Abstract
λ-cyhalothrin, a synthetic type II pyrethroid, has become increasingly popular for control of aphids, butterfly larvae, and beetles, replacing other agricultural chemicals. As a result of which, residues of this synthetic pesticide are being reported across the globe in natural water, which poses a serious threat to aquatic life. Therefore, the present study was designed to understand the toxicity effects of λ-cyhalothrin on behaviour, oxidative stress and neurotoxicity in a vertebrate aquatic model, zebrafish (Danio rerio). The fish were exposed to 0.129, 0.194 and 0.388 µg/L corresponding to 5%, 10% and 20% of 96hLC50 (1.94 µg/L) for 28 days. Upon exposure to the highest concentration (0.388 µg/L), the test animal exhibited significant alterations in behavioural patterns like number of entries to the top zone (n), decrease in average speed (m/s) and decrease in time spent in top zone (s). Moreover, the shoaling test demonstrated a significant decrease (p < 0.05) in the relative time spent by the tested fish (%) near the stimulus fish. The change in behavioural alterations might be linked to a significant decrease (p < 0.05) in the brain acetylcholine esterase activity. Furthermore, the present study also illustrates oxidative stress exerted by λ-cyhalothrin through an increase in the production of reactive oxygen species, which is again clearly depicted by a significant increase (p < 0.05) in Superoxide dismutase, Catalase and Glutathione peroxidase activities. Overall, the present study systematically demonstrates the chronic effects of λ-cyhalothrin on adult fish behaviour and physiology, which will contribute to assessing the risks of λ-cyhalothrin to organismal health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of agricultural chemicals such as pesticides and insecticides has experienced a significant increase in protecting crops from pest attacks and enhancing agricultural productivity. This trend is driven by the growing demand for food due to the increasing human population (Aktar et al. 2009; Chandola et al. 2011). Globally, 2 million tons of pesticides are currently in use, out of which 47.50% are herbicides, 29.50% are insecticides, 17.50% are fungicides, and other pesticides are 5.50% (Sharma et al. 2019). In India, 76% of the pesticides used are insecticides, compared to 44% of global use (Aktar et al. 2009). During the past 30 years, there has been a shift in the use of insecticides, with an observable decrease in the production of highly toxic compounds like organophosphates and carbamates, which has led to an increase in the use of pyrethroid insecticides worldwide (Kumar et al. 2014). However, pyrethroids are over 100 times more toxic to non-targeted aquatic organisms like fish, as these compounds are lipophilic and get easily absorbed via gills (Aydin et al. 2005).
Pyrethroids are synthetic chemical analogues of pyrethrins, which are naturally occurring insecticidal compounds produced in the flowers of chrysanthemums (Chrysanthemum cinerariaefolium). λ-cyhalothrin is a type II α-cyano synthetic pyrethroid used for the eradication of aphids, beetles, butterfly larvae, cockroaches, mosquitoes, ticks and flies at home and in agricultural fields (Arulraj et al. 2019). The half-life of λ-cyhalothrin in water and soil is reported to be 30 days and less than 30 days when exposed to sunlight (World Health Organization 1990). Commercially, λ-cyhalothrin is sold under brand names such as Karate (Emulsifiers-Creslox AE 4, Creslox AE 5, Aromax, λ-cyhalothrin), Warrior (hydrocarbons, C10-C13, aromatics, <1% naphthalene, 1, 2-benzisothiazol-3(2H)-one, λ-cyhalothrin), Scimitar (Xylene, 1,2,4-Trimethylbenzene, Cumene, Propylene Glycol, Petroleum Solvent, λ-cyhalothrin), Demand (Xylene, 1,2,4-Trimethylbenzene, Cumene, Propylene Glycol, Petroleum Solvent, λ-cyhalothrin) and Matador (Distillates- petroleum, solvent-refined light paraffinic, aromatic hydrocarbons, C10, naphthalene, λ-cyhalothrin) (Amweg et al. 2005; Oros and Werner 2005). Due to extensive use of pyrethroids across the globe, the emergence of environmental concentrations of this particular insecticide has taken a serious environmental toll (Weston et al. 2009; Domagalski et al. 2010; Stehle and Schulz 2015). Residues of pesticides can remain for a long period in the fields after application due to their decreased biodegradation properties which could be absorbed by aquatic organisms like fishes, leading to negative effects on their health and meat quality, which will ultimately affect human health (Khafaga et al. 2020). λ-cyhalothrin was detected in water at 0.11–0.14 µg/L concentrations in agricultural watersheds in Stanislaus County, California and 0.003 µg/g of dry weight in sediment samples in Imperial, Stanislaus and Placer counties (Starner 2007). Concentrations of λ-cyhalothrin in surface waters ranging from 346 ng/L in rivers of Greece (Tsaboula et al. 2016) to 797 ng/L in agricultural regions of the southern United States are already reported in the literature (Anderson et al. 2013). In India, concentrations of λ-cyhalothrin (11.7 ng/g) were detected in pond water samples in Punjab province (Bedi et al. 2016).
Previous studies have reported that λ-cyhalothrin has low toxicity to birds and mammals but causes significant behavioural changes, affecting the locomotory behaviour and a reduction in memory retention in mice (Nieradko-Iwanicka and Konopelko 2020) and the swimming behaviour in crustaceans like Daphnia (Bownik et al. 2019). λ-cyhalothrin forms a high level of free radicals, causing oxidative stress which leads to alterations in antioxidant enzyme activities and the overall physiological response of the fish towards the environment (Nnamonu et al. 2019). Studying oxidative stress in fish holds significant importance in the fields of environmental and aquatic toxicology (Konstantinou et al. 2006). This importance arises from the fact that xenobiotics including certain pesticides can induce oxidative stress in fish, by increasing the reactive oxygen species (ROS) production (Köprücü and Aydın 2004). The production and removal of ROS usually maintain a dynamic equilibrium within the body of the organism (Valavanidis et al. 2006). Antioxidant enzymes like Superoxide dismutase (SOD), and Catalase (CAT) play a vital role in eliminating ROS. However, any disruption in the equilibrium between ROS and these antioxidants leads to oxidative stress, inducing genetic and epigenetic changes at the molecular level (Afzal et al. 2023; Pokhrel et al. 2022).
Zebrafish (Danio rerio) has gained its popularity as a powerful and versatile organism in neurological diseases, and toxicological research. It is widely used as a model organism to study the behavioural changes when exposed to a toxicant as it has all the classical vertebrate neurotransmitters, a fully developed neuroendocrine system, and a diverse range of behavioural phenotypes that offer strong physiological responses to stress (Audira et al. 2018). The ability to conduct cost-effective, material-efficient, and time-saving testing through high-throughput, non-invasive analyses, sets zebrafish behavioural assays apart from other vertebrate model organisms, thus adding to their widespread adoption (Fitzgerald et al. 2019). The zebrafish exhibits a spectrum of behaviours akin to humans, including movement, responses to stress, learning, memory, and social interactions. These traits make them a promising candidate for studying cognitive decline, offering an alternative to traditional vertebrate models (Tan et al. 2022). Also, it shares similar genes with humans, with up to 70% of their genes homologous to humans, making it a relevant model organism for studying different diseases (Howe et al. 2013).
Previous studies are available regarding the acute toxicity of λ-cyhalothrin to vertebrate model zebrafish (Wang et al. 2007); however, there is a dearth of information on the behavioural effects on aquatic organisms when exposed to sublethal concentrations of this pesticide. As λ-cyhalothrin residues are present in different aquatic environments across the globe, it is extremely important to study chronic toxicity on behaviour and its relationship with brain acetylcholine esterase activity along with antioxidant enzyme activities. The best-known marker of neurotoxicity is AChE inhibition, which can be assessed by measuring the enzyme activity in the brain or whole-body homogenates of organisms following exposure (Küster and Altenburger 2006). Tracking AChE inhibition has been extensively utilized in freshwater environments as a gauge of chemical exposure and physiological impacts on exposed animals (Fulton and Key 2001). Cholinergic signalling is dependent on the synthesis and release of the neurotransmitter acetylcholine (Ach). Most of the brain regions that are innervated by cholinergic neurons play a role in learning, memory, stress response, and cognitive functions, which degenerate with exposure to neurotoxic chemicals like pesticides, resulting in alterations in behavioural biomarkers (Woolf and Butcher 2011). Therefore, the present work aimed at assessing the behavioural toxicity along with neurotoxicity and oxidative stress induced by λ-cyhalothrin in adult zebrafish when exposed to different sub-lethal concentrations for 28 days.
Materials and methods
Location of the experiment
The experiments were performed at Aquatic Toxicology Lab, Department of Aquatic Environment Management, College of Fisheries, Assam Agricultural University, Raha, Nagaon, Assam, India. The geographical co-ordinates are latitude 26°.21′.55″ N and longitude 92°.50′.67″ E.
Ethical concern
During the experiment, all the guidelines of ‘Committee for the Purpose of Control & Supervision of Experiments on Animals (CPCSEA) for Experimentation on Fishes’ by Ministry of Fisheries, Animal Husbandry & Dairying, Govt. of India (2021) were strictly followed.
Test chemicals
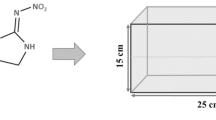
Technical grade Lambda-cyhalothrin (CAS; 91465-08-6, Purity 98%) was purchased from Sigma Aldrich (Merck Group). Acetone (100% Purity) purchased from Sigma Aldrich was used as a solvent at 0.01% in each treatment. All the other reagents used during the study were procured from HiMedia Private Ltd. Mumbai.
Experimental design
Wild-type adult zebrafish (n = 300, length=3 ± 1 cm; Weight=2.3 ± 1 gm) were purchased from a local supplier (Aquarium Miracle) located in Nagaon, Assam, India. Healthy fish without abnormalities and infection were then acclimatized in glass aquariums to laboratory conditions for 14 days prior to the experiment with constant aeration (100 L capacity aquarium). They were fed with a diet consisting of formulated feed Micromac (54% protein content), which was supplemented with brine shrimp thrice daily ad libitum. However, feeding was stopped 24 h prior to the experiment and resumed afterwards for a period of 28 days twice daily at a regular interval of 12 h. The physical and chemical parameters of the water were monitored throughout the acclimation period as per standard protocols of American Public Health Association APHA (2019) (pH = 7.5 ± 0.3, Temperature = 23 ± 2.0 °C, Dissolved Oxygen = 5.70 ± 0.10 mg/L, Total Alkalinity = 114.20 ± 3.25 mg/L, Total Hardness = 103.00 ± 2.78 mg/L, Total Ammonia = 0.05 ± 0.01 mg/L). No mortality was observed during the acclimation period.
During the experiment, the fish were randomly divided into 5 groups (C, S, T1, T2, and T3) in triplicate (n = 30 fish/group) and kept in glass aquaria containing 60 L of dechlorinated tap water. Fish were kept under a 14:10 h light: dark cycle photoperiod. The water used during the experiment was within the prescribed range of the Organization for Economic Co-operation and Development (OECD 2019). One group was kept under controlled conditions (C) which did not contain the pesticide and the other 3 groups were exposed to three sub-lethal concentrations of technical grade λ-cyhalothrin, i.e. 0.129 (T3), 0.194 (T2), and 0.388 (T1) µg/L corresponding to 5%, 10% and 20% based on the 96hLC50 (1.94 μg/L) value as reported earlier by Wang et al. (2007). Also, a solvent control (S) containing acetone (0.01%) was maintained along with the control. Water exchange was done once every 3 days to remove faecal matter and food remnants. Also, the chemical was replenished to avoid the degradation of the active ingredient and the concentration was maintained. At every 7th, 14th, 21st, and 28th day, 3 fish were randomly taken (no. of observations = 3) from each treatment and the controls for the behavioural and 5 fish (no. of observations = 5) for the oxidative stress enzymes, and neurotoxic assays.
Behavioural tests
Novel tank test
The novel tank test was performed as per Wang et al. (2015) to measure anxiety-like behaviours in response to a new environment and to compare anxiety-induced behaviour between experimental and control groups. A line was drawn horizontally on the exterior of an aquarium (100 L capacity) to divide it into two zones (the top and the bottom zone). Water was filled up to a level of 10 cm, (5 cm for the top zone and 5 cm for the bottom zone). One individual was taken and placed in the novel tank and acclimatized for half a minute (30 s). After the end of the habituation period, the fish’s behaviour was recorded for 6 min. A video camera (Nikon Z30) was set up in front of the testing tank to record the behaviour. This experiment was performed in triplicate with three independent runs. The following parameters were analyzed by using ANY-maze recording software 7.20 (Stoelting Co., Wood Dale, Illinois).
-
a.
No. of entries to the top zone (n)
-
b.
Average speed of the fish (m/s)
-
c.
Time in the top zone (s)
Shoaling test
The shoaling test was performed as per Gerlai (2003). Here, the testing tank (30 × 15 × 10 cm; length × height × width) was placed in the centre, with additional tanks (including an empty tank and a stimulus tank) on either side. Fish were placed in groups of five in a small experimental tank. The stimulus tank contained 15 individuals (stimulus fish). A vertical line was drawn in the front wall of the testing tank that separated it into two equal parts. The experimental fish were allowed to acclimate to the testing tank for a period of 30 s after which, their behaviour was video-recorded for a period of 6 min. The duration of time (%) the tested fish spent on the side of the tank closest to their conspecifics was considered a sign of shoal preference or group preference. This experiment was performed in triplicate with three independent runs.
Neurotoxic assay
AChE activity
AChE activity was measured by following the protocol of Topal et al. (2017). Whole brain homogenates of zebrafish (1%v/v) in 0.25 M ice-cold sucrose solution (pH: 7.4), using a homogenizer were taken for the assay. The homogenate was then centrifuged at 14,000 rpm for 40 min at 4 °C. The supernatant was used for the estimation of AChE activity in the brain. The enzymatic reaction occurred in a total volume of 1.0 ml containing 50 μl of 0.5 mM DNTB in 1% sodium citrate, 200 μl of 0.5 M phosphate buffer (KH2PO4/ K2HPO4; pH: 8.0), followed by 650 μl of H2O, 50 μl of tissue extract and 50 μl of 10 mM of acetylthiocholine iodide. The control did not contain acetylthiocholine iodide.
Enzyme activity was determined by reading the changes in absorbance over 5 min at a wavelength of 412 nm. One enzyme unit was defined as the amount of enzyme that catalyzes the hydrolysis of 1 μmol of acetylthiocholine iodide per minute at 25 °C. This experiment was performed in triplicate.
Antioxidant enzymes
Superoxide dismutase (SOD)
Whole body tissue homogenates were prepared and preserved in 0.25 M sucrose solution (pH: 7.4). SODs form the front line of defence against reactive oxygen species (ROS)-mediated injury, and harbour anti-inflammatory activities. Superoxide radicals are allowed to react with hydroxylamine hydrochloride to produce nitrite which in turn reacts with sulphanilamide to produce a red azo product. Superoxide dismutase activity was estimated by following the method of Das et al. (2000). The SOD activity was expressed as U/mg protein. This experiment was performed in triplicate.
SOD activity was estimated using the following formula:
Catalase (CAT)
Catalase activity was estimated by following the method of Takahara et al. (1960). Catalase is an important enzyme that acts to dissociate hydrogen peroxide (H2O2) into molecular oxygen (O2) and water (H2O) (Olson et al. 2017). The tissue homogenates prepared from 20 mg pooled whole-body tissues in 0.25 M sucrose solution were taken for the assay. The test solution contained 2.5 ml of PO4 buffer and 10–50 μl of sample (tissue extract). The blank consisted of only PO4 buffer solution. 1 ml of H2O2 was added to the test just before taking the reading. The absorbance was read using Nabi- UV/Vis Nano Spectrophotometer at 240 nm for 3 min at 15 s intervals. The activity of catalase was expressed as a micromole of H2O2 decomposed/min/mg protein. This experiment was performed in triplicate.
Glutathione peroxidase (GPx)
GPx activity was estimated by following the method described by Rotruck et al. (1973). Tissue homogenates prepared in sucrose solution (pH: 7.4) were taken for the assay. To 0.4 ml of 0.4 M sodium phosphate buffer (pH:7), 0.1 ml of 10 mM sodium azide, 0.2 ml of 4 mM reduced glutathione, 0.1 ml of 2.5 mM H2O2, 0.2 ml of enzyme extract (supernatant produced by centrifugation of homogenate mixture) and 1.0 ml of distilled water were added, made to a final incubation volume of 2.0 ml. The tubes were then incubated for 60 s. The reaction was terminated by the addition of 0.5 ml of 10% TCA (Trichloroacetic acid). After centrifugation, 2.0 ml of supernatant was added to 3.0 ml of 0.2 M phosphate buffer and 1.0 ml of DNTB reagent. The colour developed was read at 412 nm by spectrophotometer against buffer taken as blank. This experiment was performed in triplicate. The GPx activity was expressed as U/mg protein.
Total protein estimation
The total protein content for all the parameters were estimated using Erba’s Total protein kit (biuret method) and expressed in g/dl.
Statistical analysis
A Completely Randomised Block design (CRD) was followed throughout the experiment for all statistical inferences. Shapiro Wilk test was used to assess the normality of the experimental data in SPSS Software (IBM Version, 26). Graphs were drawn and statistical analyses were conducted using GraphPad Prism 8.0.2 software (GraphPad Software, San Diego, California, USA, www.graphpad.com). The results took into account the mean ± standard error (SE). As the data were parametric, a two-way factorial ANOVA was performed for the determination of the significant variations of the groups compared to the control, which was followed by Dunnett’s Multiple Comparison Test. The significance level was set at p < 0.05.
Results
Behavioural tests
Novel tank test
Number of entries to the top zone (n)
The novel tank test was performed for studying variations in the number of entries to the top zone after fish were exposed to λ-cyhalothrin for a period of 28 days. The behaviour of the fish that was exposed to the three concentrations of λ-cyhalothrin (0.388 µg/L, 0.194 µg/L, 0.129 µg/L), were recorded and calculated on the 7th, 14th, 21st and 28th day and compared to the control. The number of entries in the control were 6 ± 1.15, 7 ± 1.45, 5 ± 0.33 and 5 ± 1.15 on the 7th, 14th, 21st and 28th day respectively. The two-way ANOVA revealed that there was a significant interaction (p < 0.05) (F (9, 24) = 4.330, p < 0.05) between concentration and time. Number of entries to the top zone was the maximum and increased significantly (p < 0.05) with values (15 ± 1.45, 23 ± 1.52, 26 ± 0.55, 30 ± 0.88) in 0.388 µg/L (T1) on all the days of exposure when compared to the control but there was no significant difference in the number of entries to the top zone in the 0.194 µg/L (T2) and 0.129 µg/L (T3) treatments on the 7th and 14th day when compared to the control in the Dunnett’s post-hoc analysis. In these treatments, it showed a significant increase only on the 21st and 28th day of exposure (p < 0.05). Moreover, an overall increasing trend in all the treatments was observed on all days when compared with the control as shown in Fig. 1a.
Chronic effects of λ-cyhalothrin on the behaviour of adult zebrafish for a period of 28 days. a No. of entries to the top zone (n) by fish during 6 min. b Time spent in the top zone (s) by the fish during 6 min. c Average speed of the fish (m/s) during 6 min. d Relative duration of time (%) spent by test fish near the stimulus fish. Data are shown as the Mean ± SE. Asterisks (*) indicate significant differences at p < 0.05, Total No. of Observations, n (Replicate × Treatment) = 3 × 5 = 15
Average speed (m/s)
The average speed of the tested fish exposed to three different sublethal concentrations was recorded and calculated. The average speed of individuals in the control was found to be 0.041 ± 0.003, 0.043 ± 0.002, 0.041 ± 0.002 and 0.040 ± 0.002 on the 7th, 14th, 21st and 28th day respectively. The two-way ANOVA revealed that there was a significant interaction (F (9, 24) = 3.518, p < 0.05)) between concentration and time. Dunnett Multiple Comparison test results showed that there was no significant difference (p > 0.05) in the average speed in all the treatments on the 7th day of exposure. However, a significant decrease (p < 0.05) in the average speed of the fish individuals was observed on the 14th, 21st and 28th day of exposure in all the treatments when compared to the control. A dose and time dependent decrease pattern in the average swimming speed of the tested fish was observed from graphical representation in all the treatments when compared to the control as shown in Fig. 1c.
Time spent in the top zone (s)
Chronic exposure to λ-cyhalothrin on individual zebrafish showed alterations in the time spent in the top zone as depicted in Fig. 1b. The time spent in the control was 95.69 ± 7.96, 94.90 ± 4.08, 97.67 ± 1.38 and 100.63 ± 2.25 on the 7th, 14th, 21st and 28th day respectively. The ANOVA results revealed that there was a statistically significant interaction F (9, 24) = 58.98, p < 0.05) between concentration and time. In the 0.388 µg/L (T1), the average speed was significantly increased (p < 0.05) (195.4 ± 7.3) on the 7th day while it significantly decreased with a value of 44.6 ± 3.0 on the final day of the exposure i.e. the 28th day when compared to the control. However, there was a non-significant difference (p > 0.05) on the 14th day and 21st day. In the 0.194 µg/L (T2) the time spent at the top significantly increased (p < 0.05) when compared to the control on all days but there was decreasing pattern as days passed (178.8 ± 4.9, 176.7 ± 1.5, 152.0 ± 2.4, 84.7 ± 2.3). In the 0.129 µg/L (T3), there was a significant increase (p < 0.05) only on the 7th, 21st, and 28th day when compared to the control and it decreased with time. Overall, from graphical representation, there was a reduction in the time spent at the top in the treatment groups as days passed.
Shoaling test (% age time)
As zebrafish is a shoaling teleost and prefer to stay in shoals, we studied the changes in its shoaling behaviour when it was exposed to λ-cyhalothrin for 28 days. The percentage time spent by the test fish near the stimulus fish was recorded and measured, when exposed to three sub-lethal concentrations of λ-cyhalothrin. The ANOVA results showed that there was a significant interaction (F (9, 24) = 18.25, p < 0.05)) between the treatment and time. The time spent near the conspecifics showed a significant reduction (p < 0.05) in the percentage spent near stimulus fish (76.76% to 45.68%) in the 0.388 µg/L (T1) when compared to the control (87.64 ± 1.76, 87.71 ± 1.30, 91.33 ± 2.82, 88.63 ± 1.46) on the 7th, 14th, 21st and 28th day respectively. There was a significant reduction (p < 0.05) in the 0.194 µg/L (T2) on the 21st and 28th day (71.04 to 58.74%) and in 0.129 µg/L (T3) on the 28th day (66.20%) only as shown in Fig. 1d. From graphical inspection, a decrease in the shoaling behaviour of the fish was observed in all the treatments, in 0.388 µg/L (T1) showing the maximum decrease.
Neurotoxic assay
Acetylcholine esterase enzyme activity (AChE) (EU/mg protein)
During sublethal exposure to λ-cyhalothrin for 28 days, Dunnett Multiple Comparison test results showed significant reduction in the AChE activity in brain of zebrafish (p < 0.05) in the 0.388 µg/L (T1), 0.194 µg/L (T2) and 0.129 µg/L (T3) groups with respect to the control group (Table 1). The values in the control group were (0.512 ± 0.018, 0.571 ± 0.016, 0.592 ± 0.018 and 0.567 ± 0.035 on the 7th, 14th, 21st and 28th day respectively. A decreasing pattern was observed in all the groups with days when compared with control. The highest inhibition occurred in the 0.388 µg/L (T1) group with a reduction from 0.140 ± 0.037 to 0.099 ± 0.014 on the 28th day, followed by the 0.194 µg/L (T2) group on the 21st day. An overall decreasing trend was observed in brain tissue AChE activity of zebrafish as shown in Fig. 2.
Antioxidant enzymes
Superoxide dismutase (U/mg protein)
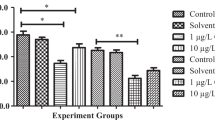
SOD in whole body of zebrafish upon chronic exposure to 3 sublethal concentrations of λ-cyhalothrin for 28 days showed significant alterations with increase in concentration and time. The two-way ANOVA results showed that there was a significant interaction (F (9, 48) = 18.06, p < 0.05)) between treatment and time. The SOD activity in the control was 6.64 ± 0.58, 6.02 ± 0.22, 6.78 ± 0.86 and 6.73 ± 0.83 on the 7th, 14th, 21st and 28th day respectively. Dunnett Multiple Comparison Test showed that there was a significant increase (p < 0.05) of 14.33 ± 0.83, 16.19 ± 0.19, 23.99 ± 0.89, 33.10 ± 1.55 in the 0.388 µg/L (T1) treatment, followed by the two treatments on all the days of exposure to the pesticide when compared to the control. Graphic representation showed that there existed an increasing pattern of SOD activity in all the treatments on all days of exposure as shown in Fig. 3a.
Chronic effects of λ-cyhalothrin on various antioxidant enzymes in adult zebrafish for a period of 28 days (a) SOD activity (b) CAT activity (c) GPx activity. Data are shown as the Mean ± SE. Asterisks (*) indicate significant differences at p < 0.05, Total No. of Observations, n (Replicate × Treatment) = 5 × 5 = 25
Catalase (U/mg protein)
The two-way ANOVA of CAT activity revealed that there was a significant interaction (F (9, 48) = 69.38, p < 0.05)) between treatment and time. The control group exhibited an enzyme activity level of 14.37 ± 0.81, 17.59 ± 0.81, 16.79 ± 1.72, 18.46 ± 0.94 at the 7th, 14th, 21st and 28th day respectively. Dunnett Multiple Comparison test results showed a significant increase (p < 0.05) in the 0.388 µg/L (T1) (54.30 ± 0.86, 76.23 ± 0.92, 86.59 ± 1.63, 116.30 ± 2.61) on the 7th, 14th, 21st and 28th day respectively when compared to the control as shown in Fig. 3b.
Glutathione peroxidase (U/mg protein)
GPx activity in the whole body of zebrafish following chronic exposure to 3 sublethal concentrations of λ-cyhalothrin for 28 days showed significant differences with increase in concentration and time. The two-way ANOVA of GPx activity revealed that there was a significant interaction (F (9, 48) = 35.44, p < 0.05)) between treatment and time. There was a significant increase in GPx activity (p < 0.05) in the highest treatment 0.388 µg/L (T1) on the 7th, 14th, 21st and 28th day when compared to the control. GPx activity in the control was found to be (2.21 ± 0.44, 3.67 ± 0.54, 3.61 ± 0.51, 3.58 ± 0.50) on the 7th, 14th, 21st and 28th day of exposure. It significantly increased (p < 0.05) from 5.85 ± 0.38 to 31.12 ± 1.55 in the 0.388 µg/L (T1) on the 7th day. After 20 days of exposure, there existed a significant increase (9.11 ± 1.05 to 21.2 ± 1.51) (p < 0.05) in the 0.194 µg/L (T2) and 0.129 µg/L (T3) (5.47 ± 0.31 to 18.2 ± 0.78) on the 21st and 28th day of exposure. However, there was a non-significant increase (p > 0.05) in the 0.194 µg/L (T2) and 0.129 µg/L (T3) treatments on the 7th and 14th day (Fig. 3c). An overall increasing pattern was observed in all the treatments with time when compared to the control. Enzyme activities data of SOD, CAT and GPx activities are presented in Table 2.
Discussion
Behaviour is a sensitive and useful toxicological indicator that can be studied to assess chronic effects on an organism. Consequently, assessing changes in behaviour and the toxicological effects through environmental risk assessment analysis proves to be a valuable approach for understanding the impact of toxicity on non-target organisms like fish (Venkateswara Rao et al. 2006).
Exposure to sublethal doses of pyrethroids often induces changes in the swimming behaviour of organisms, due to AChE inhibition which can reflect modifications in sensory and motor systems (Hopkins et al. 2003). Acetylcholine is a neurotransmitter that plays several roles at the Central Nervous System (CNS). After release, acetylcholine is rapidly removed from the synaptic cleft by acetylcholinesterase (AChE, EC 3.1.1.7), which belongs to the family of type B carboxylesterases and cleaves acetylcholine into choline and acetate. Our study revealed that the number of entries to the top zone increased in the 0.388 µg/L (T1) on all the days when compared to the control group. More specifically, the fish in the control group exhibited normal behaviour at all days of exposure. The abnormal changes in the zebrafish exposed to sub-lethal concentration of λ-cyhalothrin are time-dependent. This might be due to the inhibition of acetylcholinesterase (AChE) activity, leading to the accumulation of acetylcholine (Ach) in cholinergic synapses causing hyperstimulation. Moreover, the dopamine (DA) system is also crucial for influencing various behavioural and locomotor effects and it’s conceivable that disturbances in DA neurotransmission during a period of developmental adaptability could be a mechanism behind the locomotor alterations caused by pyrethroid exposure (Kung et al. 2015). Thus, a higher number of entries to the top indicates weaker adaptability to the new environment which increases the chance of predation (Wang et al. 2015). Any alterations in the fish’s behaviour can have consequences for its survival (Weber and Spieler 1994). Santhakumar and Balaji (2000) observed a similar trend in the surfacing to the top by Anabas testudineus exposed to Monocrotophos. Another finding explained the increase in the number of transitions to upper half in bisphenol A treated zebrafish, which resulted from the damage caused in CNS of the fish (Wang et al. 2015). Past studies have also reported a decrease in the AChE activity in zebrafish embryos when exposed to Fenpropathrin, Meothrin and Danitol (Sarmah et al. 2020). In addition, early-life exposure of rodents to permethrin was shown to impair glutamatergic signalling in vitro and in vivo (Carloni et al. 2012; Shafer et al. 2008), and another study linked overactivation of glutamatergic receptors to a depressive-like behaviour (i.e. reduced mobility) in rats (Cattani et al. 2017). Altogether, this indicates that upregulation of genes related to glutamatergic synaptic activity may support the behavioural effects observed in animals (Blanc et al. 2021).
A maximum decrease in the average speed of the fish was observed in the 0.388 µg/L (T1) group on the 28th day (time and dose-dependent) during prolonged exposure to λ-cyhalothrin. The decrease in the average speed of the fish is due to the reduction in AChE, leading to changes in the behaviour of the fish. Furthermore, this reduction in an average speed of the fish could pose significant risks to the fish in natural waters, by limiting their interaction with food organisms, predator avoidance, imbalance in its position in a shoal, and migration (Beauvais et al. 2000). The levels of neurotransmitters in the brain and the functioning of enzymes are known to be related to different behavioural states (Höglund et al. 2001). Type II pyrethroids are known neurotoxicants and have been demonstrated to cause dopaminergic neurodegeneration which alters behaviour, for example, in zebrafish larvae, deltamethrin significantly altered swimming activity which was mediated by dopaminergic dysfunction (Kung et al. 2015; Liu et al. 2018). Beauvais et al. (2000) also reported a similar type of decrease in swimming behaviour in rainbow trout when exposed to carbaryl which was explained due to reduced brain cholinesterase (ChE) activity. Our results are in line with a decrease in the swimming activity of Channa punctatus when exposed to endosulfan (Srivastava 2018). Similar reductions in average swimming speed were also reported when Gambusia affinis was exposed to sub-lethal concentrations of Chlorpyrifos (Rao et al. 2005). This was explained by the inhibition of AChE enzyme that led to the accumulation of ACh at synaptic junctions. Hence, the altered locomotor behaviour of fish could be due to the accumulation of ACh, which interrupted coordination between the nervous and muscular junctions.
Alterations in the time spent on the top zone were observed in the treatment groups on all days of exposure to λ- cyhalothrin. It increased in the 0.388 µg/L (T1) on the 7th day when compared to the control but a decrease was observed on the last day of exposure. The fish tried to explore the novel tank but gradually decreased with time due to the accumulation of acetylcholine at synaptic junctions which made the fish lethargic and increased the time spent at the bottom. The avoidance of the top area of the tank could indicate a behavioural stress response, and shelter-seeking behaviour (Schreck et al. 1997; Cantu et al. 2023). The walling behaviour (the inclination to closely associate with aquarium tank walls) is generally a stress response, which can have adverse effects on the profitability of aquaculture fish by increasing the incidences of injuries and deformities (Cobcroft and Battaglene 2009; Noble et al. 2012). Our results indicated that λ-cyhalothrin exerted an anxiogenic effect on zebrafish. This again might be due to the inhibition of the AChE enzyme which led to these behavioural changes, ultimately affecting overall fitness. A similar increase in time spent at the top was reported when zebrafish were treated with bisphenol A (Wang et al. 2015).
Group preference is very important for all shoaling teleosts like zebrafish and this behaviour can be associated with foraging, spawning security, and predator recognition (Pitcher 1993). Tamagno et al. (2023) investigated and found a reduction of shoaling behaviour when Zebrafish (Danio rerio) was exposed to household-based pyrethroids, that are prallethrin and transfluthrin to study diseases like Autism Spectrum Disorder (ASD) and Schizophrenia (SZP). The present study also showed similar reductions in shoaling behaviour in the various treatments. This is due to the decrease in AChE enzyme which leads to decreased movement and spent less time away from the stimulus fish. A reduction in the group preference may affect foraging success, mating, and decrease anti-predator benefits (Wang et al. 2015; Krause and Ruxton 2002). Loss of early predator detection, predator confusion, and risk dilution, could result in mortality due to increased predation (Pavlov and Kasumyan 2000). The mechanism of group preference is also associated with the dopaminergic system by the change in the level of dopamine (Cannon and Bseikri 2004). Consistent with our results, a decrease in the group preference was reported in zebrafish (Danio rerio) exposed to a xenoestrogen Nonylphenol (NP) (Xia et al. 2010). A similar decrease in shoaling behaviour was observed in juvenile rainbow trout (Oncorhynchus mykiss) when exposed to nonylphenol (Ward et al. 2006). Further studies should be done to study the changes in the levels of Serotonin which is involved in a range of behavioural functions like aggression and fear (Popova and Naumenko 2019).
In the present study, a maximum decrease in the AChE activity in the brain of zebrafish was observed in the 0.388 µg/L (T1) exposed to λ-cyhalothrin. This is due to the damaged CNS owing to the λ-cyhalothrin exposure. This led to excessive accumulation of acetylcholine in the synaptic cleft which disrupted the nerve activity, impairing physiological and behavioural functioning. Since the main action site of the pyrethroids is the nervous system, their action may not be restricted only to voltage-dependent Na+ channels. Our findings corroborated the observations of Vieira and Martinez (2018), where, a decrease in AChE activity in the muscle of a teleost Prochilodus lineatus at all concentrations when exposed to λ-cyhalothrin was reported. Other studies have demonstrated AChE inhibition in cyhalothrin-exposed fishes such as Oreochromis niloticus (Nile tilapia) (Amin et al. 2023) and Channa punctatus (Kumar et al. 2009).
Oxidative stress occurs when there is an imbalance between biological oxidants and antioxidants, leading to a disturbance in redox homoeostasis which might further lead to behavioural abnormalities in fish. It has been reported that increased oxidative damage can significantly impact animal performance, reducing fitness-related traits such as swimming ability and speed in evading predators as the energy is utilized in the detoxification process, which ultimately affects the behaviour of the animal (Loughland et al. 2022). SOD is the set of metalloenzymes that significantly transforms superoxide radicals to H2O2 and O2 and is the first enzyme to cope with oxyradicals (Kohen and Nyska 2002). It is an important antioxidant and comprises the important primary defence mechanism against the toxic properties of superoxide radicals in organisms (Nwani 2010). The present study demonstrated that Danio rerio exposed to λ-cyhalothrin showed concentration and time-dependent increase in SOD enzyme activities. Compared to the untreated group, there was a dose-dependent increase in antioxidant levels in the treated groups. The increase of antioxidant levels in the body of zebrafish indicates that λ-cyhalothrin and its metabolites were detoxified in the tissues by scavenging the overproduction of superoxide anions under the stress induced by λ-cyhalothrin. The findings of the present study were in accordance with that of Ezenwosu et al. (2021) who reported an increase in SOD activity in Clarius gariepinus when it was exposed to λ-cyhalothrin. Increased SOD activity was reported in Clarius gariepinus exposed to Fenthion, an organophosphate pesticide (Somdare 2015; Nnadi 2016). An increase in SOD activity in rainbow trout and Channa punctatus when exposed to Chlorpyrifos and malathion respectively was reported (Yonar 2012; Bharti and Rasool 2021). However, an increase or inhibition of antioxidant enzymes can be influenced by the intensity and duration of stressors, as well as the vulnerability of the fish species being exposed to chemicals (Oruc and Usta 2007). Changes in the levels of antioxidant enzyme activities could be used as biomarkers in different aquatic organisms (Orbea et al. 2002).
In fishes, catalase (CAT) is a very important antioxidant enzyme and is considered a sensitive indicator of oxidative stress; its activity fluctuates according to oxidative potential (Roberts and Oris 2004; Pi et al. 2010). In the present study, an elevation in CAT activity was observed in all the exposed fishes on the 7th, 14th, 21st and 28th day, where there was a maximum increase in 0.388 µg/L (T1) on the 28th day. This is due to an increase in the generation of superoxide anions and H2O2 levels. Therefore, a significant increase in the enzyme activity reflects a response to increased oxidative stress induced by λ-cyhalothrin. Ezenwosu et al. (2021) studied the effect of λ-cyhalothrin on antioxidant enzymes and gonad histoarchitecture toxicity potency in Clarius gariepinus and reported an increase in the catalase activity (CAT) as λ-cyhalothrin caused production of harmful molecules called free radicals beyond the protective capacity of the antioxidant defences. The increase in CAT activity may be due to the SOD-stimulated H2O2 production since CAT is responsible for the detoxification of hydrogen peroxide to water. A similar increase in CAT activity was observed in different tissues of rainbow trout when exposed to imidacloprid (Topal et al. 2017).
Gpx catalyzes the reduction of hydrogen peroxide and lipid peroxide (LOOH). An elevation in GPx activity following oxidative stress signifies the fish’s capacity to adapt to oxidative conditions subsequent to exposure to pollutants (Lenartova et al. 1997). In the present study, an increasing trend in GPx activity in λ-cyhalothrin exposed fish was reported and this is attributed to the neutralization of hydrogen peroxide and inhibition of ROS oxidative stress. Oxidative stress leads to an increase in GPx activity, likely indicating an adjustment to the oxidative conditions that the fish has been exposed. The GPx was seen significantly increased in 0.388 µg/L (T1) on all days of exposure followed by a significant increase in all other treatments from day 21 onwards which indicated that GPx fluctuation activities were influenced. Past studies reported that sublethal concentrations of fenthion in the brain of Oreochromis niloticus, caused elevation in GPx activity after 24 h (Piner et al. 2007). The induction of GPx activity could be related to the scavenging of H2O2 and lipid peroxides by utilizing GSH. Sayeed et al. (2003) also reported similar findings in Channa punctatus exposed to deltamethrin. It increased Glutathione peroxidase (GPx) activity in the liver and kidney but decreased the same in gill tissue.
Conclusion
The investigation demonstrated that λ-cyhalothrin, induced neurotoxicity leading to behavioural change even at sub-lethal concentrations. It showed marked deviations in behavioural patterns, and weaker adaptability to the novel environment, (both exploratory and shoaling behaviour) which were linked to the reduction of AChE activity in the brain of the zebrafish. These alterations in the behavioural patterns can have significant consequences on the overall fitness of the organism as well as at the population level of the species. Furthermore, antioxidant enzyme activities (SOD, CAT, and GPx) showed variations when compared to the untreated group, which could have also led to behavioural alterations. Further research on the relationship between antioxidant enzymes and fish behaviour with pesticide exposure can be carried out. Future research endeavours should be undertaken to conduct additional tests, such as the T-maze test, mirror biting test, and light/dark tests, to enhance our comprehension of the impact of this pesticide on fish. The present study can provide a baseline for further studies. Since λ-cyhalothrin is widely used globally, it is crucial to establish strict regulations concerning its usage, imposing restrictions and determining permissible limits. Additionally, comprehensive investigations should be conducted, encompassing molecular and genotoxicity analyses, as well as exploring other stress-related enzymes like cortisol and dopamine and examining haematological parameters.
References
Afzal S, Abdul Manap AS, Attiq A, Albokhadaim I, Kandeel M, Alhojaily SM (2023) From imbalance to impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front Pharmacol 14:1269581. https://doi.org/10.3389/fphar.2023.1269581
Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–5. https://doi.org/10.2478/v10102-009-0001-7
American Public Health Association (APHA) (2019) Standard methods for the examination of water and wastewater, 12th edn. Washington DC
Amin M, Yousuf M, Attaullah M, Ahmad N, Azra MN, Lateef M, Naeem M (2023) Cholinesterase activity as a potential biomarker for neurotoxicity induced by pesticides in vivo exposed Oreochromis niloticus (Nile tilapia): assessment tool for organophosphates and synthetic pyrethroids. Environ Technol 44(14):2148–2156. https://doi.org/10.1080/09593330.2021.2024276
Amweg EL, Weston DP, Ureda NM (2005) Use and toxicity of pyrethroid pesticides in the Central Valley, California, USA. Environ Toxicol Chem 24:966–972. https://doi.org/10.1897/04-146r1.1
Anderson TA, Salice CJ, Erickson RA, McMurry ST, Cox SB, Smith LM (2013) Effects of land use and precipitation on pesticides and water quality in playa lakes of the southern high plains. Chemosphere 92:84–90. https://doi.org/10.1016/j.chemosphere.2013.02.054
Arulraj JS, Pandurengan P, Arasan S, Gopalrajan S, Paulraj J (2019) Acute toxicity of lambda-cyhalothrin and the histopathological changes of gill and liver tissues of tilapia (Oreochromis niloticus). J Coast Res 235:238, https://www.jstor.org/stable/26820355
Audira G, Sampurna BP, Juniardi S, Liang ST, Lai YH, Hsiao CD (2018) A versatile setup for measuring multiple behaviour endpoints in zebrafish. Inventions 3:75. https://doi.org/10.3390/inventions3040075
Aydin R, Köprücü K, Dörücü M, Köprücü SŞ, Pala M (2005) Acute toxicity of synthetic pyrethroid cypermethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Aquac Int 13:451–458. https://doi.org/10.1007/s10499-005-0615-5
Beauvais L, Jones SB, Brewer SK, Little EE (2000) Physiological measures of neurotoxicity of diazinon and malation to larval rainbow trout (Oncorhynchus mykiss) and their correlation with behavioural measures. Environ Toxicol Chem 19(7):1875–1880. https://doi.org/10.1002/etc.5620190722
Bedi JS, Amit Kumar AK, Gill JPS (2016) Ignorant use of pesticides may turn to a disaster for fish farmers: a report. Haryana Vet 55(1):100–102
Bharti S, Rasool F (2021) Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish. Toxicol Rep. 8:443–455. https://doi.org/10.1016/j.toxrep.2021.02.018
Blanc M, Antczak P, Cousin X, Grunau C, Scherbak N, Rüegg J, Keiter SH (2021) The insecticide permethrin induces transgenerational behavioural changes linked to transcriptomic and epigenetic alterations in zebrafish (Danio rerio). Sci Total Environ 779:146404. https://doi.org/10.1016/j.scitotenv.2021.146404
Bownik A, Kowalczyk M, Bańczerowski J (2019) Lambda-cyhalothrin affects swimming activity and physiological responses of Daphnia magna. Chemosphere 216:805–811. https://doi.org/10.1016/j.chemosphere.2018.10.192
Cannon CM, Bseikri MR (2004) Is dopamine required for natural reward? Physiol Behav 81:741–748. https://doi.org/10.1016/j.physbeh.2004.04.020
Cantu E, Rivera M, Lacy B, Rahman MS (2023) Effects of short-term exposure to a pesticide mixture on free-swimming behavior in goldfish, Carassius auratus. J Hazard Mater Adv 11:100350. https://doi.org/10.1016/j.hazadv.2023.100350
Carloni M, Nasuti C, Fedeli D, Montani M, Amici A, Vadhana MD, Gabbianelli R (2012) The impact of early life permethrin exposure on development of neurodegeneration in adulthood. Exp Gerontol 47(1):60–6. https://doi.org/10.1016/j.exger.2011.10.006
Cattani D, Cesconetto PA, Tavares MK, Parisotto EB, De Oliveira PA, Rieg CE, Leite MC, Prediger RD, Wendt NC, Razzera G, Wilhelm Filho D (2017) Developmental exposure to glyphosate-based herbicide and depressive-like behaviour in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 387:67–80. https://doi.org/10.1016/j.tox.2017.06.001
Chandola M, Rathore S, Kumar B (2011) Indigenous pest management practices prevalent among hill farmers of Uttarakhand. Indian J Traditi Knowl http://nopr.niscpr.res.in/handle/123456789/11510
Cobcroft JM, Battaglene SC (2009) Jaw malformation in striped trumpeter Latris lineata larvae linked to walling behaviour and tank colour. Aquaculture 289(3-4):274–282. https://doi.org/10.1016/j.aquaculture.2008.12.018
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 4:45–78. http://nopr.niscpr.res.in/handle/123456789/15379
Domagalski JL, Weston DP, Zhang M, Hladik M (2010) Pyrethroid insecticide concentrations and toxicity in streambed sediments and loads in surface waters of the San Joaquin Valley, California, USA. Environ Toxicol Chem: Int J 29(4):813–23. https://doi.org/10.1002/etc.106
Ezenwosu SU, Nnamonu EI, Odo GE, Ikele BC, Ani OC (2021) Evaluation of lambda-cyhalothrin oxidative stress and gonad histoarchitecture toxicity potency in Clarias gariepinus. J Basic Appl Zool 82:1–11. https://doi.org/10.1186/s41936-020-00201-y
Fitzgerald JA, Kirla KT, Zinner CP, Vom Berg CM (2019) Emergence of consistent intra-individual locomotor patterns during zebrafish development. Sci Rep 9(1):13647. https://doi.org/10.1038/s41598-019-49614-y
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem Int J 20(1):37–45. https://doi.org/10.1002/etc.5620200104
Gerlai R (2003) Zebra fish: an uncharted behaviour genetic model. Behav Genet 33:461–468. https://doi.org/10.1023/a:1025762314250
Höglund E, Kolm N, Winberg S (2001) Stress-induced changes in brain serotonergic activity, plasma cortisol and aggressive behaviour in Arctic charr (Salvelinus alpinus) is counteracted by L-DOPA. Physiol Behav 74:381–389. https://doi.org/10.1016/s0031-9384(01)00571-6
Hopkins WA, Snodgrass JW, Staub BP, Jackson BP, Congdon JD (2003) Altered swimming performance of benthic fish (Erimyzon sucetta) exposed to contaminated sediments. Arch Environ Contam Toxicol 44:383–389. https://doi.org/10.1007/s00244-002-2030-5
Howe K, Clark M, Torroja C et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. https://doi.org/10.1038/nature12111
Khafaga AF, Naiel MA, Dawood MA, Abdel-Latif HM (2020) Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp (Cyprinus carpio). Aquat Toxicol 228:1–2. https://doi.org/10.1016/j.aquatox.2020.105624
Kohen R, Nyska A (2002) Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–650. https://doi.org/10.1080/01926230290166724
Konstantinou IK, Hela DG, Albanis TA (2006) The status of pesticide pollution in surface waters (rivers and lakes) of Greece. Part I. Review on occurrence and levels. Environ Pollut 141(3):555–570. https://doi.org/10.1016/j.envpol.2005.07.024
Köprücü K, Aydın R (2004) The toxic effects of pyrethroid deltamethrin on the common carp (Cyprinus carpio L.) embryos and larvae. Pestic Biochem Physiol 80(1):47–53. https://doi.org/10.1016/j.pestbp.2004.05.004
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford, UK
Kumar A, Rai DK, Sharma B, Pandey RS (2009) λcyhalothrin and cypermethrin induced in vivo alterations in the activity of acetylcholinesterase in a freshwater fish, Channa punctatus (Bloch). Pestic Biochem Physiol 93:96–99. https://doi.org/10.1016/j.pestbp.2008.12.005
Kumar S, Kaushik G, Villarreal-Chiu JF (2014) Scenario of organophosphate pollution and toxicity in India: a review. Environ Sci Pollut Res 23:9480–949. https://doi.org/10.1007/s11356-016-6294-0
Kung TS, Richardson JR, Cooper KR, White LA (2015) Developmental deltamethrin exposure causes persistent changes in dopaminergic gene expression, neurochemistry, and locomotor activity in zebrafish. Toxicol Sci 146(2):235–43. https://doi.org/10.1093/toxsci/kfv087
Küster E, Altenburger R (2006) Comparison of cholin-and carboxylesterase enzyme inhibition and visible effects in the zebra fish embryo bioassay under short-term paraoxon-methyl exposure. Biomarkers 11(4):341–54. https://doi.org/10.1080/13547500600742136
Lenartova V, Holovska K, Rafael Pedrajas J, Martinez Lara E, Peinado J, Lopez Barea J, Rosival I, Kosuth P (1997) Antioxidant and detoxifying fish enzymes as biomarkers of river pollution. Biomarkers 2(4):247–252. https://doi.org/10.1080/135475097231625
Liu X, Zhang Q, Li S, Mi P, Chen D, Zhao X, Feng X (2018) Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 199:16–25. https://doi.org/10.1016/j.chemosphere.2018.01.176
Loughland I, Lau GY, Jolly J, Seebacher F (2022) Rates of warming impact oxidative stress in zebrafish (Danio rerio). J Exp Biol 225(6):jeb243740
Nieradko-Iwanicka B, Konopelko M (2020) Effect of Lambda cyhalothrin on locomotor activity, memory, selected biochemical parameters, tumor necrosis factor α, and interleukin 1ß in a mouse model. Int J Environ Res Public Health 17(24):9240. https://doi.org/10.3390/ijerph17249240
Nnadi JU (2016) Effects of exposure of termex on morphological variations and oxidative stress parameters in juvenile Clarias gariepinus. Dissertation, University of Nigeria Nsukka
Nnamonu EI, Mgbenka BO, Mbegbu EC (2019) Fertility enhancing potency of omega-3 fatty acids in male rats. Braz Arch Biol Technol 62:19180374. https://doi.org/10.1590/1678-4324-2019180374
Noble C, Cañon Jones HA, Damsgård B, Flood MJ, Midling KØ, Roque A, Cottee SY (2012) Injuries and deformities in fish: their potential impacts upon aquacultural production and welfare. Fish Physiol Biochem 38:61–83. https://doi.org/10.1007/s10695-011-9557-1
Nwani CD, Lakra WS, Nagpure NS, Kumar R, Kushwaha B, Srivastava SK (2010) Toxicity of the herbicide atrazine: effects on lipid peroxidation and activities of antioxidant enzymes in the freshwater fish Channa punctatus (Bloch). Int j environ res public health 7(8):3298–3312. https://doi.org/10.3390/ijerph7083298
OECD (2019) Guidance Document on Aquatic Toxicity Testing of Difficult Test Chemicals and Mixtures. Series on Testing and Assessment No. 23, OECD, Paris.
Olson KR, Gao Y, DeLeon ER, Arif M, Arif F, Arora N, Straub KD (2017) Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox biol 12:325–339. https://doi.org/10.1016/j.redox.2017.02.021
Orbea A, Ortiz-Zarragoitia M, Solé M, Porte C, Cajaraville MP (2002) Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish from the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat Toxicol 58:75–98. https://doi.org/10.1016/S0166-445X(01)00226-0
Oros DR, Werner I (2005) Pyrethroid Insecticides: An Analysis of Use Patterns, Distributions, Potential Toxicity and Fate in the Sacramento-San Joaquin Delta and Central Valley. White Paper for the Interagency Ecological Program. SFEI Contribution 415. San Francisco Estuary Institute, Oakland, CA
Oruc EO, Usta D (2007) Evaluation of oxidative stress responses and neurotoxicity potential of diazinon in different tissues of Cyprinus carpio. Environ Toxicol Pharmacol 23:48–55. https://doi.org/10.1016/j.etap.2006.06.005
Pavlov DS, Kasumyan AO (2000) Patterns and mechanisms of schooling behaviour in fish: a review. J Ichthyol 40(2):S163
Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Andersen ME (2010) ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 244(1):77–83. https://doi.org/10.17582/journal.pjz/20181103091108
Piner P, Sevgiler Y, Üner N (2007) In vivo effects of fenthion on oxidative processes by the modulation of glutathione metabolism in the brain of Oreochromis niloticus. Environ Toxicol Int J 22(6):605–612. https://doi.org/10.1002/tox.20286
Pitcher TJ (1993) Functions of shoaling behaviour in teleosts. In: The behaviour of teleost fishes Boston, MA: Springer US, 294–337
Pokhrel H, Sarmah R, Bhagabati SK, Dutta R, Ahmed AM, Nath D, Kalita B (2022) Effects of imidacloprid on histology and mRNA levels of HSP70 and CYP1A gene to a standard non-targeted test animal, Cyprinus carpio. Indian J Anim Res 56(2):167–174. https://doi.org/10.18805/IJAR.B-4706
Popova NK, Naumenko VS (2019) Neuronal and behavioural plasticity: The role of serotonin and BDNF systems tandem. Expert Opin Ther Targets 23:227–239. https://doi.org/10.1080/14728222.2019.1572747
Rao JV, Begum G, Pallela R, Usman PK, Rao RN (2005) Changes in behaviour and brain acetylcholinesterase activity in mosquito fish, Gambusia affinis in response to the sub-lethal exposure to chlorpyrifos. Int J Environ Res Public Health 2(3):478–483. https://doi.org/10.3390/ijerph2005030013
Roberts AP, Oris JT (2004) Multiple biomarker response in rainbow trout during exposure to hexavalent chromium. Comp Biochem Physiol Part C Toxicol Pharmacol 138:221–228. https://doi.org/10.1016/j.cca.2004.08.006
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590. https://doi.org/10.1126/science.179.4073.588
Santhakumar M, Balaji M (2000) Acute toxicity of an organophosphorus insecticide monocrotophos and its effects on behaviour of an air-breathing fish, Anabas testudineus (Bloch). J Environ Biol 21(2):121–123
Sarmah R, Kanta Bhagabati S, Dutta R, Nath D, Pokhrel H, Mudoi LP, Kuotsu K (2020) Toxicity of a synthetic phenolic antioxidant, butyl hydroxytoluene (BHT), in vertebrate model zebrafish embryo (Danio rerio). Aquac Res 51(9):3839–3846
Sayeed I, Parvez S, Pandey S, Bin-Hafeez B, Haque R, Raisuddin S (2003) Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotoxicol Environ Saf 56:295–301. https://doi.org/10.1016/s0147-6513(03)00009-5
Schreck CB, Olla BL, Davis MW (1997) behavioural responses to stress. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB (ed) Fish stress and health in aquaculture. Cambridge University Press, Cambridge, UK, p 145–170. https://doi.org/10.1016/j.hazadv.2023.100350
Shafer TJ, Rijal SO, Gross GW (2008) Complete inhibition of spontaneous activity in neuronal networks in vitro by deltamethrin and permethrin. Neurotoxicology 29(2):203–12. https://doi.org/10.1016/j.neuro.2008.01.002
Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, Handa N, Thukral AK (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1:1–16. https://doi.org/10.1007/s42452-019-1485-1
Somdare PO (2015) Oxidative stress biomarkers, haematological parameters and histopathological changes in the african catfish, Clarias gariepinus exposed to an organophosphate pesticide, fenthion. Dissertation, University of Nigeria, Nsukka
Srivastava GHDN (2018) Behavioural alterations in Channa punctatus after exposure to endosulfan followed by subsequent recovery. Int J Fish Aquat Stud 6:51–55
Starner K (2007) Data queried from the Department of Pesticide Regulation Surface Water Monitoring Database
Stehle S, Schulz R (2015) Agricultural insecticides threaten surface waters at the global scale. Proc Natl Acad Sci 112:5750–5755. https://doi.org/10.1073/pnas.1500232112
Takahara S, Hamilton HB, Neel JV, Kobara TY, Ogura Y, Nishimura ET (1960) Hypocatalasemia: a new genetic carrier state. J Clin Investig 39:610–619. https://doi.org/10.1172/jci104075
Tamagno WA, Alves C, Pompermaier A, Amaral FUÍ, Freddo N, Soares SM, Barcellos LJG (2023) Household based-pyrethroids on adult zebrafish (Danio rerio) exert behavioural and cholinergic changes in different brain regions. Neurotoxicology 96:19–27. https://doi.org/10.1016/j.neuro.2023.02.011
Tan JK, Nazar FH, Makpol S, Teoh SL (2022) Zebrafish: a pharmacological model for learning and memory research. Molecules 27(21):7374. https://doi.org/10.3390/molecules27217374
Topal F, Gulcin I, Dastan A, Guney M (2017) Novel eugenol derivatives: Potent acetylcholinesterase and carbonic anhydrase inhibitors. Int J Biol Macromol 94:45–851. https://doi.org/10.1016/j.ijbiomac.2016.10.096
Tsaboula A, Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Papadopoulou-Mourkidou E (2016) Environmental and human risk hierarchy of pesticides: a prioritization method, based on monitoring, hazard assessment and environmental fate. Environ Int 91:78–93
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189. https://doi.org/10.1016/j.ecoenv.2005.03.013
Venkateswara Rao J, Begum G, Jakka NM, Srikanth K, Nageswara Rao R (2006) Sublethal effects of profenofos on locomotor behaviour and gill architecture of the mosquito fish, Gambusia affinis. Drug Chem Toxicol 29:255–267. https://doi.org/10.1080/01480540600651543
Vieira CED, dos Reis Martinez CB (2018) The pyrethroid λ-cyhalothrin induces biochemical, genotoxic, and physiological alterations in the teleost Prochilodus lineatus. Chemosphere 210:958–967. https://doi.org/10.1016/j.chemosphere.2018.07.115
Wang J, Wang X, Xiong C, Liu J, Hu B, Zheng L (2015) Chronic bisphenol A exposure alters behaviours of zebrafish (Danio rerio). Environ Pollut 206:275–281. https://doi.org/10.1016/j.envpol.2015.07.015
Wang W, Cai DJ, Shan ZJ, Chen WL, Poletika N, Gao XW (2007) Comparison of the acute toxicity for gamma-cyhalothrin and lambda-cyhalothrin to zebra fish and shrimp. Regul Toxicol Pharmacol 47:184–188. https://doi.org/10.1016/j.yrtph.2006.09.002
Ward AJ, Duff AJ, Currie S (2006) The effects of the endocrine disrupter 4-nonylphenol on the behaviour of juvenile rainbow trout (Oncorhynchus mykiss). Can J Fish Aquat Sci 63:377–382. https://doi.org/10.1139/f05-223
Weber DN, Spieler RE (1994) Behavioural mechanisms of metal toxicity in fishes. In: Malins DC, Ostrander GK (ed) Aquatic Toxicology: Molecular, Biochemical, and Cellular Perspectives. CRC Press, London, UK, p 421–467
Weston DP, Holmes RW, Lydy MJ (2009) Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut 157:287–294. https://doi.org/10.1016/j.envpol.2008.06.037
Woolf NJ, Butcher LL (2011) Cholinergic systems mediate action from movement to higher consciousness. Behav Brain Res 221(2):488–498. https://doi.org/10.1016/j.bbr.2009.12.046
World Health Organization (1990) Cyhalothrin-Environmental Health Criteria, 99; Geneva, Switzerland
Xia J, Niu C, Pei X (2010) Effects of chronic exposure to nonylphenol on locomotor activity and social behaviour in zebrafish (Danio rerio). J Environ Sci 22(9):1435–1440. https://doi.org/10.1016/S1001-0742(09)60272-2
Yonar ME (2012) The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol 32:994–1001. https://doi.org/10.1016/j.fsi.2012.02.012
Acknowledgements
The authors are thankful to Dr. Pradip Chandra Bhuyan, Dean of College of Fisheries, Raha, Assam Agricultural University, Jorhat, Assam, India and Directorate of Post Graduate Studies, Assam Agricultural University, for their constant support and guidance.
Author information
Authors and Affiliations
Contributions
DS, RS and KM conceptualized and designed the study. DS, HP, and RS acquired and analyzed the data and DS, KM, RS, SKB, ANP and DKS interpreted the data and drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
During the experiment, all the guidelines of ‘Committee for the Purpose of Control & Supervision of Experiments on Animals (CPCSEA) for Experimentation on Fishes’ by the Ministry of Fisheries, Animal Husbandry & Dairying, Govt. of India (2021) were strictly followed. All the guidelines were approved by institutional research advisory committee.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, D., Sarmah, R., Sarmah, R. et al. Lambda-Cyhalothrin induced behavioural, neurotoxic and oxidative stress on vertebrate model Danio rerio (Hamilton-Buchanan 1822). Ecotoxicology 33, 663–676 (2024). https://doi.org/10.1007/s10646-024-02763-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-024-02763-x