Abstract
Rapid expansion in urbanization and industrialization coupled with recent drought conditions has triggered unplanned groundwater development leading to severe stress on groundwater resources in many urban cities of India, particularly cities like Nanded, Maharashtra. In the quest of tapping drinking water requirement, due to recent drought conditions, people from the city are piercing through entire thickness of shallow basalt aquifers to reach productive deeper granite aquifers. Earlier reports from Nanded and surrounding districts suggest that deeper granite aquifer is contaminated with fluoride (geogenic). The study aimed to find out variations in fluoride concentration in shallow basalt (10–167 m) and deeper granite aquifers (below 167 m) and to find out the relationship between fluoride and other ions. Study suggests that concentration of fluoride in shallow basalt aquifer is within maximum permissible limits of Bureau of Indian Standards and deeper granite aquifer contains as high as 4.9 mg/l of fluoride and all samples from granite aquifers are unfit for human consumption. The groundwater from basalt aquifer is mainly Ca-HCO3−Cl type, and from granite aquifer, it is Ca-Na-Cl type. The correlation plot between F− vs. pH, Na+ and HCO3 − shows a positive correlation and an inverse relationship with Ca2+ in both aquifers. As recommendations, it is suggested that granite aquifers should not be tapped for drinking purposes; however, in drought situations, water from this aquifer should be blended with treated surface water before supplying for drinking purposes. Efforts may be made to utilize 1.35 MCM of rainwater from available rooftop, which is sufficient to cater for the needs of ~40,800 people annually. Most effective defluoridation techniques like electrolytic de-fluoridation (EDF), ion exchange and reverse osmosis may be adopted along with integrated fluorosis mitigation measures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urbanization is increasing worldwide, as in 1900, only 10% lived in cities and for the year their number is estimated to be at 50% (UN 1991). In Maharashtra, ~45% of the population lives in urban areas as compared to all India with an average of ~31%. This rapid expansion in urbanization and industrialization has triggered unplanned groundwater development leading to severe stress on groundwater resources in many urban cities of India. With depleting resources from shallow basalt aquifers (100 m), coupled with drought conditions, the users are tapping deeper granite aquifers (below 167 m), which have proved to be counterproductive economically and ecologically as it is geogenically contaminated with fluoride.
Some elements are essential in trace amount for human beings, while their higher concentration can cause toxic effects, and fluoride (F−) is one of them (CGWB 1999), and it is a conclusive fact that the concentration of F− between 0.6 and 1.0 mg/l is essential in potable water to protect tooth decay and enhance bone development, while their higher concentration may lead to fluorosis (Apambire et al. 1997; Kundu et al. 2001; BIS 2012). About 30 nations with 67 million people around the globe are facing one or another type of fluorosis, and India is one of them. In India, more than 20 states with 66 million people, of which 6 million are children (below the ages of 14 years), are suffering from fluorosis (Madhnure et al. 2007). The granite rocks contain an average of 810 μg/g of fluoride in mineral form whereas basalt rock (shallow aquifer) contains 360 μg/g (Wedepohl 1969).
The main sources of fluoride in groundwater are F−-bearing minerals present in rocks, like fluorite, fluorapatite, cryolite and apophyllite as well as F replacing OH in ferromagnesium silicates (amphiboles and micas) and soil consisting of clay minerals (Ramesam and Rajagopalan 1985; Madhnure et al. 2007). Degree of weathering and leachable F present in the rocks of an area plays an important role in deciding F content in water rather than the mere presence of F-bearing minerals in bulk soils/rocks (Ramesam and Rajagopalan 1985). The alkaline nature of groundwater with high HCO3 and moderate EC is a favourable condition for dissolution of F-bearing minerals (Saxena and Ahmed 2001; Madhnure et al. 2007).
High concentrations of fluoride in groundwater have been reported from many parts of the world, particularly India, China and Sri Lanka, (Ayoob and Gupta 2006), and many of the world’s high-fluoride areas are underlain by crystalline igneous and metamorphic rocks (i.e., parts of India, Sri Lanka, Senegal, Ghana, Cameroon, Tanzania, South Africa, the Pampean Ranges in southern South America and Scandinavia (Ayoob and Gupta 2006)). Variations in concentration of fluoride in shallow and deeper aquifers have been reported from many parts of India (Subba Rao 2006; Prem Babu et al. 2004; Madhnure et al. 2007; Madhnure et al. 2016; Reddy et al. 2010; Reddy 2014). Sayeed Juned et al. (2015) reported the occurrence of ‘F’ in the range 0.73–2.69 mg/l from southern parts of Nanded city.
The study area, Nanded, is covered by basalt rock, the thickness of which varies from ~167 to 196 m, below which granites occurs. The study area, Nanded, is facing severe drought situations in the last few years. In quest of tapping drinking water, bore wells are drilled deeper and the entire thickness of basalt is pierced through and thus groundwater from deeper granitic aquifer is tapped, and generally, granite is the source of F in groundwater. Keeping this very aspect in mind, the present study is carried out to compare groundwater quality from shallow and deeper aquifer with special reference to “fluoride”.
Study area
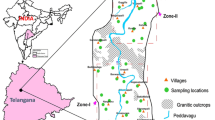
Nanded-Waghala Municipal Corporation (NWMC) is located on banks of river Godavari, falling in Survey of India toposheet 56E/8. The Municipal Corporation is spread over an area of ~44 km2 and bounded by north latitude 19° 10.015′–19° 12.445′ and east longitude 77° 16.621′–77° 20.693 at topographic elevations from 360 to 380 m above mean sea level (m amsl) (Fig. 1). The area receives ~900 mm normal annual rainfall, whereas during the years 2014 and 2015, it received only 50 and 72% rainfall to normal rainfall. As per 2011 census, the population of the town is 5.5 lakh and the total water requirement for the year 2026 is estimated to be at 118.64 million litres/day (MLD) whereas, presently, ~110 MLD of water is supplied (Rode 2009).
Hydro geological setup
Geologically, the study area is covered by basalt which is underlain by granites. Granites occur at different depths depending on the elevation in the area i.e. at 196 m on the right bank of the river at Vishnupuri and at 167 m on the left bank at Kabra Nagar (Madhnure 2014). Basalt is massive, fine grained and bluish grey to brown in colour, and each flow is separated by clay beds (intertrappean) with 0.25–3.25 m thickness and the average thickness of each flow is 24 m. The colour of the granite depends upon the colour of feldspar in it. Grey granite consisting of fluoride-bearing dark-coloured minerals occur at the upper level at deeper aquifers such as amphiboles and biotite (Fig. 2a) and pink-coloured hybrid granites from the lower level of deeper aquifers with dark-coloured amphiboles and mica (Fig. 2b). The lithological sequence from the Vishnupuri Exploratory well confirms the existence of eight flows (Fig. 3) (Madhnure 2003). Groundwater occurs under unconfined to semi-confined/confined conditions, and water levels are at shallower levels (2.3–10.4 m below ground level (m bgl)) on the right bank of river Godavari and at deeper levels (5.3–22.8 m bgl) on the left bank during the month of February 2012 (Madhnure 2004). In recent past, the water levels have still gone below this level due to more extraction coupled with droughts. Yield from basalt aquifer ranges from 10 to 325 m3/day, and from granites it ranges from 10 to 250 m3/day. Transmissivity in basalt aquifer ranges from 14 to 86 m2/day having storativity of 0.002–0.0095 (Madhnure 2004).
Analytical techniques and presentation of data
To assess the aquifer-wise variations in fluoride concentrations, samples were collected in two phases (Fig. 1).
Phase 1: Nineteen groundwater samples from shallow basalt aquifers (dug wells and shallow bore wells) and one surface water sample from river Godavari at Langar house Gurudwara were collected during February 2002 for normal analysis and two (shallow bore wells) from basalt aquifers for heavy metal analysis (Fe, Cu, Zn and Pb) (Fig. 1 and Table 1).
Phase 2: Ten groundwater samples (all bore wells) tapping shallow and deeper aquifers from five locations (with proximity with each other) were collected during the pre-monsoon season of 2015 (May) (Fig. 1 and Table 1).
Samples during phase 2 were collected immediately after encountering a fractured zone in each aquifer as per procedure suggested by Handa (1974). For preservation, storage and analysis of samples, guidelines suggested by APHA (1998) were followed. Total 12 parameters (viz., pH, TDS, TH, Ca, Na, Mg, K, HCO3, Cl−, SO4, NO3 − and F−) are analysed by using standard methods (APHA 1998).
pH and EC are measured in the field by using a portable pH metre (Hanna Instruments). The value of EC is used to calculate total dissolved solids (TDS) (HEM 1991). The sum of calcium and magnesium concentrations is normally defined as total hardness (expressed as CaCO3) (APHA 1998). Ca is determined by titration method using a standard ethylenediaminetetraacetic acid (EDTA) solution. Magnesium is estimated by the difference between total hardness and calcium (APHA 1998). Sodium and potassium are determined by a SYSTRONICS flame photometre at 589- and 766.5-nm wavelengths (APHA 1998). Chloride is determined by argentometric titration method, sulphate and nitrate by spectrophotometric method at 420 and 275 nm. Fluoride is determined by SPADNS colorimetric method (APHA 1998) and iron, copper, zinc and lead by spectrophotometric method. The ionic balance between total cation and total anion is within an acceptable range of ±5% (APHA 1998). Suitability of groundwater for drinking purposes is assessed based on the Bureau of Indian Standards (BIS 2012). Characteristics of groundwater are identified by using a Piper diagram (Hill 1940; Piper 1944), and correlation of regression between F− vs. other ions is studied by a scatter plot.
Results and discussions
In the present study, the results of phase 2 samples are considered for knowing the suitability for drinking purposes, and for fluoride distributions, phase 1 and phase 2 samples are considered.
Hydrogen ion concentration (pH)
In most natural waters, pH value is dependent on the carbon dioxide-carbonate-bicarbonate equilibrium (Karanth 1987). The pH value is less than 7 if the H+ ions exceed the OH− ions, and it is more than 7 when OH− ions exceed H+ ions (Karanth 1987).
In the groundwater from the study area, pH in basalt aquifer ranges from 8.2 to 8.5 and from 7.8 to 9 in granite aquifer. It is noticed that the average pH from shallow basalt aquifer is lower than that from deeper granite aquifer, and this may be due to recharge and flushing of shallow aquifers during the rainy season (Gupta and Sharma 1995; Gupta and Deshpande 2003; Jacks et al. 2005) and longer residence time in deeper aquifers (Handa 1975; Ramamohana Rao et al. 1993; Wodeyar and Sreenivasan 1996; Subba Rao et al. 1998; Saxena and Ahmed 2001; Subba Rao and Rao 2003; Madhnure et al. 2007; Madhnure et al. 2016).
Total dissolved solids
The concentration of TDS in groundwater depends upon the nature of rock formations, porosity and permeability, depth through which water is passing, climate and geomorphology of the area. Contamination of water by human and animal activities including septic tanks disposal and agricultural practices and mixing of different types of water also affects TDS (Handa 1975; CGWB 1998). In general, the TDS value in basaltic shallow aquifers of the Maharashtra state varies between 35 and 4200 mg/l (CGWB 1998).
In the study area, the concentration of TDS from shallow basalt aquifer ranges from 670 to 1515 mg/l (average 1008) and from deeper granite aquifers 702 to 975 mg/l (average 856). As compared to deeper granite aquifers, shallow basalt aquifers have high TDS, and this may be due to more dissolution of minerals in shallow aquifers.
Total hardness
Total hardness is the capacity of water to neutralize soap and is the sum of Ca2+and Mg2+ (Handa 1975; APHA 1998). Total hardness in groundwater from basaltic shallow aquifers of the Maharashtra state varies between 15 and 2295 mg/l (CGWB 1998). In the study area, total hardness in shallow aquifers ranges from 384 to 868 mg/l (avg 531) and in deeper aquifer it ranges from 148 to 320 mg/l (avg 210).
Calcium
The average abundance of Ca2+ in the earth’s crust is 4.9%, and in groundwater, it varies from 1 to more than 500 mg/l (APHA 1998). In most of the naturally occurring groundwater, calcium is the main cation due to its abundance in the earth’s crust and high mobility (Hem 1991). The most common forms of Ca are CaCO3 and CaMgCO3. The principal sources of calcium in groundwater are minerals present in igneous rock, especially silicates, like plagioclase and pyroxenes (Handa 1975; Hem 1991; APHA 1998). It is also present in the form of adsorbed ions on negatively charged mineral surfaces in soils and rocks. Calcium in groundwater from shallow basaltic rock formations of the Maharashtra state varies between 4 and 329 mg/l (CGWB 1998). In the study area, Ca in shallow aquifers ranges between 37 and 104 mg/l (avg 60) and in deeper aquifer it ranges from 26 to 80 mg/l (avg 51).
Magnesium
Magnesium is the 8th most abundant mineral in the solar system (Handa 1974). The average abundance of Mg in the earth’s crust is 2.1%, and in groundwater, it is more than 5 mg/l (APHA 1998). Weathering of basic igneous rocks such as dunites and pyroxenites; volcanic rocks such as basalts; metamorphic rocks like amphibolites, talc and tremolite schist; and sedimentary rocks such as dolomite is the main source of Mg2+ in the groundwater (Karanth 1987). Use of surface water for irrigation is another source of Mg2+ in groundwater (Kakkar et al. 1987; Hem 1991). In the groundwater from basaltic shallow aquifers of Maharashtra, the concentration of Mg2+ varies between 1 and 428 mg/l (CGWB 1998).
In the study area, Mg in shallow basalt aquifers ranges between 30 and 184 mg/l (avg 93) and in deeper granite aquifers it ranges from 12 to 29 mg/l (avg 20). As in most natural waters, in the study area also, the concentration of Mg is lower than Ca concentration, and this is an agreement with Hem (1991).
Sodium
The average abundance of Na+ in the earth’s crust is 2.5%; in soil, it is 0.02 to 0.62% and in groundwater it is generally 5 mg/l (APHA 1998). Silicate minerals such as labradorite ((Ca,Na)Al1-2Si3-2O8 with 30–50% Na) and augite ((Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6) in basalt are the main sources of Na+ in groundwater. The other sources are rainwater, dissolution of evaporite minerals and sodium disposal through septic tanks and industrial wastes (Handa 1975). Certain clay minerals and zeolite can increase the sodium concentration in groundwater by base exchange reaction (Karanth 1987). Sodium concentration in shallow basaltic aquifers of the Maharashtra state ranges between 3 and 644 mg/l (CGWB 1998).
In the study area, Na+ concentration in shallow aquifers ranges between 60 and 172 mg/l (avg 121) and in deeper aquifers it ranges from 167 to 210 mg/l (avg 184). Deeper granite aquifers have higher concentrations of Na than shallow basalt aquifers.
Chloride
Chloride in the form of Cl− is one of the major inorganic anions in groundwater and wastewater (APHA 1998). Hydrolysis of halite and related minerals, rainwater, irrigation and industrial effluents are the main sources of Cl− in groundwater (Handa 1975; Karanth 1987; Kakkar et al. 1987; Hem 1991; CGWB 1998). Minerals like sodalite, mica, chloro-apatite and hornblende are the other minor sources of chloride in groundwater (Karanth 1987). Abnormal concentration of Cl− in groundwater may result due to pollution of sewage wastes and planting of coconut trees (Karanth 1987). Chloride concentration in groundwater from basaltic aquifers of Maharashtra ranges between 7 and 1184 mg/l (CGWB 1998).
In the study area, concentrations of Cl− in shallow basalt aquifers vary from 90 to 446 mg/l (avg 253) and in deeper granite aquifers it varies from 216 to 290 mg/l (avg 260). The source of chloride in groundwater is mainly due to its anthropogenic nature, and the occurrence of high Cl− in deeper aquifer suggests interconnectivity between two aquifers.
Nitrate
Nitrogen present in the atmosphere reacts with rainwater and forms nitrate and ammonium ions. The incidence of high nitrate in groundwater has been observed due to pollution from anthropogenic sources, specially leaching from sewage/septic tanks (Walker 1973; Dudley 1990; Faillat and Rambaud 1991; Barnes et al. 1992; Wetselar et al. 1993; Alcocer et al. 1998; Agrawal et al. 1997). In Maharashtra, high nitrate concentration in groundwater from basaltic aquifer is highly erratic and anthropogenic activities might be responsible for their concentration (CGWB 1998).
In the study area, nitrate concentration in shallow basalt aquifers varies from 22 to 172 mg/l (avg 77) and from deeper granite aquifers it varies from 6 to 61 mg/l (avg 32). In some cases, high nitrate-bearing groundwater is associated with high concentration of chloride, which may be due to contamination of groundwater from domestic and urban wastes (Piskin 1973; Ritter and Chirnside 1984; Barnes et al. 1992; Kross et al. 1993; Madhnure 2004). Groundwater from shallow basalt aquifers contains comparatively higher concentration of nitrate than from deeper aquifer waters. However, in some cases, the concentration of nitrate in groundwater from deeper aquifers is also high, and this may be due to the hydraulic connectivity between two aquifers.
Fluoride
In the study area, F− concentration in shallow basalt aquifers varies from 0.5 to 1.2 mg/l (avg 0.8) from phase 1 samples and from phase 2 samples it varies from 1.2–1.4 mg/l (avg 1.3) in shallow basalt aquifers. In deeper granite aquifers, it ranges from 2.85 to 4.9 mg/l (avg 4.04). The comparison of F− concentration of groundwater from the shallow and deeper aquifers from the same location has indicated that deeper granite aquifers have higher concentration of F− than shallow basalt aquifers (Fig. 4), and this is in agreement with the observations made by Wodeyar and Sreenivasan (1996), Madhnure et al. (2007), and Madhnure et al. (2016). In India and other parts of the world, higher concentrations of F− have been reported from deeper aquifers (Back 1966; Apambire et al. 1997; Kruse and Ainchil 2003; Prem Babu et al. 2004; Madhnure et al. 2007; Madhnure et al. 2016). In the present study, a moderate degree of positive correlation is observed between depth vs. F− in samples collected during phase 2 (R 2 = 0.70) (Fig. 5 ). However, in some areas of the country, shallow aquifers also contain higher concentration of F− than deeper aquifers (Ramesam and Rajagopalan 1985; Subba Rao et al. 1998; Subba Rao and Rao 2003; Subba Rao 2006). Higher concentration of F− in shallow as well as deeper aquifers has also been reported from the Palghat district of Kerala state (Shaji et al. 2007).
Occurrences of heavy metals
Concentration of heavy metals (from phase 1) like iron (Fe 0.16), copper (Cu 0.051), zinc (Zn, below detectable limit) and lead (Pb 0.017) is detected in one sample (HP5), and in another sample (HP10), all parameters are below detectable limits.
Iron concentrations in shallow aquifers from basalt (phase 2) vary from 0.5 to 0.9 mg/l (avg 0.64), and from deeper granite aquifers, it varies from 0.3 to 0.9 mg/l (avg 0.57) and is beyond the permissible limits of drinking water standards i.e. 0.3 mg/l (BIS 2012).
Source of fluoride in groundwater
Fluorine is a lithophile element (those that show an affinity for silicate phases and are concentrated in silicate portion of the earth’s crust and mantle), and the average concentration of fluorine in the earth’s crust is 611 ppm (Varcia and Borgnino 2015) and in granite 810 ppm (Wedepohl 1969). A large number of minerals contain F− in their chemical formulas as a primary constituent or include it as an impurity, and among the primary minerals in igneous rocks are biotite and muscovite which may contain about 1 wt% of F−. Granite consists of minerals like K-feldspar, quartz, amphibole, biotite and muscovite of which amphibole, biotite and muscovite contain F−.
As fluoride ions have the same charge and nearly the same radius as hydroxyl ions, they may replace each other in the octahedral sheet of mineral structures (Brigatti and Guggenheim 2002). This kind of substitution is common in mica lattices, where the halogen is found at the same level as the apical oxygen of the tetrahedral sheets where they are bonded to octahedral cations, and thus, fluoride is released into groundwater. In addition to the minerals described above, granite also contains accessory minerals like fluorite and apatite with a chemical composition as CaF2 for fluorite and Ca5 (Cl, F, OH) (PO4)3 for apatite. The apatite-group minerals include three accepted species: fluorapatite (Ca10 (PO4)6F2), chlorapatite (Ca10 (PO4)6Cl2) and hydroxyl apatite (Ca10 (PO4)6OH2). Fluorapatite, in particular, is a ubiquitous accessory phase in igneous rocks (Pan and Fleet 2002). It may be noted that, prior to deep drilling by CGWB (Madhnure 2003) down to 200 m, there were no reports of tapping granite aquifers from the town.
Basalts, in addition to plagioclase feldspar (labradorite) (Ca,Na)Al1-2Si3-2O8), essentially contains amphibole mineral [(Ca,Na,K)2-3(Mg,Fe2+,Fe3+,Ti,Al)5(Si,Al)8O22(OH,F)2] which may contribute F− to the groundwater. Madhnure et al. (2011) have also reported that minerals such as amphibole, mica and zeolite are the contributory minerals for excess of F− in groundwater from shallow basalt aquifers. Similarly, calcretes from the basalts in shallow aquifer also provide a source of F− to groundwater. Near-surface exposures of basalt in the study area show that calcrete occurs as an intrusion in the form of vein or patches. Reddy et al. (2010) have reported that calcrete deposits have acted as a sink for co-precipitation and/or adsorption of fluoride in Wailapalli watershed, Nalgonda district of Telangana State.
Groundwater facies
For identification of characteristics of groundwater and chemistry, a Piper diagram is widely used as it gives the best graphical representation (Hill 1940; Piper 1944). Groundwater from shallow basalt aquifer (phase 1 and phase 2 samples, 24 in quantity) is mainly of Ca-HCO3-Cl type (12 in quantity) followed by Ca-Na-HCO3-Cl type (5), Ca-Cl type (4) and Ca-Na-Cl type (3) (Fig. 6a), whereas groundwater from deeper granite aquifer (phase 2 samples, 5 in quantity) is mainly of Ca-Na-Cl type (3) and Na-Cl type (2) (Fig. 6b).
Relationships between fluoride and major ions
The relationship between F− and four major ions (pH, Ca2+, Na+ and HCO3 −) is studied for both aquifers (Fig. 7a–h). The plot of F− vs. pH shows a moderate degree of positive correlation in shallow basalt aquifers (R 2 = 0.63) and a weak positive degree in deeper granite aquifers (R 2 = 0.28), indicating that higher alkalinity in groundwater promotes leaching of F− (Agrawal et al. 1997; Saxena and Ahmed 2001; Madhnure et al. 2007; Madhnure et al. 2016). The correlation plot between F− vs. Ca2+ shows a weak negative correlation (R 2 = 0.21 and 0.23) in both aquifers, and a similar observation was also made by Jacks et al. (2005). The plot of Na+ vs. F− shows a weak positive degree of correlation in both aquifers (R 2 = 0.034 and 0.034). Apambire et al. (1997) and Madhnure et al. (2016) have also observed that Na+ concentration increases with F−, thereby increasing the solubility of fluorite in water. The fluoride and HCO3 − pair has shown a weak positive correlation in both aquifers (R 2 = 0.08 and 0.05), and a similar relationship is also observed by Madhnure et al. (2016).
Groundwater management and augmentation options
In order to assess the management and augmentation options in the city, an assessment based on rainwater availability from rooftop and runoff water availability from open spaces and roads is made. (Madhnure 2004) estimated 1.987 MCM of rainwater available from rooftop, out of which 1.35 MCM can be recharged to groundwater or can be stored in emergency conditions, which is sufficient to cater for the needs of ~40,800 people (135 l/day/person for 245 days); Madhnure also estimated ~0.54 MCM of runoff from open spaces.
Conclusions and recommendations
Deeper granite aquifer is contaminated with high fluoride (geogenic), and shallow basalt aquifer is free from fluoride contamination, but contaminated with nitrate (anthropogenic). High concentrations of F− in deeper granite aquifer are due to source rocks containing fluoride-bearing accessory minerals, higher residence time, low recharge due to prevailing drought conditions and higher alkalinity. Due to increasing urbanization coupled with prevailing drought conditions, demand of drinking water is not satisfied by the normal recharge process resulting in desaturation of shallow aquifer particularly in the northwestern part of the city, and people are tapping deeper aquifers. As a recommendation, it is suggested to utilize 1.35 MCM of net rainwater available from rooftop along with 0.54 MCM from surface runoff with suitable rainwater-harvesting structures like recharge shafts. Fluoride-rich groundwater from deeper aquifers may be used for drinking purposes by blending with treated surface water and/or for other purposes. Most effective defluoridation techniques like electrolytic defluoridation (EDF), ion exchange and reverse osmosis may be adopted along with integrated fluorosis mitigation measures (Godfrey 2007).
References
Agrawal, V., Vaish, A. K., & Vaish, P. (1997). Groundwater quality: focus on fluoride and fluorosis in Rajasthan. Current Science, 73(9), 743–746.
Alcocer, J., Lugo, A., Marín, L. E., & Escobar, E. (1998). Hydrochemistry of waters from five cenotes and evaluation of their suitability for drinking-water supplies, northeastern Yucatan, Mexico. Hydrogeology Journal, 6(2), 293–301.
Apambire, W. B., Boyle, D. R., & Michel, F. A. (1997). Geochemistry, genesis and health implications of floriferous ground waters in the upper region of Ghana. Environmental Geology, 33, 13–24.
APHA. (1998). Standard methods for the examination of water and waste water (20th ed.pp. 10–161). Washington, DC: American Public Health Association.
Ayoob, S., & Gupta, A. K. (2006). Fluoride in drinking water: a review on the status and stress effects. Critical Reviews in Environmental Science and Technology, 36, 433–487.
Back, W. (1966). Hydrochemical facies and groundwater flow patterns in northern part of Atlantic Coastal Plain. U.S. Geological Survey Professional Paper, 498-A.
Barnes, C. J., Jacobson, G., & Smith, G. D. (1992). The origin of high nitrate ground waters in the Australian arid zone. Journal of Hydrology, 137, 181–197.
BIS. (2012). Drinking Water Specification (second revision of IS 10500:2012). Bureau of Indian Standards, Manak Bhavan, 9 Bahadur Shah Zafar Marg, New Delhi. 11p.
Brigatti M. F. and Guggenheim S. (2002). Mica crystal chemistry and the influence of pressure, temperature, and solid solution on atomistic models. In: Mottana A., Sassi F. P., Thompson, Jr. J. B. and Guggenheim S. (ed.). Micas: crystal chemistry & metamorphic petrology. Mineralogical Society of America, 1–98.
CGWB. (1998). Ground water exploration in Maharashtra and Union Territory of Dadra Nagar and Haveli. Unpub. Report, Central Ground Water Board, Ministry of Water Resources, Government of India, Nagpur, Un pub. Report, 44–46.
CGWB. (1999).High fluoride groundwater in India: occurrences, genesis and remedies. Unpub. Report Central Ground Water Board,Ministry of Water Resources, Govt. of India, 29p.
Dudley, N. (1990). Nitrates: The threat to food and water. London: Green Print. 118p.
Faillat, J. P., & Rambaud, A. (1991). Deforestation and leaching of nitrogen as nitrates into underground water in intertropical zones: The example of Côte d'lvoire. Environmental Geology and Water Sciences, 17(2), 133–140.
Gabriela Maria Varcia and Laura Borgnino. (2015). Fluoride in the context of the environment, in fluorine: chemistry, analysis, function and effects, pp.3–21 DOI:10.1039/9781782628507-00003.
Godfrey, S. (2007). Guidance manual on integrated fluorosis mitigation. NEERI, UNICEF, 98.
Gupta, S. K., & Sharma, P. (1995). An approach to tackling fluoride problem in drinking water. Current Science, 68(8) 774p.
Gupta, S. K., & Deshpande, R. D. (2003). High fluoride in groundwater of North Gujarat-Cambay region: origin, community perception and remediation. In V. P. Singh & R. N. Yadava (Eds.), Groundwater pollution (pp. 368–388). New Delhi: Allied Pub. Pvt. Ltd..
Handa, B.K. (1974). Methods of collection and analysis of water samples and interpretation of water analysis. Central Ground Water Board, Ministry of Agriculture. Technical Manual No.1.
Handa, B.K. (1975). Natural waters, their geochemistry, pollution and treatment with a chapter on saline water. Central Ground Water Board, Technical Manual No.2, Ministry of Agriculture and Irrigation, 246–252.
Hem, J.D. (1991). Study and interpretation of chemical characteristics of natural waters. Scientific pub. Jodhapur ,India, 339p.
Hill, R. A. (1940). Geochemical patterns in Coachella Valley. Transactions of the American Geophysical Union, 21, 46–49.
Jacks, G., Bhattacharya, P., Chaudhary, V., & Singh, K. P. (2005). Controls on the genesis of some high-fluoride ground waters in India. Applied Geochemistry, 20, 221–228.
Kakkar, Y.P., Lal, R., Kumar, A., Singh, B. and Dass, J. (1987) Chemistry of ground water in north-western India (Punjab, Haryana, Himachal Pradesh, Jammu and Kashmir, Union Territory of Delhi and Chandigarh. Central Ground Water Board, Ministry of Water Resources, Government of India, Chandigarh, 87p.
Karanth, K.R. (1987). Ground water assessment: development and management. Tata McGraw-Hill Education, 720p.
Kross, B. C., Hallberg, G. R., Bruner, D. R., Sherry Holmes, K., & Johnson, J. K. (1993). The nitrate contamination of private well water in Iowa. American Journal of Public Health, 83, 270–272.
Kruse, E., & Ainchil, J. (2003). Fluoride variations in groundwater of an area in Buenos Aires Province Argentina. Environmental Geology, 44, 86–89.
Kundu, N., Panigrahi, M. K., Tripathy, S., Munshi, S., Powell, M. A., & Hart, B. R. (2001). Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh District of Orissa. India Environmental Geology, 41(3–4), 451–460.
Madhnure, P. (2003). Basic data report of Exploratory well drilled at Vishnupuri village, District Nanded. Un-published report. Central Ground Water Board, Nagpur.
Madhnure, P. (2004). Impact of urbanization on the ground water regime in Nanded-Waghala City, Nanded district, Maharashtra. Un-published report. Central Ground Water Board, Nagpur. Pg61.
Madhnure, P. (2014). Groundwater exploration and drilling problems encountered in basaltic and granitic terrains of Nanded District, Maharashtra. Journal of the Geological Society of India, (84), 341–351.
Madhnure, P., Sirsikar, D. Y., Tiwari, A. N., Ranjan, B., & Malpe, D. B. (2007). Occurrence of fluoride in the groundwaters of Pandharkawada area, Yavatmal district, Maharashtra. India. Curr. Sci., 92(5), 675–679.
Madhnure, P., Malpe, D.B. and N.V. Chalapathi Rao (2011). Electron probe micro analyzer (EPMA) studies of basalt rocks from fluoride rich ground waters of Pandharkwada, Yavatmal district, Maharashtra” is published in the Journal of Applied Geochemistry Vol.13 No. 2 (2011) pp.192–197.
Madhnure, P., Malpe, D. B., & Rao, A. D. (2016). Aquifer wise seasonal variations and spatial distribution of major ions with focus on fluoride contamination, Pandharkawada block, Yavatmal district, Maharashtra, India. Journal Environmental Monitoring and Assessment, 188, 1–20. doi:10.1007/s10661-015-5027z.
Pan Y. and M. E. Fleet (2002). Compositions of the apatite-group minerals: substitution mechanisms and controlling factors. In: Kohn, M. L., Rakovan, J. and Hughes, J. M. (ed.) Phosphates. Mineralogical Society of America: pp. 13–50.
Piper, A. M. (1944). A graphic procedure in the geochemical interpretation of water analysis. Transactions of the American Geophysical Union, 25, 914–923.
Piskin, R. (1973). Evaluation of nitrate content of groundwater in Hall County, Nebraska. Ground Water, 11(6), 4–13.
Babu, P., Gonade, G., Bhai, H. Y., & Sinha, M. (2004). Fluoride contamination in groundwater in Ghatanji taluka, Yavatmal district, Maharashtra. Geological Survey of India, Special Publication, 83, 96–101.
Ramamohana Rao, N. V., Rao, N., Surya Prakash Rao, K., & Schuiling, R. D. (1993). Fluorine distribution in waters of Nalgonda District, Andhra Pradesh India. Journal of Environmental Geology, 21, 84–89.
Ramesam, V., & Rajagopalan, K. (1985). Fluoride ingestion into the natural waters of hard-rock areas, Peninsular India. Journal Geological Society of India, 26, 125–132.
Reddy, A. G. S., Reddy, D. V., Rao, P. N., & Prasad, K. M. (2010). Hydrogeochemicalcharacterization of fluoride rich groundwater of Wailapalli watershed, Nalgonda district,Andhra Pradesh,India. Environmental Monitoring and Assessment, 171, 561–577.
Reddy, A. G. S. (2014). Zeochemical evaluation of nitrate and fluoride contamination in varied hydrogeological environs of Prakasam district, Southern India. Journal for Environmental Earth Sciences, 71(10), 4473–4495. doi:10.1007/s12665-013-2841-x.
Ritter, W. F., & Chirnside, A. E. M. (1984). Impact of land use on groundwater quality in Southern Delaware. Ground Water, 22(1), 38–47.
Rode, Sanjay (2009).Drinking water supply management in Municipal Corporation of Maharashtra. Pg.31.
Shaji, E., Bindu, J., & Thambi, D. S. (2007). High fluoride in groundwater of Palaghat district Kerala. Current Science, 92(2), 240–245.
Saxena, V., & Ahmed, S. (2001). Dissolution of fluoride in groundwater: a water-rock interaction study. Environmental Geology Journal, 40(9), 1084–1087. doi:10.1007/s002540100290.
Sayeed Juned, A., Bhosle Arjun B, Lolage Yogesh P., Zafar S Khan and Bembrekar Shivraj K. (2015). Seasonal variation of fluoride content in ground water and its environmental impact at South Nanded City in Maharashtra. Special Vol on ‘Clean Earth-Green Earth’, SRTM University’s Research Journal of Science Vol.no.1, pp.25–29.
Subba Rao, N., Krishna Rao, G., & John Devadas, D. (1998). Variation of fluoride in groundwater of crystalline terrain. Journal of Environmental Hydrology, 6(paper 3), 1–5.
Subba Rao, N., & Rao, A. T. (2003). Fluoride in groundwater in a developing area of Guntur district, Andhra Pradesh India. Journal of Applied Geochemistry, 5(2), 94–100.
Subba Rao, N. (2006). Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environmental Geology, 49, 413–429. doi:10.1007/s00254-005-0089-9.
UN. (1991). Year book of the United Nations (Vol. 45, 317). New York: Department of Public Information United Nations.
Walker, W. H. (1973). Ground-Water Nitrate Pollution in Rural Areas. Ground Water, 11(5), 19–22.
Wedepohl, K. H. (Ed.). (1969). Handbook of geochemistry (Vol. II-1). Berlin: Springer.
Wetselar, R., Fox, J. J., Smith, G. D., Rum, A. M., Moermanto, R.J. & Ahmed, I. (1993). Ground water nitrate in East Java, Indonesia. Australian Geological Survey Organization, Journal Geology and Geophysics, 14, 273–277.
Wodeyar, B. K., & Sreenivasan, G. (1996). Occurrence of fluoride in the ground waters and its impact in Peddavankahalla Basin, Bellary District, Karnataka—a preliminary study. Journal Current Science, 70(1), 71–74.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandith, M., Kaplay, R., Potdar, S.S. et al. Impact of urbanization coupled with drought situations on groundwater quality in shallow (basalt) and deeper (granite) aquifers with special reference to fluoride in Nanded-Waghala Municipal Corporation, Nanded District, Maharashtra (India). Environ Monit Assess 189, 428 (2017). https://doi.org/10.1007/s10661-017-6098-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6098-9