Abstract
In this study, the effects of deltamethrin on antioxidant status and oxidative stress biomarkers in digestive gland and gill of freshwater mussel, Unio elongatulus eucirrus, was examined. Deltamethrin was applied at concentrations of 25, 50, 100, 200, 400, 800 and 1,600 μg L−1, for 1, 24, 48, 72 and 96 h. With increasing deltamethrin concentrations, the mussels exposed duration 1–96 h significantly increased lipid peroxidation, which might be associated to decreased levels of reduced glutathione and catalase activity in digestive gland and gill of freshwater mussel (p < 0.05 for each cases). Negative correlations were observed between the lipid peroxidation and glutathione or catalase levels after deltamethrin exposure, indicating a protective role of glutathione and catalase against lipid peroxidation, suggesting the use of these antioxidants as a potential biomarker of toxicity associated with contaminant exposure in freshwater mussels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The increasing use of synthetic pesticides is improving worldwide pollution risks. The synthetic pyrethroids are among the most potent and effective insecticides available (Casida et al. 1983), accounting for more than 30% of the world market in insecticides. They are less persistent and less toxic to mammals and birds. One of the pyrethroids that have found wide acceptability is deltamethrin. It is extensively used in agriculture and forestry because of its high activity against a broad spectrum of insect pests (Glickman and Lech 1982).
Pesticides applied to the land may be washed into surface waters and may kill or at least adversely influence the life of aquatic organisms (Köprücü et al. 2006). Synthetic pyrethroids have been found to be highly toxic to fish (Datta and Kaviraj 2003; Köprücü and Aydın 2004), zooplankton communities and some beneficial aquatic arthropods (Tidou et al. 1992). Effects of deltamethrin on nervous, haematological and respiratory systems in fishes are reported (Golow and Godzi 1994). Deltamethrin have proved to alter the filtration and pumping activity of mussels (Kontreczky et al. 1997). The environmental fate and effects of synthetic pyrethroid insecticides have been summarized by Hill (1989).
Study of deltamethrin-induced oxidative stress and its influence on various antioxidants of fish and other aquatic organisms could provide useful information on the ecotoxicological consequences of deltamethrin use. Several studies have shown that the antioxidants of fish and mussels could be used as biomarkers of exposure to aquatic pollutants. A review of the literature reveals that there is a paucity of information on deltamethrin-induced oxidative stress and its effects on various enzymatic antioxidants in freshwater mussels (Charissou et al. 2004; Almeida et al. 2005; Parvez and Raisuddin 2006).
In recent years, there has been considerable interest in the use of biochemical indices within bivalve molluscs. Mussels have a number of properties which make them useful sentinels for chemical pollution. By filtering large quantities water, mussels may be exposed to large quantities of pollutants even when their concentrations are quite small (Robillard et al. 2003).
In Turkey, deltamethrin is a commonly used pesticide for pest control in the agricultural fields around freshwater reservoirs. Therefore, the present study has aimed to investigate effects of deltamethrin on antioxidant status and oxidative stress biomarkers in digestive gland and gill of freshwater mussel, Unio elongatulus eucirrus. An attempt has also been made to access usefulness of these parameters as biomarkers of exposure to deltamethrin.
Materials and Methods
In the present study, a semi-static acute toxicity bioassay was performed according to the standard method (APHA 1985). Deltamethrin was applied at sub-acute doses of 25, 50, 100, 200, 400, 800 and 1,600 μg L−1, for 1, 24, 48, 72 and 96 h.
Deltamethrin, (S)-alpha-cyano-3-phenoxybenzyl (1R, 3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropancarboxylate, was obtained from Roussel Uclaf, in form of DECIS 2.5 EC (purity 2.5%, dissolved in 97.5% acetone).
Adult specimens of freshwater mussels, weighing 24–26 g and total length 5.8–6.1 cm, were obtained from the Pertek Region of Keban Dam Lake in Turkey. They were brought to the laboratory and acclimatized to laboratory conditions for 7 days. Water temperature in the aquaria was maintained at 12 ± 1°C using a heater and the mussels were subjected to a 12 h photoperiod using fluorescent lights. Mussels were fed with trout feed during adaptation, but they were not fed during the last 24 h of adaptation and throughout the duration of the test. Before starting the test, all experimental aquaria (280 L) were cleaned and filled with 270 L of dechlorinated tap water. Ten mussels were transferred into each aquarium. Five replicates were used in the control and test series. The experimental water was kept in the tank for 24 h before deltamethrin was added.
Water quality characteristics in the control units were determined according to APHA (1985). Dissolved oxygen and pH were determined by a digital oxygen meter and a pH meter. The mean quality parameters of water used for preparation of test solutions were as follows; dissolved oxygen 8.3 ± 0.4 mg L−1, pH 7.1 ± 0.2, alkalinity 138 ± 12 mg L−1 and total hardness 187 ± 15 mg L−1 as CaCO3.
Seven different concentrations of deltamethrin and a control with five replicates were used in the test series. Two control groups received acetone at a concentration used in the dilution of the maximum deltamethrin concentration. Exceeding aeration was applied to the aquarium for 2 h in order to obtain a homogeneous concentration of the toxic compound, and then ten mussels were transferred into each aquarium. Mortality was assessed at 1, 24, 48, 72 and 96 h after the start and dead mussels were removed immediately.
After tissue samples rinsing with cold 0.09% NaCl solution and filtering to remove fluid, the tissue samples were weighed meticulously. The homogenization of tissues was carried out in a Sorvall homogenizer with a buffer containing 1.15% KCl to obtain 1:10 (w/v) whole homogenate. The homogenates were centrifuged at 18,000g (+4°C) for 30 min to determine malondialdehyde, reduced glutathione concentrations and catalase activities. The concentrations of malondialdehyde as a marker of lipid peroxidation were determined according to the method of Ohkawa et al. (1979) based on the reaction with thiobarbituric acid, and were expressed as nmol/g tissue. Catalase activity was determined according to the method of Aebi (1974). Tissue glutathione concentration was measured by an assay using the dithionitrobenzoic acid recycling method described by Ellman (1959). Protein concentrations were measured according to Lowry et al. (1951).
Statistical analyses were performed with the SPSS 10.1 computer program (SPSS Inc. Chicago, IL, USA). The results were expressed as mean ± SE. Significant differences between groups were analyzed by one-way analysis of variance and Duncan’s post hoc test where appropriate. The significance of the results was ascertained at p < 0.05.
Results and Discussion
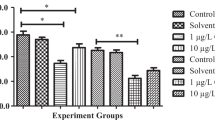
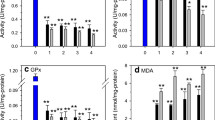
The results show that with increasing deltamethrin concentrations, the mussels exposed duration 1–96 h significantly increased lipid peroxidation (Table 1), which might be associated to decreased levels of reduced glutathione (Table 2) and catalase (Table 3) activity in digestive gland and gill of freshwater mussel (p < 0.05 for each cases). Negative correlations were observed between the lipid peroxidation and glutathione or catalase levels after deltamethrin exposure, indicating a protective role of glutathione and catalase against lipid peroxidation, and suggesting the use of these antioxidants as a potential biomarker of toxicity associated with contaminant exposure in freshwater mussels.
Deltamethrin is known to after cell metabolism in various ways, with a potential genotoxic risk, including DNA damage and micronucleus induction in human lymphocytes (Scassellati et al. 1994). The present findings implicate a role of oxidative stress and free radical formation in these effects. Studies in rodents have demonstrated that absorbed deltamethrin is readily metabolized and excreted, elimination is achieved within 2–4 days. The tissue residue levels are reported to be generally very low, except in fat, where slightly higher residues occurred. The major metabolic reactions ascribed to deltamethrin metabolism are oxidations mediated by the microsomal monooxygenase system. The degredation pathways in cows, poultry, and fish are almost similar to those in rodents (IPCS 1989). However, comparative in vivo and in vitro metabolic studies have shown that fish have a lower capacity to metabolize and eliminate pyrethroid insecticides (Glickman and Lech 1982). This is reflected in the present investigation, where deltamethrin induced peroxidative damage in digestive gland and gill in particular during a short-term sub-acute exposure regimen.
The extent of lipid peroxidation is determined by the balance between the production of oxidants and the removal and scavenging of those oxidants by antioxidants (Filho 1996). Lipid peroxidation has been extensively used as a marker of oxidative stress (Huggett et al. 1992). In the present investigation, we also showed that lipid peroxidation estimation could provide useful information about the exposure to aquatic pollutants. However, the response may be nonspesific in terms of type of pollutants.
Use of glutathione and catalase as biomarkers of exposure to organic xenobiotics has gained credence in aquatic pollution biomonitoring (Almeida et al. 2005). The activity of glutathione and catalase was found to be significantly decreased in digestive gland and gill. This decrease in catalase activity could be due to flux of superoxide radicals, which have been reported to inhibit catalase activity (Pandey et al. 2001). Reduced glutathione is the man nonprotein thiol and one of the main reductants found in cell (Siegers 1989). It possesses antioxidant properties and its protective role against oxidative stress induced toxicity in aquatic animals is well established (Hasspielar et al. 1994).
Our results show decrease in glutathione and catalase levels in digestive gland and gill of freshwater mussel. This demonstrates a protective response in freshwater mussel toward exposure to an oxidative stress inducing deltamethrin. However, the induction pattern of glutathione and catalase under long-term exposure conditions needs to be investigated.
References
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods enzymatic analysis. Academic Press, New York
Almeida EA, Bainy ACD, Dafre AL, Gomes OF, Medeiros MHG, Mascio PD (2005) Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. J Exp Mar Biol Ecol 318:21–30. doi:10.1016/j.jembe.2004.12.007
APHA (1985) Standard methods for the examination of water and wastewater, 16th edn. American Public Health Association, Washington, DC
Casida JE, Gammon DW, Glickman AH, Lawrence LJ (1983) Mechanism of pyrethroid insecticides. Annu Rev Pharmacol Toxicol 23:413–418. doi:10.1146/annurev.pa.23.040183.002213
Charissou AM, Cossu-Leguille C, Vasseur P (2004) Relationship between two oxidative stress biomarkers, malondialdehyde and 8-oxo-7,8-dihydro-2-deoxyguanosine, in the freshwater bivalve Unio tumidus. Sci Total Environ 322:109–122. doi:10.1016/j.scitotenv.2003.09.028
Datta M, Kaviraj A (2003) Acute toxicity of the synthetic pyrethroid deltamethrin to freshwater catfish Clarias gariepinus. Bull Environ Contam Toxicol 70:296–299. doi:10.1007/s00128-002-0190-7
Ellman G (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Filho DW (1996) Fish antioxidant defences—a comparative approach. Braz J Med Biol Res 29:1735–1742
Glickman AH, Lech JJ (1982) Differential toxicity of trans-per-methrin in rainbow trout and mice. II. Role of target organ sensitivity. Toxicol Appl Pharmacol 66:162–171. doi:10.1016/0041-008X(82)90281-2
Golow AA, Godzi TA (1994) Acute toxicity of deltamethrin and dieldrin to Oreochromis niloticus (LIN). Bull Environ Contam Toxicol 52:351–354. doi:10.1007/BF00197820
Hasspielar BM, Behar JV, DiGiulio RT (1994) Glutathione dependent defense in channel catfish (Ictalurus punctatus) and brown bullhead (Ameuirus nebulosus). Ecotoxicol Environ Saf 28:82–90. doi:10.1006/eesa.1994.1036
Hill IR (1989) Aquatic organisms and pyrethroids. Pestic Sci 27:429–465. doi:10.1002/ps.2780270408
Huggett RJ, Kimerle RA, Mehrle PM, Bergman HL (1992) Biochemical, physiological and histological markers of anthropgenic stress. Lewis Publishers, Boca Raton, FL
IPCS (1989) Deltamethrin, international programme on chemical safety. Health and Safety Guide No. 30. United Nations Environment Programme, International Labour Organization, World Health Organization, Geneva
Kontreczky CS, Farkas A, Nemcsok J, Salanki J (1997) Short- and long-term effects of deltamethrin on filtering activity of freshwater mussel (Anodonta cygnea L.). Ecotoxicol Environ Saf 38:195–199. doi:10.1006/eesa.1997.1575
Köprücü K, Aydın R (2004) The toxic effects of pyrethroid deltamethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Pestic Biochem Physiol 80:47–53. doi:10.1016/j.pestbp.2004.05.004
Köprücü ŞS, Köprücü K, Ural MS (2006) Acute toxicity of the synthetic pyrethroid deltamethrin to fingerling European catfish, Silurus glanis L. Bull Environ Contam Toxicol 76:59–65. doi:10.1007/s00128-005-0889-3
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. doi:10.1016/0003-2697(79)90738-3
Pandey S, Ahmad I, Parvez S, Bin-Hafeez B, Haque R, Raisuddin S (2001) Effect of endosulfan on antioxidants of freshwater fish Channa punctatus block. I. Protection against lipid peroxidation in liver by copper pre-exposure. Arch Environ Contam Toxicol 41:345–352. doi:10.1007/s002440010258
Parvez S, Raisuddin S (2006) Copper modulates non-enzymatic antioxidants in the freshwater fish, Channa punctata (Bloch) exposed to deltamethrin. Chemosphere 62:1324–1332. doi:10.1016/j.chemosphere.2005.07.025
Robillard S, Beauchamp G, Laulier M (2003) The role of abiotic factors and pesticide levels on enzymatic activity in the freshwater mussel Anadonta cygnea at three different exposure sites. Comp Biochem Physiol C 135:49–59
Scassellati SG, Moretti M, Villarini M, Angeli G, Pasquini R, Monarca S, Scarselli R, Crea MG, Leonardis C (1994) An evaluation of toxic and genotoxic risk from work-related exposure to chemical compounds. Prev Oggi 6:125–138
Siegers CP (1989) Glutathione and glutathione dependent enzymes. Progr Pharmacol Clin Pharmacol 7:171–180
Tidou AS, Moreteau JC, Ramade F (1992) Effects of lindane and deltamethrin on zooplankton communities of experimental ponds. Hydrobiologia 232:157–168. doi:10.1007/BF00017475
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Köprücü, S.Ş., Yonar, E. & Seker, E. Effects of Deltamethrin on Antioxidant Status and Oxidative Stress Biomarkers in Freshwater Mussel, Unio elongatulus eucirrus . Bull Environ Contam Toxicol 81, 253–257 (2008). https://doi.org/10.1007/s00128-008-9474-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-008-9474-x