Abstract
Hydrogeochemistry and groundwater quality of the Kadava River Basin have been performed by analyzing 40 groundwater samples for pre- and post-monsoon seasons of 2011 through different geostatistical techniques. Analyses showed that the concentrations of Mg2+ exceeded Ca2+ in both the seasons suggesting silicate weathering as a source of ions. Average Ca + Mg values contribute 73.53% of total cations and signify the major supply of mafic minerals. Elevated TDS and TH limit drinking potential and other beneficial uses indicate permanently hard water. The average concentrations of the anions were found within Indian PL except NO3, attributed to use of fertilizers. The spatial distribution maps show the EC, Mg, Na, Cl, and SO4 to be generally higher for both the seasons in flood plain deposits than denuded landforms and middle-level/high-level plateaus containing fractured basalt aquifers. It is confirmed that silicate weathering is the dominant process followed by carbonate weathering. Inputs of TDS, Cl, SO4, Mg, Na, and NO3 are influenced by rainfall and human activities. Groundwater sample numbers 20, 37, and 38 have been severely impacted from agricultural runoff as these samples are located along the surface water flow path. The high loading of TDS and TH is controlled by the Mg, Na, Cl, and SO4 ions. Elevated concentration of Na over Ca represents the ion exchange between Ca and Na. High contents of Mg, Na, Cl, and SO4 corroborate that the groundwater is influenced by anthropogenic sources. This study is an insight for water managers to address groundwater pollution problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater has become a more important and reliable source of fresh water for potable and agricultural uses in India in the last few decades. Access to safe drinking water remains a vital requirement, as in India, where 30% and 90% of the urban and rural population still depend on both surface and groundwater resources (Kumar et al. 2005). In recent years, public reliance on groundwater in India for drinking and irrigation use has been amplified due to the deterioration in quality and inadequate quantity of surface water resources. In India, the net groundwater availability is 399 billion cubic meters (m3). The annual groundwater draft is 231 billion cubic meters (bcm); 213 bcm is used for irrigation and 18 bcm is utilized for domestic and industrial use (CGWB 2006). As per international norms, if per capita water availability is < 1700 m3/year, then the country is categorized as being in a state of water stress; if it is < 1000 m3/year, the nation is designated as a water scarce country. In India, it estimated that per capita water availability was 2309 m3 in year 1991 and it was depleted to 1902 m3 in year 2001. It is projected to reduce in year 2025 and 2050 by 1401 m3 and 1191 m3, respectively (Kumar et al. 2005). The SERI (2009) reported that most part of India will face a severe water scarcity by 2050.
The geochemical composition of groundwater is mainly influenced by natural factors such as wet and dry deposition of atmospheric salts, precipitation, evapotranspiration, soil matrix, rock-water interaction, residence time, etc., and anthropogenic factors which include human activities related to surface runoff and groundwater recharge from agricultural uses and the generation and disposal of industrial wastes, leachate from solid waste dumping, on-site sanitation systems, and disposal of domestic waste, etc. (Todd 1980; Tóth 1999; Sefie et al. 2018; Karroum et al. 2017; Devic et al. 2014; Barbieri et al. 2014; Mukate et al. 2017). In developing countries like India, urbanization, industrialization, agricultural chemicals, population pressure, lack of water conservation measures, and over extraction of groundwater are posing serious threats to the groundwater quality and quantity as supplied by the aquifer system (Singh et al. 2006; Rezaei and Hassani 2018; Rao 2014; Mukate et al. 2015; Panaskar et al. 2016; Wagh et al. 2016a, b; Pawar et al. 2014; Vasant et al. 2016). The consumption of water sourced from contaminated aquifers has raised many health issues in recent decades (Barbieri et al. 2017). The consumption of groundwater with high content of fluoride, nitrate, and arsenic has created human health risks for millions of people in India (Wagh et al. 2017a; Pandith et al. 2017). Thus, it is important to assess the groundwater quality to reduce the human health impacts and to protect and maintain groundwater quality while minimizing natural and anthropogenic contaminants.
The Deccan Volcanic Province (DVP) of peninsular India of Cretaceous-Eocene age is one of the most vertically and laterally extensive geological formations on Earth having well-documented chemostratigraphic evaluations (Krishnan 1982; Beane et al. 1986). Also, it is the largest and most prominent among the Phanerozoic volcanic sequences of India occupying a central place in global tectonic models of breakup and assembly of the Gondwana supercontinent (Ramakrishnan and Vaidyanadhan 2008). Pawar and others (Pawar et al. 2008) investigated the geochemical incongruity in groundwater of the Panjhara River Basin and concluded that it is influenced by silicate mineral dissolution, cation exchange, and precipitation reactions and anthropogenic factors. Hydrogeochemistry of the Koyana River Basin has been studied by Naik and others (Naik et al. 2009), and they noted highly mineralized deeper aquifers compared to the less mineralized shallow aquifers which were dominated by alkaline earth metals and weak acids. The groundwater quality has a high salinity hazard in the lower reaches of the Koyana River Basin due to the use of fertilizers. The groundwater quality assessment study undertaken in Nanded tehsil depicts that the deterioration in water quality mainly occurred near human settlement and in industrial areas (Wagh et al. 2016b; Panaskar et al. 2016). In groundwater of Karha River Basin, the elevated salinity is attributed to the process of mineralization, as well as climatic, geomorphologic, geologic, and anthropogenic factors. The fluctuating monsoonal rainfall promotes mineralization under natural conditions; however, in the lower reaches of the river system, the anthropogenic factors contributing to salinity are related to the overuse of irrigation water and fertilizers (Kale and Pawar 2012). In general, several studies in the DVP emphasize groundwater movement from recharge area to discharge area modified by hydro-geochemical processes along the water migration pathway, and the variations in geochemistry could be the result of hydrogeological, hydro-geomorphologic, and anthropogenic factors (Pawar and Nikumbh 1999).
Most of these studies were carried out in large river basins for characterizing the surface water and groundwater quality affected mainly from numerous factors including varied climatic conditions and lithological heterogeneity (Singh et al. 2006; Naik et al. 2008; Raju et al. 2016). To ascertain the groundwater quality in a small river basin like the Kadava River Basin, one needs to recognize the hydro-geochemical mechanisms controlling the groundwater quality in basaltic terrain due to relative uniform lithology and climatic variations. The Kadava River is a major tributary of Godavari River and originates in the Sahyadri Hills of Western Maharashtra. In the Kadava River Basin, the upper reaches of the river average 800 mm of annual rainfall (Chandwad and Dindori tehsil), and in the lower reaches, the average annual rainfall is (Niphad tehsil) reduced to 700 mm. Rainfall is the main direct recharge and irrigation water is the indirect recharge source of groundwater (Rao et al. 2017). A larger portion of the population in the basin lives in isolated villages and farm houses where agriculture is the prime occupation. Shallow dug wells and deeper bore wells are commonly used for drinking and irrigation with the principal crops grown including sugarcane, grapes, onions, and a variety of vegetables. To enhance crop yield, the application of chemical fertilizers and pesticides to the agricultural fields is a common practice, which facilitates the downward leaching of contaminants along with recharge water. As a result, groundwater quality is degraded and poses a serious threat to human health.
The shallow alluvium aquifers are more vulnerable to contamination than the basaltic aquifers due to generally high permeability of river deposits. In the case of the study area, very limited research has been undertaken on the groundwater quality and hydrogeochemistry; however, studies by a few federal government agencies and individuals reported nitrate contamination in groundwater (Wagh et al. 2017a, b, 2018a, b). Groundwater is primarily used for drinking and domestic needs in the Kadava River Basin, so groundwater quality is closely associated with human health. Therefore, continuous monitoring and characterization of groundwater quality are required in the study area to prevent further groundwater deterioration in the future and groundwater issues affecting human health. However, the link between human health and groundwater geochemistry and water quality was not recognized widely until recently. Therefore, this requires hydro-geochemists to distinguish the impact of natural and anthropogenic factors on groundwater quality. The purposes of the study are (i) to assess the physico-chemical characteristics of groundwater with a view to drinking water evaluation, (ii) to investigate the major ion chemistry of shallow and deeper level groundwater aquifers to comprehend the hydro-geochemical processes that impact the quality of groundwater, (iii) to determine the factors controlling groundwater chemistry through geostatistical techniques, and (iv) to use of GIS packages to evaluate the spatio-temporal variation among cations and anions. To recognize the geogenic and anthropogenic sources affecting the hydrochemistry, several techniques have been used. The characterization of groundwater in this study has been investigated through identification of hydrochemical facies: ion exchange, rock weathering, evaporation, etc. Geostatistical methods were used to recognize the processes controlling the groundwater composition. The GIS-based point interpolation technique has been used to express the spatial extent of pollutants. In sum, combining these techniques will provide reliable information on the source and history of water contaminants, which may assist water resource managers and water regulators to develop basin management plans to improve groundwater quality in semi arid environment.
Study area
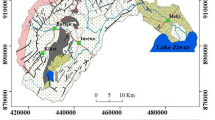
The study area is located within the Deccan Trap Hydrologic province, administratively situated in Chandwad and Niphad Tehsil of Nashik District, Maharashtra. Geographically, it lies between latitude 19°55′N to 20°25′N and longitude 73°55′E to 74°15′E with total area of 1053 km2 (Fig. 1). The Kadava River is the tributary of Godavari River which originates in Western Ghat mountain ranges locally known as Sahyadri Hills. The Kadava River flows in northwest to southeast direction to the confluence with Godavari at Nandur-Madmeshwar dam in Niphad Tehsil. The area receives 700 mm rainfall from South-West monsoonal winds (June to September) and rainfall decreases gradually from north to south. The study area is characterized as a semi-arid climate with temperature ranges from 5 to 42 °C in winter and summer season, respectively, and relative humidity ranges from 43 to 62% (CGWB 2014). Along the banks of river, alluvium deposits (20 to 25 m depth) are found in Niphad tehsil. The soils in the study area are classified as lateritic black soil, reddish brown soil, coarse shallow reddish black soil, and medium light brownish black soil. The black soil contains high levels of alumina and carbonates of calcium and magnesium with variable concentrations of potash, nitrogen, and phosphorous (CGWB 2014). A major part of the study area has flat topography with gentle slopes inclined towards the southeast. Most of the study area is covered by black soil and is used for agricultural production, primarily for grapes, sugarcane, and onions. The agricultural land with varied cropping patterns is spread along the water courses of the Kadava River, the Palkhed Canal, and the Nandur-Madhmeshwar dam. The study area is composed of various geomorphologic units (Fig. 2a). Photos show lithology and well condition (Fig. 2b, c). The North Region composed of high level and middle level plateaus of > 900 m and 550 to 900 m elevation, respectively. The Central and North East Region is experiencing denudation processes. The South Region and part of Central Region are influenced by floodplain hydrologic processes where the underlying alluvium is drained by the Kadava and Godavari Rivers (GSI 2001).
a Geo-morphological map of the study area (after GSI 2001). b (left) Surrounded by a vineyard, the well shows the black soil around 1 m (3 ft) deep in the New Flood Plain region near Shivare Village in the Chandwad Tehsil, underlain by weathered basalt. The arrow is 1 m (3 ft). c (right) A dug well from Palkhed Village in the older flood plain in Niphad Tehsil. The weathered portion of the well (arrow in lower right) is covered with concrete to stabilize wall. The underlying basalt (arrow near center of photo) is compacted and stable. The well is about 6 m (20 ft) wide and 20 m (66 ft) deep. d Lithologic Column for the Kadava River Basin
Hydrogeological settings
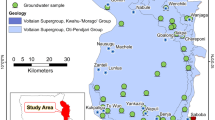
Geologically, the study area is a part of South East Deccan Volcanic Province, underlain by basalt of Upper Cretaceous to Lower Eocene age and composed of ‘pahoehoe’ and ‘aa’ lava flows (GSI 2001). The study area comprises hard rock (basalt) of the Sahyadri Group and soft rock (alluvium) which occurs in patches on the floodplains (Fig. 3). The Sahyadri Group in this area consists of the Kalsubai Subgroup which contains geologic markers (M1 and M2). Thin beds M1 and M2 developed on the top of the Salher Formation and Lower Ratangarh Formation, respectively. The Salher formation comprises 6 to 7 lava flows, which are predominantly pahoehoe-type features. This formation has volcanic tuffs which are found between the lava flows along the contacts in a few places. The entire lava pile is classified into three formations of this portion of the Sahyardri Group: the lowest unit in the area is the Salher Formation, the middle unit is the Lower Ratangarh Formation (also called Ratangad), and the highest unit is the Upper Ranangarh Formation. These formations are divided by megacryst flows of maximum thickness of 300 m and are expressed along the Godavari, Girna, and Kadava Rivers of Nashik Districts. The upper part of Salher Formation is marked by megacryst (M1) pahoehoe flow that contains size of 1 to 5 cm phenocrysts of plagioclase feldspar. The megacryst horizon is regionally quite extensive and acts as an excellent geologic marker horizon in the lava succession. Lower Ratangarh Formation thickness varies from 300 to 450 m and comprises 7 compound pahoehoe flows. These formations are mainly occurring in Dindori, Satana, Nashik, and Kalwan areas of the Nashik District. The rocks are characterized as fine to medium grained, hard, compact, and phyric in nature. A megacryst (M2) flow containing phenocrysts of plagioclase of size ranging 3 to 7 cm marks the upper contact of the Lower Ratangarh Formation. Lower Ratangarh Formation is the youngest in the lava succession in the district comprising six compound pahoehoe flows containing olivine phenocrysts. Thin beds of tuff varying in thickness from 0.2 to 1.0 m are characterized as reddish brown to dark brown color, fine grained containing angular fragments of basalts and lath of plagioclase feldspar. Mineralogically, the lava flows are quartz and hypersthene normative tholeiites, with olivine tholeiites. It shows 1:200 towards East-South-East of Niphad and 1:415 near Yeola (GSI 2001). A lithostratigraphic column (Fig. 2d) shows the portion of the basalt section found in the study area for the Kadava River Basin.
The groundwater occurs mostly in upper weathered and fractured parts down to 20 to 25 m in depth under unconfined, semi-confined to confined conditions. The yields of the wells are variable, related to permeability and transmissivity of the aquifer and also vary with the well location, diameter, and depth of the well. In the basalt formation, the yield of dug wells tapping upper phreatic aquifer at the depth of 12 to 15 m below ground surface ranges from 45 to 90 m3/day and bore wells drilled down to 50 to 70 m depth and yields varies from 18 to 68 m3/day, depending on local hydrology. Alluvium occurs in discontinuous patches along the bank and floodplains of the Kadava River. Alluvium is composed of sand, gravel, and kankar which occur as thin layers. The alluvium thickness ranges from 7 to 21 m. Groundwater in the alluvium deposits is found under semi-confined and confined conditions whereas the water yield from dug wells and bore wells ranges from 13 to 22 m3/day (CGWB 2014).
Material and methodology
In order to understand the seasonal hydro-geochemical status of Kadava River Basin, 40 groundwater samples were collected from representative shallow dug wells (36) and deeper bore wells (4) during the pre-monsoon and post-monsoon seasons of 2011. The surface location of the 40 wells relates to geomorphic features. New flood plains, old flood plains, denudational landforms, and middle level plateaus had 16, 7, 6, and 7 shallow dug wells, respectively, for a total of 36 wells. Deeper bored wells were located in the new flood plains (2), denudational landforms (1), and middle level plateaus (1) for a total of four wells. The groundwater samples were collected in pre-cleaned (1 l capacity) inert plastic containers. Prior to the sample collection each sampled well was subjected to water evacuation for 2 to 3 min to eliminate the influence from stored well water. The location coordinates were recorded with the help of Global Positioning System (GPS) device (Explorist 500). The pH and electrical conductivity (EC) were recorded on site by using a handheld digital meter (Multi-Parameter PCS Tester 35). The plastic sampling containers were sealed and labeled properly and brought to the analytical laboratory and stored at 4 °C for further analysis. The major cations analyzed included Ca, Mg, Na, and K; and anions: CO3, HCO3, SO4, NO3, and Cl. The cation and anions were analyzed in the analytical laboratory of School of Earth Sciences, SRTM University, Nanded by following the standard methods of American Public Health Association (APHA 2005). The physicochemical parameters like calcium (Ca), magnesium (Mg), carbonate (CO3), bicarbonate (HCO3), and chloride (Cl) were analyzed by gravimetric analysis by standard methods of APHA. The sodium (Na) and potassium (K) ions were analyzed by using flame photometer (ELICO CL 361). Also, sulfate (SO4) and nitrate (NO3) were estimated by using UV spectrophotometer (Shimadzu UV-800). The total dissolved solids (TDS) is calculated from EC by multiplying with factor 0.65 (Hem 1985). The total hardness (TH) as CaCO3 is calculated by Ca (meq) + Mg (meq) × 50. The ion balance errors (IBE) are within ± 10% which is supposed to be acceptable and calculated by using Eq. 1. The relationship between cations and anions illustrated in Fig. 4 confirms the relatively good ion balance and reliability of measured values. More information on IBE and acceptable water quality is found in the literature (Berner and Berner 1987; Edmond et al. 1995). The study area base map was prepared with the assistance of the Survey of India based on topographic sheets (46 L/3, 46 L/4, 46 H/15, and 46 H/16 on scale 1:50,000), digitized in ArcGIS 9.3v Software. Spatial distribution maps were prepared in ArcGIS software by performing the Inverse Distance Weightage (IDW) technique. The statistical analysis executed through MS-Excel, Statistical Package for Social Sciences (SPSS 22.0), and R software.
Results and discussions
The summary of the probe showing range, average, and comparison with the Bureau of Indian Standards (BIS) for the groundwater samples of pre-monsoon and post-monsoon 2011 season is summarized in Tables 1 and 2. The field measured parameters (pH, EC, TDS), and laboratory measured cations and anions were evaluated to determine the seasonal hydrochemical deviation in the Kadava River Basin.
pH, EC, and TDS
In the study area, pH values ranged from 7.8 to 8.9 (pre-monsoon) and 7.7 to 8.6 (post-monsoon) which indicate groundwater is fairly alkaline. This nature is attributed to the loss of CO2, and the precipitation and dissolution of minerals from within the basalt (Pawar and Kale 2006). Generally, elevated pH (alkaline waters) does not impact human heath, but it can alter the taste of the water and it shows a close association with ionic constituents of the water (Wagh et al. 2016a, b). The BIS (2012) has set the safe limit of pH for potable use at 6.5 to 8.5. In this study, 15% (sample numbers 9, 32, 34, 36, 39, 40) and 2.5% (sample no 19) of the samples exceeded the permissible pH limit in pre-monsoon and post-monsoon season 2011 (Tables 1 and 2). The spatial irregularity is observed in the Southern and Northern parts of the study area and may be influenced by saturation and dilution phenomenon (Fig. 5). Electrical conductivity (EC) is related to the amount of total dissolved salts in water, which, in turn, may suggest an elevated inorganic pollution load in the water (Morrison et al. 2001). The average value of EC is high in pre-monsoon (2508.5 μS/cm) samples compared with lower post-monsoon (2134.55 μS/cm) values for average EC. The value of EC increases with temperature and varies with the amount of geologically available soluble salts. The BIS has not prescribed any safe limit for EC; however, the World Health Organization (WHO 2011) designated 1500 μS/cm EC as the permissible limit. The samples compared with WHO standards confirm that 30 (75%) of the pre-monsoon groundwater samples and 25 (62.5%) of the post-monsoon groundwater samples exceed the permissible limit in pre-monsoon and post-monsoon season EC data (Tables 1 and 2). In view of spatial coverage, the EC values are elevated in the downstream portion of the Kadava River along the groundwater flow path where alluvium deposits encountered (Fig. 6). The total dissolved solids (TDS) are an important parameter for drinking water quality and irrigation suitability due to the contained ionic constituents (Davies and DeWiest 1966). The TDS has a wide range in the study area of 530.4 to 5044.0 mg/l in pre-monsoon and 466.7 to 5200 mg/l in post-monsoon 2011 season (Tables 1 and 2). The average concentration of TDS is higher in pre-monsoon (1630.53 mg/l) than the post-monsoon 2011 season (1387.46 mg/l), due to the semi-arid climate, lithology, and local agricultural and anthropogenic practices. The permissible limit of the TDS for drinking water is 2000 mg/l set by the BIS (2012). The physiochemical analysis confirms that all the groundwater samples exceed the desirable limit in both the pre-monsoon and post-monsoon 2011 season (except sample no. 34 in post-monsoon season). In pre-monsoon season, 27.5% of the groundwater samples, and in post-monsoon season 17.5% of the groundwater samples surpass the permissible limit set by the BIS. The elevated TDS is attributed to the percolation of salts, dissolution of minerals from the basaltic aquifer system and agricultural inputs. Consumption of groundwater with such an elevated TDS level may lead to gastrointestinal irritation. The lower catchment of the Kadava River Basin contains the intense agriculture zone which exhibits elevated concentrations of TDS in both the pre-monsoon and post-monsoon seasons in 2011.
Cations (Ca, Mg, Na, K)
The calcium content ranges from 12.02 to 130.40 mg/l (avg. 52.89 mg/l) and 15.2 to 99.86 mg/l (avg. 45.86 mg/l) in pre-monsoon and post-monsoon season, respectively. The BIS has set the permissible limit 200 mg/l for calcium contents in drinking water. As per the BIS standards, all the groundwater samples from both the pre-monsoon and post-monsoon seasons are within permissible limit; hence, beneficial use for drinking purposes. Conversely, four samples in pre-monsoon samples (numbers 8, 26, 28, 30) and post-monsoon samples (numbers 10, 25, 26, 28) in the 2011 season are beyond the BIS desirable limit (75 mg/l) (see Tables 1 and 2). Variations in calcium content in pre-monsoon and post-monsoon seasons of 2011 suggest the precipitation of calcite in pre-monsoon season (Pawar et al. 2008). The calcium values are elevated in a few areas of Central and Southern regions due to local geological influences (Fig. 7). The magnesium content varies widely from 28.32 to 285.37 mg/l in pre-monsoon and 19.8 to 265.5 mg/l in post-monsoon samples with an average of 102.79 mg/l and 77.60 mg/l in pre-monsoon and post-monsoon samples, respectively. In the study area, 45% (pre-monsoon) and 17.5% (post-monsoon) groundwater samples exceeded the permissible limit (100 mg/l) of the BIS. The high content of magnesium is encountered in patches downstream (Fig. 8) of the Kadava River Basin catchment area owing to geological control and the presence of the Thakurwadi Formation containing picritic horizons (MgO of 3.5% to 11.86%) (Beane et al. 1986). Average Ca + Mg value (meq/l) contributes 73.53% of total cations and signifies the major supply of mafic minerals such as olivine and pyroxene from weathering of basalt in the study area. The contribution of Mg in groundwater from basalt formations is diluted due to the rainwater recharge (Pawar et al. 2008). Within each of the 40 wells, 35 (88%) of the pre-monsoon and 29 (73%) of the post-monsoon and have higher magnesium concentrations than calcium concentrations indicating the abundance of olivine and pyroxene in the aquifer media as the primary source of Mg (Rabemanana et al. 2005). The prescribed permissible limit of total hardness (TH) for drinking water is 600 mg/l (BIS 2012). Analytical results confirm that total hardness content of 27.5% (sample numbers 2, 8, 10, 17, 19, 25, 26, 28, 31, 37, 38) and 10% (sample numbers 20, 28, 37, 38) groundwater samples exceed the permissible limit (600 mg/l) of drinking in the pre-monsoon and post-monsoon seasons of 2011, respectively (see Tables 1 and 2). The sodium content of groundwater samples ranges from 15.6 to 583.4 mg/l with an average 102.45 mg/l for pre-monsoon samples and 25.2 mg/l to 403.7 mg/l with an average 96.27 mg/l for post-monsoon season samples. The basalt weathering and dissolution of soil salts are redeposited by evaporation. The basalt-derived salts are the prime sources of sodium in groundwater and sodium exhibits high solubility behavior (Stallard and Edmond 1983). The increased sodium concentration is likely derived from the Salher Formation. The Salher Formation contains plagioclase feldspar and forms the solution between anorthite (CaAl2Si3O8) and albite (NaAlSi3O8) having a mole ratio of different cations and anions (Garrels and Christ 1967). The sodium is accumulated in the Southern Region of the study area due to the hydrogeomorphologic conditions such as slope and groundwater flow path (Fig. 9). The high intake of sodium in drinking water may cause human health effects such as high blood pressure, arteriosclerosis, edema, hyperosmolarity, vomiting, cerebral and pulmonary edema, muscular stiffness and twitching (Prasanth et al. 2012; Varade et al. 2014). The K content is mainly influenced by anthropogenic activities such as agriculture, which relies on K-rich fertilizers; however, its diminutive content is due to a lack of naturally occurring K-bearing minerals within the study area. The cation concentration in the study are a shows the following order of Mg > Ca > Na > K.
Anions (Cl, SO4, NO3, F, CO3, and HCO3)
Anions in the groundwater of Kadava River Basin are reflecting the influence of geochemical processes and anthropogenic inputs. In the study area, chloride is the primary anion based on concentration followed by HCO3, SO4, and NO3. The average concentrations of all the anions found in the study area are within permissible limits set by the BIS, except nitrate. The chloride is contributed from natural and anthropogenic processes such as weathering of chloride minerals such as halite, domestic waste, fertilizers, septic tanks, and leachate from landfills sites (Loizidou and Kapetanios 1993). As compared with prescribed BIS permissible limit (1000 mg/l), the chloride contents of all the 40 groundwater samples are seen to be within the permissible limit except sample number 38. However, 11 (27.5%) pre-monsoon groundwater samples (sample numbers 8, 17, 20, 23, 28, 31, 32, 33, 37, 38, and 39), 5 (12.5%) post-monsoon groundwater samples (sample numbers 20, 33, 37 to 39) exceed the desirable BIS limit for chloride (250 mg/l) (see Tables 1 and 2). The chloride content depicts the spatio-temporal equality in the Southern Region in both wet and dry seasons caused by leaching of fertilizers and animal waste coupled with surface runoff from agricultural fields (Fig. 10). According to the BIS, 200 mg/l is the highest desirable concentration of sulfate and 400 mg/l is the permissible concentration of sulfate in water used for drinking. Almost all of the groundwater samples in the study area are within the permissible limit for sulfate, except 7.5% of pre-monsoon samples (sample numbers 31, 33, 38) and 2.5% of post-monsoon samples (sample number 38) (see Tables 1 and 2). The sulfate content in groundwater is contributed from gypsum-containing fertilizers used to alter the physicochemical properties of soil (Todd 1980). The spatiotemporal map of sulfate depicts that the lower reaches of catchment area has been affected while the northern region is affected at a few places in both seasons (Fig. 11). The lower reaches of the Kadava River Basin have a prolonged agriculture growing period in fields in the alluvium region. This agricultural area has sufficient water for irrigation but the soil and water contain lower levels of plant nutrients. Due to the limited nutrient availability, farmers apply fertilizers, some of which contain sulfate.
The nitrate content varies from 19.31 to 68.62 mg/l and 31.40 to 66.15 mg/l in pre-monsoon and post-monsoon samples, respectively, while the average concentration is elevated in post-monsoon samples (49.97 mg/l) more than the pre-monsoon samples (48.63 mg/l). The analytical results of nitrate from the 40 wells compared with BIS standards confirm that 52.5% and 65% samples exceed the permissible limit (45 mg/l) of BIS in pre-monsoon and post-monsoon seasons, respectively (see Tables 1 and 2). The elevated nitrate concentration corresponds to nitrogen-phosphorus-potassium (NPK) complex fertilizers, organic fertilizers like solid and liquid manure, i.e., animal waste and compost and inorganic fertilizers includes urea, superphosphate, and potash in proportion to enhance crop yield of sugarcane, grapes, and vegetables; hence, it acts as a potential source of nitrate (Wagh et al. 2017a). Generally, after nitrogen containing fertilizer application, soil microorganism transforms nitrogen into ammonium nitrogen and nitrate nitrogen which used for growth by the plant and excess nitrogen will leach through soil, leads to groundwater contamination (Frate 2007). Consequently, the leaching of nitrate into groundwater mainly depends on solubility, mobility, acidity/alkalinity, and decomposition properties of particular fertilizer (Houzim et al. 1986). The spatiotemporal map of nitrate illustrates the uneven distribution in both the seasons due to the change in cropping pattern and use of fertilizers (Fig. 12).
Excess fluoride beyond 1.5 mg/l (BIS 2012) can cause fluorosis. The analytical results confirm that except for pre-monsoon sample number 14 which contained 2 mg/l fluoride, all the groundwater samples have safe levels of fluoride for drinking as they show a fluoride content below the permissible limit (Tables 1 and 2). The fluoride content source is anthropogenic like phosphate fertilizer use in agricultural field.
Hydro-geochemical evaluation
To develop the hydro-geochemical evolution within an area, it is important to know the major ion chemistry of shallow and deeper level groundwater and to understand the active hydro-geochemical processes occurring along the flow path from upstream to downstream within a groundwater basin. During groundwater transportation within a basin, the chemical composition of groundwater chemically and biologically reacts with and is altered through the naturally occurring rock-water interactions such as biological and chemical reactions in soil and groundwater, various types of weathering as well as significant impacts by anthropogenic activities (Todd 1980). Therefore, it is essential to understand the groundwater geochemistry to identify the contributing sources through hydro-geochemical facies, rock-water interactions, and dissolution and precipitation reactions.
Piper diagram
Arthur Piper (Piper 1944) developed the Piper diagram (or Piper Trilinear plot) which uses two triangles and one diamond shaped field to graphically represent water chemistry. First lower left side triangle is related to cation, on right side is for anion and third triangle placed above these two is used to plot an overall chemical composition of groundwater. The concept of hydro-geochemical facies is used to understand and classify water composition, through specialized charts and diagrams used for visualizing the trends of groundwater chemistry and interpretation for decisive flow pattern and source identification along with chemical history of groundwater samples. The Piper diagram (Fig. 13) can demonstrate anomalies in chemical composition of groundwater samples. The plot for the study area shows that in majority of the groundwater samples (95%), alkaline earth (Ca + Mg) concentrations exceed the alkali (Na + K) elements in both the pre-monsoon and post-monsoon seasons in 2011 while in only 5% of the alkali elements exceeded the alkaline earth concentrations. In all the groundwater samples, strong acids (SO4 + Cl) dominate over weak acids (CO3 + HCO3) in both seasons (Table 3).
Gibbs diagram
Ronald T. Gibbs developed the Gibbs diagram (Fig. 14) which is mainly used to represent the source of chemical constituents in groundwater related to the dominance of precipitation, rock, and evaporation (Gibbs 1970). The ratios for cations and anions, i.e., Na/(Na + Ca) and Cl-/(Cl + HCO3) and of the groundwater samples when plotted against relative values of total dissolved solids (TDS) showed the evaporation dominance process in most of the samples from pre-monsoon and post-monsoon season of 2011, which are affecting the groundwater quality in the study area. From this pattern, it is confirmed that most of the groundwater samples from vicinity of the agricultural area, evaporation causes salinity to increase by increasing Na and Cl with relation to the increase of TDS. Also, anthropogenic inputs like agricultural fertilizers, mixed canal and river water, and irrigation return flows also influence the evaporation by the increasing Na+ and Cl−, and thus TDS is increased. This clearly demonstrates that apart from the natural source, artificial factors, namely, anthropogenic activity, decide and dominate the change in chemical composition of groundwater (Hem 1985; Karanth 1987). The study area is characterized as being within an arid and semi-arid region where rainfall was lower than the average in 2011 and 2012; hence, the evaporation process becomes dominant in controlling the groundwater chemistry. Figures 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, and 27 show scatter plots of the ratio of various hydro-geochemical parameters.
Ion exchange process
The plot of Ca + Mg vs HCO3 + SO4 (Fig. 15) is used to represent the ion exchange and different weathering processes. The 1:1 equiline is maintained by the dissolution of calcite, dolomite, and gypsum (Cerling et al. 1989; Fisher and Mullican III 1997). The significant contribution from silicate weathering and required demand of HCO3 + SO4 is balanced by alkalis Na + K (Datta and Tyagi 1996). The plot showing positive correlations (r = 0.76 and 0.65) in pre-monsoon and post-monsoon season of 2011 indicating the ion exchange process is dominant in the groundwater. The ionic concentrations falling above the equiline indicate carbonate and silicate weathering. In post-monsoon season, HCO3 is in excess of Ca + Mg possibly pointing to the process of ion exchange reaction (Rajmohan and Elango 2004). The Ca + Mg vs Cl + SO4 (Fig. 16) suggests that majority of the samples in pre- and post-monsoon samples are clustered due to ion exchange process; it may suggest that excessive Cl and SO4 are contributed from agricultural runoff. The Ca vs Na plots show weak correlation (r2 = 0.058 and 0.0362) suggesting input from a common source (plagioclase feldspar) and that Na substitutes for Ca in groundwater (Fig. 17). Calcrete deposits are observed in the soil profiles, dug well sections piercing through weathered basalt and alluvium. These secondary carbonates are primarily characterized by calcite (Dessai and Warrier 1987). Further, dissolution of calcium carbonate as calcrete is the accompanying source of Ca and HCO3 in the groundwater (Pawar et al. 2008). Equation 2 summarizes the reaction:
The weak correlation of Ca + Na vs HCO3 represents the dissolution of plagioclases is small and does not affect the groundwater (Fig. 18).
Rock weathering process
The scatter plot of total cations (TZ+) vs Na + K (Fig. 19) shows a strong positive correlation in pre-monsoon and post-monsoon season data which confirms a positive correlation with total cations. It is confirmed that silicate weathering is the dominant process which controls the groundwater geochemistry. The logarithmic bivariate plot of HCO3/Na and Mg/Na vs Ca/Na is used to infer the type of weathering (Figs. 20 and 21). Figure 21 shows the areas of the chart indicative of specific types of weathering: evaporite weathering, silicate weathering, and carbonate weathering. In this study, the plotted data suggest silicate weathering as the primary type of weathering. The higher solubility of sodium over calcium increases the sodium concentration, and therefore, the Ca/Na ratio is expected to be low inferring that silicate weathering is the dominant geochemical process (Gaillardet et al. 1999; Raju et al. 2016). The plot of Ca/Na vs HCO3/Na showed silicate weathering prevailing in the study area. However, data from pre-monsoon and post-monsoon groundwater samples point towards carbonate weathering as a secondary weathering process. The presence of lime kankar supports the concept of carbonate weathering.
In the pre-monsoon and post-monsoon groundwater samples, the correlations between Ca + Mg vs Na + K are weak. The ratio suggests that there is an addition of Ca and Mg ions in groundwater sourced from the weathering of silicate rocks (basalt) in the area. The positive correlation coefficients between Ca and Mg confirm the role of Fe-Mg bearing silicates in basalt as their source of Ca and Mg ions (Fig. 22). The ratio of Ca + Mg vs HCO3 (Fig. 23) shows a weak correlation in pre-monsoon and post-monsoon 2011 groundwater samples. This ratio is significant and is used to know the origin of Ca and Mg in groundwater. The ratio of less than 0.5 may be due to the depletion of Ca and Mg or weathering of minerals like pyroxenes and amphiboles (Sami 1992), representing the fresh recharge of groundwater. In the present study, almost all groundwater samples are above 1.0 which is not attributed to the reduction of HCO3 as carbonic acid, indicating that the HCO3 is not formed under alkaline conditions which suggests that the reverse ion exchange process is dominant (Spears 1986). The ratio of 1.0 also provides evidence to suggest that the alkaline elements (calcium and magnesium) may be derived from silicate and carbonate minerals (Mahato et al. 2016).
The scatter plot of Na + K vs Cl + SO4 (Fig. 24) illustrates that there is an increase of alkali elements due to the presence of sodium sulfate, potassium sulfate, sodium chloride, and potassium chloride in the soil system (Bhardwaj et al. 2010). The weak correlation between Mg vs HCO3 (Fig. 25) and Ca + Mg vs HCO3 (Fig. 23) suggests minor weathering of olivine and pyroxene minerals contributing to the increased total ionic strength; therefore, it is likely due to anthropogenic inputs such as agricultural waste and domestic activities. The plot of (Ca + Mg)-(HCO3 + SO4) vs (Na + K)-Cl (Fig. 26) confirms the active cation exchange process. The groundwater samples plotting close to zero are not affected by ion exchange processes while those plotting on the slope of the line (− 1) (Fig. 26) will readily exchange ions (Jankowski et al. 1998; Kortatsi 2006, 2007).
Evaporation process
The Na/Cl ratio (Fig. 27) is used to identify the salinity mechanism in the semi-arid and arid environment (Sarin et al. 1989; Sami 1992; Singh et al. 2005). The halite maintains the ratio to 1 by releasing Na and Cl ions (Hounslow 2018). The groundwater ratio of Na/Cl indicates a good correlation in pre-monsoon and post-monsoon groundwater samples during 2011. The higher value of Na/Cl ratio suggests that the possible source of Na is silicate weathering and a lower ratio of less than 1 indicates the possibility of ion exchanges of Na with for Ca and Mg in clay particles (Tiwari and Singh 2014).
Geostatistical analysis
The geostatistical analysis was performed using MS Excel and R programming software to reduce and organize the data with similar hydrochemical characteristics. A total of 14 physicochemical parameters were used for correlation analysis, principle component analysis, and cluster analysis.
Correlation matrix
The correlation matrix is used to determine the degree of correlation among the various physicochemical water quality variables which influence groundwater quality in the study area. The establishment of the relationship between two variables is confirmed with the help of correlation coefficient studies. The correlation analysis involving statistical calculations was first devised by Karl Pearson (1896) and gives a measure of how well one parameter predicts the value of another parameter (Krumbein and Graybill 1965). Based on the values of correlation coefficient “r,” the correlation between two parameters is plotted on an XY scatter diagram with the overall correlation can be termed as positive or negative. Due to its significance in indicating the ions which control its water chemistry, correlation analysis is recognized as a common and useful statistical tool for water quality studies (Box 1978; Chapman 1996). A positive strong correlation can show the same sources of particular ions which can be natural or anthropogenic in origin and mobility while weak correlation suggests that the sources of ions are independent from each other (Islam et al. 2017). The correlation matrices for 14 physicochemical parameters of pre-monsoon and post-monsoon groundwater samples from 2011 were prepared, and their results are tabulated (Tables 4 and 5). The variables showing a correlation coefficient (r > 0.7) are considered to be strong, where (r) values between 0.5 and 0.7 indicate a moderate correlation, while r < 0.3 is weak correlation.
Table 4 demonstrates that a positive correlation of EC with TDS (r = 1), TH (r = 0.70), Cl (r = 0.87), Mg (r = 0.75), Na (r = 0.77), and SO4 (r = 0.63) which reflects that the dissolution of salts increases the electrical process of weathering (Wagh et al. 2016a). Inputs of TDS, Cl, SO4, Mg, Na, and NO3 are influenced by rainfall and human activities prevailing in the study area. The subsequent components like TDS with TH (r = 0.70), Mg (r = 0.75), Na (r = 0.77), and SO4 (r = 0.63) show strong correlation. TH exhibits a strong positive correlation with Mg (r = 0.97), Na (r = 0.50), Cl (r = 0.77), and SO4 (r = 0.66). Also, Mg with Cl (r = 0.81), HCO3 (r = 0.62), Na (r = 61), and SO4 (r = 0.65) shows positive correlation. Na and Cl have a strong correlation (r = 0.88). Cl and SO4 have a moderate correlation (r = 0.59), and CO3 correlates positively with HCO3 (r = 0.63). Also, Ca, Mg, Na, and Cl and EC, TH, and TDS have shown a significant association for irrigation purposes.
Table 5 confirms that pH and potassium are negatively correlated with all the physicochemical parameters. Electrical conductivity (EC) has shown a significant correlation with TDS, TH, Mg, Na, Cl, F, CO3, HCO3, and SO4 (r = 1; 0.92; 0.91; 0.86; 0.97; 0.57; 0.60; 0.74; 0.81), respectively, in post-monsoon groundwater samples. TDS has a strong positive correlation with TH (r = 0.92), Mg (r = 0.91), Na (r = 0.86), and SO4 (r = 0.81). Total hardness (TH) is strongly correlated with magnesium (r = 0.97), sodium (r = 0.72), chloride (r = 0.92), and sulfate (r = 0.85). Magnesium (Mg) has a good positive correlation with sodium (r = 0.78), chloride (r = 0.92), sulfate (r = 0.80), and a moderate correlation with fluoride (r = 0.51). Sodium has a strong correlation with chloride (r = 90) and a moderate association with fluoride, bicarbonate, and sulfate. Chloride is positively correlated with fluoride (r = 0.58) and sulfate (r = 0.79).
Cluster analysis
The cluster analysis (CA) is applied to identify groups or clusters of similar sites on the basis of like-characteristics within a class and dissimilarities between different classes (Lattin et al. 2003). Cluster analysis comprises a series of multivariate methods which are used to identify the true groups of data sets, i.e., the similar objects fall in the same class (Danielsson et al. 1999). The groups are divided into the level of similarity and the dendrogram is developed where observations are merged. In pre-monsoon groundwater samples, four clusters have been identified while in post-monsoon groundwater samples, only three clusters were noted (Fig. 28). In both the pre-monsoon and post-monsoon, groundwater sample numbers 20, 37, and 38 form a separate cluster representing that these samples have been severely impacted from agricultural runoff as these samples are all located along the same flow path. Figure 28 illustrates the classification of samples in these clusters. There are four clusters in pre-monsoon groundwater samples while three clusters are noted in the post-monsoon groundwater samples of 2011.
Principal component analysis
In order to know the contributing factors of physicochemical parameters affecting the groundwater quality, R-mode factor analysis was applied to the hydrochemical data to classify the different groups based on inherent qualities of parameters. R-mode factor analysis examines the relationship among variables by analyzing a matrix of simple correlation coefficients for all pairs of variables considered (Saager and Sinclair 1974). Factor analysis is performed with Kaiser Varimax rotation to differentiate the factors without changing the data structure which helps to reduce the contribution of less significant parameters affecting water quality (Mertler and Vannatta 2005) (Fig. 29). The Scree plot is used to know the number of principle component groups to be retained to identify the physicochemical behavior of groundwater (Fig. 30). Based on the Scree plot results, eigenvalues are greater than one and five factors in the pre-monsoon groundwater samples and three factors in the post-monsoon groundwater samples are extracted which contributes 84.06% and 71.82% of the total variance in pre-monsoon and post-monsoon data, respectively. Table 6 summarizes the correlation of various parameters in pre-monsoon and post-monsoon 2011 samples.
After the rotation varimax method was performed, it seemed that first factor (factor 1) accounts for 42.46% of the total variance and is positively loaded with EC, TDS, TH, Mg, Na, Cl, and SO4 in pre-monsoon season. The Na and Cl show the highest principal component (PC) loadings due to their higher solubility (Rao 2014). The high loading of TDS (0.926) is controlled by the Mg, Na, Cl, and SO4 ions, while TH (0.768) is controlled by the Mg, Cl, and SO4 that suggests the permanent type of water hardness which has been demonstrated by a high positive loading of TDS. The high loading of Na over Ca represents the ion exchange between Ca and Na (Drever 1988). The high loadings of Mg, Na, Cl, and SO4 corroborate that the groundwater is influenced by anthropogenic sources. The second factor (factor 2) shows CO3 (0.886) and HCO3 (0.862) with 15.46% of total variance suggesting that groundwater is recharged with fresh water. The infiltrated water reacts with soil CO2 forming H2CO3. The long-term irrigation practices also increase the alkalinity caused by HCO3 (Rao 2014). Therefore, factor 2 is considered to be alkalinity controlled. The third factor (factor 3) shows high loadings of calcium (0.931) and TH (0.409) with 10.94% of the total variance indicating a small amount of dissolution of calcite minerals. The fourth factor (factor 4) is dominated by high loadings of potassium (0.884) and fluoride (0.696) with 8.02% of the total variance owing to the application of fertilizers and agrochemicals, thus reflecting anthropogenic origin. The fifth factor (factor 5) is influenced by nitrate loading (0.791) illustrating the leaching of agricultural and animal wastes, fertilizers, and pesticides in the study area. In post-monsoon samples, it is observed that first factor (factor 1) accounts the relationships between EC, TDS, TH, Mg, Na, Cl, F, HCO3, and SO4 with 50.46% of total variance. The high positive loading of EC and TDS (0.975) is due to the percolation of contaminants along with recharge water supported by high loadings of HCO3. The high loadings of Na, Cl, SO4, and HCO3 show that groundwater quality is controlled by salinity and alkalinity deriving factors. The second factor is positively loaded of CO3 (0.531) and pH (0.442) with 13.246% of total variance. This association suggests that aquifers are recharged through rain water which controls the alkalinity of water. The third factor is with high loadings of F (0.543) with 8.11% of total variance suggesting that the presence of fluoride is related to fertilizers.
Conclusion
The integration of groundwater chemistry, geostatistical analysis, and spatio temporal analysis were applied to elucidate the natural and anthropogenic processes altering the hydro-chemical composition of groundwater in an area of intensive agriculture activity in the Kadava River Basin. Groundwater in the study area is permanently hard water, having high TDS or TH which may limit drinking potential and other beneficial uses in those wells. TH or TDS were above Indian PL in 40% of samples in pre-monsoon season. With dilution, post-monsoon samples contained TH or TDS which exceeded the Indian PL in only 18% of the samples. Anthropogenic activities have likely contributed to elevated NO3, which exceeded Indian PL in 53% of pre-monsoon, and 65% of post-monsoon samples. Therefore, it is proposed that management of nitrogen-based fertilizers should be implemented and nitrate level in groundwater must be monitored at regular intervals to prevent the potential risk. It is observed that only 7.5% and 2.5% of pre- and post-monsoon samples exceed the PL of sulfate such as high content in groundwater that is contributed from gypsum-containing fertilizers. Chloride, although below the Indian PL except for one in pre-monsoon sample, is likely related to anthropogenic activities. The spatial distribution map depicts that Southern region is affected mostly due to the leaching of contaminants along with natural drainage from agricultural fields. The Piper plot showed that in 95% of the groundwater samples, alkaline earth concentrations exceed the alkali element concentrations, while only 5% of the alkali element concentrations exceeded the alkaline earth concentrations. Moreover, strong acids dominate over weak acids in all the groundwater samples. High loading of TDS is controlled by the Mg, Na, Cl, and SO4 ions, while TH is associated with Mg, Cl, and SO4 attributing permanent hardness. The high loadings observed Na over Ca in PC I represent the ion exchange process. In post-monsoon season, high loadings of Mg, Na, Cl, and SO4 corroborate that the groundwater is influenced by anthropogenic sources. The positive loading of CO3 and pH suggests that aquifers are recharged through rain water which controls the alkalinity of water. Concentrations of Mg2+ exceeded Ca2+ in 88% of the pre-monsoon samples and 73% in the post-monsoon samples. In groundwater samples, the correlation between Ca + Mg vs Na + K is weak, suggesting addition of Ca and Mg ions in groundwater sourced from the weathering of silcate-rich basalts in the area. The scatter plots, correlation matrix, and cluster analysis of groundwater sample suggest that certain geogenic processes (ion exchange, silicate mineral weathering, and evaporation) and specific anthropogenic processes (agricultural runoff, animal waste, and domestic waste) are the dominant factors controlling the groundwater chemistry of the study area. In general, groundwater composition is principally controlled by dissolution of silicate minerals; pre-monsoon variability is attributable to the post dissolution changes due to precipitation and anthropogenic processes. The study has identified variations in water quality within the Kadava River Basin, related to a variety of natural and anthropogenic factors. Geochemical evaluation with data analysis combined with GIS provide tools for water managers to address different beneficial uses of specific groundwater supply wells, depending on the local water requirements, to prioritize point-of-use water treatment, if appropriate, to suggest well deepening or well abandonment, if needed, or to recommend best management practices for agricultural or industrial activities to reduce anthropogenic factors. Also, central water supply system, potable water treatment, and appropriate sanitation facilities and proper sealing of dug and bore wells are recommended. Furthermore, the outcomes of this study may be used as background information of major ions in groundwater and their influencing factors to research scholars and decision makers to improve the groundwater quality in the intensive agriculture area, semi-arid, arid climate condition.
References
APHA A (2005) WPCF, standard methods for the examination of water and wastewater. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Barbieri M, Sappa G, Vitale S, Parisse B, Battistel M (2014) Soil control of trace metals concentrations in landfills: a case study of the largest landfill in Europe, Malagrotta, Rome. J Geochem Explor 143:146–154
Barbieri M, Nigro A, Petitta M (2017) Groundwater mixing in the discharge area of san Vittorino plain (Central Italy): geochemical characterization and implication for drinking uses. Environ Earth Sci 76(11):393
Beane JE, Turner CA, Hooper PR, Subbarao KV, Walsh JN (1986) Stratigraphy, composition and form of the Deccan basalts, Western Ghats, India. Bull Volcanol 48(1):61–83
Berner EK, Berner RA (1987) Global water cycle: geochemistry and environment. Prentice-Hall, Upper Saddle River
Bhardwaj V, Singh DS, Singh AK (2010) Water quality of the Chhoti Gandak River using principal component analysis, ganga plain, India. J Earth Syst Sci 119(1):117–127
BIS (2012) Indian standards specification for drinking water, BIS: 10500: 2012. http://www.cgwb.gov.in/ Documents/WQ-standards.pdf
Box GE (1978) Statistics for experimenters: an introduction to desing, data analysis, and model building. Probability and mathematical statistics
Cerling TE, Pederson BL, Von Damm KL (1989) Sodium-calcium ion exchange in the weathering of shales: implications for global weathering budgets. Geology 17(6):552–554
CGWB (2006) Central ground water board. Dynamic ground water resources of India –As on March 2004:1–120
CGWB (2014) Central Groundwater Board. http://cgwb.gov.in/district_profile/maharashtra/nashik.pdf
Chapman D (Ed) (1996) On the behalf of UNESCO, WHO. UXEP. Water quality assessments-a guide to use biota, sediments and water in environmental monitoring. F & F Spoil, London, Chapter 9
Danielsson Å, Cato I, Carman R, Rahm L (1999) Spatial clustering of metals in the sediments of the Skagerrak/Kattegat. Appl Geochem 14(6):689–706
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Davies SN, DeWiest RJ (1966) Hydrogeology. Wiley, New York, pp 1–463
Dessai AG, Warrier S (1987) Mineralogy and geochemistry of the calcretes in alluvial sediments from Pune, India. J Geol Soc India 29(6):584–593
Devic G, Djordjevic D, Sakan S (2014) Natural and anthropogenic factors affecting the groundwater quality in Serbia. Sci Total Environ 468:933–942
Drever JI (1988) The geochemistry of natural waters. Prentice hall, Englewood cliffs, NJ. The geochemistry of natural waters, 2nd edn. Prentice Hall, Englewood Cliffs, NJ
Edmond JM, Palmer MR, Grant B, Stallard RF (1995) The fluvial geochemistry and denudation rate of the Guayana shield in Venezuela, Colombia, and Brazil. Geochim Cosmochim Acta 59(16):3301–3325
Fisher RS, Mullican WF III (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, trans-Pecos, Texas, USA. Hydrogeol J 5(2):4–16
Frate C (2007) Nitrogen transformation in soil University of California Cooperative Extension, Tulare County http://cdrf.org/wp-content/uploads/2012/01/11.7_Nitrogen_Transformations-final.pdf. Accessed 15 Sept 2014
Gaillardet J, Dupré B, Louvat P, Allegre CJ (1999) Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem Geol 159(1–4):3–30
Garrels RM and Christ CL (1967) Equilibres des minéraux et de leurs solutions aqueuses. Gauthier-Villars
Geological Survey of India (GSI) (2001) District resource map. GSI, Nashik District
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170(3962):1088–1090
Hem JD (1985). Study and interpretation of the chemical characteristics of natural water (Vol. 2254). Department of the Interior, US Geological Survey
Hounslow A (2018) Water quality data: analysis and interpretation. CRC press, Boca Raton
Houzim V, Vavra J, Fuska J, Pekny V, Vrba J, Stibral J (1986) Impact of Fertilizers and Pesticides on Groundwater Quality. In: Gastany G, Groba E, Rominj (eds) Impact of Agricultural Activities on Groundwater. IAH Publ., Hannover 332 p
Islam ARMT, Ahmed N, Bodrud-Doza M, Chu R (2017) Characterizing groundwater quality ranks for drinking purposes in Sylhet district, Bangladesh, using entropy method, spatial autocorrelation index, and geostatistics. Environ Sci Pollut Res 24(34):26350–26374
Jankowski J, Acworth RI and Shekarforoush S (1998) Reverse ion exchange in a deeply weathered porphyritic dacite fractured aquifer system, Yass. In New South Wales Australia. In: GB Arehart, JR Hulston (eds) Proceedings of 9th international symposium on water–rock interaction, Taupo, New Zealand, Vol 30, pp 243–246
Kale SS, Pawar N (2012) Origin of salinity in the groundwater of semi-arid Karha Basin, Maharashtra. Memoir Geol Soc India 80:145–166
Karanth KR (1987) Ground water assessment: development and management. Tata McGraw-Hill Education, New York City
Karroum M, Elgettafi M, Elmandour A, Wilske C, Himi M, Casas A (2017) Geochemical processes controlling groundwater quality under semi arid environment: a case study in Central Morocco. Sci Total Environ 609:1140–1151
Kortatsi BK (2006) Hydrochemical characterization of groundwater in the Accra plains of Ghana. Environ Geol 50(3):299–311
Kortatsi BK (2007) Hydrochemical framework of groundwater in the Ankobra Basin, Ghana. Aquat Geochem 13(1):41–74
Krishnan MS (1982) Geology of India and Burma, 6th edn. CBS publisher and distributors, New Delhi
Krumbein WC, Graybill FA (1965) An introduction to statistical models in geology. McGraw-Hill, New York City
Kumar R, Singh RD, Sharma KD (2005) Water resources of India. Curr Sci:794–811
Lattin JM, Carroll JD, Green PE (2003) Analyzing multivariate data. Thomson Brooks/Cole, Pacific Grove, CA
Loizidou M, Kapetanios EG (1993) Effect of leachate from landfills on underground water quality. Sci Total Environ 128(1):69–81
Mahato MK, Singh PK, Tiwari AK, Singh AK (2016) Risk assessment due to intake of metals in groundwater of east Bokaro coalfield, Jharkhand, India. Exposure and Health 8(2):265–275
Mertler CA and Vannatta RA (2005) Advanced and multivariate statistical methods. Glendale
Morrison G, Fatoki OS, Persson L, Ekberg A (2001) Assessment of the impact of point source pollution from the Keiskammahoek sewage treatment plant on the Keiskamma River-pH, electrical conductivity, oxygen-demanding substance (COD) and nutrients. Water SA 27(4):475–480
Mukate SV, Panaskar DB, Wagh VM, Pawar RS (2015) Assessment of groundwater quality for drinking and irrigation purpose: a case study of Chincholikati MIDC area, Solapur (MS), India. SRTMUs J Sci 4(1):58–69
Mukate S, Panaskar D, Wagh V, Muley A, Jangam C, Pawar R (2017) Impact of anthropogenic inputs on water quality in Chincholi industrial area of Solapur, Maharashtra, India. Groundw Sustain Dev:359–371. https://doi.org/10.1016/j.gsd.2017.11.001
Naik PK, Tambe JA, Dehury BN, Tiwari AN (2008) Impact of urbanization on the groundwater regime in a fast growing city in Central India. Environ Monit Assess 146(1–3):339–373
Naik PK, Awasthi AK, Anand AVSS, Behera PN (2009) Hydrogeochemistry of the Koyna River basin, India. Environ Earth Sci 59(3):613–629
Panaskar DB, Wagh VM, Muley AA, Mukate SV, Pawar RS, Aamalawar ML (2016) Evaluating groundwater suitability for the domestic, irrigation, and industrial purposes in Nanded tehsil, Maharashtra, India, using GIS and statistics. Arab J Geosci 9(13):615
Pandith M, Kaplay RD, Potdar SS, Sangnor H, Rao AD (2017) Impact of urbanization coupled with drought situations on groundwater quality in shallow (basalt) and deeper (granite) aquifers with special reference to fluoride in Nanded-Waghala municipal corporation, Nanded District, Maharashtra (India). Environ Monit Assess 189(9):428
Pawar NJ, Kale VS (2006) Waterfall tufa deposits from the Deccan Basalt Province, India: implications for weathering of basalts in the semi-arid tropics. Z Geomorph NF 145:17–36
Pawar NJ, Nikumbh JD (1999) Trace element geochemistry of groundwater from Behedi basin, Nasik district, Maharashtra. J Geol Soc India 54:501–514
Pawar NJ, Pawar JB, Kumar S, Supekar A (2008) Geochemical eccentricity of ground water allied to weathering of basalts from the Deccan Volcanic Province, India: insinuation on CO 2 consumption. Aquat Geochem 14(1):41–71
Pawar RS, Panaskar DB, Wagh VM (2014) Characterization of groundwater using groundwater quality index of Solapur industrial belt, Maharashtra, India. Int J Res Eng Technol 2(4):31–36
Pearson K (1896) Mathematical contributions to the theory of evolution. III. Regression, heredity, and panmixia. Philosophical Transactions of the Royal Society of London Series A, Containing Papers Of A Mathematical Or Physical Character 187:253–318
Peng ZX, Mahoney J, Hooper P, Harris C, Beane J (1994) A role for lower continental crust in flood basalt genesis? Isotopic and incompatible element study of the lower six formations of the western Deccan traps. Geochim Cosmochim Acta 58(1):267–288
Piper, A.M., (1944). A graphic procedure in the geochemical interpretation of water-analyses Eos, Transactions. Am Geophys Union 25(6):914–928
Prasanth SS, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2(3):165–175
Rabemanana V, Violette S, De Marsily G, Robain H, Deffontaines B, Andrieux P, Bensimon M, Parriaux A (2005) Origin of the high variability of water mineral content in the bedrock aquifers of southern Madagascar. J Hydrol 310(1–4):143–156
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River basins, Southern India. Environ Geol 46(1):47–61
Raju NJ, Patel P, Reddy BSR, Suresh U, Reddy TVK (2016) Identifying source and evaluation of hydrogeochemical processes in the hard rock aquifer system: geostatistical analysis and geochemical modeling techniques. Environ Earth Sci 75(16):1157
Ramakrishnan M and Vaidyanadhan R (2008) Geology of India (Vol. 1). Geological society of India
Rao NS (2014) Spatial control of groundwater contamination, using principal component analysis. J Earth Syst Sci 123(4):715–728
Rao NS, Marghade D, Dinakar A, Chandana I, Sunitha B, Ravindra B, Balaji T (2017) Geochemical characteristics and controlling factors of chemical composition of groundwater in a part of Guntur district, Andhra Pradesh, India. Environ Earth Sci 76(21):747
Rezaei A, Hassani H (2018) Hydrogeochemistry study and groundwater quality assessment in the north of Isfahan, Iran. Environ Geochem Health 40(2):583–608
Saager R, Sinclair AJ (1974) Factor analysis of stream sediment geochemical data from the mount Nansen area, Yukon territory, Canada. Mineralium Deposita 9(3):243–252
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, eastern cape, South Africa. J Hydrol 139(1–4):27–48
Sarin MM, Krishnaswami S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the ganga-Brahmaputra river system: weathering processes and fluxes to the bay of Bengal. Geochim Cosmochim Acta 53(5):997–1009
Sefie A, Aris AZ, Ramli MF, Narany TS, Shamsuddin MKN, Saadudin SB, Zali MA (2018) Hydrogeochemistry and groundwater quality assessment of the multilayered aquifer in Lower Kelantan Basin, Kelantan, Malaysia. Environ Earth Sci 77(397). https://doi.org/10.1007/s12665-018-7561-9
SERI (2009) State of Environment Report India (SERI). Ministry of Environment and Forests, Government of India, p 179
Singh AK, Mondal GC, Singh PK, Singh S, Singh TB, Tewary BK (2005) Hydrochemistry of reservoirs of Damodar River basin, India: weathering processes and water quality assessment. Environ Geol 48(8):1014–1028
Singh KP, Malik A, Mohan D, Singh VK, Sinha S (2006) Evaluation of groundwater quality in northern indo-Gangetic alluvium region. Environ Monit Assess 112(1–3):211–230
Spears DA (1986) Mineralogical control of the chemical evolution of groundwater. Solute processes. Wiley, Chichester, p 512
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res Oceans 88(C14):9671–9688
Tiwari AK, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 83(3):329–343
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
Tóth J (1999) Groundwater as a geologic agent: an overview of the causes, processes, and manifestations. Hydrogeol J 7(1):1–14
Varade AM, Yenkie R, Shende R, Kodate J (2014) Drinking water quality assessment studies for an urbanized part of the Nagpur District, Central India. J Environ Sci Eng 56(1):53–64
Vasant W, Dipak P, Aniket M, Ranjitsinh P, Shrikant M, Nitin D, Manesh A and Abhay V (2016) GIS and statistical approach to assess the groundwater quality of Nanded tehsil,(MS) India. In Proceedings of first international conference on information and communication Technology for Intelligent Systems: volume 1. Springer, Cham, pp 409–417
Wagh VM, Panaskar DB, Varade AM, Mukate SV, Gaikwad SK, Pawar RS, Muley AA, Aamalawar ML (2016a) Major ion chemistry and quality assessment of the groundwater resources of Nanded tehsil, a part of Southeast Deccan Volcanic Province, Maharashtra, India. Environ Earth Sci 75(21):1418
Wagh VM, Panaskar DB, Muley AA, Mukate SV, Lolage YP, Aamalawar ML (2016b) Prediction of groundwater suitability for irrigation using artificial neural network model: a case study of Nanded tehsil, Maharashtra, India. Modeling Earth Systems and Environment 2(4):196
Wagh, V.M., Panaskar, D.B. and Muley, A.A., (2017a). Estimation of nitrate concentration in groundwater of Kadava river basin-Nashik district, Maharashtra, India by using artificial neural network model. Model Earth Syst Environ 3(1):36
Wagh VM, Panaskar DB, Muley AA, Mukate SV (2017b) Groundwater suitability evaluation by CCME WQI model for Kadava River basin, Nashik, Maharashtra, India. Model Earth Syst Environ 3(2):557–565
Wagh V, Panaskar D, Muley A, Mukate S, Gaikwad S (2018a) Neural network modelling for nitrate concentration in groundwater of Kadava River basin, Nashik, Maharashtra, India. Groundw Sustain Dev 7:436–445. https://doi.org/10.1016/j.gsd.2017.12.012
Wagh VM, Panaskar DB, Mukate SV, Gaikwad SK, Muley AA, Varade AM (2018b) Health risk assessment of heavy metal contamination in groundwater of Kadava River basin, Nashik, India. Model Earth Syst Environ 4(3):969–980
World Health Organization (WHO) (2011) Guidelines for drinking water quality, library cataloguing-in-publication data, 4th ed. NLM classification: WA. World Health Organization, Geneva, p 675
Acknowledgements
The authors would like to express sincere gratitude to the Director, School of Earth Sciences, SRTM University, Nanded for providing necessary facilities to carry out the present research work. Also, authors are grateful to anonymous reviewers for their meticulous assessment and significant comments which are helpful to strengthen the manuscript quality.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagh, V.M., Panaskar, D.B., Jacobs, J.A. et al. Influence of hydro-geochemical processes on groundwater quality through geostatistical techniques in Kadava River basin, Western India. Arab J Geosci 12, 7 (2019). https://doi.org/10.1007/s12517-018-4136-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-4136-8