Abstract
In this study, the effects of cypermethrin on antioxidant status, oxidative stress biomarkers, behavior, and mortality in the freshwater mussel Unio elongatulus eucirrus were examined. Cypermethrin was applied at concentrations of 5, 10, 20, 40, 80, and 160 µg/L, for 1, 24, 48, 72, and 96 h. With increasing cypermethrin concentrations, the mussels showed significantly (P < 0.05) increased lipid peroxidation (MDA), which might be associated with decreased levels of reduced glutathione (GSH) and catalase (CAT) activity in the digestive glands and gills of the mussels. Negative correlations were observed between the MDA and the GSH and CAT levels after cypermethrin exposure, indicating a protective role of GSH and CAT against lipid peroxidation, and suggesting the use of these antioxidants as a potential biomarker of toxicity associated with contaminant exposure in freshwater mussels. In addition, at 5 µg/L, cypermethrin had no effect on the duration of the active and rest periods. All higher concentrations caused inhibition of the filtration activity by reducing the active periods and lengthening the rest periods. The active periods shortened as the cypermethrin concentrations increased, the reduction being 90% of the control at 160 µg/L. Rest periods were longest (237% longer than the control) at 160 µg/L. The 48, 72, and 96 h LC50 values of cypermethrin for mussels were determined as 96.50, 77.96, and 59.20 µg/L, respectively (P < 0.05). The results indicate that the insecticide cypermethrin has a harmful effect not only on nontarget aquatic arthropods and fish, but also on mollusks, although the sensitivity of mussels is less than that of fish. Being a general toxicant for aquatic life, cypermethrin should be used with great caution in agriculture to protect natural waters from contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increased use of synthetic pyrethroid pesticides is increasing worldwide pollution risks. One of the pyrethroids that has found wide acceptability is cypermethrin. It is extensively used in agriculture and forestry because of its high activity against a broad spectrum of insect pests [1, 2]. Pesticides applied to the land may be washed into surface waters and may kill or at least adversely affect the life of aquatic organisms [3, 4]. Synthetic pyrethroids have been found to be highly toxic to fish [5, 6], zooplankton communities [7], and some beneficial aquatic arthropods [8]. The environmental fate and effects of synthetic pyrethroid pesticides have been summarized by Hill [3]. According to environmental quality standards, the maximum allowable concentration is 1 ng/L [5].
The characterization of the quality of freshwater systems requires specially designed biological methods for assessing the health status of biota. Biomarkers are known to be useful tools for measuring environmental health [9]. Biomarkers of toxicity, such as malondialdehyde, have been proposed to appraise the health status of exposed species. Malondialdehyde reflects membrane degradation in a variety of pathological conditions [10]. Among biomarkers of stress, the alteration of membrane phospholipids through lipid peroxidation is considered to be one of the key events in oxidative damage. Oxidative stress usually characterizes chemically induced toxicity [11, 12]. An increase in malondialdehyde levels can thus reflect degradation of an environmental site.
Study of cypermethrin-induced oxidative stress and its influence on various antioxidants of fish and other aquatic organisms could provide useful information on the ecotoxicological consequences of cypermethrin use. Several studies have shown that the levels of antioxidants in fish and mussels could be used as biomarkers of exposure to aquatic pollutants [12–15]. A review of the literature reveals that there is a paucity of information on pesticide-induced oxidative stress and its effects on various enzymatic antioxidants in freshwater mussels [10, 16–18].
To protect against reactive oxygen species, cells possess specific antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which decompose superoxide anion radical (SOD) and hydrogen peroxide (CAT and GPx). Glutathione S-transferases (GST) catalyze the conjugation of glutathione (GSH) to electrophilic xenobiotics and oxidized components. Moreover, complementary enzymes such as glutathione reductase (GR) and glucose-6-phosphate dehydrogenase (G6PDH) produce GSH and NADPH to maintain the cellular antioxidant status [16].
In recent years, there has been considerable interest in the use of biochemical indices within bivalve molluscs. Mussels have a number of properties that make them useful sentinels for chemical pollution. By filtering large quantities of water, mussels may be exposed to large quantities of pollutants even when the concentrations are quite small [11, 15].
In Turkey, cypermethrin, (R,S)-alphacyano-3-phenoxybenzyl (1R,S)-cis/trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate, is a commonly used pesticide in the agricultural fields around freshwater reservoirs. Therefore, the present study aimed to investigate the effects of cypermethrin on antioxidant status, oxidative stress biomarkers, behavior, and mortality in the freshwater mussel Unio elongatulus eucirrus. An attempt has also been made to determine the usefulness of these parameters as biomarkers of exposure to cypermethrin.
Materials and methods
Exposure test
In the present study, adult specimens of the freshwater mussels Unio elongatulus eucirrus, weight 23–25 g and total length 5.6–5.9 cm, were obtained from the Pertek Region of Keban Dam Lake in Turkey. They were brought to the laboratory and acclimatized to laboratory conditions for 7 days. Water temperature in the aquaria was maintained at 12 ± 1°C using a heater, and the mussels were subjected to a 12-h photoperiod using fluorescent lights. Mussels were fed with trout feed during adaptation, but they were not fed during the last 24 h of adaptation and throughout the duration of the test. Before starting the test, all experimental aquaria (280 L) were cleaned and filled with 270 L of dechlorinated tap water.
Cypermethrin was obtained from Novartis, in the form of Polytrin 200 EC (purity 20%, dissolved in 80% acetone). A semi-static toxicity bioassay was performed according to the standard method [19]. Cypermethrin was prepared from a stock solution weighed in a glass bottle and transferred to a volumetric flask containing experimental water. Dilutions of the defined stock solution were used for tests described below. The stock solutions were renewed every 12 h. Cypermethrin was applied at concentrations of 5, 10, 20, 40, 80, and 160 µg/L, for 1, 24, 48, 72, and 96 h. Six different concentrations of cypermethrin and a control with five replicates were used in the test series. The control group received acetone at a concentration used in the dilution of the maximum cypermethrin concentration. Aeration was applied to the aquaria for 2 h in order to obtain a homogeneous concentration of the toxic compound, and then 20 mussels were transferred into each aquarium. Mortality was assessed at 1, 24, 48, 72, and 96 h after the start, and dead mussels were removed immediately. Behavioral changes of test animals were closely followed and recorded. The results were evaluated by comparing the mean durations of the open and closed positions of the valves before cypermethrin treatment and during the action of the chemical. The mean durations of the active (open) and rest (closed) periods in the control were taken as 100% for each animal, and the experimental data are expressed as percentage deviations from the control [20].
The experimental water was kept in the tank for 24 h before cypermethrin was added. Water quality characteristics in the control units were determined according to the APHA [19]. Dissolved oxygen and pH were determined by a digital oxygen meter and a pH meter. The mean quality parameters of water used for preparation of test solutions were as follows: dissolved oxygen 8.4 ± 0.3 mg/L, pH 7.2 ± 0.2, alkalinity 136 ± 9 mg/L, and total hardness 225 ± 12 mg/L as CaCO3.
Biological assays
Tissue samples were washed with 0.9% NaCl and kept at −20°C until analyzed. Tissues were weighed, rinsed with ice-cold deionized water, cut into small pieces, and then dried on a filter paper. The tissues were homogenized using a phosphate buffer solution containing 1.15% KCl to obtain 1:10 w/v, depending upon the variable to be measured. The homogenates were centrifuged at 18,000 × g for 30 min at +4°C to determine malondialdehyde (MDA) and reduced glutathione (GSH) concentrations and catalase (CAT) activities. The concentrations of MDA as a marker of lipid peroxidation were determined according to the method of Ohkawa et al. [21] based on the reaction with thiobarbituric acid and are expressed as nanomoles per gram of tissue. CAT activity was determined according to the method of Aebi [22]. Tissue GSH concentration was measured by an assay using the dithionitrobenzoic acid recycling method described by Ellman [23]. Protein concentrations were measured according to Lowry et al. [24].
Statistics
Statistical analyses were performed with the SPSS 10.1 computer program (SPSS, Chicago, IL, USA). The results are expressed as mean ± standard error. Significant differences between groups were analyzed by one-way analysis of variance and Duncan’s post-hoc test where appropriate. In addition, the data for active and rest periods were subjected to t-test. Changes are expressed as percentages of the control. The data obtained from the cypermethrin toxicity tests were evaluated using the Probit Analysis Statistical Method. The LC50 values were calculated.
Results
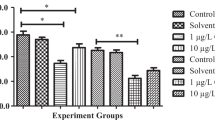
In this study, with increasing cypermethrin concentrations, the mussels significantly increased MDA (Table 1) and decreased GSH (Table 2) and CAT (Table 3) activity in digestive glands and gills (P < 0.05, for each case). Negative correlations were observed between the MDA and the GSH and CAT levels after cypermethrin exposure. The highest MDA levels and the lowest GSH and CAT activity in digestive glands and gills of freshwater mussels were measured after 48 h of exposure to cypermethrin (P < 0.05, for all cases).
In addition, the valve movement representing the activity of the adductor muscles was recorded (Table 4). The durations of the active and rest periods in the control differed significantly. In general, the active periods lasted 13.5 h, while the rest periods lasted 10.5 h. Application of cypermethrin to the water led to a concentration-based reduction in the duration of the active periods. A concentration of 5 µg/L had no observable effect, but 10 µg/L caused a nearly 21% reduction, and the reduction reached 90% at 160 µg/L. The duration of the rest periods also changed in response to cypermethrin. At 5 µg/L, there was a 4% increase in the duration of the rest periods, and at higher concentrations (10–160 µg/L) further lengthening of the rest periods was observed. The longest rest periods (237% as compared with the control) occurred at 160 µg/L. It was also observed that there was lightening in the color of the gills in the mussels.
Cumulative mortality of the freshwater mussels exposed to concentrations of cypermethrin is presented in Table 5. Only 1% mortality in the lowest concentration of cypermethrin (5 µg/L) was observed. The toxicity of cypermethrin on mussels increased with increasing concentration. For example, for mussels exposed to 10 µg/L cypermethrin, only 21% had died at 96 h, whereas 61% were dead at 1 h when exposed to the 160 mg/L concentration. The 1 h and 24 h LC50 values could not be calculated because the mortality was not over 50%. However, the 48, 72, and 96 h LC50 values of cypermethrin for mussels were determined to be 96.50, 77.96, and 59.20 µg/L, respectively. There were significant differences in LC50 values obtained for different times of exposure (P < 0.05).
Discussion
Pyrethroid and organophosphorus pesticides may induce oxidative stress, leading to generation of free radicals and alteration in antioxidants, oxygen free radicals, the scavenging enzyme system, and lipid peroxidation. Several studies have demonstrated oxidative stress induced by organophosphorus pesticides in rats and humans. Lipid peroxidation is also evident in rat brains and human erythrocytes. Organophosphorus pesticide-induced seizures have been reported, associated with oxidative stress [25].
Studies in rodents have demonstrated that absorbed pyrethroids are readily metabolized and excreted, and elimination is achieved within 2–4 days. The tissue residue levels are reported to be generally very low, except in fat, where slightly higher residues occurred. The major metabolic reactions ascribed to pyrethroid metabolism are oxidations mediated by the microsomal monooxygenase system. The degradation pathways in cows, poultry, and fish are similar to those in rodents [1, 26].
However, comparative in vivo and in vitro metabolic studies have shown that fish have a lower capacity to metabolize and eliminate pyrethroid insecticides [27]. This is reflected in the present investigation, where cypermethrin induced peroxidative damage in digestive glands and gills in particular during a short-term sublethal exposure regimen.
The extent of lipid peroxidation is determined by the balance between the production of oxidants and the removal and scavenging of those oxidants by antioxidants [28]. Lipid peroxidation has been extensively used as a marker of oxidative stress [29]. In the present investigation, it was shown that lipid peroxidation (MDA) estimation could provide useful information about the exposure to aquatic pollutants. However, the response may be nonspecific in terms of type of pollutants. In this study, the levels of MDA were found to be significantly increased, which might be associated with decreased GSH and CAT activities in digestive glands and gills of mussels after cypermethrin exposure.
Use of GSH and CAT as biomarkers of exposure to organic xenobiotics has gained credence in aquatic pollution biomonitoring [14, 16]. In the present study, the activity of glutathione and catalase was found to be significantly decreased in digestive glands and gills after cypermethrin exposure. This decrease in catalase activity could be due to flux of superoxide radicals, which have been reported to inhibit CAT activity [30]. Reduced GSH is the main nonprotein thiol and one of the main reductants found in cells. GSH possesses antioxidant properties, and its protective role against oxidative stress-induced toxicity in aquatic animals is well established [31–33].
Effects of insecticides on lipid peroxidation have been evaluated in several studies. In the study of Gohary et al. [33], the effect of deltamethrin on testicular apoptosis in rats and the protective effect of nitric oxide synthase inhibitor was investigated. Plasma levels of both nitric oxide and lipid peroxides were measured as MDA, and the MDA levels were found to be significantly increased in deltamethrin-treated animals in this study.
Shadnia et al. [34] studied genotoxicity and oxidative stress in workers who formulate organophosphorus pesticides. Results indicated that chronic exposure to organophosphorus pesticides was associated with increased activities of CAT, SOD, and GSP-Qx in erythrocytes. In the study of Akhgari et al. [35], the effect of subchronic exposure to malathion in the production of oxidative stress was evaluated in male Wistar rats. Administration of malathion (100, 316, 1,000, and 1,500 ppm) for 4 weeks increased CAT and SOD activities, as well as MDA concentrations in red blood cells and liver. Seth et al. [36] studied the effects of propoxur on lipid peroxidation. In that study they found that propoxur increased MDA levels and altered glutathione levels. Gromov et al. [37] investigated effects of deltametrin and dichlorvos on memory processes, and the activity of antioxidant enzymes such as SOD and CAT in brain and blood of female rats. The enzyme activities were not significantly changed in blood, but CAT activity was lowered in brain 3 h after administration.
Oxidative stress and lipid peroxidation were determined by measuring the MDA concentration [25]. In the present study, MDA levels were found to increase with high concentrations. These findings about MDA levels are compatible with other study results.
In addition, the durations of the active and rest periods in the control were significantly different, in agreement with the literature [38]. The experiment revealed that the lasting presence of cypermethrin in the water also influences central neural regulation. The mechanism of contraction and relaxation of the adductor muscles, forming the basis of the periodic activity, is controlled by the central nervous system, and the external influences resulting in the modulation of the opening and closing of the valves are exerted through neural mechanisms from the ganglia [20, 39]. As the present experiment indicates, under cypermethrin exposure the regulatory mechanism operates first with reduction of the active periods and elongation of the rest periods. Chronic application of cypermethrin may affect the central neural (possibly both membrane and synaptic) processes, which in turn results in alteration of the effector system regulating the contraction and relaxation of the adductor muscles, and leads to shortening of the active periods and elongation of the rest periods. The effect of cypermethrin at concentrations of 10 µg/L or more can be interpreted in this experiment as depressive to the filtering behavior of mussels. It is an adverse action: upon reduction of the filtering activity, the feeding, respiration, and excretory processes are reduced, affecting both the living processes of the mussels and their water-cleaning function, which is an important component with respect to the maintenance of a healthy aquatic ecosystem.
The USDA National Agricultural Pesticide Impact Assessment Program’s document reports that cypermethrin causes acute toxicity in fish in laboratory tests at an average concentration of 1.8–8.2 µg/L [5], for example, the 96 h values are 1.2 µg/L for Salmo trutta, 2 µg/L for Salmo salar, 2.2 µg/L for Tilapia nilotica, and 6 µg/L for Oncorhynchus mykiss. Clark et al. [8], examining cypermethrin toxicity in other aquatic organisms, reported a 96 h LC50 value of cypermethrin for the grass shrimp Palaemonetes pugio to be 0.016 µg/L. In the present study, the 96 h LC50 value of cypermethrin for freshwater mussels was determined as 59.20 µg/L, and the toxicity of cypermethrin on mussels increased with increasing concentration. However, the toxicity of cypermethrin on mussels did not increase with increasing times of exposure. The results indicate that the insecticide cypermethrin has a harmful effect not only on fish [5] and nontarget aquatic arthropods [8], but also on mollusks, although the sensitivity of the freshwater mussel is less than that of fish.
The results obtained from this study show significantly increased lipid peroxidation (MDA) levels and decreased GSH and CAT activities in digestive glands and gills of freshwater mussels after exposure to increasing cypermethrin concentrations. Negative correlations were observed between the MDA levels and the GSH and CAT activities. This suggests a protective response in freshwater mussels toward exposure to an oxidative stress-inducing cypermethrin. The experimental data obtained with freshwater mussels can be considered as a useful reference for comparisons with biomarker responses of organisms living in polluted environments. It is concluded that cypermethrin contamination is dangerous to the ecosystem, and this fact should be taken into consideration when this insecticide is used in agriculture or in the control of mosquito populations.
References
Casida JE, Gammon DW, Glickman AH, Lawrence LJ (1983) Mechanism of pyrethroid insecticides. Annu Rev Pharmacol Toxicol 23:413–418
Glickman AH, Lech JJ (1982) Differential toxicity of trans-per-methrin in rainbow trout and mice. II. Role of target organ sensitivity. Toxicol Appl Pharmacol 66:162–171
Hill IR (1989) Aquatic organisms and pyrethroids. Pestic Sci 27:429–465
Polat H, Erkoç FU, Viran R, Koçak O (2002) Investigation of acute toxicity of beta-cypermethrin on guppies, Poecilia reticulata. Chemosphere 49:39–44
US EPA (1989) Pesticide fact sheet number 199: cypermethrin. Office of Pesticides and Toxic Substances, Washington DC
Aydın R, Köprücü K, Dörücü M, Şimşek Köprücü S, Pala M (2005) Acute toxicity of synthetic pyrethroid cypermethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Aquacult Int 13:451–458
Bradbury SP, Coats JR (1989) Comparative toxicology of the pyrethroid insecticides. Rev Environ Contam Toxicol 108:133–177
Clark JR, Patrick JM, Moore JC, Lores EM (1987) Waterborne and sediment-source toxicities of six organic chemicals to grass shrimp (Palaemonetes pugio) and amphioxus (Branchiostoma caribaeum). Arch Environ Contam Toxicol 16:401–407
Chapman D (1996) Water quality assessments: a guide to the use of biota, sediments and water in environmental monitoring, 2nd ed. Spon, London
Charissou AM, Cossu-Leguille C, Vasseu P (2004) Relationship between two oxidative stress biomarkers, malondialdehyde and 8-oxo-7,8-dihydro-2-deoxyguanosine, in the freshwater bivalve Unio tumidus. Sci Tot Environ 322:109–122
Livingston DR, Martinez PG, Michel X, Narbonne JF, O’Hara S, Ribera D, Winston GW (1990) Oxyradical production as a pollution-mediated mechanism of toxicity in the common mussel Mytilus edulis and other molluscs. Funct Ecol 4:415–424
Cossu C, Doyotte A, Jacquin MC, Babut M, Exinger A, Vasseur P (2000) Antioxidants biomarkers in freshwater bivalves, Unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicol Environ Saf 45:106–121
Livingston DR (1993) Biotechnology and pollution monitoring: use of molecular biomarkers in the aquatic environment. J Chem Technol Biotechnol 57:195–211
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Rausiddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctata Bloch. Ecotoxicol Environ Saf 56:295–301
Jeferson LF, Trivella DBB, Trevisan R, Dinslaken DF, Marques MRF, Bainy ACD, Dafre AL (2006) Antioxidant status and stress proteins in the gills of the brown mussel Perna perna exposed to zinc. Chem Biol Interact 160:232–240
Almeida EA, Bainy ACD, Dafr AL, Gomes OF, Medeiros MHG, Mascio PD (2005) Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. J Exp Mar Biol Ecol 318:21–30
Ferreira M, Moradas-Ferreira P, Reis-Henriques MA (2005) Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichthys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat Toxicol 71:39–48
Parvez S, Raisuddin S (2006) Copper modulates non-enzymatic antioxidants in the freshwater fish Channa punctata (Bloch) exposed to deltamethrin. Chemosphere 62:1324–1332
APHA (1989) Standard methods for the examination of water and wastewater, 17th ed. American Public Health Association, Washington DC
Salanki J (1994) Mussel behaviour in the indication of water pollution. In: Salanki J, Jeffrey D, Hughes GM (eds) Biological monitoring of the environment: a manual of methods. CAB International, Wallingford, pp 131–136
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Ellman G (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A (2004) Pesticides and oxidative stress: a review. Med Sci Monit 10:141–147
IPCS (1989) Health and safety guide no. 30: deltamethrin. United Nations Environment Programme, International Labour Organization, World Health Organization, Geneva
Glickman AH, Weitman SD, Lech JJ (1982) Differential toxicity of trans-permethrin to rainbow trout and mice. I. Role of biotransformation. Toxicol Appl Pharmacol 66:153–161
Filho DW (1996) Fish antioxidant defences—a comparative approach. Braz J Med Biol Res 29:1735–1742
Huggett RJ, Kimerle RA, Mehrle PM, Bergman HL (1992) Biochemical, physiological and histological markers of anthropogenic stress. Lewis, Boca Raton
Pandey S, Ahmad I, Parvez S, Bin-Hafeez B, Haque R, Raisuddin S (2001) Effect of endosulfan on antioxidants of freshwater fish Channa punctatus Block. I. Protection against lipid peroxidation in liver by copper pre-exposure. Arch Environ Contam Toxicol 41:345–352
Hasspielar BM, Behar JV, DiGiulio RT (1994) Glutathione dependent defense in channel catfish (Ictalurus punctatus) and brown bullhead (Ameuirus nebulosus). Ecotoxicol Environ Saf 28:82–90
Şimşek Köprücü S, Yonar E, Seker E (2008) Effects of deltamethrin on antioxidant status and oxidative stress biomarkers in freshwater mussel, Unio elongatulus eucirrus. Bull Environ Contam Toxicol 81:253–257
Gohary M, Awara WM, Nassar S, Hawras S (1999) Deltamethrin-induced testicular apoptosis in rats: the protective effects of nitric oxide synthase inhibitor. Toxicol 132:1–8
Shadnia S, Azizi E, Hosseini R, Khoei S, Fouladdel S, Pajoumand A, Jalali N, Abdollahi M (2005) Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol 24:439–445
Akhgari M, Abdollahi M, Kebryaeezadeh A, Hosseini R, Sabzevari O (2003) Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum Exp Toxicol 22:205–211
Seth V, Banerjee BD, Bhattacharya A, Chakravorty AK (2000) Lipid peroxidation, antioxidant enzymes, and glutathione redox system in blood of human poisoning with propoxur. Clin Biochem 33:683–685
Gromov LA, Seredi PI, Syrovatskaia LP, Ovinova GV, Filenenko MA (1993) Free radical mechanisms of memory disorders of toxic origin and experimental therapy of the condition. Patol Fiziol Eksp Ter 4:24–26
Salanki J, Glaizner B, Labos E (1970) On the temporal organization of periodic and rhythmic activity in fresh-water mussel (Anodonta cygnea L.). Biol Rhythm Res 1:123–134
Salanki J, Varanka I (1978) Effect of some insecticides on the periodic activity of the freshwater mussel (Anodonta cygnea L.). Acta Biol Hung 29:173–180
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Köprücü, K., Yonar, S.M. & Şeker, E. Effects of cypermethrin on antioxidant status, oxidative stress biomarkers, behavior, and mortality in the freshwater mussel Unio elongatulus eucirrus . Fish Sci 76, 1007–1013 (2010). https://doi.org/10.1007/s12562-010-0293-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-010-0293-8