Abstract
Concerted efforts are being made to understand the current and past processes that have shaped Antarctic biodiversity. However, high rates of new species discoveries, sampling patchiness and bias make estimation of biodiversity there difficult. Antarctic continental shelf benthos is better studied in the Ross, Weddell and Scotia seas, whilst the Amundsen Sea has remained biologically unexplored largely because of severe ice conditions year-round. Here we report results from examination of the first benthic biological samples taken from the Amundsen Sea. We compare relative abundance, taxonomic richness and faunal composition of isopod families, and genera and species within two example families (i.e. Desmosomatidae and Nannoniscidae) from the Amundsen Sea with complementary sampling from the Scotia Sea. Benthic samples were taken from inner and outer Pine Island Bay (eastern Amundsen Sea) sites using an epibenthic sledge at 500 m. Similar samples were also collected from 15 Scotia arc sites at 160- to 500-m depths. The relative abundance of isopods in the Amundsen Sea samples was high and surprisingly less variable than across samples in the Scotia Sea. The abundance structure of isopods at family level was compared across different Antarctic seas. We found that in the Amundsen, Scotia and Ross Seas two families dominated abundance. In contrast, isopod abundance reported in the literature from Weddell Sea samples was much more evenly distributed across families. The Amundsen continental shelf isopod fauna appears to be rich, with 96% of individuals belonging to currently undescribed species. Most of the genera have either been described or found elsewhere, but for many of these genera it is the first time they have been recorded away from the Antarctic continental slope or deep sea. The Amundsen Sea assemblages differed greatly from the Scotia Sea sites in terms of both composition and (species and generic) richness. This was largely due to high consistency between samples compared with the highly variable Scotia Sea samples. Thorough biological analyses implementing well-structured geographic sampling regimes and the application of phylogeographic analyses on a variety of taxa are required to further explore the geographic structure of biodiversity and the evolutionary history of the Amundsen Sea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The continental shelves around Antarctica are very deep (depressed to 1,000 m in places) and geographically extensive (hundreds of kilometres wide). The marine fauna of these shelves is rich in species for many higher taxa, if compared with temperate and non coral-reef tropic areas (Clarke and Johnston 2003; Gutt et al. 2004). Antarctic continental shelf fauna has several unique features: the ectothermic community is typically slow growing and developing, it has few errant skeleton-breaking predators, and several key groups are absent or very rare, such as sharks, brachyuran crabs, acorn barnacles, and non-indigenous species (Aronson and Blake 2001; Tavares and de Melo 2004). Besides the nature of the communities living there, Antarctic continental shelves differ strikingly from non-polar continental shelves in that they are naturally (rather than anthropogenically) highly disturbed environments subjected to intensive ice scouring (e.g. Gutt and Piepenburg 2003; Smale et al. 2008). They are also unusual in their physical constancy (e.g. in terms of temperature and salinity) and great connectivity with deeper waters and seabed, for example, due to lack of thermocline and production of Antarctic bottom water (see Carmack 1977).
There have now been many decades of biological sampling on the continental shelf around the Scotia arc, Ross and Weddell Seas. However, even within these seas there are areas (such as around the East Antarctic Peninsula and Bouvet Island) where few samples have been taken and species accumulation curves are still very steep (e.g. Arntz et al. 2006). At one of the best studied localities, the continental shelf of the South Orkney Islands (Scotia Sea), new samples increase total recorded richness at about seven species per trawl (Barnes et al. 2008). Other seas around Antarctica, such as the Bellingshausen Sea and those along the East Antarctic coastline, have been reasonably well sampled in a few places but overall their shelf biodiversity is still poorly known. In the Amundsen Sea, a large area spanning hundreds of kilometres and >40 degrees of longitude of non-peninsular West Antarctica, no benthic or pelagic shelf samples have ever been taken, so the biodiversity of this region is completely unknown. Previously, the closest samples had been taken in the South Bellingshausen Sea by scientific cruises of the Spanish BENTART (e.g. Saiz et al. 2008) or the United States Eltanin programmes.
Isopod crustaceans can be a major component of the Antarctic fauna, though they seem to be particularly rich in the Southern Ocean deep sea (see Brandt et al. 2007a). Isopod taxonomy has been studied in the Southern Ocean (defined as areas south of the Polar Front, see Clarke and Johnston 2003) for more than a century (e.g. von Suhm, 1876; Brandt 1991; Castello 2004; Brandt et al. 2007b), yet surprisingly little work has focused on assessing shelf biodiversity and distribution patterns across taxonomic levels and geographic regions. Despite differences in taxonomic resolution, sampling devices and protocol amongst studies, current literature shows that Southern Ocean isopods can be considered rich at local and regional scales and across taxonomic levels from family to species, though with considerable variability (Brandt 2004; Choudhury and Brandt 2007; Kaiser et al. 2007). Although it is clear that in many places around Antarctica the rate of new species discovery and description is still high, perhaps the most limiting aspect of our understanding of Southern Ocean biodiversity to date is the scale at which most variability in richness occurs (see Kaiser et al. 2007). The extent to which patterns in peracarids are reflected in other taxa is not clear but certainly there is a wide literature on Antarctic richness and variability in abundance and richness across taxa (see, e.g., Clarke and Johnston 2003; Gutt and Starmans 2003; Saiz et al. 2008).
Using a widely studied taxon in initial investigations of poorly sampled areas provides a potentially more powerful insight due to greater comparability with other areas. High ubiquity, abundance and taxonomic richness of peracarid crustaceans, such as isopods, across marine habitats and depths has led to their frequent use as a model group for investigating biodiversity structure and distribution patterns in macrobenthic communities (e.g. Castello 2004; Brandt et al. 2007b; Choudhury and Brandt 2007). As they are generally brooders and direct developers (with the exception of few parasitic taxa), it is assumed that benthic isopods are limited in their dispersal ability. Thus, peracarids should be ideal for investigations of geographic and bathymetric distributions.
The current study, based on the BIOPEARL (Biodiversity, Phylogeny, Evolution and Adaptive Radiation of Life in Antarctica) 2 cruise to the Amundsen Sea, reports isopod biodiversity from the first benthic collections in the least explored Antarctic continental shelf. We compare levels of relative abundance and taxonomic richness as well as faunal composition across three taxonomic levels (family, genus and species) at 500-m depth with samples taken from a better-sampled shelf, the Scotia Sea. Due to subglacial erosions (Lowe and Anderson 2002), the near year-round cover of ice and high number of icebergs in the Amundsen Sea, we expected that soft sediments would be infrequent and that isopod (and generally macrobenthic) abundance would be lower than in the Scotia Sea. We considered that relative abundance would vary considerably and similarly across spatial scales (i.e. be variable in distribution) as in deeper waters of the region (Kaiser et al. 2007). Through rarity of suitable habitat, poor surface-generated productivity and frequent ice-driven disturbance we suggested that richness would be low. Finally, we expected that the taxa present would probably resemble assemblages in the nearby Antarctic Peninsula, given proximity and both areas being influenced by overlying Circumpolar Deep Water.
Material and methods
Study area
Amundsen Sea
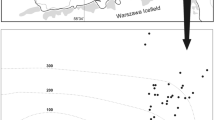
The eastern Amundsen Sea (Pine Island Bay, Fig. 1a,b) is a highly dynamic area across evolutionary and ecological time scales (due to major glaciations and deglaciations, intense iceberg scouring, primary productivity, meltwater flow, changes in overlying water mass and differences in salinity and temperature). The seabed, especially close to the continent edge (Fig. 1b), was shaped by past (glacial) and present ice conditions, resulting in a mosaic of cavities, drainage channels, valleys and furrows, and the topography smoothes towards the continent edge. The Amundsen Sea (Fig. 1b) is characterised by almost perennial sea ice cover and ice shelves still cover large parts of the continental shelf there. The continental shelf is generally wide, reaching >200 km off Thurston Island and 450 km off the ice front of Pine Island Glacier (Lowe and Anderson 2002). The bathymetry of the Amundsen continental shelf appears to be complex (Lowe and Anderson 2002; unpublished BIOPEARL 2 cruise data; Fig. 2). The inner shelf of Pine Island Bay is quite uneven and exhibits many depressions exceeding 500-m depth (Lowe and Anderson 2002). Some of these troughs reach nearly 1,700 m and were probably formed by glacial drainages (Lowe and Anderson 2002). Towards the outer shelf the water depth decreases (to 350-450 m) and the topography becomes smoother.
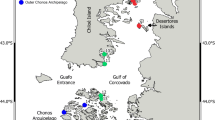
a The sample region of West Antarctica, the Scotia and Amundsen Seas, showing the epibenthic sledge stations of BIOPEARL 1 (JR 144) cruise (white circles): Livingston and Deception Is. (two sites), Elephant I., Powell Basin (South Orkney Is.), Southern Thule (South Sandwich Is.), South Georgia, Shag Rocks. b The boxed area in a at a larger scale showing detail of Amundsen Sea sites (black circles): Pine Island Bay and the position of epibenthic sledge stations sampled during the BIOPEARL 2 (JR 179) cruise
The central Amundsen Sea is important in that it represents an area for exchange of water masses between the Pacific and Atlantic oceans (Grotov et al. 1998) due to the flow of the Antarctic Circumpolar Current (ACC). The oceanography of the Amundsen shelf waters is generally less complex than that of the Ross and Weddell Seas as it lacks shelf and bottom water formation. Hence, water temperatures there are lower than measured in the adjacent Bellingshausen Sea but warmer than Ross Sea waters (Thoma et al. 2008). A westward coastal current dominates the current system of the Amundsen Sea shelf and slope. The coastal current is suggested to derive from the Antarctic Polar Slope Current, transporting cold waters from the Weddell into the Amundsen Sea (Grotov et al. 1998). However, in contrast to the cold bottom water generated there, the continental shelf of the Amundsen Sea is also periodically and unpredictably flooded by warmer Circumpolar Deep Water (Thoma et al. 2008).
Scotia Sea
The Scotia Sea (Fig. 1a), one of the most productive areas in the Southern Ocean, is bounded by the Scotia arc, and combines archipelagos, seamounts, and submerged banks separated by relatively young (30 Ma) deep seafloor (Barker 2001). Islands and shelves of the Scotia arc differ greatly in, for example, age, origin (e.g. oceanic, continental, volcanic), size and distance to the continent. These differences in, for example, geological history, degree of isolation and topography between Scotia arc islands, have important ramifications on the ecology and evolution of their fauna. The remote South Sandwich forearc, a combination of (active and non-active) volcanic islands and seamounts, are one of the youngest features within the Scotia arc (<5 Ma, Barker 2001). In contrast, South Georgia, Shag Rocks, Elephant Island and the South Orkney Islands are ancient continental fragments (see Thomson 2004). The size of shelf areas varies considerably between Scotia arc locations. South Sandwich Islands shelves are typically very narrow and surrounding waters rapidly increase to bathyal and abyssal depth, whilst South Georgia and South Orkney Is. (to the south) shelves extend to tens and hundreds of kilometres; thereby the depth of the shelf break ranges from 225 (Elephant I.) to about 500 m (South Orkney Is.). The major current system crossing Scotia Sea shelves and slopes is the ACC. Driven by westerly winds the ACC surrounds Antarctica from west to east, passing the South Shetland, South Orkney and South Sandwich Is. at its southernmost boundary (cf. Orsi et al. 1995). Scotia arc locations to the south [such as South Shetland (including Elephant, Deception and Livingston Is.), South Orkney (including Powell Basin) and South Sandwich Is.] represent a transition area between Weddell and Scotia Sea (deep and shallow) waters; these areas are also characterised by winter sea ice cover and an increased iceberg frequency compared with other Scotia arc locations to the north.
Sampling protocol
Our study is based on benthic shelf samples collected by two different cruises (JR 144 and JR 179) with RRS James Clark Ross in Austral summer 2005/06 and 2007/08 using an epibenthic sledge (EBS). During JR 179 (BIOPEARL 2), five 500-m samples were taken at four different sites (102, 101 and 100 kilometres apart) in- and outside Pine Island Bay, eastern Amundsen Sea (Table 1, Fig. 1b). For comparison, we report taxonomic richness and relative abundance of macrobenthos taken at 15 stations at shelf depth (160-500 m) along the island chain of the Scotia arc sampled during JR 144 (BIOPEARL 1 cruise, Table 1). We refer to macrobenthos as organisms retained by 500-µm mesh size. The biodiversity of shelf isopods and other macrobenthos have been recently reported for some localities sampled during this expedition [i.e. Southern Thule I. (ST), see Kaiser et al. 2008, and the South Orkney Is. (PB), see Barnes et al. 2008].
During both cruises (JR 144 and JR 179) benthic samples were taken using an EBS (for a detailed description see Brenke 2005). Briefly, the EBS consists of a 500-µm epi- (lower) and a supra- (upper) net, each with an opening of 100-cm width and 33-cm height. Both nets end up in a cod end with a mesh size of 300 µm. The EBS was hauled over the seabed at 1 knot for 10 min. Calculation of the trawling distance (d) followed equation (4) in Brenke (2005), ranging from 388 to 648 m (Table 1). On deck, samples were immediately fixed in 96% undenaturated pre-cooled (at -20°C) ethanol and kept at –20°C for at least 48 h. All metazoans were sorted to groups of higher taxonomic level (i.e. phylum, class). Individuals of Malacostraca were further identified to order and, within these, isopods were identified to family level. The discrimination into genera and species of two model families, Desmosomatidae and Nannoniscidae (both asellotan isopod taxa), was continued in the laboratory using a stereomicroscope. These taxa were chosen as they are ubiquitous from continental shelves to the deep sea in the polar regions (e.g. Svavarsson et al. 1993; Brandt 2004; Choudhury and Brandt 2007). Species were either assigned to previously known species or, for those considered to represent undescribed species, given a numerical or letter code (in the case of the BIOPEARL 1 or 2 expeditions respectively). Individuals that were too badly damaged (e.g. Austroniscus sp. indet) to be assigned to any genus or species were not included in the analysis and were not counted as a distinct species. As epi- and supra-nets are not considered to collect different faunas (Brenke 2005), both were treated as one sample.
Data analysis
Multivariate statistical analyses implemented in the PRIMER 6.0 package (Clarke and Gorley 2006) were performed on the basis of presence/absence data only. Similarities in composition between (all) stations were analysed across different taxonomic levels (family, genus and species) using Bray-Curtis similarities. Non-metric multidimensional scaling (nMDS) was computed to investigate faunistic similarities between stations.
Comparison of the dominance structure, abundance levels and richness between Scotia and Amundsen Sea samples were based on 500-m samples only (Table 1). Only five Scotia Sea samples were included in the analysis to ensure a comparable sampling size. These samples were randomly chosen. Cumulative number of species and generic accumulation curves were created as a function of the number (n) of individuals for Amundsen and Scotia Sea samples. Family dominance curves were plotted (using SigmaPlot 10) for each of five Amundsen and Scotia Sea sites and compared with each of five samples from the Ross (cf. Choudhury and Brandt 2007) and Weddell Sea (cf. Brandt 2004). For comparison, standardised abundance values (to 1,000 m) were used. Ross Sea samples were taken by a Rauschert dredge and depth ranged from 410-515 m (Choudhury and Brandt 2007), while the Weddell Sea samples were collected by an EBS and depth ranged from 204-743 m (Brandt 2004). Using a one-way analysis of variance (ANOVA) implemented in the statistical package MINITAB 15 differences in isopod abundance levels between seas were tested. Shannon J’ (evenness) and Shannon H’ (diversity) were calculated on family level and species level using PRIMER 6.0. Differences in taxonomic richness, evenness and diversity between seas were statistically tested across taxonomic levels using a one-way ANOVA.
Results
The relative abundance of isopods in the Amundsen Sea was very high and much less variable than across samples in our similar Scotia Sea samples. Two families, Munnopsidae and Desmosomatidae, dominated isopod abundance in the Amundsen Sea. We found high family richness and, within two model families, species richess of isopods on the Amundsen Sea shelf. Strikingly, 96% of these species were currently undescribed—the highest proportion ever recorded from a shelf anywhere. Although all genera have either been described or found elsewhere, for many of these genera it is the first time they have been recorded away from the Southern Ocean slope or deep sea.
Abundance
The EBS is not a quantitative sample apparatus (Brenke 2005), but there was notable variability in number of individuals. The relative abundance of isopods varied by two orders of magnitude between Scotia Sea shelf sites (Table 2). In contrast, isopods were consistently abundant in all Amundsen Sea 500-m samples (Table 2). The proportion of macrobenthos, in terms of relative abundance, constituted by isopods varied more in the Scotia Sea 500-m samples than in the Amundsen Sea, but insignificantly so. Rather than predicted variability in relative abundance across scales, the number of individuals was high in the Amundsen Sea and increased from the shelf-break to the innermost sites (Fig. 2).
Similar isopod families were represented across samples and seas. In the Scotia Sea, the relative abundance of each family differed (by up to two orders of magnitude, Table 3) as much between samples as between Scotia and Amundsen Sea samples (one-way ANOVA, all F < 5, all P > 0.06). In the Amundsen Sea the relative abundance of each family was less variable and two families (Munnopsidae and Desmosomatidae) dominated the samples (Table 3). Abundance of desmosomatids was variable in the Scotia arc samples, with five samples being represented by less than five individuals and one site with 70 (Deception I., see Table 4), while in the Amundsen Sea values were similarly high. The Amundsen and Scotia Sea samples were similar (munnopsids were ubiquitous and abundant whilst nannoniscids were just present). Paramunnidae were only poorly represented, and desmosomatids were more abundant in the Amundsen Sea samples (one-way ANOVA, F = 15, P = 0.003) (Table 3).
In the Amundsen Sea the most abundant family comprised 41.5-80.1% of isopods and the two most dominant families made up 70.3-89.9% of isopods (Table 4). Comparison of family abundance structure of isopod assemblages in our Amundsen and Scotia Sea samples with those in the Ross and Weddell Sea (using data from the literature; Brandt 2004, Choudhury and Brandt 2007) showed some similarities (Fig 3). Ross Sea family abundances showed strong similarities with those in the Scotia and Amundsen samples but with all families being more abundant (i.e. similar slope of lines but axes intercepts shifted to the right in Fig. 3). Weddell Sea abundances were intermediate between Amundsen, Scotia and Ross seas but the top two families were much less dominant, i.e. in Weddell Sea samples isopod abundance was more evenly distributed. Comparison of relative abundances at genus and species levels showed similar patterns to family level in the Amundsen Sea samples. In Desmosomatidae for example, the genus Mirabilicoxa (and within this genus a single species) dominated all samples, comprising between 51.6 and 93.1 % of total Desmosomatidae (Table 4). Evenness (Shannon J’) of isopod assemblages (family level) did not differ between seas but ranged from 0.37-0.64 (Amundsen Sea) to 0.21-0.93 (Scotia Sea) (one-way ANOVA, F 1 = 0.21, P = 0.657).
Abundance of isopod families with site sample and sea ranked from the least to the most dominant family (y-axis). Abundance data are summarised per area/sea and plotted against (log) family abundance per sea, within sea variablity in abundance is marked with a (standard) error bar. Families are similarly abundant in lines with little x-axis displacement, whereas greater x-axis shows more dominance by more abundant families. The seas are Scotia Sea (grey circle), Amundsen Sea (black circle), Weddell Sea data (white diamond) from Brandt (2004) using epibenthic sledge; Ross Sea data (grey triangle) from Choudhury and Brandt (2007) using Rauschert dredge
Isopod richness
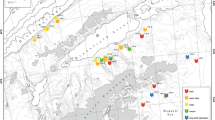
Taxonomic richness of Amundsen Sea samples was high at all sites and across taxonomic levels (Fig. 4a,b). Family level richness, as with abundance, was more variable between samples in the Scotia than the Amundsen Sea (Fig. 4a), but not significantly different (ANOVA, F = 0.56, P = 0.587). Similarly, family diversity (Shannon H’) did not differ between the Amundsen and Scotia samples (one-way ANOVA, F = 1.06, P = 0.329), varying from 0.74-1.60 in the Amundsen Sea and from 0.63-1.49 in the Scotia Sea. However, we did find significantly higher species and generic richness (in desmosomatids and nannoniscids) in the Amundsen Sea samples (species level: one-way ANOVA, F = 18.51, P = 0.003; generic level: one-way ANOVA, F = 21.44, P = 0.002). Generic richness of Amundsen Sea samples was 3.7-times higher than in the Scotia Sea (Fig. 4b), while Amundsen Sea species richness was 3.8-times higher than in Scotia Sea assemblages. Species diversity was not significantly different between Scotia and Amundsen Sea sample (one-way ANOVA, F = 2.79, P = 0.134) probably due to higher eveness in Scotia Sea assemblages (ranging from 0.54-1.00 in Scotia Sea samples and from 0.29-0.70 in Amundsen Sea samples). The occurrence of families, genera and species of isopods identified from EBS samples is shown in tables 3 and 4, respectively. Species accumulation of the two example families (Desmosomatidae and Nannoniscidae) did not reach asymptote in either Amundsen or Scotia Sea samples (Fig. 5). The rate of accumulation of species was similar in samples from both seas but differed for genera. Genera were accumulated more slowly in the Scotia Sea and did not approach asymptote, whereas it appeared that within the Amundsen Sea we found most genera for the sample area or habitat (Fig 5).
Family and generic (in Desmosomatidae and Nannoniscidae) richness of isopod crustaceans at each of five Scotia arc and Amundsen Sea 500-m sites. LI-500 Livingston Island, PB-500 Powell Basin, ST-500b Southern Thule, SR-500 Shag Rocks, SG-500 South Georgia. PB from Barnes et al. (2008), ST data from Kaiser et al. (2008)
The Amundsen Sea shelf has not been sampled previously, so every species found was a new record at each relevant taxonomic level for this area. In contrast, typically less than a third of the species found represent new records for the area in the better-studied Scotia Sea. Of the 40 desmosomatid and nannoniscid species found across samples, 34 have not yet been described. A description of one of these species (Regabellator sp. 2, see Table 4) is currently in progress. Unsurprisingly, the proportion of species new to science was higher in Amundsen Sea samples than those from the Scotia Sea, i.e. 96% of the Amundsen Sea species have not yet been described compared with 66.7% in the Scotia Sea (Table 5). Only one species of Nannoniscidae (i.e. Nannoniscus bidens; Vanhöffen 1914) in our Amundsen Sea samples had been recorded anywhere previously (Table 4). However, most of the desmosomatid and nannoniscid species belonged to established Southern Ocean genera apart from gen. nov. 1 (see Table 4). Our finding of gen. nov. 1 is the first Southern Ocean record, but it may be a congeneric of a similar as yet undescribed genus found from the Arctic, north Atlantic and Mediterranean seas. Remarkably, our samples yielded eight genera that had not been recorded from the Antarctic continental shelf (see Table 4). Additionally, our samples established new southern limits for 11 genera and 25 species, and new northern limits for two species (Table 4).
Faunal similarity
Patterns of faunal similarity differed across taxonomic (family, genus, and species) scale in our Amundsen Sea and Scotia Sea shelf samples. The nMDS ordinations we performed all had low associated stress values, indicating that the two-dimensional plots (see Fig. 6a-c) were good representations of these data in multidimensional space. At the family level, nMDS plots showed no clear pattern with geography or with bathymetry (Fig. 6a). For example, family composition differed as much between samples taken 103-km apart as those collected 100-km apart. Family level composition of Amundsen shelf samples was essentially a subset of the variability encompassed by Scotia shelf samples. At the generic level (in desmosomatids and nannoniscids), the Amundsen Sea samples were more distinct but clustered with one Scotia shelf sample (200 m at Shag Rocks, see Fig. 6b). Analysis of the similarity in desmosomatid and nannoniscid species composition showed samples from Amundsen and Scotia seas clustered separately (Fig. 6c). As with familial and generic levels, species level dispersion was greater (more variable) between samples from the Scotia than the Amundsen Sea.
To investigate how much variability in similarity could be explained by distance between samples we plotted (generic) similarity of data against distance apart (using longitude/latitude distance calculator, http://www.nhc.noaa.gov/gccalc.shtml). There was a significant relationship (see Fig. 7), in which much (r 2 = 63.3 %) of the variability in generic composition across samples was potentially explained by simply distance between samples. The samples we collected in the Amundsen Sea dominated those taken close together and thus any ‘Amundsen Sea effect’ could bias this relationship. However, without Amundsen Sea data excluded, we still found a significant and strong (r 2 = 51.9%) relationship with distance. Species similarity of samples with distance (apart) was significant but explained only <16% of variability (regression associated ANOVA, F 1 = 15.4, P < 0.001). Excluding Amundsen Sea data did not change the significance level but reduced variance explained to <11%. Thus, the distance between samples is important to explain differences in generic composition but not to explain species composition in isopod assemblages on the continental shelf.
Bray-Curtis similarity of generic composition of samples with distance (km) apart. Data are from comparisons within and between Scotia and Amunden Sea sites (see Table 1). Amundsen vs Amundsen comparison data are shown in dark grey
Discussion
Biological sampling around Antarctica is, like the distribution of organisms, very patchy. Benthic sampling has been highly geographically and bathymetrically biased, with most samples taken on the Scotia, Weddell and Ross Sea shelves (Clarke et al. 2008). The largest knowledge gap of Southern Ocean benthos is the Amundsen Sea and adjacent waters of the Pacific sector of the Southern Ocean (see SCAR-MarBIN, De Broyer and Danis 2008). Such differences in sample effort have a major impact on perceived patterns of richness and biases apparent distributions of biodiversity (see Clarke and Johnston 2003; Griffiths et al. 2009). For example, the impoverished biodiversity around Bouvetøya (Bouvet Island) could be theorised as due to island youth, small size and great isolation, but just five benthic samples taken there recently more than doubled the number of species known there (Arntz et al. 2006). Its fauna may, indeed, be found to be impoverished due to age, size and isolation, but the paucity of sampling is such that we do not have enough information to assess this yet. Even within the Scotia arc (one of the best studied shelf areas within the Southern Ocean and amongst the better studied globally) there are areas which are poorly sampled, such as some of the South Sandwich archipelago. Recent benthic sampling found ≥20% of families, genera and species were new to the South Sandwich Islands (Kaiser et al. 2008, Brandt et al. unpublished ANDEEP data), so taxon accumulation curves are steep.
A surprisingly rich isopod fauna on the Amundsen Sea continental shelf
Lowe and Anderson (2002) characterised the seismic profile of the inner shelf of Pine Island Bay as mainly crystalline bedrock due to glacial erosion. It was thus a reasonable premise that soft-sediments of significant thickness would be rare, but we found considerable soft substrata at all our sample sites. We had hypothesised that macrobenthic richness would be depauperate because of expected sediment rarity (macrobenthic isopods, being mostly detritivores require a sediment layer) and presumed intense scouring in the Amundsen Sea. In contrast, isopod richness in our Amundsen Sea samples was high across all taxonomic levels studied (family, genus and species), relative to similar samples collected elsewhere. Scotia Sea biodiversity is considered high for a polar sea (see Clarke and Johnston 2003; Barnes et al. 2008) but isopod richness was not significantly different to that in our Amundsen Sea samples at familial level. Furthermore our results showed Scotia samples were significantly less rich than those from the Amundsen Sea at generic and species levels in desmosomatids and nannoniscids (see Fig. 4, Table 4). How general this result is will require specialists to investigate richness across a range of differing taxa and dispersal strategies. It is likely that this shelf area is still being recolonised from major glaciation (Thatje et al. 2005) and (on shorter time scales) icescours. Although some isopods can be fairly mobile (e.g. munnopsids), most of those investigated are poor or non-swimmers (see Hessler and Strömberg 1989) and brooding direct developers and thus likely to be poor dispersers. Yet in Pine Island Bay they are hundreds of kilometres from the nearest continental slope, suggesting either better than predicted dispersal (e.g. via Circumpolar Deep Water) in our model groups or perhaps survival in refuges on the shelf during glacial maxima.
Multibeam sonar images of the Amundsen shelf have revealed complex bottom topography (unpublished BIOPEARL 2 cruise data). There are irregular and abundant plough marks (caused by icescours), even at 500-m depth (Lowe and Anderson 2002; Dowdeswell and Bamber 2007). High habitat heterogeneity (such as scours, gouges and furrows) is widely considered to promote biodiversity (e.g. Tews et al. 2004, but see Cummings et al. 2006 and discussions therein). Thus, our finding of high taxonomic richness may be driven, at least in part by strong habitat heterogeneity, three-dimensional structures and the resulting varition in niches. Isopod richness was consistently high across taxonomic scales in the Amundsen Sea, while levels were more variable in the Scotia Sea. Sea temperatures, water masses, sea ice extent and duration, and other oceanographic features vary considerably across the Scotia arc and even casual observations are enough to see that there is also considerably more variability in guilds (such as higher predators) there than around the continental margins of Antarctica. So, biological variability seems to match environmental variability. Furthermore taxonomic richness of at least some Antarctic shelf faunas (e.g. bryozoans) show a strong negative correlation to distance from nearest continent margin (Barnes 2008), which would also explain higher variability in the Scotia arc than the Amundsen Sea.
Prior to the current study, seven genera and 13 (described) species of desmosomatids and nannoniscids were known from the Antarctic continental shelf (cf. Schotte et al. 1995 onwards; Castello 2004). Our Amundsen and Scotia Sea samples added eight genera and 34 species: that is doubling and almost quadrupling the number of genera and species, respectively. This high rate of taxon discovery might in part be due to past undersampling of macro- and meiofaunal shelf organisms because of using either large mesh sizes (e.g. Agassiz trawl) or sampling very small areas (such as box- or multicorer). Expeditions using towed apparatus with small mesh sizes (≤500 µm) on the continental shelf, such as BIOPEARL, BENTART, EASIZ or the recent ITALICA cruise (e.g. Zimmermann and Brandt 1992; Choudhury and Brandt 2007; Rehm et al. 2007; Kaiser et al. 2008) are likely to keep increasing the known shelf macrofaunal species considerably. Even in the Ross Sea, one of the better studied Antarctic continental shelves, a recent investigation of the macrofauna more than doubled the number of isopod and cumacean species known there (Rehm et al. 2007; Choudhury and Brandt unpublished data).
Gutt et al. (2004) estimated that 17,000 species occur on the Antarctic continental shelf, but calculated this on the basis of 16 mega- and larger macrofaunal samples collected by different apparatus from the Weddell Sea. Highly diverse groups, such as the polychaetes, amphipods or isopods, were either not considered or just partly included in that study. Furthermore, by using Bottom or Agassiz trawls an entire (smaller) size class was neglected, thus leading to a probable drastic underestimation. Yet, trawled apparatus with smaller mesh sizes, such as epibenthic sledge or Rauschtert dredge, are also biased in that they only poorly sample meio- or infauna (e.g. Hilbig 2004). A further significant source of error in such estimates is cryptic speciation in some taxa, e.g. isopods (Held and Wägele 2005), bivalves (Linse et al. 2007) and crinoids (Wilson et al. 2007).
High rate of novelty in Amundsen Sea samples
Recently Brandt et al. (2007) highlighted that many of the species (and most of the isopods) from recent collections in the Southern Ocean deep sea were not merely new records for the area but previously undescribed. Such a finding would be rare for a major continental shelf area; but any habitat, environment or site which has been little or never sampled would be expected to contain new species, especially if the area was large, topographically complex or isolated. The shelf of the Amundsen Sea is exceptionally wide with complex basins and trenches within (Fig. 2). However, despite apparent connectivity, most Antarctic shelves have many endemic species (see SCAR-MarBIN database, De Broyer and Danis 2008). Like gastropods (see Linse et al. 2006), many isopods are known from just a few sites and are thought to be rare, highly restricted or very patchy in distribution (Brandt et al. 2007b; Kaiser et al. 2007). Although all the isopod genera we recorded have been previously found elsewhere, for many of these genera it is the first time they have been recorded away from the Southern Ocean slope or deep sea (cf. Brandt et al. 2007a).
The fauna of Antarctica’s continental shelves is becoming moderately well known (see Clarke and Johnston 2003). International databases [e.g. The Admiralty Bay Benthos Biodiversity Database (ABBED)] and literature show that some Antarctic shelf areas (Clarke et al. 2008) have been intensively sampled. Thus, the fact that virtually all the Amundsen shelf desmosomatid and nannoniscid isopods were previously undescribed was remarkable. Whether these species represent unique species for this area requires thorough analyses and comparison with material from previous cruises (e.g. ITALICA, ANDEEP, BENTART; see, e.g., Castello 2004; Choudhury and Brandt 2007, Brandt et al. 2007a). In the Scotia Sea, new records of isopod families, genera and species were 0%, 3.1% and 3.2% for our shelf samples at the South Shetland Is., respectively (cf. Castello 2004). Equivalent values for the South Orkney Is. were 26.7%, 26.7% and 25.6% (cf. Barnes et al. 2008) and for the South Sandwich Is. were 44.4%, 20% and 22.2% (Brandt 1991; Kaiser et al. 2008; Brandt et al. unpublished ANDEEP data) for family, genus and species levels, respectively. It is also notable that so many undescribed species were found in just five Amundsen Sea samples, but this may not reflect discovery rates that will be found in other taxa once examined by taxonomic experts. Evidence for this comes from a recent study of the (otherwise well studied) Ross Sea benthos, which found >50 species currently undescribed in just 18 samples (Choudhury and Brandt unpublished data). Clearly rates of undescribed species discovery vary across taxa, and are likely to be lower in those groups which are well represented in the shallows, large, with charismatic features and attract larger taxonomist communities than obscure, small, cryptic infauna. Even with isopods this gradient can be seen; for example, Zimmermann and Brandt (1992) reported that in the Weddell Sea 47% of munnopsids were new species compared with 91% of desmosomatids (Table 5). The high rate of new species discovery in desmosomatids and nannoniscids may reflect their size and habit rather than impoverishment (but see Brandt 2004; Choudhury and Brandt 2007). Only in the deep sea are rates of new species discovery comparable with the Amundsen shelf (Table 5; Brandt et al. 2007b).
Consistently high abundance on the Amundsen shelf
It was quickly apparent that macrobenthos was abundant in the Amundsen Sea. In an area of rapidly calving glaciers and rarely visited due to ice conditions, we had strong a-priori reasons for hypothesising low and variable abundance that is typical of recovery from ice scour (Gutt and Piepenburg 2003; Smale et al. 2008). However, the relative abundance of isopods in our Amundsen Sea samples was very high and much less variable than across samples in other seas. Isopod abundance was higher (Table 1), varied by less (a factor of three) and more predictable (Fig. 2) than expected. Any potential explanation of consistently high and predictable abundance is likely to be complex and involve primary productivity, ice scour frequency, sediment characteristics and probably recolonisations (from glacial maxima). Clearly disturbance is not as catastrophically frequent as we expected. However, even in the shallows, where turbulence might aid recolonisation and higher food concentrations should promote growth, recovering abundance to pre-scour levels takes years (Smale et al. 2008). Isopods are seemingly not particularly mobile (i.e compared with broadcasters), though Smale et al. (2008) showed them to recover abundance as rapidly than other taxa (or more so) on the shallow shelf of the West Antarctic Peninsula. Consistent high abundance of any organism requires a strong food supply and on the Amundsen shelf, perhaps due to primary production associated around the many icebergs or in coastal polynyas. Smith et al. (2007) found that free-drifting icebergs in the Weddell Sea can act as important areas of enhanced primary productivity due to iron enrichment of adjacent surface waters. Although the Amundsen Sea has high iceberg densities (see www.natice.noaa.gov), this often results in decreased local productivity (due to shading; see Smith et al. 2007). More important for primary production, and potential explanations of high faunal abundances in the Amundsen Sea, might be the occurrence of coastal spring polynyas, which enhance phytoplankton blooms and secondary production (e.g. Arrigo and Van Dijken 2003). Mean annual primary production in coastal (post-) polynyas in the Pine Island Bay area of the Amundsen Sea (and parts of the Ross Sea) are the highest around Antarctica (Arrigo and Van Dijken 2003). The increase in abundance from the shelf-break towards the coast of Antarctica (Fig. 2) remains surprising as primary productivity is typically maximal at the marginal sea-ice edge (Thomas et al. 2008). We suggest a priority for examination of new samples should be confirmation of this tentative trend of increased abundance towards the continent margin.
The high and non-variable isopod abundances in our Amundsen samples showed no obvious signal of strong (icescour) disturbance, but closer examination of patterns at family level did. The two isopod families which we found to be (by far) most abundant in the Amundsen Sea were the munnopsids and desmosomatids—amongst the more mobile of isopod families (e.g. Hessler and Strömberg 1989). Their dominance of assemblages on the Amundsen Sea is consistent with the view of the area being highly disturbed (see also Choudhury and Brandt 2007). That very few isopod families dominated abundance in the Amundsen Sea was similar to patterns in the Scotia and Ross seas (Fig. 3 and see Choudhury and Brandt 2007). In this respect the Weddell Sea samples were anomalous, with many isopod families being similarly abundant (Brandt 2004; Fig. 3). A similar family-abundance contrast occurs in the fish community of ‘undisturbed’ Weddell Sea sites, while ice-scoured sites were dominated by a single taxon (cf. Knust and Schröder 1999) and thus resemble Amundsen and Scotia Sea isopod patterns (Fig. 3). So, the few samples we have looked at are probably not representative for an entire region (e.g. Amundsen Sea), but the faunal signals appear to be strong enough to suggest regular (e.g. ice-scour) disturbance.
In the current study we focussed on overall and familial patterns of abundance but the latter was reflected in patterns at generic and species levels in that one genus (and within this genus, one species) dominated all samples in the Amundsen Sea (Table 4). This is relevant to discussions of disturbance signals as highly impacted areas are often dominated by few species (e.g. Knust and Schröder 1999; Gutt and Piepenburg 2003), whilst eveness is greater in ‘undisturbed’ areas. The similarity of familial, generic and species abundance patterns (higher taxon surrogacy, see Balmford et al. 1996) contrasted with taxonomic composition patterns.
Faunal similarity of Amundsen vs other Antarctic shelves depends on taxonomic level
We hypothesised that, despite new species records, the composition of Amundsen shelf assemblages would resemble those in seas nearby (e.g. West Antarctic Peninsula). Similarities (Barnes and DeGrave 2001) and strong differences (Ragua-Gil et al. 2004) have been found between adjacent Antarctic seas at differing depths. Comparing faunal similarities across three taxonomic levels in the Scotia and Amundsen seas, we found that spatial patterns varied critically depending on the taxonomic level used. Isopod familial composition did not partition by sea, but generic and species were distinct across seas (Fig. 6). Whilst the distinctness of Amundsen benthic faunal composition from other seas mirrored results of Antarctic regional faunas elsewhere (e.g. Ragua-Gil et al. 2004), potential explanations did not. In contrast to our findings (see Fig. 7), the distance between samples was unimportant in explaining faunal differences between and within the Weddell Sea and West Antarctic Peninsula, either on the shelf (Ragua-Gil et al. 2004) or deeper (Kaiser et al. 2007) (Fig. 7). Our finding of poor familial surrogacy for generic or species patterns contrasts with faunal patterns in the shallows of Antarctic shelves, where Stark et al. (2003), for example, found that family or suborder represented patterns on the species level well. We did find that some generic patterns reflected those at species level well (Fig. 6b, c), but this is unlikely to be useful in comparing regions due to, for example, typically very different levels of generic versus species endemism (e.g. Griffiths et al. 2009). Asellotan isopod families are mostly cosmopolitan and (desmosomatid and nannoniscid) genera typically show wide geographic distributions (see e.g. Schotte et al. 1995 onwards). Thus, it is not suprising that species are the most informative biogeographic unit to look at.
Many Scotia Sea shelf areas are separated by continental slope depths (in contrast to the Amundsen Sea shelf, which is continuous). Given shelf fragmentation, age and differences in substratum origin (e.g. volcanic vs microcontinental crust) between Scotia arc sites, greater variability in species composition there than between Amundsen Sea samples is unsurprising. We found little (<17%) similarity in isopod species composition between seas and the only described species shared with the Scotia Sea (N. bidens, Table 4) seems to have a circumpolar distribution (Kaiser et al., unpublished data). Circumpolar distributions have been proposed for many Antarctic benthic species (e.g. Ragua-Gil et al. 2004), although recent discoveries of species complexes, even in ‘highly’ dispersive taxa (e.g. antedoniid crinoids) is challenging this view (Wilson et al. 2007).
Concluding remarks
The eastern Amundsen Sea (Pine Island Bay) is unusual in many ways but mostly not in the ways that we expected and hypothesised. The rich and abundant isopod fauna is undoubtedly associated with the development of widespread and thick soft sediments that we found, but probably also with high overlying productivity and a potentially lower than expected frequency of ice-mediated disturbance. It seems highly likely that topographic and oceanographic complexity in the Amundsen Sea (thus niche diversity) is also an important factor underpinning anomalous taxonomic richness there. We suggest that this surprisingly high isopod richness we report will also be found in other taxa there. That so many of the species we found were previously undescribed was surprising, but reflected (taxonomic and geographic) sampling bias. Consistent high abundance and taxonomic richness typically requires stability (low disturbance), yet the most abundant isopod families by far were those which were fairly mobile (for isopods), implying high disturbance. Isopod data presented here paradoxically provides strong evidence of stability (high taxonomic richness) and, conversly, dynamism (abundance structure). Data which have stronger spatial structure and which cross multiple unrelated taxa will be needed to tease apart seemingly contrasting biodiversity patterns and explain ecological and evolutionary scale colonisation of the Amundsen Sea. It seems certain that the investigation of the fauna there will reveal much more than a faunal inventory of just another Antarctic sea.
The influence of Circumpolar Deep Water, the Pine Island and Thwaites Glaciers in this region are the fastest retreating and melting of Antarctic glaciers, causing massive freshening (Jacobs et al. 1996). Therefore, the Amundsen Sea may prove one of the most important shelf areas on the planet to interpret organisms and community responses to (past and present) climate change. If the major scientific cruises planned for the area employ well-structured sampling regimes and application of molecular phylogeographic techniques, as well as utilising the strong potential for comparison with well-studied adjacent shelves and faunas, the least known shelf on Earth could quickly become a vital ecological barometer of change.
References
Arntz WE, Thatje S, Linse K et al (2006) Missing link in the Southern Ocean: sampling the marine benthic fauna of remote Bouvet Island. Polar Biol 29:83–96. doi:10.1007/s00300-005-0047-8
Aronson RB, Blake DB (2001) Global climate change and the origin of modern benthic communities in Antarctica. Am Zool 41:27–39. doi:10.1668/0003-1569(2001)041[0027:GCCATO]2.0.CO;2
Arrigo KR, van Diejken GL (2003) Phytoplankton dynamics within 37 Antarctic coastal polynya systems.. J Geophys Res 108(C8):27–1–15
Balmford A, Green MJB, Murray MG (1996) Using higher-taxon richness as a surrogate for species richness: I. Regional tests. Proc R Soc Lond B Biol Sci 263(1375):1267–1274. doi:10.1098/rspb.1996.0186
Barker PF (2001) Scotia Sea regional tectonic evolution: implications for mantle flow and palaeocirculation. Earth Sci Rev 55:1–39. doi:10.1016/S0012-8252(01)00055-1
Barnes DKA (2008) A benthic richness hotspot in the Southern Ocean: slope and shelf cryptic benthos of Shag Rocks. Antarct Sci 20:263–270
Barnes DKA, DeGrave S (2001) Ecological biogeography of southern polar encrusting faunas. J Biogeogr 28:359–365. doi:10.1046/j.1365-2699.2001.00562.x
Barnes DKA, Kaiser S, Griffiths HJ et al (2008) Marine, intertidal, fresh-water and terrestrial biodiversity of an isolated polar archipelago. J Biogeogr. doi:10.1111/j.1365-2699.2008.02030.x
Brandt A (1991) Zur Besiedlungsgeschichte des antarktischen Schelfes am Beispiel der Isopoda (Crustacea, Malacostraca). Ber Polarforsch 98:1–240
Brandt A (2004) Abundance, diversity and community patterns of Isopoda (Crustacea) in the Weddell Sea and Bransfield Strait. South Ocean. Antarc Sci 16(1):5–10
Brandt A, Brix S, Brökeland W, Choudhury M et al (2007a) Deep-sea isopod biodiversity, distribution and endemism in the Atlantic sector of the Southern Ocean—results from the ANDEEP I-III expeditions. Deep Sea Res Part II Top Stud Oceanogr 54:1760–1775. doi:10.1016/j.dsr2.2007.07.015
Brandt A, Gooday AJ, Brandao SN et al (2007b) First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 447(7142):307–311. doi:10.1038/nature05827
Brenke N (2005) An epibenthic sledge for operations on marine soft bottom and bedrock. J Mar Technol Soc 39(2):10–19
Carmack EC (1977) Water characteristics of the Southern Ocean south of the Polar Front. In: Angel M (ed) A voyage of discovery. Pergamon Press, Oxford, pp 15–41
Castello J (2004) Isopods (Crustacea, Isopoda) from the Spanish “Bentart-94/95” expeditions to the South Shetland Islands (sub-Antarctic). Polar Biol 28:1–14
Choudhury M, Brandt A (2007) Composition and dirtsibution of benthic isopod (Crustacea, Malacostraca) families off the Victoria-Land Coast (Ross Sea, Antarctica). Polar Biol 30:1431–1437. doi:10.1007/s00300-007-0304-0
Clarke A, Johnston NM (2003) Antarctic marine benthic diversity. Oceanogr Mar Biol 41:47–114
Clarke A, Griffiths HJ, Linse K et al (2008) How well do we know the Antarctic marine fauna? A preliminary study of macroecological and biogeographical patterns in Southern Ocean gastropod and bivalve molluscs. Divers Distrib 13:620–632
Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth
Cummings V, Thrush S, Norkko A et al (2006) Accounting for local scale variability in benthos: implications for future assessments of latitudinal trends in the coastal Ross Sea. Antarct Sci 18(4):633–644. doi:10.1017/S0954102006000666
De Broyer C, Danis B (2008). SCAR-MarBIN: The Antarctic Marine Biodiversity Information Network. World Wide Web electronic publication. Available online at http://www.scarmarbin.be/. Accessed 15 Sep 2008
Dowdeswell JA, Bamber JL (2007) Keel depth of modern Antarctic icebergs and implications for sea-floor scouring in the geological record. Mar Geol 243:120–131. doi:10.1016/j.margeo.2007.04.008
Grotov AS, Nechaev DA, Panteleev GG et al (1998) Large-scale circulation in the Bellingshausen and Amundsen seas as a variational inverse of climatological Data. J Geophys Res 103(C6):13011–13022. doi:10.1029/98JC00449
Griffiths HJ, Barnes DKA, Linse K (2009) Towards a generalized biogeography of Southern Ocean benthos. J Biogeogr 36:162–177. doi:10.1111/j.1365-2699.2008.01979.x
Gutt J, Piepenburg D (2003) Scale-dependent impact on diversity of Antarctic benthos caused by grounding of icebergs. Mar Ecol Prog Ser 253:77–83. doi:10.3354/meps253077
Gutt J, Starmans A (2003) Patchiness of the megabenthos at small scales: ecological conclusions by examples from polar shelves. Polar Biol 26:276–278
Gutt J, Sirenko BI, Smirnov IS et al (2004) How many macrozoobenthic species might inhabit the Antarctic shelf ? Antarct Sci 16:11–16. doi:10.1017/S0954102004001750
Held C, Wägele J-W (2005) Cryptic speciation in the giant Antarctic isopod Glyptonotus antarcticus (Isopoda: Vavilfera: Chaetiliidae). Sci Mar 69(2):175–181. doi:10.3989/scimar.2005.69s2175
Hessler RR, Strömberg JO (1989) Behavior of janiroidean isopods (Asellota), with special reference to deep-sea genera. Sarsia 74:145–159
Hilbig B (2004) Polychaetes of the deep Weddell and Scotia Seas-composition and zoogeographical links. Deep Sea Res Part II Top Stud Oceanogr 51:1817–1825. doi:10.1016/j.dsr2.2004.07.015
Jacobs SS, Hellmer HH, Jenkins A (1996) Antarctic ice sheet melting in the Southeast Pacific. Geophys Res Lett 23(9):957–960. doi:10.1029/96GL00723
Kaiser S, Barnes DKA, Brandt A (2007) Slope and deep-sea abundance across scales: Southern Ocean isopods show how complex the deep sea can be. Deep Sea Res Part II Top Stud Oceanogr 54:1776–1789. doi:10.1016/j.dsr2.2007.07.006
Kaiser S, Barnes DKA, Linse K, Brandt A (2008) Epibenthic macrofauna associated with the shelf and slope of a young and isolated Southern Ocean island. Antarct Sci 20:281–290. doi:10.1017/S0954102008001107
Knust R, Schröder A (1999) Effects of iceberg scouring on the fish community and the role of Trematomus spp as predator on the benthic community in early successional stages. Ber Polarforsch 301:22–25
Linse K, Griffiths HJ, Barnes DKA et al (2006) Biodiversity and biogeography of Antarctic and Sub-Antarctic Mollusca. Deep Sea Res Part II Top Stud Oceanogr 53:985–1008. doi:10.1016/j.dsr2.2006.05.003
Linse K, Cope T, Lörz A-N et al (2007) Is the Scotia Sea a centre of Antarctic marine diversification? Some evidence of cryptic speciation in the circum-Antarctic bivalve Lissarca notorcadensis (Arcoidea: Philobryidae). Polar Biol 30(8):1059–1068. doi:10.1007/s00300-007-0265-3
Lowe AL, Anderson JB (2002) Reconstruction of the West Antarctic ice sheet in Pine Island Bay during the Last Glacial Maximum and its subsequent retreat history. Quat Sci Rev 21:1879–1897. doi:10.1016/S0277-3791(02)00006-9
Orsi AH, Whitworth HI, Nowlin WD Jr (1995) On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Res Part I Oceanogr Res Pap 42:641–673. doi:10.1016/0967-0637(95)00021-W
Ragua-Gil JM, Gutt J, Clarke A et al (2004) Antarctic shallow-water megaepibenthos: shaped by circumpolar dispersion or local conditions ?. Mar Biol (Berl) 144:829–839. doi:10.1007/s00227-003-1269-3
Rehm P, Thatje S, Mühlenhardt-Siegel U et al (2007) Composition and distribution of the peracarid crustacean fauna along a latitudinal transect off Victoria Land (Ross Sea, Antarctica) with special emphasis on the Cumacea. Polar Biol 30(7):871–881. doi:10.1007/s00300-006-0247-x
Saiz JI, García FJ, Manjón-Cabeza ME et al (2008) Community structure and spatial distribution of benthic fauna in the Bellingshausen Sea (West Antarctica). Polar Biol 31:735–743. doi:10.1007/s00300-008-0414-3
Schotte M, Kensley BF, Shilling S (1995 onwards) World list of marine, freshwater andterrestrial Crustacea Isopoda. National Museum of Natural History Smithsonian Institution, Washington D.C. http://www.nmnh.si.edu/iz/isopod/. Accessed 18 Nov 2008
Smale DA, Barnes DKA, Fraser KPP et al (2008) Benthic community response to iceberg scouring at an intensely disturbed shallow water site at Adelaide Island, Antarctica. Mar Ecol Prog Ser 355:85–94. doi:10.3354/meps07311
Smith KL Jr, Robison BH, Helly JJ et al (2007) Free-Drifting Icebergs: Hot Spots of Chemical and Biological Enrichment in the Weddell Sea. Science 317:478–482. doi:10.1126/science.1142834
Stark JS, Riddle MJ, Simpson RD (2003) Human impacts in soft-sediment assemblages at Casey Station, East Antarctica: Spatial variation, taxonomic resolution and data transformation. Austral Ecol 28(3):287–304. doi:10.1046/j.1442-9993.2003.01289.x
von Suhm R (1876) Preliminary report on crustacean during the cruise of A.M.S. Challenger in the Southern Seas. Proc R Soc Lond 24:1–591
Svavarsson J, Strömberg JO, Brattegard T (1993) The deep-sea asellote (Isopoda, Crustacea) fauna of the northern seas: Species composition, distributional patterns and origin. J Biogeogr 20(5):537–555. doi:10.2307/2845725
Tavares M, de Melo GAS (2004) Discovery of the first known benthic invasive species in the Southern Ocean: the North Atlantic spider crab Hyas araneus found in the Antarctic Peninsula. Antarct Sci 16:129–131. doi:10.1017/S0954102004001877
Tews J, Brose U, Grimm V et al (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31(1):79–92
Thatje S, Hillenbrand CD, Larter R (2005) On the origin of Antarctic marine benthic community structure. Antarctic ice sheet melting in the Southeast Pacific. Trends Ecol Evol 20:534–540. doi:10.1016/j.tree.2005.07.010
Thoma M, Jenkins A, Holland D, Jacobs S (2008) Modelling Circumpolar Deep Water intrusions on the Amundsen Sea continental shelf.. Geophys Res Lett 35:L18602. doi:10.1029/2008GL034939
Thomas DN (2008) Frozen Oceans. Natural History Museum, London
Thomson MRA (2004) Geological and palaeenvironmental history of the Scotia Sea region as a basis for biological interpretation. Deep Sea Res Part II Top Stud Oceanogr 51:1467–1487. doi:10.1016/j.dsr2.2004.06.021
Vanhöffen E (1914) Die Isopoden der Deutschen Suedpolar-Expedition 1901-1903. Deutsche Südpolar-Expedition 1901-1903, 25 (Zoologie) 7:447-598
Wilson NG, Hunter RL, Lockhart SJ et al (2007) Multiple lineages and absence of panmixia in the “circumpolar” crinoid Promachocrinus kerguelensis from the Atlantic sector of Antarctica. Mar Biol (Berl) 152:895–904. doi:10.1007/s00227-007-0742-9
Zimmermann A, Brandt A (1992) New results of the “EPOS” leg 3 cruise to Antarctica: Horizontal and vertical distribution of isopods (Crustacea) in the eastern Weddell Sea. Polar Biol 12(1):121–128. doi:10.1007/BF00239972
Acknowledgements
We are greatful to Drs. K. Linse and P. Enderlein for organising and running the cruise, and to the officers and crew of RRS James Clark Ross, whose patience, accuracy and timing have made this work possible. Many thanks to M. Choudhury for making data available, and to P. Rothery for statistical support and to H.J. Griffiths for his help editing the figures. P. Fretwell kindly provided the maps. Drs. W.P. Goodall-Copestake and C.D. Hillenbrand are thanked for helpful discussions. We are grateful to three anonymous referees for valuable comments on an earlier draft of this manuscript. S. Kaiser acknowledges grants of a SCAR fellowship, the German Science foundation (DFG, under contract no. Br 1121/26-4) and the German Academic Exchange Service (DAAD). This study is a contribution to CAML and the SCAR-EBA programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaiser, S., Barnes, D.K.A., Sands, C.J. et al. Biodiversity of an unknown Antarctic Sea: assessing isopod richness and abundance in the first benthic survey of the Amundsen continental shelf. Mar Biodiv 39, 27–43 (2009). https://doi.org/10.1007/s12526-009-0004-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-009-0004-9