobacilli isolated from the gut of Indian people. Mol Biol Rep 39, 7887–7897 (2012). https://doi.org/10.1007/s11033-012-1633-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1633-9