Abstract
We investigated the ability of fluorodeoxyglucose positron emission tomography (FDG PET) imaging to serially monitor macrophage content in a rabbit model of atherosclerosis. Atherosclerosis was induced in rabbits (n = 8) by a combination of atherogenic diet and balloon denudation of the aorta. At the end of nine months, the rabbits were randomized to a further six months of the same atherogenic diet (progression group) or normal diet (regression group). In vivo uptake of FDG by the thoracic aorta was measured using aortic uptake-to-blood radioactivity ratios at the start and end of the randomized period. A significant increase in FDG uptake of the progression group after continued cholesterol feeding (aortic uptake-to-blood radioactivity: 0.57 ± 0.02 to 0.68 ± 0.02, P = 0.001), and a corresponding fall in FDG uptake of the regression group after returning to a normal chow diet (aortic uptake-to-blood radioactivity ratios: 0.67 ± 0.02 to 0.53 ± 0.02, P < 0.0001). FDG PET can quantify in vivo macrophage content and serially monitor changes in FDG activity in this rabbit model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerotic disease is one of the leading causes of morbidity and mortality in western society [1, 2]. Atherosclerotic plaque disruption with superimposed thrombosis is the main cause of the acute coronary syndromes. Acute thrombosis on disrupted atherosclerotic plaque also plays a role in atherosclerosis progression [3]. While atherosclerosis burden remains an important factor in progression of disease, the composition and vulnerability of plaque have emerged as being crucial determinants for the development of the thrombus-mediated acute coronary syndromes [4]. Recent investigations have focused on the central role of macrophages in converting a stable, quiescent plaque to an unstable one with rupture and fissuring [5, 6]. Macrophages have been implicated in weakening the fibrous cap of the plaque due to the secretion of matrix-metalloproteinase (MMP) [7, 8]. MMPs are a family of enzymes that degrade extracellular matrix components, particularly collagen, elastin, and proteoglycans, thereby possibly serving to thin the collagen skeleton of the fibrous cap, leading to plaque rupture and fissuring [9].
Imaging techniques that can characterize the plaque and its components will potentially allow risk stratification in asymptomatic, as well as symptomatic, patients with coronary artery disease and potentially select the most appropriate therapies to help attenuate this risk. In particular, quantification of macrophage burden might help to assess plaque vulnerability and risk for rupture. A positron emitting radiotracer, fluorine-18-2-deoxy-2-glucose ([18F]FDG), an analog of d-Glucose, is a useful marker of high metabolic activity commonly found in tumor-associated macrophages [10, 11]. We and others have shown the feasibility of FDG PET imaging to assess macrophage content [12–14]. Recently, a small study has demonstrated attenuation of plaque inflammation in humans with simvastatin utilizing this imaging technique [15]. The purpose of this study was to assess progression and/or regression of macrophage content in atherosclerotic plaques in an experimental rabbit model using this non-invasive technique.
Methods
Experimental model
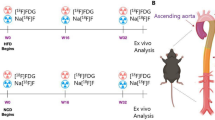
A total of eight New Zealand white rabbits were studied after atherosclerosis induction. Figure 1 illustrates the timeline of the experiment. Induction of atherosclerosis was achieved with combination of a moderate atherogenic diet (0.2% cholesterol) for 9 months and double balloon denudation of the aorta 1 week and 13 weeks after diet initiation. At the end of the induction period, the rabbits were randomized to one of two treatment groups: the progression group that received the same atherogenic diet for six more months (n = 4), and the regression group (n = 4) that received a normal diet for 6 months. FDG PET imaging was performed at the commencement and end of the randomized period, after which all animals were sacrificed. The pathological correlation between FDG-PET and macrophage content in these groups has been published previously [14].
Timeline of experiment. All eight rabbits underwent atherosclerosis induction with 9 months of atherogenic diet (0.2% cholesterol) and balloon denudation of the thoracic aorta at week 1 (W1 BD) and week 13 (W13 BD). The rabbits were randomized to a further 6 months of either the same atherogenic diet or normal diet. FDG PET imaging was performed at 9 months and 15 months on all rabbits
Balloon denudation
All procedures were performed under general anaesthesia by intramuscular injection of ketamine (20 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (10 mg/kg, Bayer Corporation, Shawnee Mission, KA). Aortic denudation of the aorta from the aortic arch to the iliac bifurcation was performed by withdrawal, with moderate resistance, of a 4Fr Fogarty embolectomy catheter introduced through the iliac artery and passed into the aortic arch. Catheter insertion and inflation were repeated for four passes, after which the catheter was removed, the femoral artery tied, and the incision closed as previously described [14, 16–18]. All experiments were approved by the Mount Sinai School of Medicine animal management program, under accreditation from the American Association for the Accreditation of Laboratory Animal Care (AALAC).
FDG-PET imaging of rabbits
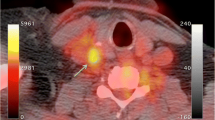
Immediately prior to the FDG-PET imaging, all rabbits underwent magnetic resonance imaging (MRI) for the purpose of localization of the anatomical structures on the PET images. After fasting the previous night, the rabbits were anesthetized as described above. Then, they were immobilized with a body-fitting thermosetting plastic holder compatible with both the MRI and PET scanning systems, thus assuring the exact positioning of the rabbits for both imaging studies. The rabbits were laser-aligned on the xiphisternum in both scanning modalities. After initial gradient echo scout images to identify the thoracic aorta, 2D-time of flight imaging was performed to exactly localize the aorta. Thereafter, the anesthetized rabbits were immediately taken to the PET scanner in the holders. Imaging was then performed using a GE 2048-plus brain dedicated PET scanner. The axial field of view was 10.5 cm and the measured in-plane resolution was 4.5 mm in the center of the transverse planes. A 30-min transmission scan was performed for the whole descending aorta of the rabbit. Each rabbit was injected via a marginal ear vein with 1–2 mCi of FDG. FDG (T 1/2 = 110 min) was purchased form PET-NET System (New York, NY). The PET imaging was performed 30 min after injection. A 30-min emission scan was then obtained. The data was reconstructed with standard filtered back projection and attenuation correction. Using an in-house software system, each PET slice was co-registered with its corresponding MRI slice guided by anatomical landmarks and the aortic arch. Thus anatomical structures identified by MRI were correlated with the FDG uptake by a process of image superimposition, as previously described (Fig. 2); [14].
Demonstration of the co-registration of PET and MR images allowing the exact determination of FDG uptake in the aorta. These images are from the same animal showing MRI (a), the resultant fusion (b) and FDG PET (c). The yellow arrows highlight the position of the aorta and the white arrows the position of the heart
Blood sampling
Arterial blood samples were collected from an auricular artery at 15, 30, 45, and 60 min post-injection of FDG. Blood samples were weighed. The radioactivity was counted in a gamma counter (Minaxi Auto-Gamma 5000 Series Gamma Counter, Packard, USA) and decay corrected, as previously described [14].
PET image and data analysis
The PET images were transferred to an IBM-compatible computer for further analysis. Aortic uptake was expressed as concentration (μCi/ml), and for the correction of blood pool activity, FDG uptake was expressed as the ratio of aortic uptake-to-blood radioactivity, as previously validated [14]. Slices were 6 mm apart by taking a 5 × 5 mm2 ROI. Serial comparisons were made with FDG uptake using the techniques described. A total of 25 consecutive axial slices, beginning from the distal aortic arch, were analyzed in the descending aorta pre and post dietary modification per animal. This resulted in a total of 100 pairs of data for each group of rabbits.
All FDG PET analyses were performed by a single operator blinded to the dietary randomization.
Statistical analysis
Data is presented as mean ± standard error of the mean. To account for multiple samplings at different sites from the same rabbit, the “svy: regress” procedure was used (STATA 9.1, College Station TX). Paired students t-testing was performed on the matched aortic slices pre and post dietary modification (Statview, Abacus Corp) and the data graphically presented in “box and whisker” formats. A P value < 0.05 was considered statistically significant.
Results
Serum lipid profiles
The serum total cholesterol at baseline was normal (34 ± 23 mg/dl) but was significantly increased (949 ± 141 mg/dl) at the end of atherosclerosis induction at 9 months. The serum total cholesterol at 15 months in the progression group and the regression group were 936 ± 340 and 27 ± 6 mg/dl, respectively.
Histopathology
The combination of the atherogenic diet for 9 months and balloon denudation of the aorta induced a significant thickening of the arterial wall due to the increase in lipid and fibrotic components. The induced atherosclerotic lesions were rich in macrophage areas as indicated by RAM-11 staining (Fig. 3).
In vivo axial PET image showing the uptake of FDG in the thoracic aorta (black arrow) in a control rabbit (a), in a rabbit with mild atherosclerosis (b) and in a rabbit with more advanced atherosclerosis showing a higher uptake of FDG (c). Corresponding histopathology stained with RAM-11 of a, b, c showing no macrophages (d) some macrophages (e) and abundant macrophages (f), respectively
[18F]FDG uptake
Using all of the 25 aortic slices pre and post dietary modification per animal, there were significant differences in aortic uptake-to-blood radioactivity value between the progression and regression groups at commencement (0.57 v 0.67, P = 0.003) and end of randomization (0.68 v 0.53, P < 0.001) as determined by unpaired t-testing.
There was a significant increase in the aortic uptake-to-blood radioactivity value in the progression group after a further 6 months of cholesterol feeding (0.57 ± 0.02 to 0.68 ± 0.02, P = 0.001) (Fig. 4).
“Box and Whisker” plot comparing the results of the aortic-to-cardiac uptake activity ratios, pre- and post-continued cholesterol feeding in the atherosclerosis progression group. We can appreciate the significant increase in values representing increased FDG PET uptake and thus increased macrophage content from the pre to post continued cholesterol feeding. The error bars (“whiskers”) represent the 10th and 90th percentiles, respectively, and the top and bottom of the box the 25th and 75th percentiles, respectively. The line in the box represents the median value of the aortic-to-cardiac uptake activity ratios for the given group
Similarly, there was a corresponding fall in the aortic uptake-to-blood radioactivity values in the regression group after returning to a normal chow diet for 6 months. (0.67 ± 0.02 to 0.53 ± 0.02, P < 0.0001) (Fig 5).
“Box and Whisker” plot comparing the results of the aortic-to-cardiac uptake activity ratios, pre- and post- cholesterol feeding in the atherosclerosis regression group. We can appreciate the significant decrease in values representing FDG PET uptake and thus decreased macrophage content from the pre to post continued cholesterol feeding. The error bars (“whiskers”) represent the 10th and 90th percentiles, respectively and the top and bottom of the box the 25th and 75th percentiles respectively. The line in the box represents the median value of the aortic-to-cardiac uptake activity ratios for the given group
Discussion
We have demonstrated, the ability of [18F]FDG PET to monitor regression and/or progression of macrophage content in an experimental model.
Radionuclide imaging of atherosclerosis uses the pathophysiology of the atherosclerotic process to deliver radiopharmaceuticals to the lesion [19]. Therefore, different constituents of atherosclerosis, including radiolabeled lipoproteins [20, 21], monocytes [22], smooth muscle cells [23], platelets [24, 25] and fibrinogen [26] have been utilized as potential plaque-imaging agents. However, in vivo detection has been difficult to achieve for different reasons. Among these reasons, are the facts that the absolute and specific uptake of radiotracer in atherosclerotic lesions is low and that the blood-pool activity is very high due to slow plasma clearance of the radiotracer [19].
The most important mechanism responsible for the sudden and unpredictable onset of acute thrombosis is plaque rupture [2, 27]. The risk of plaque rupture depends on its composition rather than its size. Ruptures occur preferentially in plaques containing a soft, lipid-rich core that is covered by a thin and inflamed cap of fibrous tissue [4]. Compared with intact caps, the ruptured ones usually are thinner and contain less collagen, fewer smooth muscle cells and many more macrophages. Disrupted fibrous caps usually are heavily degraded and infiltrated by macrophages foam cells [5, 9]. These macrophages are activated, indicating ongoing inflammation at the site of plaque disruption [28]. In human coronaries, there is a correlation between plaque fibrous cap macrophage density and the presence of thin cap fibrous atheroma as detected by optical coherence tomography [29]. There is also a correlation between plaque macrophage density and symptom severity in patients with acute coronary syndromes [30].
Visualization of the vessel wall and the plaque itself rather than the lumen is necessary for the identification of such vulnerable atherosclerotic plaques. Different imaging modalities for assessing plaque composition including magnetic resonance (MR) imaging, angioscopy, vascular ultrasonography and infrared imaging analysis have been developed to discriminate wall components in normal and atheromatous arteries [31–34]. Magnetic resonance techniques appear to be able to non-invasively identify and discriminate the components of complex atherosclerotic lesions both in ex vivo and in vivo settings [33, 34]. However, no non-invasive imaging technique has been shown to identify in vivo macrophages until recently [12–14]. Previous attempts to detect activated macrophages utilized 111In–IgG. Although this was a valid tracer for detection of activated macrophages in tissues ex vivo, it was impossible to detect lesions in the aorta in vivo in rabbits because of a relatively low target-to-background ratio [35].
Fluorine-18-2-deoxy-2-glucose ([18F]FDG) has been shown to be a useful tumor-detecting agent [11, 36]. The mechanism of accumulation of this tracer into the malignant tissue is due to the enhanced rate of glucose utilization by neoplastic cells [37]. Due to increased metabolic demand for glucose, the hexokinase (a key enzyme for glycolysis) activity is increased. 2-Deoxy-2-glucose has been shown to be a substrate for hexokinase [38]. Therefore 2-deoxyglucose analog [18F]FDG is a particularly appropriate imaging agent for tumors. Recent work using microautoradiography has demonstrated that macrophages showed a high uptake of [18F]FDG [10].
In this study, uptake of FDG was assessed in the same rabbit at two time points and a serial comparison between the same atherosclerotic aortic segments made for each rabbit. We were able to monitor and identify significant changes in FDG uptake between the progression and regression groups. Although this may reflect serial changes in macrophage activity alone, we and others have previously shown FDG uptake to correlate with macrophage content [12, 14].
Limitations
Due to the lack of availability of a PET/CT system, MR imaging was used to co-register FDG uptake with the corresponding anatomical sites. We have validated this technique previously [14], but potentially PET/CT systems would allow a simpler and potentially more accurate co-registration to be performed. This study included a relatively small number of animals. Although we have identified significant changes in FDG uptake between the groups, statistical regression to the mean cannot entirely be excluded. A cross-over design may have added further to the potential of FDG PET in serial monitoring of changes in this atherosclerotic model.
We were not able to quantify plaque burden in this study as the MRI images were used purely for localization. However, we have previously demonstrated atherosclerotic burden progression and regression utilizing high resolution MRI with histopathological correlation in the same animal model [17].
Summary
[18F]FDG-PET scanning in this animal model of atherosclerosis has allowed us to serially monitor macrophage content. Thus FDG PET could potentially be used to serially monitor changes in atherosclerotic plaque macrophage content in response to therapies such as lipid lowering over time.
References
Worthley SG, Helft G, Zaman AG et al (2000) Atherosclerosis and the vulnerable plaque–pathogenesis: part I. Aust N Z J Med 30:600–607
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126. doi:10.1056/NEJM199901143400207
Gronholdt ML, Dalager-Pedersen S, Falk E (1998) Coronary atherosclerosis: determinants of plaque rupture. Eur Heart J 19(Suppl C):C24–C29
Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92:657–671
Libby P (1995) Molecular bases of the acute coronary syndromes. Circulation 91:2844–2850
Berliner J, Navab M, Fogelman A et al (1995) Atherosclerosis: basic mechanisms: oxidation, inflammation, and genetics. Circulation 91:2488–2496
Shah PK, Falk E, Badimon JJ et al (1995) Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 92:1565–1569
Galis ZS, Muszynski M, Sukhova GK et al (1994) Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extracellular matrix digestion. Circ Res 75:181–189
Lendon C, Davies M, Born G et al (1991) Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 87:87–90. doi:10.1016/0021-9150(91)90235-U
Kubota R, Kubota K, Yamada S et al (1994) Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med 35:104–112
Som P, Atkins HL, Bandoypadhyay D et al (1980) A fluorinated glucose analog, 2-fluoro-2-deoxy-d-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med 21:670–675
Ogawa M, Ishino S, Mukai T et al (2004) (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 45:1245–1250
Rudd JH, Warburton EA, Fryer TD et al (2002) Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105:2708–2711. doi:10.1161/01.CIR.0000020548.60110.76
Zhang Z, Machac J, Helft G et al (2006) Non-invasive imaging of atherosclerotic plaque macrophage in a rabbit model with F-18 FDG PET: a histopathological correlation. BMC Nucl Med 6:3. doi:10.1186/1471-2385-6-3
Tahara N, Kai H, Ishibashi M et al (2006) Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 48:1825–1831. doi:10.1016/j.jacc.2006.03.069
Helft G, Worthley SG, Fuster V et al (2001) Atherosclerotic aortic component quantification by noninvasive magnetic resonance imaging: an in vivo study in rabbits. J Am Coll Cardiol 37:1149–1154. doi:10.1016/S0735-1097(01)01141-X
Helft G, Worthley SG, Fuster V et al (2002) Progression and regression of atherosclerotic lesions: monitoring with serial non-invasive magnetic resonance imaging. Circulation 105:993–998. doi:10.1161/hc0802.104325
Worthley SG, Helft G, Fuster V et al (2000) Serial in vivo MRI documents arterial remodelling in experimental atherosclerosis. Circulation 101:586–589
Vallabhajosula S, Fuster V (1997) Atherosclerosis: imaging techniques and the evolving role of nuclear medicine. J Nucl Med 38:1788–1796
Lees RS, Lees AM, Strauss HW (1983) External imaging of human atherosclerosis. J Nucl Med 24:154–156
Lees AM, Lees RS, Schoen FJ et al (1988) Imaging human atherosclerosis with 99mTc-labeled low density lipoproteins. Arteriosclerosis 8:461–470
Virgolani I (1989) Radiolabeling autologous monocytes with 111In oxine for reinjection im patients with atherosclerosis. Prog Clin Biol Res 355:271–274
Narula J, Petrov A, Bianchi C et al (1995) Noninvasive localization of experimental atherosclerotic lesions with mouse/human chimeric Z2D3 F(ab′)2 specific for the proliferating smooth muscle cells of human atheroma. Imaging with conventional and negative charge-modified antibody fragments. Circulation 92:474–484
Minar E, Ehringer H, Dudczak R et al (1989) Indium-111-labeled platelet scintigraphy in carotid atherosclerosis. Stroke 20:27–33
Davis HH, Siegel BA, Sherman LA et al (1980) Scintigraphic detection of carotid atherosclerosis with indium-111-labeled autologous platelets. Circulation 61:982–988
Mettinger KL, Larsson S, Ericson K et al (1978) Detection of atherosclerotic plaques in carotid arteries by the use of 123I-fibrinogen. Lancet 1:242–244. doi:10.1016/S0140-6736(78)90485-3
Fuster V, Badimon L, Badimon J et al (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes. N Eng J Med 326(242–250):310–318
van der Wal AC, Becker AE, van der Loos CM et al (1994) Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89:36–44
Raffel OC, Tearney GJ, Gauthier DD et al (2007) Relationship between a systemic inflammatory marker, plaque inflammation, and plaque characteristics determined by intravascular optical coherence tomography. Arterioscler Thromb Vasc Biol 27:1820–1827. doi:10.1161/ATVBAHA.107.145987
MacNeill BD, Jang IK, Bouma BE et al (2004) Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol 44:972–979. doi:10.1016/j.jacc.2004.05.066
Brezinski M, Tearney G, Nalwalk JW et al (1997) Assessing atherosclerotic plaque morphology: comparison of optical coherence tomography and high frequency intravascular ultrasound. Heart 77:397–403
Casscells W, Hathorn B, David M et al (1996) Thermal detection of cellular infiltrates in living atherosclerotic plaques: possible implications for plaque rupture and thrombosis. Lancet 347:1447–1451
Worthley SG, Helft G, Fuster V et al (2000) High resolution ex vivo magnetic resonance imaging of in situ coronary and aortic atherosclerotic plaque in a porcine model. Atherosclerosis 150:321–329. doi:10.1016/S0021-9150(99)00386-X
Toussaint J, LaMuraglia G, Southern J et al (1996) Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 94:932–938
Demacker PN, Dormans TP, Koenders EB et al (1993) Evaluation of indium-111-polyclonal immunoglobulin G to quantitate atherosclerosis in Watanabe heritable hyperlipidemic rabbits with scintigraphy: effect of age and treatment with antioxidants or ethinylestradiol. J Nucl Med 34:1316–1321
Di Chiro G, DeLaPaz RL, Brooks RA et al (1982) Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology 32:1323–1329
Weber G (1977) Enzymology of cancer cells (second of two parts). N Engl J Med 296:541–551
Renner ED, Plagemann PG, Bernlohr RW (1972) Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem 247:5765–5776
Acknowledgments
This work was supported by grants from the National Heart Foundation of Australia (SA Branch) (S.G.W.), the French Federation of Cardiology (G.H.) and the National Health and Medical Research Council of Australia (G.Y.H.L., No: 497809).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Worthley, S.G., Zhang, Z.Y., Machac, J. et al. In vivo non-invasive serial monitoring of FDG-PET progression and regression in a rabbit model of atherosclerosis. Int J Cardiovasc Imaging 25, 251–257 (2009). https://doi.org/10.1007/s10554-008-9377-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-008-9377-2