Abstract
The biological processes within atherosclerotic plaques are known to be critically important to the progression and rupture of plaques. Among them inflammation is thought to be a key participant in all stages of atherosclerosis, from plaque initiation to progression and atherothrombosis. Accordingly, imaging of inflammation may provide important insights into the atherosclerotic milieu.
18F-fluorodeoxyglucose positron-emission tomography/computed tomography (FDG-PET/CT) imaging, originally developed as an imaging modality to evaluate oncologic processes, has evolved into a robust and reproducible modality for the quantification of arterial inflammation. Studies have shown that FDG uptake significantly correlates with arterial wall macrophage infiltration, high-risk morphological features of atherosclerotic plaques, expression of proinflammatory genes, and increased risk for future atherothrombotic events. Moreover, arterial FDG uptake is modifiable by anti-atherosclerotic treatments especially those with anti-inflammatory properties such as statins. Accordingly, FDG-PET/CT imaging is increasingly used as a surrogate imaging biomarker to monitor therapeutic efficacy of anti-atherosclerotic agents.
In this chapter, we review the basic cellular and molecular aspects of FDG-PET/CT imaging. Thereafter, we discuss the role of PET/CT imaging in noninvasive imaging of arterial inflammation, prediction of risk for progression of atherosclerosis, and risk assessment for subsequent atherothrombotic events. Furthermore, we discuss the utility of PET/CT imaging to evaluate the efficacy of new anti-atherosclerotic pharmacotherapies and review the results from recent imaging trials that employed PET/CT for this purpose. Lastly, we review the current state of the art and future directions for PET/CT imaging, with a review of coronary artery imaging, characterization of new targets, and recent improvements in camera and tracer technologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Human Immunodeficiency Virus
- Visceral Adipose Tissue
- Framingham Risk Score
- Coronary Plaque
- Cardiac Sarcoidosis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
A large body of evidence demonstrates that evaluation of coronary stenosis per se provides a relatively crude assessment of future risk of plaque rupture and cardiovascular events. This fact was emphasized, nearly 20 years ago, by a series of studies that evaluated the relationship between presence of coronary stenosis and subsequent atherothrombosis [1]. The study demonstrated that most culprit lesions do not cause significant luminal narrowing prior to cardiovascular events (Fig. 15.1). While plaques with greater degrees of luminal stenosis are more likely to progress to occlusion and/or give rise to infarction, most atherothrombotic events result from rupture of the plaques which are mildly to moderately obstructive, since the less obstructive plaques usually far outnumber severely obstructive plaques [1]. Accordingly, evaluation of coronary stenosis provides inadequate information in terms of risk assessment for subsequent cardiovascular events. The delineation of additional atherosclerotic features, such as vessel wall structure and biology, may provide important insights [2].
Degree of coronary stenosis and subsequent cardiovascular events. In the coronary arteries, less obstructive plaques outnumber severely obstructive plaques. Vascular occlusions and cardiovascular events evolve most frequently from plaques that are only mildly to moderately obstructive months to years before infarction (Adapted with permission from a review article by Falk et al. [1])
The paradigm of simultaneous assessment of structure and biology has been successfully performed for over a decade in the field of oncology. Two landmark papers in 2003 demonstrated that combined molecular and structural imaging, using PET and CT, is capable to localize and stage tumors more accurately compared to structural imaging alone [3, 4]. These observations led to the vast proliferation of PET/CT scans and have since fundamentally transformed the practice of oncology. In the study of cardiovascular disorders, the similar paradigm of simultaneous evaluation of structure and biology of atherosclerosis is relatively new and rapidly evolving.
Several features of atherosclerotic plaques that can be visualized noninvasively through imaging have been shown to be associated with a heightened risk of subsequent atherothrombosis [5]. These features include positive remodeling, high lipid content (low-attenuation plaque), thin fibrous cap, evidence of ulceration, intra-plaque hemorrhage, and microcalcification [5, 6]. The biological processes within plaques are also known to be critically important to the progression and rupture of plaques. Among them, inflammation is thought to be a key participant in all stages of atherosclerosis, from plaque initiation to plaque progression to atherothrombosis [7, 8]. Accordingly, imaging of inflammation may also provide important insights into the atherosclerotic milieu.
2 FDG-PET/CT Imaging of Oncologic Processes and Inflammation
The commonly employed PET/CT imaging agent, 18F-fluorodeoxyglucose (FDG), is clinically used for identification of neoplasms, screening of tumor metastases, and assessment of response to antineoplastic therapies. Additionally, FDG-PET/CT imaging has been demonstrated to be clinically useful for imaging of various sources of inflammation, including evaluation of device infection, fever of unknown origin, and sarcoidosis. Studies have shown that among the inflammatory cells, macrophages tend to have high uptake of FDG which is comparable to the amount of FDG uptake by tumor cell lines. Moreover, further investigations which were designed to reveal the source of intra-tumoral FDG uptake have demonstrated that the tumor-associated macrophages (TAMs) demonstrate even higher FDG uptake than the tumor cells [9] (Fig. 15.2). Accordingly, a substantial portion of the clinical utility of FDG-PET/CT scan for oncologic imaging relates to the uptake of FDG by macrophages.
High uptake of glucose analogs in tumor-associated macrophages. The uptake of tritium deoxyglucose ([ 3 H] DG) is demonstrated over time in intra-tumoral cellular components of mammary carcinoma in mice. [3H] DG uptake is determined by microautoradiographic silver grain counting (Adapted with permission from Kubota et al. [9])
The molecular mechanisms underlying FDG uptake by inflammatory cells have been well studied. FDG is a glucose analog that, similar to glucose, enters the cells through glucose transporters (GLUT), a group of membrane proteins that facilitate the transport of glucose over the plasma membrane. Within cells, FDG is phosphorylated by hexokinase to produce FDG-6-phosphate. Similarly, glucose is phosphorylated by the similar enzyme to become glucose-6-phosphate. Since the glucose-6-phosphatase enzyme is absent in most cells (except hepatocytes), the phosphorylated form of glucose/FDG cannot efflux from the cell. Glucose-6-phosphate, which is the natural substrate for the glycolysis pathway, becomes further phosphorylated and participates in glycolysis. However, FDG-6-phosphate is unable to participate further in glycolysis and thus accumulates within cells; hence, FDG accumulates in the cells and the quantity is in accordance with the rate of glycolysis (Fig. 15.3).
Glycolytic flux in activated macrophages. (a) Metabolic flux (glucose uptake) is demonstrated in macrophages stimulated through the classic activation (induced by lipopolysaccharide and interferon-γ) and alternative activation pathways (induced by IL-4, IL-10, and IL-13). The time course of glucose consumption was determined by sampling the culture medium at periods of 2 h. (b) Gene set enrichment analysis. The normalized enriched scores are shown in the bars. Positive scores indicate upregulated genes, whereas negative normalized enriched scores correspond to downregulated genes (Modified with permission from Rodriguez-Prados et al. [10])
A broad body of research extending over three quarters of a century shows that myeloid cells including macrophages are highly dependent on glycolysis for energy metabolism [11, 12]. In resting macrophages, ATP is generated through the glycolytic and mitochondrial pathways in roughly equal proportions. Activated macrophages manifest a substantial upregulation of ATP production, the vast majority of which is produced through glycolysis. Further, the manner of macrophage stimulation has a significant impact on glycolytic flux [13]. While innate or classic activation (proinflammatory activation or M1 polarization) leads to increased glycolysis, alternative activation (anti-inflammatory activation or M2 polarization) does not [13]. Additionally, with M1 polarization, there is an upregulation of genes which encode glycolytic enzymes, which is accompanied by a downregulation of the genes that encode tricarboxylic acid cycle (TCA) and electron transport chain (ETC) proteins. The opposite occurs with M2 polarization (Fig. 15.4). In congruence with these findings, compared to resting macrophages, deoxyglucose uptake is increased in M1-polarized macrophages but is decreased in M2-polarized macrophages (Fig. 15.5) [14]. It should be noted that these findings are not without controversy. One study suggested that hypoxia, more than proinflammatory activation, drives macrophage glycolysis [16]. However, multiple groups have demonstrated that hypoxia itself is an important driver of both macrophage proinflammatory activation and glycolysis [17]. Moreover, it has been reported that across varying oxygen tensions, including hypoxic conditions, and through varying macrophage stimulatory conditions, a tight relationship exists between glycolytic flux and macrophage proinflammatory activation (measured as TNF alpha production) [18]. Accordingly, the glycolytic rate of macrophage-rich tissues (as assessed by FDG uptake) may provide an index of state of proinflammatory activation.
Intracellular 3H-FDG accumulations in human macrophages. Compared to un-stimulated macrophages, classically stimulated macrophages (M1 polarized) accumulate higher amounts of FDG, whereas in the alternatively stimulated macrophages (M2 polarized), the amount of FDG uptake is lower than un-stimulated macrophages (Modified with permission from Satomi et al. [14]. (Asterisks indicate statistical significance)
Metabolic trapping of fluorodeoxyglucose. Fluorodeoxyglucose (FDG) enters the cells through glucose transporters (GLUT) and becomes phosphorylated by hexokinase to produce FDG-6-phosphate. Similarly, glucose is phosphorylated by the similar enzyme to become glucose-6-phosphate. Glucose-6-phosphate, which is the natural substrate for the glycolysis pathway, becomes further phosphorylated and participates in glycolysis. However, FDG-6-phosphate is unable to participate further in glycolysis and thus accumulates within cells; hence, FDG accumulates in the cells and the quantity is in accordance with the rate of glycolysis (Adapted with permission from Rudd et al. [15])
Given FDG accumulation in inflammatory cells, it follows that it may be useful for imaging inflammation. Indeed, in addition to its use as a tracer to characterize oncologic processes, FDG is clinically used for imaging of inflammation and infection. The clinical use of FDG-PET/CT for imaging of inflammation has substantially grown over the years, and recent guidelines provided by the Society of Nuclear Medicine and Molecular Imaging as well as the European Association of Nuclear Medicine suggest the clinical use of FDG imaging of inflammation and infection [19]. The current major inflammatory indications for FDG-PET/CT imaging include evaluation of fever of unknown origin, suspected spinal infections, vasculitides, osteomyelitis, sarcoidosis, device and prosthetic infections, and endocarditis [20].
3 FDG-PET/CT Imaging of Arterial inflammation
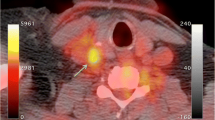
Imaging of atherosclerotic inflammation using FDG-PET was initially reported in humans in 2002 by Rudd et al. [21]. In their landmark study of individuals with recent strokes, they observed higher FDG uptake in the wall of the symptomatic carotid artery (compared to the contralateral carotid artery plaques; Fig. 15.6). In an analysis of an excised carotid plaque that was incubated with tritiated deoxyglucose, the authors furthermore observed that deoxyglucose localized to foamy macrophages within the lipid core, suggesting that the FDG signal may be reporting on macrophages. Thereafter, several groups reported in animal models that arterial FDG uptake correlates with inflammation which was confirmed by histology [22, 23]. Further, it was soon after shown in humans that PET imaging of FDG uptake within human carotid arteries provided an index of atherosclerotic plaque inflammation [24]. In that study, the authors measured the FDG uptake in carotid plaques of individuals who subsequently underwent carotid endarterectomy. They assessed the correlation between FDG uptake and the macrophage content, measured as CD68 staining by histopathology, on the same plaques that were excised during endarterectomy. In that study, the authors observed a significant correlation between FDG uptake and the macrophage content (Fig. 15.7). Several groups have replicated this observation, and some extended the concept by demonstrating that FDG uptake in the arterial wall also relates to the expression of proinflammatory genes [25, 26].
FDG-PET/CT imaging of arterial inflammation. The top row (from left to right) shows PET, contrast-enhanced CT, and co-registered PET/CT images. These images are obtained from a 63-year-old man who had experienced two episodes of left-sided hemiparesis. The bottom row demonstrates a low level of FDG uptake in an individual with an asymptomatic carotid stenosis (Modified with permission from Rudd et al. [21])
FDG-PET imaging and macrophage content of atherosclerotic plaques. Axial positron-emission tomography (PET) images from two patients are demonstrated: Patient A manifested low FDG uptake in the region of the carotid plaque, whereas patient B had a high FDG uptake in that region. The region of the excised carotid plaque is noted with arrows. In patient A, the corresponding trichrome-stained histologic specimen demonstrates a collagen-rich plaque with low lipid content, and CD68 staining on the high-powered images demonstrates limited macrophage infiltration. In patient B, the corresponding trichrome-stained histologic specimen demonstrates a complex plaque with a necrotic core, and the CD68 staining demonstrates intense macrophage infiltration. The boxes in the trichrome-stained images indicate the regions corresponding to the high-powered CD68 stains. The scatter plots demonstrate that carotid plaque FDG uptake in carotid plaques significantly correlated with histologic measure of inflammation in the corresponding sections taken during carotid endarterectomy (Modified with permission from Tawakol et al. [24])
The accumulation of FDG within the arterial wall is inhomogeneous, as would be expected given the inhomogeneous distribution of atherosclerotic plaques and various stages of different atherosclerotic plaques. Figueroa et al. observed that arterial FDG uptake is especially prominent within the plaques that have morphological features suggestive of high-risk potential [27]. In their study, they recruited patients who were scheduled for carotid endarterectomy and performed arterial FDG-PET/CT and contrast-enhanced CT imaging prior to the procedure. They characterized plaque morphology using CT of the carotid arteries and defined high-risk morphological features as plaques with ulceration, low attenuation (which indicates lipid-rich locations), or positive remodeling. These features were selected based on a wealth of clinical data suggesting that those features are associated with an increased risk of subsequent plaque rupture and stroke. Additionally, following carotid endarterectomy, the carotid plaques were examined histopathologically. The authors demonstrated that the distribution of FDG uptake within the carotid arteries was heterogeneous; however, the amount of the FDG that localized to the arterial wall was associated with presence of atherosclerotic plaques. Moreover, they demonstrated that arterial inflammation as assessed by FDG uptake was higher in plaques with high-risk morphological features and depends on the number of high-risk features that each plaque contains (Fig. 15.8). Similarly, the macrophage staining present within those plaques (as measured histopathologically after endarterectomy) also correlated with the number of high-risk morphological features. Accordingly, FDG uptake relates to both inflammation and the presence of high-risk morphological features, which are in turn interrelated.
Distribution of FDG uptake correlates with high-risk morphology of atherosclerotic plaques. Axial and coronal sections of computed tomography (CT) and positron-emission tomography/computed tomography (PET/CT) are demonstrated. Top row demonstrates that FDG uptake is higher in the regions of carotid artery with high-risk morphology (HRM) in the atherosclerotic plaques. Low-attenuation plaques (LAP) with positive remodeling (PR) are considered as HRM. The bottom row demonstrates that a predominantly calcified plaque without high-risk morphological feature (HRM) is associated with lower FDG uptake (Modified with permission from Figueroa et al. [27])
4 Relationship Between Arterial Inflammation, Disease Progression, and Risk of Cardiovascular Events
Arterial inflammation has been shown to be predictive of subsequent plaque progression in human studies. Abdelbaky et al. observed that arterial wall locations with underlying inflammation (by FDG-PET/CT) subsequently manifested greater arterial calcification (quantified by CT imaging), compared to the locations with less inflammation [28] (Fig. 15.9). In that study, the authors observed that arterial FDG uptake was a strong predictor of subsequent arterial calcification (odds ratio [95 % CI] = 2.59 [1.18–5.7]) after adjusting for other cardiovascular risk factors (e.g., age, hypertension, and dyslipidemia). Similarly, Fayad et al. demonstrated in a large prospective study that arterial segments with increased arterial inflammation (by PET/CT) experienced more volumetric expansion (measured by MRI) over the ensuing 18 months compared to segments with reduced inflammation [29]. These studies present insights about the local consequences of arterial inflammation and thus provide a plausible explanation for observed increased risk of atherothrombotic events in individuals with heightened arterial inflammation.
Arterial inflammation and progression of atherosclerosis. This study evaluated the relationship between baseline arterial inflammation and subsequent calcification of the atherosclerotic plaques. The authors used a within-patient analysis where the segmental FDG uptake was expressed relative to the mean FDG uptake for the entire aorta within which that segment resides (as the percent difference between the individual segment SUV and the mean SUV of the whole aorta). They demonstrated that within each patient, the relative baseline SUV was higher in segments that manifested subsequent calcification. This held true in subset analyses of segments with calcium present at baseline (P = 0.007) and segments without calcium at baseline (P = 0.001) (Adapted with permission from Abdelbaky et al. [28])
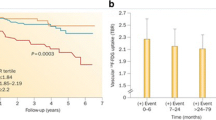
Measures of arterial inflammation may provide an index of the risk of subsequent atherothrombotic events. In an early retrospective study of individuals who had undergone FDG-PET/CT imaging for clinical (mostly oncologic) indications, Rominger et al. initially demonstrated that arterial inflammation (FDG uptake) was predictive of event-free survival over a 40-month period [30]. However, that particular study did not identify the degree to which imaging of arterial inflammation may add to risk prediction over the standard risk prediction tools such as the Framingham Risk Score (FRS). Indeed, studies have demonstrated an association between FRS and arterial inflammation (FDG uptake) [31]. Recently, one study evaluated the degree to which imaging measures of arterial inflammation (FDG-PET/CT imaging) may refine assessment of risk of cardiovascular beyond the FRS calculation [32]. In a study of 513 individuals who had undergone imaging for clinical indications but who were deemed to be free of active oncologic disease, FDG-PET/CT images were retrospectively analyzed. The authors of that study observed that arterial inflammation (measured as FDG uptake) independently predicted the risk of subsequent cardiovascular disease events. Moreover, they reported a net reclassification improvement of 27.5 % over FRS, with 12.7 % of events correctly reclassified and 14.8 % of nonevents correctly reclassified using noninvasive imaging (Fig. 15.10). Additionally, an association between vascular inflammation and the timing of cardiovascular events was observed, whereby individuals with events occurring within 6 months of imaging had the highest amount of FDG uptake in the index imaging. In contrast, the individuals with more remote events had lower initial arterial inflammatory signals, while those who never developed cardiovascular events had the lowest FDG uptake. In a separate small prospective study of 60 individuals with ischemic stroke, Marnane et al. sought to evaluate whether carotid FDG uptake after a stroke was associated with the risk of short-term (90 days) stroke recurrence. They found that in a Cox regression model including age and degree of stenosis (50–69 % or 70 %), mean plaque FDG uptake was the only independent predictor of stroke recurrence (adjusted hazard ratio, 6.1; 95 % CI, 1.3–28.8; p = 0.02) [33]. While these studies suggest a promising role for PET/CT imaging in risk assessment and stratification in various patient populations, large prospective studies are needed to confirm these findings.
Quantification of arterial inflammation improves the risk assessment of cardiovascular events. Arterial FDG uptake (target-to-background ratio, TBR) is demonstrated to have a significant association with the risk of subsequent cardiovascular events. Moreover, authors showed that it improves the reclassification by 27 % when combined with the Framingham Risk Score (FRS) (Modified with permission from Figueroa et al. [32])
5 PET/CT Measures of Arterial Calcification
Large studies have demonstrated that measures of coronary artery calcification, such as the coronary calcium score (assessed by CT), provide potent prediction of subsequent incident cardiovascular events [34]. Molecular imaging has been used to identify calcification of atherosclerotic plaques as well. 18F-sodium fluoride (18F-NaF), which localizes formation of hydroxyapatite crystals in very early stages, has been suggested as a tracer for vascular imaging. Recently, Joshi et al. demonstrated that PET/CT imaging using 18F-NaF might introduce a promising role for this noninvasive imaging modality to identify and localize high-risk coronary plaques [35] (Fig. 15.11). However, the relationship between 18F-NaF uptake and the risk of cardiovascular events is yet undefined. Furthermore, it is still unknown whether arterial 18F-NaF uptake provides an independent measure of risk when added to CT measures of calcium deposition such as the Agatston score. Dweck et al. have employed 18F-NaF PET/CT imaging to identify active calcification and disease progression in aortic valve [36]. It is worth noting that these studies are performed in small patient populations and require larger studies to confirm their findings.
PET/CT imaging of coronary arteries. 18F-sodium fluoride (18 F-NaF) is a tracer that localizes formation of hydroxyapatite crystals in very early stages. (a) Significant stenosis of proximal segment of left anterior descending coronary artery (LAD) by invasive angiography; (b) high uptake of 18F-NaF in the same coronary segment; (c) culprit lesion (red arrow; LAD) in a patient with non-ST elevation myocardial infarction and bystander non-culprit lesion (white arrow; circumflex artery) that were both stented during the admission; (d) only the culprit lesion had increased 18F-NaF uptake PET/CT. (e) Box plot demonstrates that 18F-NaF uptake was significantly higher in culprit coronary lesions compared with non-culprit lesions (Modified with permission from Joshi et al. [35])
6 Utility of Noninvasive Imaging of Arterial Inflammation to Assess Effectiveness of Therapies
Arterial inflammation (FDG uptake using FDG-PET/CT imaging) is rapidly modifiable by interventions. Two preclinical studies evaluated the rate of change in arterial inflammation (using FDG-PET/CT) after pharmacotherapy. In one study, atherosclerotic inflammation decreased substantially within 2 days after administration of an anti-inflammatory drug [37]. Conversely, arterial FDG uptake increased within 3 days after administration of a proinflammatory cytokine [38]. Taken together, these preclinical findings show that arterial inflammation can be rapidly modified through anti-atherosclerotic pharmacotherapy.
Arterial inflammation (FDG uptake) in the atherosclerotic plaques must be relatively stable and reproducible in order to be qualified as a monitoring tool for assessment of anti-atherosclerotic treatment effects. Rudd et al. demonstrated in 20 individuals with clinical cardiovascular disease that imaging measure of arterial inflammation by FDG-PET/CT scan is reproducible [39]. They prospectively enrolled study subjects and performed PET imaging at two timepoints 2 weeks apart and concluded that in patients with stable atherosclerosis who received stable treatment, the FDG signal was highly reproducible (Intraclass Correlation Coefficients of 0.79–0.92 and interobserver agreements of 0.92–0.97). Accordingly, the high sensitivity of FDG-PET/CT imaging for detection of treatment effects, along with its reproducibility under stable conditions in humans, provides rationale for use of FDG-PET/CT imaging to assess anti-inflammatory therapies.
FDG-PET/CT has been used to study anti-atherosclerotic therapeutic interventions in several human studies. Tahara et al. published the first human study to evaluate the impact of a pharmacologic intervention on arterial inflammation as measured by FDG-PET/CT imaging [40]. Using a single-center open-label study design, they compared simvastatin (5–20 mg) to diet management. By 3 months, arterial inflammation (FDG uptake) was significantly reduced in the simvastatin group compared to the group that was assigned to the diet management (Fig. 15.12). More recently, the anti-inflammatory effect of statins on arterial inflammation was studied in a multicenter double-blind trial [41]. In that study, 83 adults who were at risk or with established clinical atherosclerosis and were not taking high-dose statins were randomized to atorvastatin 10 mg versus 80 mg. Arterial FDG-PET/CT imaging was performed at baseline, 4 weeks, and 12 weeks after randomization. The authors reported that high-dose atorvastatin (80 mg daily) resulted in a significant relative reduction in arterial inflammation compared to atorvastatin 10 mg and that the reductions from baseline were seen as early as 4 weeks after randomization (Fig. 15.13). Most recently, the effect of non-pharmacologic lipid lowering on arterial inflammation was evaluated. In a study of individuals with familial hypercholesterolemia (FH), lipid lowering by lipoprotein apheresis resulted in a substantial reduction in arterial inflammation (FDG-PET/CT imaging) within 1 week of the intervention [42].
Determination of simvastatin treatment effect by FDG-PET/CT imaging. Representative FDG-PET images at baseline and after 3 months of simvastatin therapy (posttreatment) are compared with dietary intervention. Baseline images demonstrate FDG uptake within a common carotid plaque (square). A substantial reduction in plaque. FDG uptake is seen after 3 months of simvastatin therapy (top). The bottom panel demonstrates per-subject data for baseline and posttreatment values in the diet and simvastatin groups. There was a significant reduction in vascular FDG uptake after simvastatin therapy but not after dietary modification. NS not significant (Modified with permission from Tahara et al. [40])
Changes in arterial inflammation following statin therapy. In this multicenter study, statin therapy induced a rapid reduction in arterial inflammation. This figure provides the change in FDG uptake (measured as target-to-background ratio, TBR, in the most diseased segment, MDS) at 4 and 12 weeks after initiation of therapy with high- and low-dose atorvastatin. At 12 weeks, inflammation (TBR) in the MDS of the index vessel was significantly reduced from baseline with atorvastatin 80 mg, but not with atorvastatin 10 mg (Adapted with permission from Tawakol et al. [41])
Additionally, FDG-PET/CT imaging has been used to study the effect on arterial inflammation of therapies that do not substantially impact serum LDL. Mizoguchi et al. studied the effect of pioglitazone (15–30 mg) versus glimepiride (0.5–4.0 mg) on arterial FDG uptake in glucose intolerant or diabetic patients who had carotid atherosclerosis [43]. They found that while both treatments comparably reduced fasting glucose concentrations and hemoglobin A1C values, pioglitazone but not glimepiride reduced arterial inflammation. Indeed, an anti-atherosclerotic effect of pioglitazone has been suggested by clinical endpoint studies as well, whereby several trials have suggested that the use of pioglitazone in diabetic patients is associated with a reduced incidence of atherothrombotic events [44, 45]. Hence, for statins as well as pioglitazone, findings of reduced arterial inflammation in FDG-PET/CT imaging trials are consistent with the findings of clinical endpoint trials.
Recently, multicenter FDG-PET/CT studies have been performed to evaluate therapeutic efficacy of novel pharmacologic agents. The results of two large clinical endpoint studies have recently been published and provided an opportunity to compare the results of the imaging trials to those of the clinical endpoint trials. The first novel drug was dalcetrapib, a cholesteryl ester transfer protein (CETP) modulator. The dal-PLAQUE trial used multimodality imaging to evaluate the safety and efficacy of dalcetrapib on atherosclerotic disease [29]. In that phase 2b double-blind multicenter trial, 130 individuals with or at risk for coronary heart disease were randomized to placebo versus dalcetrapib. FDG-PET/CT imaging was performed at baseline and 6 months after randomization. In that study, there was no significant difference in the primary FDG-PET/CT imaging endpoint between dalcetrapib and placebo (p = 0.51). The subsequently published dal-OUTCOMES trial evaluated the effect of dalcetrapib versus placebo by the composite outcome of death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, unstable angina, or cardiac arrest with resuscitation in nearly 16,000 individuals with the recent acute coronary syndromes [46]. The authors reported that while dalcetrapib was associated with a 40 % increase in HDL cholesterol, it did not reduce the risk of recurrent cardiovascular events.
The second novel therapeutic agent to be evaluated separately in an FDG-PET/CT imaging trial as well as in clinical endpoint trials was rilapladib, an inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2). In a multicenter trial, FDG-PET/CT imaging was used to evaluate the effect of rilapladib on arterial inflammation in over 80 individuals randomized to rilapladib (250 mg once daily) versus placebo for 3 months [28]. While rilapladib significantly reduced Lp-PLA2 activity by up to 80 %, it did not reduce arterial inflammation (FDG uptake). The recent clinical endpoint trial evaluating the related Lp-PLA2 antagonist, darapladib, similarly showed no significant reduction in clinical events. The STABILITY trial of nearly 16,000 individuals with stable atherosclerotic disease showed no beneficial effect of darapladib to reduce the primary composite endpoint of cardiovascular death, myocardial infarction, or stroke [47].
Thus for all four drug classes for which there are both FDG-PET/CT data and clinical endpoint data (atorvastatin, darapladib, dalcetrapib, and pioglitazone), the imaging trial findings have been consistent with the results from clinical endpoint studies. Accordingly, the use of FDG-PET/CT imaging may provide a pathway for predicting the potential efficacy of cardiovascular therapeutics using a relatively small number of patients and with observation periods of 1–3 months.
7 Imaging of Arterial Inflammation to Develop Physiologic Insights
Noninvasive imaging of arterial inflammation has proven to be useful for developing pathophysiologic insights while studying patient populations. FDG-PET/CT imaging has been used to study diseases that have been hypothesized to be associated with arterial inflammation. Kim et al. demonstrated that arterial inflammation is increased in individuals with type 2 diabetes mellitus or impaired glucose tolerance compared to healthy controls [31]. Others have reported increased arterial inflammation in patients with chronic obstructive pulmonary disease (COPD) and patients with mild stable asthma [48, 49]. Further, in individuals with mild asthma, the degree of arterial inflammation relates to the severity of asthma as assessed by pulmonary function testing such that there was an inverse correlation between arterial FDG uptake and forced expiratory volume in the first second (FEV1) and peak expiratory flow (PEF). These studies support the hypothesis that the increased risk of cardiovascular events in these disease states might be in part related to higher arterial inflammation.
It has been previously demonstrated that patients with rheumatoid arthritis (RA) have higher risk of myocardial infarction. A suggested mechanism is that joint inflammation potentiates systemic inflammation, which in turn accelerates arterial inflammation and the risk of atherothrombosis. One study recently evaluated whether adult patients with RA have increased arterial inflammation and whether synovial activity correlates with arterial inflammation [50]. In that study, 66 individuals underwent FDG-PET/CT imaging: 33 individuals with and 33 individuals without RA (matched by age and gender). The individuals with RA had substantially increased levels of arterial inflammation even after adjusting for well-established risk factors of atherosclerosis such as FRS. Moreover, the authors in that study showed that arterial inflammatory signal strongly correlated with the degree of synovial activation assessed with FDG-PET/CT imaging. Recently, Mäki-Petäjä et al. demonstrated that treatment with an antitumor necrosis-α therapy reduces arterial inflammation in individuals with RA within 8 weeks [51]. Taken together, these studies suggest that arterial inflammation in rheumatoid arthritis is closely linked to synovial disease activity and that anti-inflammatory therapies that have primarily targeted synovial disease are effective at reducing arterial inflammation as well. Several groups are now assessing whether more aggressive targeting of synovial disease activity in rheumatoid arthritis patients would result in a reduction in arterial inflammation.
FDG-PET/CT imaging has been used to gain important physiologic insights in studying atherosclerosis in association with human immunodeficiency virus (HIV) infection. It has long been established that HIV is associated with an increased risk of cardiovascular disease events, which is not fully accounted for by traditional risk factors [52, 53]. Several lines of evidence support the concept that chronic immune cell activation may promote arterial inflammation leading to an increased high-risk plaque phenotype in patients with HIV. Subramanian et al. used FDG-PET/CT imaging to assess arterial inflammation in individuals who are infected with HIV [54]. To do so, the authors prospectively recruited 81 individuals, 27 of whom were HIV infected without any known cardiovascular diseases. In their study, they employed FDG-PET/CT imaging to compare arterial inflammation within individuals with versus without HIV who were matched by FRS. They found that arterial inflammation was higher in HIV versus FRS-matched control subjects but was similar when compared to patients with established atherosclerosis. Moreover, they demonstrated that the arterial inflammatory signal correlated with levels of the monocyte activation marker CD163. In a separate study, the interrelationship between arterial inflammation and high-risk morphological features of coronary atherosclerotic plaques was assessed in HIV-infected individuals [55]. In that study, the authors recruited 41 subjects with HIV who were on stable antiretroviral therapy and had asymptomatic coronary atherosclerotic plaques on coronary CT angiography. Thereafter, the investigators tested the hypothesis that the degree of arterial inflammation (FDG uptake) is related to high-risk plaque morphology. They demonstrated that among HIV-infected individuals with higher degrees of aortic inflammation, there was an increased prevalence of coronary plaques manifesting high-risk morphological features compared to patients with lower degrees of arterial inflammation. Taken together, these data suggest that arterial inflammation is increased in HIV-infected individuals, is related to markers of monocyte activation, and is associated with the presence of high-risk coronary plaque subtypes. Several studies are currently ongoing to assess whether anti-inflammatory strategies would reduce arterial inflammation as well as the burden of high-risk coronary plaque features in individuals with HIV.
8 Extra-arterial FDG-PET/CT Inflammatory Imaging
FDG-PET/CT imaging is routinely used clinically for evaluation of extravascular inflammation. Notably, FDG-PET/CT imaging is used routinely for evaluation of sarcoidosis, a cardiac inflammatory disorder. Lee et al. demonstrated in a preclinical model that FDG uptake in myocardial tissue correlates with the density of proinflammatory monocytes [56]. While this observation provides rationale for evaluating cardiac inflammatory disorders such as sarcoidosis, it must be noted that the uptake of FDG by noninflammatory cells such as myocytes can be substantial. However, because myocytes preferentially use fatty acids rather than glucose as the energy source, myocardial uptake of FDG can be suppressed through dietary manipulation, allowing imaging of the inflamed tissues associated with sarcoidosis. Indeed, FDG-PET/CT imaging to assess cardiac sarcoidosis has become a routine procedure. Blankstein et al. demonstrated that cardiac PET enhances prognostic assessments of patient with suspected cardiac sarcoidosis [57]. They showed that the presence of focal FDG uptake allows identification of patients who are at higher risk of death or potentially fatal arrhythmias (such as ventricular tachycardia). More recently, it has been shown in a longitudinal cohort that reduction in FDG uptake on serial FDG-PET/CT imaging is associated with improved left ventricular ejection fraction in patients of sarcoidosis [58]. The authors have suggested that serial PET scanning may be useful to guide monitoring and titration of immunosuppressive therapy in patients with sarcoidosis to improve or prevent heart failure. Taken together, an emerging literature suggests a potential clinical role for FDG-PET/CT imaging in the assessment of cardiac sarcoidosis.
FDG-PET/CT imaging has also been used to study the interrelationships between arterial inflammation and the inflammatory processes in other tissues. For example, there has been substantial interest in understanding the potential link between periodontal disease (a common inflammatory disorder of gingiva) and atherosclerosis, since several studies have shown that individuals with periodontal disease are at an increased risk for atherosclerosis. One clinical study showed (using FDG-PET/CT imaging) that periodontal FDG uptake correlates with the extent of carotid atherosclerotic inflammation (by carotid histopathological analysis) [59]. A more recent clinical trial demonstrated that periodontal FDG uptake correlates with alveolar bone loss seen on periodontal CT imaging which is a well-established imaging surrogate for severity of periodontal inflammation. Moreover, that study demonstrated that high-dose atorvastatin therapy results in a reduction in periodontal inflammation and that the improvements in periodontal inflammation closely correlate with improvements in arterial inflammation. Those findings highlight a potentially novel pleiotropic effect of statins and raise the possibility that a portion of the benefit of statins on atherosclerosis may in part be related to their effect on extra-arterial inflammation.
Along similar lines, visceral adiposity is also known to be associated with arterial inflammation as well as an increased risk of atherothrombosis. Studies have shown that the volume of visceral adipose tissue (VAT) is strongly correlated with metabolic risk factors [60]. Other studies evaluated the metabolic activity of VAT. Christen et al. demonstrated that FDG-PET/CT imaging can be used to characterize the biological activity of visceral fatty tissue [61]. They demonstrated that FDG uptake is higher in VAT compared to subcutaneous adipose tissue. Additionally, they showed that FDG uptake in VAT is increased after exposure to TNF-α. Figueroa et al. used FDG-PET/CT imaging to demonstrate that arterial inflammation correlates with the metabolic activity of VAT [62]. Further, they demonstrated that the metabolic activity of VAT (FDG uptake) predicts subsequent risk of cardiovascular disease events. Two groups have recently evaluated the impact of systemic pharmacotherapies on VAT activity. Elkhawad et al. showed that a p38 mitogen-activated protein kinase (p38MAPK) inhibitor reduces the metabolic activity (FDG uptake) in visceral but not subcutaneous adipose tissue (relative to placebo) [63]. Similarly, another study demonstrated that pioglitazone but not glimepiride reduces visceral adipose tissue activity [64]. These studies suggest that imaging of visceral adipose tissue may yield important physiologic insights and provide a pathway to identify potentially fruitful therapeutics.
9 FDG-PET/CT Imaging of the Coronary Arteries and Future Directions
Imaging of arterial inflammation has been most commonly performed in the large vascular beds (aorta and carotids arteries). The vast majority of the validation studies have been performed in the carotid circulation, while most of the human arterial FDG-PET/CT imaging trials have focused on the aorta and carotid arteries. Nevertheless, there is substantial interest to use this imaging modality for assessment of atherosclerotic plaque inflammation in the coronary arteries since the majority of myocardial infarctions and sudden cardiac deaths result from the rupture of coronary plaques. However, several factors provide substantial obstacles to performing coronary FDG-PET/CT imaging. Those challenges include (1) the significant motion of the coronary arteries, (2) the relatively small size of coronary plaques, and (3) the high uptake of FDG by adjacent myocardial cells. Despite these limitations, several groups have provided early observations of FDG-PET/CT imaging of coronary plaques.
One of the earliest reports of FDG-PET/CT imaging of coronary arteries was provided by Dunphy et al. In that study, patients with (vs. without) a history of coronary artery disease (CAD) had a fourfold increased likelihood of manifesting high left main coronary FDG uptake [65]. Similarly, Wykrzykowska et al. studied 32 patients with CAD who underwent FDG-PET/CT imaging after active dietary suppression of myocardial FDG uptake (via a low-carbohydrate high-fat meal the night before imaging and administration of a vegetable oil drink in the morning prior to imaging) [66]. In that study, myocardial suppression was adequate in 63 % of the patients, and they identified coronary FDG uptake in roughly half of the patients. In a subsequent study, Rogers et al. performed the first prospective study evaluating coronary FDG uptake after myocardial infarction [67]. In that study, 10 individuals with and 15 subjects without ACS underwent both FDG-PET/CT imaging and coronary angiography. The authors observed higher arterial inflammation (FDG uptake) in association with culprit lesions compared to stable plaques (Fig. 15.14). Furthermore, coronary FDG uptake correlated with C-reactive protein levels. While others have likewise reported higher coronary FDG uptake after acute myocardial infarction [68], not all groups evaluating FDG uptake in the coronary arteries observed increased FDG localization to culprit lesions. Joshi et al. recently evaluated FDG ability to localize to coronary plaques [35]. In that study, no active methods (such as an oil-rich drink or heparin) were used on the day of the study to suppress the myocardial FDG uptake. Unsurprisingly, the authors reported that patchy myocardial uptake affected the majority of vessel territories and thus limited their evaluation of coronary FDG uptake. That study serves as an important reminder that substantial challenges remain for more widespread application of coronary FDG imaging.
FDG-PET/CT imaging of coronary arteries. Upper panel demonstrates three representative images from patients who presented with chest pain syndromes. The left top panel represents a patient with an acute coronary syndrome (ACS), and the arrow points to the stent placement site in the culprit lesion which has a substantial FDG uptake. The middle top panel represents a patient with a stable angina who had a stent placement at the culprit lesion. The FDG uptake associated with that lesion is relatively modest. The right top panel shows a remotely stented lesion for comparison. The bottom panels demonstrate quantitative values for FDG uptake and indicate that the post-ACS patients had higher FDG uptake in association with culprit lesions. The fact that FDG uptake in the stented stable angina group was not higher following the stent placement suggests that the increased FDG uptake in the post-ACS group is not the result of stenting procedure alone, but rather is due to the coronary syndrome (Adapted with permission from Rogers et al. [67])
The challenges notwithstanding, several groups have been investigating the effect of pharmacotherapies on coronary FDG uptake. Nitta et al. recently reported that pioglitazone decreases coronary artery inflammation measured with FDG-PET/CT imaging [69]. They studied 50 individuals with type 2 diabetes or impaired glucose tolerance that were randomized to pioglitazone versus glimepiride daily. The study subjects were imaged with FDG-PET/CT at baseline and 16 weeks after randomization. The authors analyzed the FDG uptake in the left main coronary artery. In that study, they demonstrated that FDG uptake in the coronary artery was substantially reduced in the pioglitazone group compared to the glimepiride group (p = 0.03). Hence, initial studies suggest that pharmacotherapies with beneficial anti-atherosclerotic properties have the potential to modulate coronary FDG uptake, at least as assessed within the left main coronary artery. No study thus far has reported on changes in FDG uptake in more distal portions of the coronary tree where the vast majority of culprit coronary lesions exist.
More routine and extensive evaluation of coronary plaque inflammation will require several technological advances. Firstly, the challenges caused by substantial coronary motion will need to be overcome. To that end, several groups are investigating techniques to minimize the impact of coronary motion on the blurring of measured tracer activity. The PET images are obtained in List Mode, in which the data are acquired in multiple acquisition frames or bins. Thereafter, those bins are grouped according to external monitoring events based on electrocardiographic and/or respiratory cycles. However, simple dual respiratory and cardiac gating would result in loss of over 80 % of the acquired imaging data and would thus lead to an excessively low target-to-background ratio. Hence, alternative approaches are required to reduce the motion artifact without compromising image quality. One such approach that has been developed is the motion frozen technique [70]. This approach corrects for cardiac motion by tracking the left ventricle through all cardiac phases and then shifts the counts from most phases of the cardiac cycle into the end diastolic position by means of nonlinear image wrapping. Another approach to correct for motion uses the original projection data in a joint motion compensation and reconstruction process [71]. This process, termed Data-domain Cardiac Shape Tracking and Adjustment for Respiration (D-CSTAR), uses analysis of motion by evaluating the CT data to estimate the motion of PET activity and reconstructs the image without discarding any of the acquired PET data bins. Both general approaches have been shown to improve quantification methods, and both are being applied, in the research settings, for coronary PET/CT acquisitions. However, the intensive computing that is required to implement these techniques limits their utility at this time.
Another important advance that may allow for more routine coronary PET imaging is improvements in camera technology. In general, quantification of tracer activity, using PET, is possible due to the unique properties of positron-emitting tracers. A beta particle (positron) emitted from a tracer travels a short distance in tissue before encountering an electron and participating in an annihilation event. This annihilation event leads to the generation of two high-energy photons that are emitted at exactly 180° apart. In standard PET imaging, a detector system looks for photons that are separated by 180° when they are coincidentally (nearly simultaneously) detected by the detector system. Using the concept of coincidental detection, nearly simultaneously detected pairs of photons arriving 180° from one another are accepted as “true events,” while non-coincidentally detected photons or those arriving at angles other than 180° apart are rejected (since they might have been subjected to scatter). This thus allows for the generation of a line of response between those two accepted photons to indicate the location of the line of origin of the annihilation event. As more pairs of photons are emitted from that tissue location, several intersecting lines will then pinpoint the origin of the annihilation events and the activity can be quantified. Time of flight (TOF) takes this concept further to additionally improve the quantification of PET signal. The TOF systems are able to not only identify photons that arrive at 180° apart but also to resolve the slight time differences between them. By doing so, the system need not to assume that the photons were admitted along a single line, but instead will calculate the difference in the time of flight between the two photons and derive a more precise location for where the photons were initially generated. Hence, TOF allows for even more precise quantification of PET activity. As TOF technology improves, quantification of PET signal will continue to improve as well.
Two additional advances that may expedite the development of coronary PET imaging are advances in PET/MR technologies and development of novel tracers. PET/MR is currently becoming widely available and provides new opportunities and challenges for evaluating the coronary tree. While MR is generally less well suited for coronary imaging compared to CT (due to its reduced temporal and spatial resolution), MR does provide certain advantages. One such advantage is that the MR acquisition may be derived simultaneously to the PET. Hence theoretically, attenuation correction (AC) algorithms that use MR data to correct the PET data may be superior to those derived from CT. The net impact on coronary PET imaging of MR-derived AC (relative to CT-based AC) is still under investigation.
Improvements in tracer technology may provide the most impactful advance in arterial PET/CT imaging. Several novel tracers targeting inflammation are currently under development. One such tracers (studied in clinical trials) targets the benzodiazepine receptors (which is upregulated in inflamed tissues) [72]. Another tracer which has been tested in preclinical models targets the adenosine receptor (which is also richly expressed in inflamed tissues) [73]. Moreover, novel tracers have been developed to differentially target M2-polarized (vs. M1-polarized) macrophages. One such tracer, F18 radiolabeled fluorodeoxymannose (FDM), targets the mannose receptor, which is highly expressed in M2-polarized macrophages [74]. It should be noted, however, that none of the tracers targeting inflammation are currently FDA approved, with the exception of FDG. Nonetheless, the eventual development and approval of tracers targeting inflammation may prove to be transformative for imaging atherosclerosis and in particular for imaging of coronary inflammation.
10 Conclusion
Measuring arterial inflammation is feasible using FDG-PET/CT imaging. The approach has been shown to correlate well with plaque inflammation in humans, is reproducible, and is rapidly modifiable by anti-inflammatory interventions. However, several obstacles limit application of PET imaging to the coronary arteries. Improvements in camera technologies, in reconstruction methods, and in novel tracers may provide a means to overcome these obstacles. Nonetheless, currently feasible assessment of large arterial inflammation (aorta and carotids) has been shown to enhance assessment of clinical risk, provide a better understanding of therapeutic efficacy of novel drugs, and may provide a window into inflammation within the coronary tree.
References
Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92(3):657–71.
Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–7.
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med. 2003;348(25):2500–7.
Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290(24):3199–206.
Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54(1):49–57.
Kitagawa T, Yamamoto H, Horiguchi J, Ohhashi N, Tadehara F, Shokawa T, et al. Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging. 2009;2(2):153–60.
Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74.
Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–9.
Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med. 1994;35(1):104–12.
Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605–14.
Fleischmann W, Kubowitz F. Über den Stoffwechsel der Leukocyten. Biochem Z. 1927;181:385.
Garedew A, Henderson SO, Moncada S. Activated macrophages utilize glycolytic ATP to maintain mitochondrial membrane potential and prevent apoptotic cell death. Cell Death Differ. 2010;17(10):1540–50.
Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605–14.
Satomi T, Ogawa M, Mori I, Ishino S, Kubo K, Magata Y, et al. Comparison of contrast agents for atherosclerosis imaging using cultured macrophages: FDG versus ultrasmall superparamagnetic iron oxide. J Nucl Med. 2013;54(6):999–1004.
Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55(23):2527–35.
Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, et al. Hypoxia but not inflammation augments glucose uptake in human macrophages: implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol. 2011;58(6):603–14.
Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–65.
Singh P, Tawakol A, Mojena M, Pimentel-Santillana M, Emami H, Vucic E, et al. Close interrelationship between macrophage pro-inflammatory activation and energy metabolism in an atherosclerotic environment. Meeting of American Heart Association, Dallas. 2013.
Jamar F, Buscombe J, Chiti A, Christian PE, Delbeke D, Donohoe KJ, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med. 2013;54(4):647–58.
Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol. 2013;2013:623036.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–11.
Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12(3):294–301.
Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50(6):959–65.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–24.
Pedersen SF, Graebe M, Fisker Hag AM, Hojgaard L, Sillesen H, Kjaer A. Gene expression and 18FDG uptake in atherosclerotic carotid plaques. Nucl Med Commun. 2010;31(5):423–9.
Graebe M, Pedersen SF, Borgwardt L, Hojgaard L, Sillesen H, Kjaer A. Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg. 2009;37(6):714–21.
Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, et al. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5(1):69–77.
Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, et al. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6(5):747–54.
Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378(9802):1547–59.
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50(10):1611–20.
Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, et al. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging. 2010;3(2):142–8.
Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6(12):1250–9.
Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71(5):709–18.
Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45.
Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383(9918):705–13.
Dweck MR, Jenkins WS, Vesey AT, Pringle MA, Chin CW, Malley TS, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7(2):371–8.
Lobatto ME, Fayad ZA, Silvera S, Vucic E, Calcagno C, Mani V, et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm. 2010;7(6):2020–9.
Singh P, Tawakol A, Mojena M, Pimentel-Santillana M, Fayad Z, Rudd J, et al. GM-CSF enhances glycolytic activity in macrophages in vitro and improves detection of atherosclerotic inflammation in vivo. San Francisco: Meeting of American College of Cardiology; 2013.
Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49(6):871–8.
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48(9):1825–31.
Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62(10):909–17.
van Wijk D, Sjouke B, Figueroa A, Emami H, van der Valk F, MacNabb H, et al. Non-pharmacologic lipoprotein apheresis reduces arterial inflammation in patients with familial hypercholesterolemia: a pilot study. J Am Coll. Cardiol. 2014 (in press).
Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging. 2011;4(10):1110–8.
Erdmann E, Dormandy JA, Charbonnel B, Massi-Benedetti M, Moules IK, Skene AM. The effect of pioglitazone on recurrent myocardial infarction in 2,445 patients with type 2 diabetes and previous myocardial infarction: results from the PROactive (PROactive 05) Study. J Am Coll Cardiol. 2007;49(17):1772–80.
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–89.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702–11.
Coulson JM, Rudd JH, Duckers JM, Rees JI, Shale DJ, Bolton CE, et al. Excessive aortic inflammation in chronic obstructive pulmonary disease: an 18F-FDG PET pilot study. J Nucl Med. 2010;51(9):1357–60.
Vijayakumar J, Subramanian S, Singh P, Corsini E, Fontanez S, Lawler M, et al. Arterial inflammation in bronchial asthma. J Nucl Cardiol. 2013;20(3):385–95.
Emami H, Vijayakumar J, Subramanian S, Vucic E, Singh P, MacNabb M, et al. Arterial inflammation in rheumatoid arthritis correlates with joint inflammation. Meeting of American Heart Association, Dallas. 2013.
Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC, et al. Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473–80.
Mehta N, Reilly M. Atherosclerotic cardiovascular disease risk in the HAART-treated HIV-1 population. HIV Clin Trials. 2005;6(1):5–24.
Rasmussen LD, Engsig FN, Christensen H, Gerstoft J, Kronborg G, Pedersen C, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS (London, England). 2011;25(13):1637–46.
Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–86.
Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66(2):164–71.
Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59(2):153–63.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329–36.
Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, et al. Reduction in (18)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21(1):166–74.
Fifer KM, Qadir S, Subramanian S, Vijayakumar J, Figueroa AL, Truong QA, et al. Positron emission tomography measurement of periodontal 18F-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J Am Coll Cardiol. 2011;57(8):971–6.
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48.
Christen T, Sheikine Y, Rocha VZ, Hurwitz S, Goldfine AB, Di Carli M, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010;3(8):843–51.
Figueroa AL, MacNabb M, Abdelbaky A, Corsini E, Lavender Z, Kaplan R, et al. Visceral adipose tissue activity independently predicts the risk of future cardiovascular events. Meeting of American Heart Association, Dallas. 2013.
Elkhawad M, Rudd JH, Sarov-Blat L, Cai G, Wells R, Davies LC, et al. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc Imaging. 2012;5(9):911–22.
Kodama N, Tahara N, Tahara A, Honda A, Nitta Y, Mizoguchi M, et al. Effects of pioglitazone on visceral fat metabolic activity in impaired glucose tolerance or type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98(11):4438–45.
Dunphy MP, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med. 2005;46(8):1278–84.
Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, et al. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50(4):563–8.
Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3(4):388–97.
Cheng VY, Slomka PJ, Le Meunier L, Tamarappoo BK, Nakazato R, Dey D, et al. Coronary arterial 18F-FDG uptake by fusion of PET and coronary CT angiography at sites of percutaneous stenting for acute myocardial infarction and stable coronary artery disease. J Nucl Med. 2012;53(4):575–83.
Nitta Y, Tahara N, Tahara A, Honda A, Kodama N, Mizoguchi M, et al. Pioglitazone decreases coronary artery inflammation in impaired glucose tolerance and diabetes mellitus: evaluation by FDG-PET/CT imaging. JACC Cardiovasc Imaging. 2013;6(11):1172–82.
Suzuki Y, Slomka PJ, Wolak A, Ohba M, Suzuki S, De Yang L, et al. Motion-frozen myocardial perfusion SPECT improves detection of coronary artery disease in obese patients. J Nucl Med. 2008;49(7):1075–9.
Ambwani S. Joint cardiac and respiratory motion correction and super resolution reconstruction in coronary PET/CT. International symposium on biomedical imaging, Chicago. 2011.
Cuhlmann S, Gsell W, Van der Heiden K, Habib J, Tremoleda JL, Khalil M, et al. In vivo mapping of vascular inflammation using the translocator protein tracer 18F-FEDAA1106. Mol Imaging. 2014;13(0):1–10.
Elmaleh DR, Fischman AJ, Tawakol A, Zhu A, Shoup TM, Hoffmann U, et al. Detection of inflamed atherosclerotic lesions with diadenosine-5′,5′″-P1, P4-tetraphosphate (Ap4A) and positron-emission tomography. Proc Natl Acad Sci U S A. 2006;103(43):15992–6.
Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, et al. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20(2):215–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Emami, H., Tawakol, A. (2015). PET/CT Imaging of Inflammation and Calcification. In: Aikawa, E. (eds) Cardiovascular Imaging. Springer, Cham. https://doi.org/10.1007/978-3-319-09268-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-09268-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09267-6
Online ISBN: 978-3-319-09268-3
eBook Packages: MedicineMedicine (R0)