Abstract
The importance of inflammation to atherothrombosis has led to the pursuit of noninvasive imaging methods to measure inflammation within the arterial wall. There is substantial evidence supporting the use of 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) imaging for evaluation of atherosclerotic plaque inflammation. However, coronary imaging with this technique has been limited, due to several technical hurdles. Nonetheless, early experiences in coronary FDG-PET imaging have been encouraging. This review outlines the development of vascular PET imaging and its potential use for evaluation of coronary artery disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although substantial effort and resources have been directed toward the detection and treatment of atherosclerotic disease, coronary heart disease remains the leading cause of death in United States. While the identification of coronary narrowing has been a mainstay of coronary disease diagnosis, stenosis severity is a relatively poor predictor of future acute coronary events (ACS) [1]. Imaging methods used clinically, such as perfusion imaging or invasive coronary angiography, provide information about stenosis severity while offering little information about the characteristics of the underlying plaques. On the other hand, several plaque characteristics beyond stenosis, such as the presence of a thin fibrous cap, a lipid-rich necrotic core, and inflammation, are closely linked to ACS. Accordingly, delineation of those additional plaque characteristics is desirable, and may provide useful diagnostic information.
18F-flourdeoxyglucose positron emission tomography (FDG-PET) has long been utilized to detect metabolically active tumors and recently has been investigated for imaging atherosclerotic plaques, providing an assessment of plaque inflammation. More recently, FDG-PET imaging has been used to characterize inflammation in the coronary tree. This review will focus on the history, current state of the art, and future directions of coronary FDG-PET imaging.
Inflammation and Atherosclerosis
Over the past several decades, our understanding of the atherosclerotic process has shifted, from the belief that it is a disease of steady lipid deposition, to a realization that atherosclerosis is a highly active process (Fig. 1) underpinned by inflammation [2]. Preclinical studies have defined an important mechanistic link between inflammation and atherothrombosis. Macrophages are thought to be responsible for augmenting the inflammatory process in the plaques by producing reactive oxygen species and modifying the lipoproteins resulting in chemo-attraction of more leukocytes to the site of atheroma. This in turn increases the cellular inflammatory burden causing the release of metalloproteinases, leading to breakdown of extracellular matrix and depletion of collagen with subsequent thinning of fibrous cap, rendering the plaque vulnerable for rupture and atherothrombosis [3, 4]. To make matters worse, the inflammatory milieu increases the production of pro-coagulant factors and makes it more likely that a plaque rupture will result in coronary occlusion [5]. Several epidemiologic studies have defined the link between plasma biomarkers of inflammation, including cell adhesion molecules, cytokines, pro-atherogenic enzymes, and C-reactive protein (CRP), with future cardiovascular risk in a variety of clinical settings [6]. Among the inflammatory markers, CRP has emerged as a strong predictor of cardiovascular risk in apparently healthy individuals, patients undergoing elective revascularization procedures, and patients presenting with ACS [7, 8]. Pathological studies have shown that plaques that have caused sudden death (culprit lesions) typically have a large necrotic core infiltrated by macrophages and T-lymphocytes and with a thin overlying fibrous cap [9]. Further studies demonstrated a correlation between degree of macrophage infiltration and lesion progression [10]. Accordingly, the important link between inflammation and atherothrombosis is supported by a wealth of pre-clinical, epidemiologic, and histopathologic data.
Development of an atherothrombotic lesion. A simplified depiction of the development of an atherothrombotic lesion, starting with a normal blood vessel (far left) to a ruptured plaque with superimposed thrombus (far right). AHA, American Heart Association; ICAM1, intercellular adhesion molecule 1; LDL, low-density lipoprotein; MMP, matrix metalloproteinase; VCAM1, vascular cell adhesion molecule 1. (Adapted from Sanz and Fayad [67])
PET Imaging of Inflammation
PET imaging is widely used in clinical evaluation of oncologic disease, and is considered to be the gold standard for tumor detection. The capability to detect tumors using FDG-PET lies in part within the fact that tumors are typically highly metabolically active, allowing them to accumulate FDG. Interestingly, much of the FDG associated with tumors actually accumulates within the macrophages that are associated with the tumor. In vitro, FDG uptake by macrophages is similar to that of tumor cells [11]. Further, careful radiomicroscopy of tumor lesions demonstrates that there may be more FDG accumulation within the peri-tumorous macrophages than in the tumor cells themselves. Hence, the success of clinical FDG-PET for tumor imaging can be attributed in large part to the uptake of FDG by macrophages, and in turn, the ability of PET to measure that macrophage activity [12, 13].
The realization that FDG imaging can be directed toward atherosclerosis imaging is a relatively recent phenomenon. FDG uptake within the vasculature has long been noted incidentally, during interpretation of oncologic imaging studies. High vascular FDG uptake has been reported in vascular inflammatory conditions such as giant cell arteritis and Takayasu’s arteritis [14]. Over the last decade, however, substantial effort has been devoted to the evaluation of FDG uptake within atherosclerotic plaques.
The uptake of FDG within inflamed atherosclerotic plaques relates to the high glycolytic rate of macrophages. FDG is a glucose analogue that enters cells through the glucose transporter protein (GLUT) [15]. Macrophage and inflammatory cells within the plaque are in constant demand for energy to fuel processes such as cellular motility and phagocytosis. Moreover, they have substantially higher glucose uptake when compared to noninflammatory cells within the plaque [16–18]. The relatively hypoxic milieu within plaques favors anaerobic metabolism, which in turn forces the macrophages to metabolize glucose over free fatty acids (which require oxygen to be metabolized). Thus, glucose is regarded as the main fuel for inflammatory cells inside the plaque [19–22]. Once FDG enters the macrophage, it becomes phosphorylated to FDG-6-phosphate, which unlike glucose-6-phosphate, cannot be further metabolized into the glycolytic pathway (Fig. 2). This allows for accumulation within cells, a phenomenon called metabolic trapping. Hence, accumulation of FDG provides an index of macrophage glycolytic activity.
FDG uptake by cells and subsequent metabolic trapping. GLUT, glucose transporter. (Adapted with permission from Rudd et al. [66])
PET Imaging of Inflamed Atherosclerotic Plaques: Preclinical Models
Several animal studies have evaluated vascular FDG uptake in models of atherosclerosis. Numerous studies have shown a strong correlation between FDG uptake and macrophage infiltration in rabbit models of atherosclerosis [23–25]. One study showed that highly inflamed atherosclerotic lesions accumulate 20 times more FDG than noninflamed arteries and that there is no relationship between FDG uptake and plaque size, and an inverse relationship with density of the plaque smooth muscle cells [25]. Aziz et al. [26] demonstrated in a rabbit model of triggered plaque rupture that the sites of subsequent rupture are the same sites that have the highest FDG uptake prior to triggered rupture.
Several studies have employed FDG-PET imaging to assess the effect of therapeutic interventions on atherosclerotic inflammation. Ogawa et al. [27] reported one of the first such studies, wherein they demonstrated a reduction in aortic FDG uptake after 3 months’ treatment with Probucol, an anti-inflammatory agent, thereby suggesting a role for FDG-PET as a noninvasive method to assess the effect of interventions on atherosclerotic plaque biology. Subsequently, several additional interventional studies have been reported in animal models, using FDG-PET [28–30].
PET Imaging of Atherosclerotic Plaques: Human Experience
Several studies have compared plaque uptake of FDG to risk factors for atherosclerotic diseases. Risk factors that have been associated with increased FDG uptake include advancing age [31, 32], male gender [33], diabetes [34], the metabolic syndrome, as well as increased Framingham risk scores (Fig. 3.) [34].
FDG uptake correlates with Framingham risk score. (Adapted with permission from Kim et al. [34])
The uptake of FDG within atherosclerotic plaques has been correlated with several systemic biomarkers of inflammation, such as CRP [33, 35]. Another study evaluated the levels of systemic levels of matrix metalloproteinase 1 after carotid stenting and showed that the FDG uptake within the target lesion correlated with the serum metalloproteinase level after the intervention [36].
Importantly, the FDG-PET signal has been linked to atherothrombotic events. Rudd et al. [37] showed that symptomatic carotid artery lesions had higher FDG uptake than asymptomatic lesion. In another study, Rominger et al. [38••] evaluated vascular FDG uptake in 334 patients with cancer, and demonstrated that high FDG uptake is a strong predictor of cardiovascular risk factors (Fig. 4). Paulmier et al. correlated high FDG uptake with cardiovascular events prior to or within 6 months after FDG-PET scans. Rogers et al. [35] demonstrated increased aortic FDG uptake in patients with recent ACS compared to controls with stable coronary syndromes.
Increased FDG uptake is associated with subsequent atherothrombotic events. Kaplan-Meier cumulative event-free survival curve: mean target-to-background ratio (TBR) using mean TBR cutoff of 1.7. (Modified from Rominger et al. [38••])
To underpin the biological relevance of the vascular FDG signal, several clinical trials have assessed the correlation between inflammation and FDG uptake. One such study showed a strong correlation between FDG uptake and macrophage infiltration of histologic sections obtained from patients who underwent carotid endarterectomy for carotid artery stenosis [39–41]. More recently [39, 40], carotid FDG uptake has been shown to correlate with the expression of several inflammatory genes within the plaque tissue subsequently removed at endarterectomy, such as the inflammatory cytokine interleukin 18 (IL-18), the macrophage-specific marker CD68, and proteinases such as cathepsin K and matrix metalloproteinase 9 (MMP-9).
Several trials have shown that the arterial FDG signal is reproducible. FDG-PET imaging had excellent short-term (14 days) interscan reproducibility with good interobserver agreement [42, 43••, 44]. Another study demonstrated a high 3 months interscan reproducibility in the contralateral carotid arteries in patients who had carotid endarterectomy [45]. Given the reproducibility of the signal, it becomes feasible to evaluate therapeutic interventions. Accordingly, Lee et al. [46] examined vascular FDG uptake before and after aggressive risk factor modification and demonstrated a 65% reduction in the number of vascular regions that accumulated FDG at 17 months. There, the change in FDG uptake correlated with the change in HDL cholesterol. Tahara et al. [47] documented a decrease in FDG uptake in patients who received simvastatin for 3 months compared to patients who had dietary interventions only. This was correlated with an increase in the HDL in the simvastatin group (Fig. 5). Currently, several multicenter, double-blinded, randomized placebo-controlled trials are being conducted using FDG-PET imaging to assess the effect of several novel therapies on plaque inflammation [48].
Effects of simvastatin on FDG uptake in atherosclerotic plaque inflammation. Representative FDG-PET images at baseline and after 3 months of treatment (post-treatment) with dietary intervention versus simvastatin. Baseline images demonstrate FDG uptake within a common carotid plaque (square). A substantial reduction in plaque FDG uptake is seen after 3 months of simvastatin (top). The bottom panel demonstrates per-subject data for baseline and post-treatment values in the diet and simvastatin groups. There was a significant reduction in vascular FDG uptake after simvastatin therapy but not after dietary modification. NS, not significant; SUV, standardized uptake value. (Modified from Tahara et al. [47]; with permission)
Coronary PET Imaging: Initial Experience
While imaging of large vessels has provided a wealth of information on the pathobiology of atherosclerotic disease, imaging of the coronary arteries, the locus of most clinically significant atherothrombotic events, remains the ultimate goal of noninvasive atherosclerosis imaging. However, coronary FDG-PET imaging has lagged behind large-vessel PET imaging due to increased technical demand needed for coronary imaging relative to large-vessel imaging.
Despite those obstacles, reports over the last several years provide hope that coronary plaque imaging with PET may be feasible. Perhaps the first account of coronary uptake of FDG was provided by Dunphy et al. [49]. In that report, a patient with suspected esophageal malignancy underwent dietary manipulation prior to PET imaging in order to suppress myocardial uptake of FDG so that the peri-cardiac structures could be better-delineated. As a result, the radiologists were able to identify several FDG hot spots in the location of the left anterior descending artery and suggested that this activity might be derived from active coronary plaques. A case report commented on incidental FDG uptake in left main coronary artery, which was found to be anatomically correspondent to a noncalcified plaque on subsequent coronary CT angiography [50].
Despite the incidental observations of FDG uptake in association with the coronary tree, it is widely acknowledged that multiple practical hurdles stand before coronary FDG-PET imaging. To begin with, myocardial cells have been known to avidly utilize glucose under normal aerobic conditions, despite the fact that free fatty acids are the major substrate for myocardial metabolism [51]. This will result in a low target-to-background ratio and interfere with analysis of FDG uptake. Another problem is the relatively small size of coronary plaques when compared to larger arteries. The smaller coronary plaques would be expected to result in lower measured FDG uptake and, due to the lower absolute number of macrophages than, say, a bulky carotid plaque. Furthermore, the relatively limited (3–5 mm) spatial resolution of PET introduces a phenomenon termed partial volume error when small structures are imaged, wherein the FDG activity derived from a lesion that is smaller than the resolution of PET is averaged over several adjacent pixels. As a result of these two additional issues, the signal-to-noise ratio would be further reduced. Furthermore, there is considerable cardiac motion, particularly in the distant vessels, which may lead to blurring of plaque FDG uptake.
Coronary PET Imaging: State of the Art
Despite those limitations, several groups have identified ways to overcome some of the hurdles facing PET imaging of coronary artery plaques. In line with the report of Dunphy et al. [49], several investigators have employed dietary manipulation to limit competing myocardial uptake of FDG. Dietary manipulation takes advantage that the myocardium will preferentially consume fatty acids over glucose, while macrophages in the anaerobic environment of atherosclerotic plaques preferentially use glucose. Accordingly, it has been shown that a fatty acid meal prior to FDG-PET imaging results in a substantial reduction of myocardial FDG uptake without significantly affecting nonmyocardial FDG uptake [52, 53]. One study demonstrated that patients who had pre-imaging high-fat low-carbohydrate meals had less myocardial FDG uptake when compared to patients who fasted prior to imaging [49]. Another study documented coronary FDG uptake in patients who underwent FDG-PET-CT imaging with a prior high-fat diet, and demonstrated colocalization with multiple extracardiac sites of atherosclerosis [54].
In a study designed specifically to evaluate coronary uptake of FDG, Wykrzykowska et al. [55] studied 32 cancer patients who underwent PET-CT imaging after dietary preparation who also underwent cardiac catheterization. In that study, myocardial suppression of FDG uptake was successful in the majority of patients, and foci of FDG uptake within the coronary tree was seen in 15 of 30 patients. Further, the investigators reported a trend to a significant correlation with angiographic disease (P = 0.07; 80 vessels examined) [55].
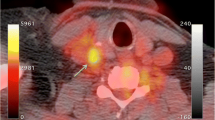
A potential limitation of the aforementioned studies is that there was little evidence that the observed FDG hotspots were related to atherosclerotic plaque activity per se. In order to test the hypothesis that FDG uptake localizes to inflamed coronary lesions, Rogers et al. [35] assessed whether FDG uptake in plaques deemed responsible for recent ACS is greater than in plaques in patients with stable coronary disease. Additionally, to exclude FDG uptake secondary to lesion manipulation, they compared FDG uptake in plaques recently stented for ACS with plaques recently stented for stable coronary syndromes. Accordingly, they performed a prospective study on 25 patients with coronary heart disease who underwent cardiac FDG-PET and cardiac CT angiography. Patients received a high-fat low-carbohydrate diet prior to imaging to suppress myocardial glucose uptake. Images were coregistered, and FDG uptake was measured at the site of the culprit lesion deemed clinically responsible for the presenting syndrome by virtue of locating the stent deployed to treat the syndrome. They reported higher FDG uptake in the ascending aorta and left main coronary arteries of patients who presented with ACS when compared to patients who had stable angina. They also demonstrated a higher FDG uptake in the ACS culprit lesions when compared to lesions stented for stable angina, and this was correlated with CRP and other serum inflammatory biomarker levels (Fig. 6) [35].
Upper panels show three representative images from patients presenting with chest pain syndromes. The left top panel is from a patient with an acute coronary syndrome (ACS). The arrow points to the stent placed at the site of the culprit lesion. Substantial FDG uptake is seen at that site. The middle top panel is from a patient with a stable coronary syndrome. A stent placed at the culprit lesion is seen. The FDG uptake associated with that lesion is modest. The right top panel shows a remotely stented lesion for comparison. The bottom panels demonstrate group values for FDG uptake. Patients with ACS had higher FDG uptake in association with culprit lesions. The fact that FDG uptake was not higher after stent placement for ACS compared to stent placement for stable syndromes suggests that the increased FDG uptake is not the result of stenting alone, but rather is due to the coronary syndrome. (Adapted from Rogers et al. [35]; with permission)
Despite the encouraging initial reports of coronary FDG-PET imaging, several important limitations need to be acknowledged. Firstly, cardiac motion leads to substantial blurring of the signal in the distal coronary tree, and substantially limits the reproducibility of the measurement there [56•]. Current methods for signal gating (which could otherwise correct for the blurring of the PET signal) result in the loss of too much signal and hence cannot be used. For now, coronary PET imaging is best focused on the left main coronary artery, which is tethered to the aorta and hence experiences less motion compared to the distal coronaries. Furthermore, the co-registration of PET images to coronary CT angiograms requires substantial technical expertise, and cannot be realistically used for clinical care. Accordingly, clinical application of this technique is not currently feasible.
Future Directions
PET vascular imaging will continue to evolve to provide better evaluation of plaque biological processes. This in turn may open new opportunities to identify novel therapies and provide better risk stratification of patients. However, additional advances in technology are needed if the dream of routine PET imaging of coronary vessels is to be realized.
High on the list of required advances is the production of new tracers that might localize more specifically to inflamed atherosclerotic plaques while providing a relatively high target-to-background ratio. To that end, several groups are evaluating novel tracers other than FDG for their ability to target plaque inflammation [57–60]. Additionally, other important biological processes within plaques, such as apoptosis and neovascularization, are being targeted by specific tracers [61, 62].
The greatest gains in coronary PET imaging will likely come from technological breakthroughs in imaging technologies. PET-CT systems are constantly being refined and provide improved resolution and enhanced co-registration with structural images. The recent development of PET-MRI systems provides additional opportunities for even better image co-registration (by virtue of co-incidental image acquisition of the PET and MRI, compared to the serial acquisition of PET and CT imaging). This in turn might enable better correction of partial volume errors. Additionally, novel methods to correct coronary motion, such as those reported by Suzuki et al. [63], might prove useful for coronary PET imaging.
Conclusions
Refinements in the understanding of the pathobiology of atherosclerosis along with the emergence of novel diagnostic and therapeutic approaches may provide a new opportunity to reduce morbidity and mortality associated with cardiovascular disease [64]. FDG-PET imaging is poised to play a potentially important role by providing information on the biology of atherosclerotic lesions, thereby complementing the structural information that can be derived from other imaging technologies. Such biological assessment of atherosclerotic plaques with PET may evolve into a clinical application as a risk assessment tool for cardiovascular disease, and may facilitate the evaluation of anti-atherosclerotic drugs. However, prior to clinical application, several aspects must be first addressed. There is a need for standardization of imaging protocols and quantification of FDG uptake, and a need for development of large prospective clinical trials to evaluate the prognostic significance of the vascular FDG signal [65]. Once these initial goals are met, then intensification of efforts to develop coronary PET techniques will follow.
References
Papers of particular interest, published recently have been highlighted as: • Of importance •• Of major importance
Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–71.
Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74.
Kovanen PT. Mast cells and degradation of pericellular and extracellular matrices: potential contributions to erosion, rupture and intraplaque haemorrhage of atherosclerotic plaques. Biochem Soc Trans 2007;35:857–61.
Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. J Intern Med 2008;263:517–27.
Libby P. Multiple mechanisms of thrombosis complicating atherosclerotic plaques. Clinical Cardiology 2000;23 Suppl 6:VI-3-7.
Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med 2002;252:283–94.
Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res 2001;89:763–71.
Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol 2003;41:37S–42S.
Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276–82.
Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–25.
Deichen JT, Prante O, Gack M, Schmiedehausen K, Kuwert T. Uptake of [18F]fluorodeoxyglucose in human monocyte-macrophages in vitro. Eur J Nucl Med Mol Imaging 2003;30:267–73.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992;33:1972–80.
Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med 1994;35:104–12.
Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries: a new observation. Clin Nucl Med 2001;26:314–9.
Shepherd PR, Kahn BB. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med 1999;341:248–57.
Leppanen O, Bjornheden T, Evaldsson M, Boren J, Wiklund O, Levin M. ATP depletion in macrophages in the core of advanced rabbit atherosclerotic plaques in vivo. Atherosclerosis 2006;188:323–30.
Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol 1999;19:870–6.
Mayr M, Sidibe A, Zampetaki A. The paradox of hypoxic and oxidative stress in atherosclerosis. J Am Coll Cardiol 2008;51:1266–7.
Evans WH, Karnovsky ML. The biochemical basis of phagocytosis. IV. Some aspects of carbohydrate metabolism during phagocytosis. Biochemistry 1962;1:159–66.
Weisdorf DJ, Craddock PR, Jacob HS. Granulocytes utilize different energy sources for movement and phagocytosis. Inflammation 1982;6:245–56.
Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol 2009;218:7–29.
Strauss HW, Dunphy M, Tokita N. Imaging the vulnerable plaque: a scintillating light at the end of the tunnel? J Nucl Med 2004;45:1106–7.
Ogawa M, Ishino S, Mukai T, et al. 18F-FDG accumulation in atherosclerotic plaques: Immunohistochemical and PET imaging study. J Nucl Med 2004;45:1245–50.
Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med 2009;50:959–65.
Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 2005;12:294–301.
Aziz K, Berger K, Claycombe K, Huang R, Patel R, Abela GS. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation 2008;117:2061–70.
Ogawa M, Magata Y, Kato T, et al. Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med 2006;47:1845–50.
Davies JR, Izquierdo-Garcia D, Rudd JH, et al. FDG-PET can distinguish inflamed from non-inflamed plaque in an animal model of atherosclerosis. Int J Cardiovasc Imaging 2010;26:41–8.
Worthley SG, Zhang ZY, Machac J, et al. In vivo non-invasive serial monitoring of FDG-PET progression and regression in a rabbit model of atherosclerosis. Int J Cardiovasc Imaging 2009;25:251–7.
Zhao Y, Kuge Y, Zhao S, Strauss HW, Blankenberg FG, Tamaki N. Prolonged high-fat feeding enhances aortic 18F-FDG and 99mTc-annexin A5 uptake in apolipoprotein E-deficient and wild-type C57BL/6J mice. J Nucl Med 2008;49:1707–14.
Bural GG, Torigian DA, Chamroonrat W, et al. FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging 2008;35:562–9.
Joly L, Djaballah W, Koehl G, et al. Aortic inflammation, as assessed by hybrid FDG-PET/CT imaging, is associated with enhanced aortic stiffness in addition to concurrent calcification. Eur J Nucl Med Mol Imaging 2009;36:979–85.
Rudd JH, Myers KS, Bansilal S, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging 2009;2:107–15.
Kim TN, Kim S, Yang SJ, et al. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 2010;3:142–8.
Rogers IS, Nasir K, Figueroa AL, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging 2010;3:388–97.
Wu YW, Kao HL, Chen MF, et al. Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase-1. J Nucl Med 2007;48:227–33.
Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–11.
•• Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 2009;50:1611–20. This article provides evidence that patients with large blood vessels that contain foci of high FDG uptake have a high risk of subsequent atherothrombotic events.
Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg 2009;37:714–21.
Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun 2010;31:423–9.
Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–24.
Rudd JH, Myers KS, Bansilal S, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007;50:892–6.
•• Rudd JH, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 2008;49:871–8. This paper demonstrates that PET measures of vascular FDG uptake are highly reproducible.
Izquierdo-Garcia D, Davies JR, Graves MJ, et al. Comparison of methods for magnetic resonance-guided [18-F]fluorodeoxyglucose positron emission tomography in human carotid arteries: reproducibility, partial volume correction, and correlation between methods. Stroke 2009;40:86–93.
Font MA, Fernandez A, Carvajal A, et al. Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci 2009;14:3352–60.
Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med 2008;49:1277–82.
Tahara N, Kai H, Ishibashi M, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 2006;48:1825–31.
clinicaltrials.gov. (Accessed at clinicaltrials.gov.)
Dunphy MP, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med 2005;46:1278–84.
Alexanderson E, Slomka P, Cheng V, et al. Fusion of positron emission tomography and coronary computed tomographic angiography identifies fluorine 18 fluorodeoxyglucose uptake in the left main coronary artery soft plaque. J Nucl Cardiol 2008;15:841–3.
Bing RJ, Fenton JC. Cardiac Metabolism. Annu Rev Med 1965;16:1–2.
de Groot M, Meeuwis AP, Kok PJ, Corstens FH, Oyen WJ. Influence of blood glucose level, age and fasting period on non-pathological FDG uptake in heart and gut. Eur J Nucl Med Mol Imaging 2005;32:98–101.
Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics 1999;19:61–77
Williams G, Kolodny GM. Retrospective study of coronary uptake of 18F-fluorodeoxyglucose in association with calcification and coronary artery disease: a preliminary study. Nucl Med Commun 2009;30:287–91.
Wykrzykowska J, Lehman S, Williams G, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med 2009;50:563–8.
• Rogers IS, Nasir K, Figueroa AL, et al. Feasibility of 18-fluorodeoxyglucose imaging of the coronary arteries: Comparison between patients with acute coronary syndromes and stable angina. JACC Cardiovascular Imaging 2010; 3;388–397. This article demonstrates with FDG-PET imaging that atherosclerotic inflammatory activity is increased in coronary culprit lesions after ACS.
Elmaleh DR, Fischman AJ, Tawakol A, et al. Detection of inflamed atherosclerotic lesions with diadenosine-5′,5‴-P1,P4-tetraphosphate (Ap4A) and positron-emission tomography. Proc Natl Acad Sci U S A 2006;103:15992–6.
Laitinen I, Saraste A, Weidl E, et al. Evaluation of alphavbeta3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging 2009;2:331–8.
Matter CM, Wyss MT, Meier P, et al. 18F-choline images murine atherosclerotic plaques ex vivo. Arterioscler Thromb Vasc Biol 2006;26:584–9.
Laitinen IE, Luoto P, Nagren K, et al. Uptake of 11C-choline in mouse atherosclerotic plaques. J Nucl Med 2010;51:798–802.
Calcagno C, Cornily JC, Hyafil F, et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol 2008;28:1311–7.
Isobe S, Tsimikas S, Zhou J, et al. Noninvasive imaging of atherosclerotic lesions in apolipoprotein E-deficient and low-density-lipoprotein receptor-deficient mice with annexin A5. J Nucl Med 2006;47:1497–505.
Suzuki Y, Slomka PJ, Wolak A, et al. Motion-frozen myocardial perfusion SPECT improves detection of coronary artery disease in obese patients. J Nucl Med 2008;49:1075–9.
Muller JE, Tawakol A, Kathiresan S, Narula J. New opportunities for identification and reduction of coronary risk: treatment of vulnerable patients, arteries, and plaques. Journal of the American College of Cardiology 2006;47:C2–6.
Sheikine Y, Akram K. FDG-PET imaging of atherosclerosis: Do we know what we see? Atherosclerosis 2010;211:371–80.
Rudd JH, Narula J, Strauss HW, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? Journal of the American College of Cardiology 2010;55:2527–35.
Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature 2008;451:953–7.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelbaky, A., Tawakol, A. Noninvasive Positron Emission Tomography Imaging of Coronary Arterial Inflammation. Curr Cardiovasc Imaging Rep 4, 41–49 (2011). https://doi.org/10.1007/s12410-010-9062-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12410-010-9062-4