Abstract
The coastal areas of Bangladesh have poor accessibility to fresh drinking water and the groundwater is not suitable for drinking, cooking, and other domestic uses due to high levels of salinity and potentially toxic elements. The current study focuses on understanding of the distribution of some physicochemical parameters (temperature, pH, EC, TDS, and salinity) and chemical elements (Fe, Mn, Zn, Ca, Mg, Na, K, Cu, Co, Pb, As, Cr, Cd, and Ni) with health perspective in drinking water from the southwestern coastal area of Bangladesh. The physicochemical properties of the water samples were examined with a multiparameter meter, while the elemental concentrations were analyzed using atomic absorption spectrometer. Water quality index (WQI) and irrigation indices were utilized to determine the drinking water quality and irrigation feasibility, respectively, whereas hazard quotients (HQs) and hazard index (HI) were used to assess the probable pathways and the associated potential risks to human health. The concentrations of some toxic elements in measured samples were relatively higher compared to drinking water guidelines, indicating that ground and surface water are not apt for drinking and/or domestic uses. The multivariate statistical approaches linked the source of the pollutants in the studied water body mostly to the geogenic origin including saline water intrusion. WQI values ranged from 18 to 430, reflecting excellent to unsuitable categories of water quality. The assessment of human health risks due to exposure to contaminated water demonstrated both carcinogenic and non-carcinogenic health risks in the exposed residents of the study area. Therefore, appropriate long-term coastal area management strategies should be adopted in the study region for environmental sustainability. The findings of this research will be supportive in understanding the actual situation of fresh drinking water in the area for policymakers, planners, and environmentalists to take effective necessary measures to ensure safe drinking water in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
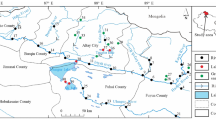
Worldwide freshwater resources are deteriorating due to the rapid growth of population, over-extraction, unplanned urbanization, rapid industrialization, undesired effluent discharges, atmospheric deposition, land cover transformation in watersheds, intense agrochemical utilization, and climate change concerns (Kim et al. 2018). Consequently, about 844 million people have currently been facing drinking water problems worldwide due to the presence of hazardous contaminants, and they do not have access to good-quality drinking water (WHO and UNICEF 2017). One of the key requisites to attain the UN’s Sustainable Development Goals (SDGs) by 2030 for ensuring food security, good public health, and livelihoods is safe drinking water (Sojobi 2016). Normally, people in developing countries like Bangladesh use groundwater (GW) as a primary source of potable water (Hasan et al. 2007; Zahid et al. 2008). But in the coastal areas, GW is saline in most cases and is not suitable for drinking, cooking, and other household activities as well as for agricultural practices (Khan et al. 2011; Bahar and Reza 2010; Talukder et al. 2016; Salehin et al. 2018; Chakraborty et al. 2019; Murshed et al. 2019; Kormoker et al. 2020; Sun et al. 2022; Rashid et al. 2022a, 2023). However, in some places, salinity in water is comparatively low and people use it for drinking and other purposes. Where GW salinity exceeds the tolerable limit, people in Bangladesh use rain-fed pond (RFP) water (Fig. 1b) for drinking as an alternative (Islam et al. 2014). In many places, poor people developed pond sand filter (PSF) (Fig. 1c) systems to eliminate the salinity and other contaminants from pond water (Islam et al. 2014) which were then eventually used for drinking.
a) Location map of the study area (southwestern Bangladesh) and b–g) different sources of drinking water: b) rain feed pond (RFP), c) pond sand filter (PSF), d) harvesting rainwater (HRW) collected in plastic tanks, e) HRW collected in water house, f) extracted groundwater (GW) filtered using modern water filtering machine and sell to the locals, and g) saline water withdrawn from the pond and waiting for the rainy season to fill up the pond through rainwater to be used for drinking purposes
In recent years, saline water intrudes into the fresh pond water and other surface water sources in the coastal area owing to frequently occurring storm surges and tidal surge natural events, leading to polluting pond water sources for drinking (Penning-Rowsell et al. 2013; Rashid et al. 2022a, 2023). To solve this problem, people living in the coastal regions also take another adaptation; they remove the imposed saline water from the desired ponds and wait for the rainy season to fill up the ponds with rain water (Fig. 1g). Recently, people in the southwestern coastal parts of Bangladesh (Shyamnagar and Assasuni Upazilas of Satkhira District) have faced serious problems due to the shortage of potable GW (Rashid et al. 2022a, 2023). Therefore, people of this region collect and store rainwater during the rainy season through a rainwater harvesting system (HRW). They also store the rainwater in plastic containers/tanks as well as man-made water houses (Fig. 1d, e) for long-term storage for drinking purposes. Presently, some people in the area installed modern water filtering machines and filter the extracted GW (PGW) (Fig. 1f) and sell it to the local dwellers; however, most of the people are outside of this benefit due to their restraint. The reality is that most people in the area use RFP and PSF water for their drinking and household activities. Therefore, the supply of fresh and safe potable water is a vital issue for the mass people (exceeding 50 million) in the littoral region of Bangladesh (770 people per square kilometer) (BBS 2011; Khan et al. 2011; Rahman et al. 2018; Murshed et al. 2019; Sun et al. 2022).
Several studies reported that elemental abundances in HRW surpassed the permissible levels fitting for human health (Magyar et al. 2008; Simmons et al. 2001). The risk due to the drinking of the RFP water is also deeply alarming as farmers utilize synthesized fertilizers, insecticides, and pesticides in agricultural lands which are eventually washed out by rainwater into the nearby ponds and consequently, caused pollution. Various toxic elements are readily released from both anthropogenic and geogenic sources. Arsenic has also been identified in the RFP and PSF water of Bangladesh (Howard et al. 2006).
High levels of physicochemical parameters, trace elements, and major cations in water deteriorate the water quality for drinking purposes. Therefore, it is inevitable to assess the water quality based on the level of major ions and trace elements, their sources, distributions, and extent of health hazards to control water pollution and to implement pollution source control and prevention strategies. Several works have demonstrated the crisis of potable water due to the severe vulnerabilities to coastal salinity as well as elemental toxicity (BGS and DPHE 2001; Islam et al. 2016, 2017a, b; Rahman et al. 2021a, b, 2017; Huang et al. 2015; Das et al. 2017; Sarkar and Vogt 2015; Saha et al. 2017, 2018; Rakib et al. 2019; Ghosh et al. 2020; Hasan et al. 2021). Very little research (e.g., Islam et al. 2014) was concerned about major ions and trace element abundances in potable water from diverse water resources (e.g., RFP, PSF, GW, HRW, and PGW) of the southwestern coast of Bangladesh. However, no detailed study has been conducted so far on the concentrations of potentially toxic elements, their sources, and distributions in drinking water from diverse water sources in southwestern littoral regions of Bangladesh, considering GW geochemistry, irrigation feasibility, and the degree of human health risks. The present study evaluated the preliminary human-health adversity of ingestion and dermal absorption of toxic elements from RFP, PSF, GW, HRW, and PGW, for the first time. The motives of the current research are to (1) examine some physicochemical properties such as temperature, pH, EC, TDS, salinity, and the chemical elements, viz., Fe, Mn, Zn, Ca, Mg, Na, K, Cu, Co, Pb, As, Cr, Cd, and Ni in GW, RFP, PSF, HRW, and PGW; (2) compare the level of the measured parameters with those of guideline values for drinking water along with the existing literature; (3) categorize the source(s) of contamination in water by multivariate statistical approaches; (4) assess geochemistry and irrigation feasibility of water from multiple resources; and (5) evaluate the health risks due to the exposure of toxic element–contaminated water in the residents (both adults and children) of the study area. The novel aspect of this work is that it is the first inclusive investigation of toxic elements, their sources, and distributions in drinking water of the study area which will address the long-term potable water crisis and human health safety in the littoral region of Bangladesh along with the irrigation feasibility study. Therefore, the output of this research work will be supportive for coastal scientists, planners, and policymakers to take effective measures for coastal area management in improving safe water access and thus achieving the UN SDGs.
Experimental
Study area
Bangladesh possesses one of the largest deltas in the world, known as the Ganges–Brahmaputra-Meghna (GBM) delta. This delta is more or less flat, sloping gently toward the south to merge into the Bay of Bengal which produces a wide coastal area of about 710 km long and occupies an area of ~ 47,201 km2. The mean elevation of this coast is about 1–3 m from sea level (Khan 2001) where about 50 million people are living. It consists of one of the richest ecosystems in the world characterized by mangrove forests and fertile soils. However, it is one of the most susceptible coasts due to the combined consequences of climate change originating from global warming and sea level rise (SLR) (IPCC 2007; Rashid et al. 2022a). Globally, Bangladesh is ranked as the third most susceptible country in terms of the number of affected people with respect to SLR and increasing temperatures. Apart from these, other natural hazardous events, namely, saline water intrusion, tidal water movements, fisheries destruction, coastal erosion, drainage congestion, waterlogging, damage to infrastructures, land subsidence, crop failure, loss of biodiversity, and so on, lead to large-scale damages to crops, landlessness, fragmentation of community cohesion, unemployment, challenged life and livelihoods of the coastal communities, and continuously create sufferings for the coastal inhabitants (Pethick and Orford 2013; Ahsan et al. 2009, 2022; Rashid and Mahmud 2011; Islam et al. 2011; Rashid et al. 2013, 2022b).
The study area is located between the latitudes 22° 10′ and 22° 40′ N and longitudes 89° 00′ and 89° 20′ E in the southwestern coastal part of Bangladesh (Shyamnagar and Assasuni Upazilas of Satkhira District) and near the Sundarbans mangrove forest (Fig. 1a). It comprises natural levee deposits of paleo and the present Ganges River system (Rashid et al. 2014) and the intertidal and upper tidal plain of different connecting rivers in the coastal area of Bangladesh. The surficial deposits of the area are clayey silt and silty clay textures (Rashid et al. 2014). The study area falls under a humid tropical monsoon climate. The monthly temperature varied between 10 and 35 ℃, and the yearly average rainfall varied between 150 and 2500 mm. The study area is one of the salinity-impacted areas in Bangladesh with a serious lack of potable salinity-free water (Rashid 2023; Rashid et al. 2023). The local people in the area are struggling very much for drinking water and also employing several adaptation strategies for their survival in the hostile environment.
Sample collection, processing, and analysis
The drinking water was sampled from the Shyamnagar and Assasuni Upazillas of Satkhira District situated in the southwestern coastal parts of Bangladesh (Fig. 1) in February 2021. Sampling locations were marked with a handheld Global Positioning System (Germin GPS MAP® 78 s). The samples were collected from multiple sources such as rain-fed pond (RFP), pond water filtered through the sand filter (PSF), groundwater (GW), harvesting rainwater (HRW), and groundwater extracted through filtering using a modern water filtering machine (PGW) (Fig. 1b–f). A total of 33 composite water samples were collected from the study area in pre-cleaned 1-L plastic bottles following our earlier reports (Abedin et al. 2023; Rahman et al. 2022; Ahmed et al. 2021; Hasan et al. 2021). Each sample was collected in a triplicate fashion and mixed them well to obtain a total of 33 composite samples from the entire study area. The details of the sample collection points are shown in Fig. 1. From the selected RFP, 7 composite water samples (triplicate samples from each pond with different water depths) were collected. For PSF samples, 6 composite samples were collected from the 6 randomly selected PSF sites. For collecting GW samples, 14 hand tubewells of both shallow (up to 60 m depth) and deep (> 60 m depth) were randomly selected (Fig. 1a) and 14 composite samples (triplicate samples were collected with ~ 5-min intervals of well pumping) were collected from 14 tubewells. For collecting HRW and PGW samples, 3 of each HRW and PGW sites were also randomly selected, and 3 composite samples were collected from HRW and 3 from PGW sites. For elemental analysis, all the collected water samples from different sources (RFP, PSF, GW, PGW, HRW) were immediately acidified with 2 mL conc. HNO3 (2 mL/L) to prevent the loss of its content concentration (Rahman et al. 2022). The collected samples were then brought to the analytical laboratory as soon as possible and reserved at 4 ℃ until elemental analysis.
The physicochemical parameters such as temperature, pH, electrical conductivity (EC), total dissolved solids (TDS), and salinity of multiple sourced water samples were measured on-site. The water temperature and pH were monitored by a calibrated thermometer and portable pH meter (calibrated with pH 4 and 7), respectively. The EC, TDS, and salinity of samples were determined by using a multimeter (HQ40D, HACH, UK) which was calibrated with respective standard solutions obtained from Fluka Analytical, Sigma-Aldrich, Germany. To avoid the contamination of the samples, all physicochemical measurements were performed in triplicate and the mean results were considered. The study area is prone to salinity intrusion. So, the salinity of the samples was carefully measured on-site to delineate the salinity distribution in the surface water as well as shallow and deep aquifer water of the study area.
The concentrations of 14 chemical elements, viz., Fe, Mn, Zn, Ca, Mg, Na, K, Cu, Co, Pb, As, Cr, Cd, and Ni, for all samples were measured by several arrangements of atomic absorption spectrometers (AAS) (models: a. AA240FS, b. GTA 120-AA240Z, c. SpectrAA 220, Varian, Australia), except for Na and K which were estimated by a flame photometer (model: PFP7, Jenway, UK). While determining the As concentration in the water samples, the electric hydride vapor generation technique of AAS was employed which uses an electro-thermal temperature controller for producing atomic vapor in the analysis. The elements such as Fe, Mn, Zn, Mg, and Cu were analyzed using air/acetylene flame, while the Ca was analyzed using air/nitrous oxide/acetylene flame. The elements Co, Pb, Cr, Cd, and Ni were analyzed using a graphite furnace equipped with an auto sampler. The metals Na and K were analyzed using natural gas. For elemental determinations, water samples were prepared through the acid digestion method (Siddique et al. 2022; Ahsan et al. 2019). A 100 mL of sample was digested with 4–5 mL of analytical grade conc. HNO3 (68% pure, Fluka Analytical, Sigma-Aldrich, Germany) on an electrically heated hot plate at around 100–120 ℃. The final volume of the sample was made 100 mL in a calibrated volumetric flask using deionized (DI) water (EC < 0.5 µS/cm) and analyzed by AAS. The multiple procedural blank samples were also prepared for contamination and quality checks. All water samples were examined at the ISO/IEC 17025:2017-accredited laboratory, Institute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka, Bangladesh. The digested samples were used for the analysis of elements by AAS following the APHA method (APHA 2017) and our previous report (Siddique et al. 2020; Hasan et al. 2020; Habib et al. 2020). During analytical measurements, the concentration of elements was plotted against absorbance to generate calibration curves for individual elements. The working standard solutions prepared from the individual stock standard solution of 1000 mg/L (Fluka Analytical, Sigma-Aldrich, Germany) through dilution with DI water were used for the calibration curve construction, and the concentration of each element was determined against the generated curve. During the analysis, the samples were post-diluted or pre-concentrated depending on the abundance of the elements in the samples. All samples were analyzed in a triplicate fashion and the mean values were reported for each element. The experimental parameters of the element-specific hallow cathode lamp used in the AAS instrument during the measurements along with the limit of quantification, the limit of detection, calibration range, and measurement uncertainty for all the analyzed elements are summarized in Table S1.
Quality assurance and quality control in elemental analysis
Good analytical laboratory practices were assured to generate highly reliable analytical data for water samples as the analytical chemistry laboratory (INARS, BCSIR, Dhaka, Bangladesh) where we analyzed our samples maintained an ISO/IEC 17025:2017 accreditation (“general requirements for the competence of testing and calibration laboratories”) from the Bangladesh Accreditation Board (BAB) for 88 water quality parameters. Starting from the sampling to analysis, adequate precautions were taken to avoid all kinds of contaminations. In the laboratory, the samples and standards were prepared using high-quality DI water (EC: < 0.5 µS/cm and resistivity: 18 MΩ cm at 25 ℃) and analytical grade conc. HNO3 (68% pure, Fluka Analytical, Sigma-Aldrich, Germany). Only acid-cleaned (10% v/v HNO3) and calibrated glassware such as a pipette and volumetric flask were used throughout the experiments. The certified reference materials (CRM, obtained from Fluka Analytical, Sigma-Aldrich, Germany) as traceable to the National Institute of Standards and Technology, USA, and prepared according to the ISO/IEC 17025 and ISO guide 34 were used for the generation of calibration curves with high linearity (≥ 0.99) for individual elements. The CRM was also used for checking the instrument’s performance (sensitivity check), and accuracy and precession of analysis. The data production reliability of the instrument was further checked by the analysis of the independent check standards of the elements obtained from a different source (Scharlau, Spain) than CRM. The precession of the analysis was also verified by the multiple analysis of the same water samples (relative standard deviation, RSD less than 5%) selected randomly. The matrix effect of the water samples was evaluated through the analysis of spiked samples and the recoveries for all elements were within the 93–104% with ± 5% RSD. During the sample analysis, the CRM, instrumental method blank, sample blank for water samples, and spiked samples were analyzed in proper sequence following the established internal laboratory “standard operating procedure” to assure the generation of high-quality, reliable, accurate, and precise data. All samples including the CRM, water samples, blanks, and spiked samples were analyzed by the AAS instrument in a triplicate fashion to report the mean values (RSD < 5%). Following the ISO/IEC 17025 management systems, all analysis was performed by only validated and trained analysts as responsible for the analysis of elements in the water samples by AAS. To ensure the production of internationally accepted data on elements, the laboratory also made intra- and inter-laboratory comparisons for the elemental analysis and participated international proficiency testing program every year with successful completion and passed.
Data illustrations
Spatial distributions of analyzed water quality parameters were illustrated by GIS-based interpolation (ArcGIS 10.2) where the “inverse distance weighting” technique was used. However, analytical data were further evaluated by multivariate statistical approaches such as principal component, cluster, and correlation analyses using SPSS software (version 20) to ascertain their associated patterns and source of origin (Hossain et al. 2022; Siddique et al. 2021; Hasan et al. 2021).
Water quality index (WQI)
The WQI subjects to the theoretical and mathematical expressions that transform determined water-quality data into a single number which demonstrates the level of water quality (Horton 1965). Estimation of WQI involves three-stepped processes which are concise in Eq. (1) (Siddique et al. 2021; Islam et al. 2020).
where Ci, Si, wi, qi, Wi, and SIi are the concentrations of each parameter, standard values (obtained from WHO 2011 and ECR 1997; Table 1), relative weightage (Table S2), water-quality rating variable, relative weight, and sub-index of ith parameters, respectively.
Irrigation indices
Irrigation indices, e.g., sodium adsorption ratio (SAR: Richard 1954), soluble sodium percentage (SSP: Todd 1980), Kelley’s ratio (KR: Kelley 1963; Paliwal 1967), magnesium hazard ratio (MHR: Raghunath 1987), total hardness (TH: Raghunath 1987), and total dissolved solids (TDS: Islam et al. 2018) as calculated using Eqs. (2)–(7) are utilized to assess the water quality for irrigation.
Health risk estimation
Health risk assessment is an extensively used method for screening the potential adversity of exposure to a particular chemical (USEPA 1989). The exposure doses for direct ingestion (ADDingestion) and dermal absorption (ADDdermal) can be calculated as follows (USEPA 2004):
where the terminologies (Cw, IR, EF, SA, ET, ED, BW, AT, Absg, and Kp) and the reference values are essentially the same as that of Ahmed et al. (2021) and are explained in Table S3. Non-carcinogenic risk (NCR) can be calculated from hazard quotients (HQ) for both ingestion and dermal exposure by Eqs. (10)–(12),
where RfDingestion and RfDdermal represent reference doses for specific elements (µg kg−1 day−1). Then, the total potential NCR can be estimated from the hazard index (HI),
NCR can be considered, only when HQ and HI are > 1 (USEPA 2004). However, CR for specific elements can be estimated by Eq. (14),
where SF is the oral cancer slope factor (mg/kg/day)−1 (Ahmed et al. 2021). The tolerable range (1 × 10−6 to 1 × 10−4) of CR was taken from USEPA (2004).
Results and discussion
Concentrations and spatial distributions of water quality parameters in the water of the study area
The descriptive statistics of the physicochemical parameters and chemical elements of drinking water collected from the study area are shown in Table 1 along with their guideline values and literature data. The water quality parameters of water samples point out the suitability of water for drinking purposes. The mean temperature and pH in HRW were higher compared to those of other potable water sources (Table 1; Fig. S1a). The rainwater pH typically varies from 4.5 to 6.5, which rises slightly while falling on the rooftop and stored in a water tank. Lower pH in rainwater may change owing to the mixing of other chemical constituents from air/roof catchment/storage tank. The potential cause for higher pH in HRW may be due to the use of a ferrocement roof for collecting rainwater and the leaching of calcium oxide from the roof to the storage tank (Handia 2005; Karim 2010; Islam et al. 2014). An optimal pH level in potable water is essential for disinfecting the chlorine which is commonly used for drinking water treatment (Macomber 2001).
The mean EC of RFP, PSF, GW, HRW, and PGW samples were 2424, 1568, 2335, 777, and 73 µS cm−1, respectively (Table 1; Fig. S1b). The conductivity in RFP, PSF, and GW was > 1000 µS cm−1 (Table 1) which was greater compared to the tolerable limit (1000 µS cm−1) for drinking water (ECR 1997). The maximum EC of RFP, PSF, and GW was 7.8, 2.9, and 4.3 times greater compared to the Bangladesh standard limit (ECR 1997), respectively. Conductivity is directly related to the contents of dissolved salts in water and, hence, to the total dissolved solids (TDS). EC is one of the most valuable and normally measured water-quality parameters where an abrupt change in the conductivity of water indicates contamination (Miller et al. 1988). The mean TDS concentrations of RFP, PSF, GW, HRW, and PGW samples were found at 711, 789, 866, 378, and 34 mgL−1 with ranging from 286 to 1505, 257 to 1502, 2 to 1310, 370 to 385, and 34 to 35 mgL−1, respectively (Table 1; Fig. S1c). The maximum TDS concentrations of RFP, PSF, and GW were 1.51-, 1.5-, and 1.3-fold higher than the Bangladesh standard, respectively. The mean salinities of RFP, PSF, GW, HRW, and PGW samples were 0.72, 0.79, 1.21, 0.37, and 0.03 ppt, respectively (Table 1; Fig. S1d). For salinity > 0.5 ppt, the water is identified as brackish (Gray and Cohen 2011). The maximum salinity of RFP, PSF, and GW was 3.1, 3.1, and 4.62 times higher than the freshwater limit, correspondingly.
The salinity distribution maps showed that the surface water was more saline in the southeastern segment of the study area (Fig. S2). Water from the shallow aquifer was also more saline in the same part and followed a similar pattern to the main rivers, indicating that the shallow aquifer is recharging from the saline river water (Hasan et al. 2021; Rashid et al. 2023). However, the deep aquifer is more saline in the southwestern part of the study area. The salinity intrusion magnitude in the groundwater aquifers of the coastal area governs by a rational balance of the seawater and freshwater flow (Bhattacharya 2015). Lam et al. (2021) and Salehin et al. (2018) stated that the decrease in the flow of the lower Ganges during the dry season pushes seawater inland into rivers and canals through vertical penetration into close land which results in the salinization of soil and groundwater in southwestern Bangladesh. Salehin et al. (2018) stated that excessive groundwater withdrawal, which has lowered the groundwater level and rising sea levels has caused seawater to occupy coastal aquifers from the ocean. Floods and frequent storm surges related to saline water from the sea further spoil the situation (Kabir and Golder 2017; Salehin et al. 2018; Ashrafuzzaman et al. 2022; Rashid 2023; Rashid et al. 2022a, 2023). The upward revocation of the groundwater lessens the probability of freshwater holding back the intrusion of salinity. This is an important concern in the studied coastal region and the groundwater quality in the area is deteriorating considerably due to the seawater intrusion impact leading to potential implications on the environment and human health.
In Bangladesh, many coastal community’s intake of salt is up to 16 gd−1 from only 2 L of normal potable water (Vineis et al. 2011) which was far higher than the acceptable daily dietary intake of salt (intake limit: < 5 g; Nishida et al. 2004). The access intake of salt may cause health dilemmas, including hypertension, severe respiratory diseases, diarrheal diseases, skin diseases, and miscarriage in pregnant women in the study area of Bangladesh (Ministry of Environment and Forest 2006). Safe Drinking Water Foundation (SDWF 2018) reported that 80% of all physiological problems were triggered by unsafe potable water. Nevertheless, nowadays the overall situation of the paucity of potable water has been more deteriorated due to the intrusion of salinity in the littoral areas of Bangladesh.
Sighting along with the elemental abundances (Table 1; Figs. S1 and S3) in the water samples revealed that the mean concentrations of Fe in RFP and GW samples were significantly higher compared to those of other sources of drinking water in this study (Fig. S1e). The maximum concentrations of Fe in RFP and GW were 32.6- and 15.9-fold higher than the Bangladesh standard, respectively. The abundance of Fe in the study area is of geogenic origin. Humic substrates can reduce Fe3+ to Fe2+ and form stable Fe2+-complexes which in turn inhibit the Fe2+oxidation (while contacting with air), thus raising the Fe abundances in water (Livens 1991). The availability of Fe in the GW samples of the study area can further be attributed to the dissolution of siderite (FeCO3) minerals (Islam et al. 2019; Rahman et al. 2022). The existence of chlorite (ClO2−, geochemically modified form of ferromagnesian minerals) in the shallow aquifer of the area may be another probable reason for Fe abundance in the water, especially in the GW aquifer (Zahid et al. 2009; Carretero and Kruse 2015; Rahman et al. 2022). Higher Fe abundances in drinking water can create health risks since an excess Fe-accumulation is associated with the formation of oxygen free-radical, which are assumed to be carcinogenic (Stevens 1990). Islam et al. (2014) also found high Fe concentration in RFP water. However, the mean abundance of Fe was significantly lower in PSF water similar to an earlier study by Islam et al. (2014). Therefore, PSF reduced Fe concentrations significantly. Hence, uptaking the PSF water instead of RFP water can lessen the health risks from Fe exposure.
The maximum concentrations of Mn in RFP and GW were 2.02 and 1.48 times higher than the Bangladesh standard. The mean contents of Mn in RFP water samples were significantly greater compared to those of other sources of potable water (Fig. S1f). The mean contents of Mn were much lower in PSF water (Fig. S1f). Therefore, PSF reduced Mn concentrations significantly similar to Fe concentrations. Hence, health risks from Mn exposure can be reduced by consuming PSF water instead of RFP water. Naturally, Mn occurs in the pedosphere that may leach out into the surface water (WHO 2011). Therefore, Mn in RFP and PSF water can originate from the soil-surface runoff.
The mean Zn concentrations from different potable water samples evaluated in the present study were lower compared to the WHO (2011) and ECR (1997). Though, the concentrations in HRW were higher than in other sources of water (Fig. S1g). Ward et al. (2010) identified the roof catchments as the potential source of Zn in harvested rainwater where high levels of Zn enter into the water by the erosion of Zn-covered roofs (Gromaire et al. 2002; Islam et al. 2014).
The measured Ca contents for RFP, PSF, GW, HRW, and PGW samples were found to be ranged from 33.1 to 187, 36.1 to 98.0, 6.5 to 195, 2.6 to 2.8, and 5.25 to 5.29 mgL−1 with the mean concentrations of 89.0, 77.0, 111, 2.70, and 5.27 mgL−1, respectively (Table 1; Fig. S1h). The maximum concentrations of Ca in RFP, PSF, and GW were 2.5-, 1.3-, and 2.6-fold higher than the Bangladesh standard, respectively. The measured maximum concentrations of Mg in RFP, PSF, and GW were 1.2, 1.3, and 1.5 times greater compared to ECR (1997) recommendation, respectively. The mean contents of Ca and Mg in HRW and PGW were particularly low (Table 1; Figs. S1h and S3a). Such a paucity of Mg and Ca in drinking water triggers numerous diseases in the dwellers who utilized this water for drinking and other domestic purposes. Rainwater has the probable scarcity of some essential chemical elements such as Ca and Mg (Uba and Aghogho 2000). Furthermore, Mg-loss from the food is generally higher while cooking the food in Mg-poor water (Haring and Delft 1981). The Mg and Ca deficiencies in potable water of HRW and PGW could cause deficiency of these elements since Ca and Mg are considered bio-essential metals. Yang et al. (2006) demonstrated through an epidemiological study that Mg from potable water can reduce the risk of death from cardiovascular diseases, although the major portion of Mg-intake in human physiology comes from food instead of only drinking water. Nevertheless, for individuals with Mg deficiency in drinking water in the coastal area of Bangladesh, water-borne Mg can make a significant impact on their total consumption.
The mean concentrations of Na in the GW sample were higher than the recommended values (200 mgL−1) proposed by WHO (2011) and ECR (1997). The maximum concentrations of Na in RFP, PSF, and GW were 2.1-, 1.9-, and 2.4-fold higher than the Bangladesh standard, respectively. The maximum concentration of K in RFP water is 1.5 times higher compared to the ECR (1997) guideline. The mean concentrations of Cu and Co from different potable water samples evaluated in the present study were lower compared to the WHO (2011) and ECR (1997) guidelines (Table 1; Fig. S3d, e), indicating these waters suffer Cu deficiency. The maximum concentrations of Pb in RFP, PSF, GW, and HRW were 1.4, 1.6, 1.4, and 1.4 times higher than the Bangladesh standard, respectively. Higher Pb concentration in RFP and HRW may be originated from agricultural fertilizer and runoff from the galvanized iron roof of the study area (Simmons et al. 2001). The chemical elements, viz., Cr, Cd, and Ni, are absent in all samples (i.e., below the detection limit and not shown in Table 1).
The analyzed parameters of the collected RFP and GW samples are compared with those of relevant previous reports in Bangladesh and some other countries in Tables 1, S4, and S5. As compared in Table 1, the values of EC were found higher than in most of the previous studies on surface water. The concentrations of Fe, Mn, Zn, K, Cu, Co, Pb, and As are almost comparable with the previous literature. However, the concentrations of Ca and Mg are much higher than the compared literature, indicating contribution from saline water. The concentration of Pb in the surface water of this work (0.06 mgL−1) was analogous to some of the regions in Bangladesh. Only the surface water of the Dhaka region presented remarkably high Pb abundance (Table S5). The surface water of Pakistan, China, Thailand, Cambodia, and South Korea possesses a lower Pb concentration than the water of Bangladesh, but surface water bodies in Nepal and Srilanka had significantly higher Pb content than the present study. On the other hand, As content in the surface water of the study area (0.009 mgL−1) was comparable to some other coastal cities (Chattogram and Khulna) in Bangladesh. However, the other part of the country had a high As concentration, except Rajshahi city. China and Cambodia possess lower As levels in their surface water compared to Bangladesh (Table S5). Mn level in the water samples of the present work was higher than in the water of India, Nepal, Srilanka, China, and Thailand. Cobalt in surface water in the studied area had a lower concentration than in the other parts of the country. However, the finding was comparable to Cambodia (Table S5). Copper concentration in this study was noticeably lower than in the other parts of Bangladesh. However, except for Pakistan, all other Asian countries possess lower Cu levels compared to that of the present study in Bangladesh. Zinc concentration in the present study was comparable to most Asian countries. Depending on the above comparative study, it was noticed that the concentration of the studied elements in water samples was reported to be considerably higher compared to the other Asian countries. It delineates that the pollution level of toxic elements in the water samples is threatening in the southwestern coastal area of Bangladesh, and thus, proper measures should be adopted to lessen it immediately.
The spatial distributions of water quality parameters of RFP and GW are shown in Figs. 2 and 3, respectively. In RFP water, the distribution of salinity, TDS, and EC possess significant positive correlations across the study area. Similarly, homogeneous spatial distribution patterns were observed for salinity, TDS, EC, Fe, Mn, Zn, Ca, Mg, Na, K, Cu, Co, and Pb, except for As. In GW, the distributions of salinity, EC, and TDS also showed significant positive correlations across the region similar to RFP. Homogeneous patterns of spatial distribution were noticed for salinity, TDS, EC, Mg, Na, and Pb. However, distribution patterns of As, Fe, Mn, Zn, Ca, K, Cu, and Co are different from other parameters. The overall distribution pattern implied that the salinity, trace elements, and other ions have some association to enter the water of the study area.
Pollution source identification
The inter-relationships among water quality parameters can be utilized to evaluate their probabilistic sources (Hasan et al. 2021; Siddique et al. 2021) as well as the hydro-geochemical nature of the water sources (Islam et al. 2019). Multivariate statistical approaches including principal component, cluster, and correlation analysis were employed to know the associated patterns of the analytical datasets of the water quality parameters for groundwater (GW) and rain-fed pond (RFP) water only since these water sources were not treated like other water sources which were treated by several techniques, and thus, original content of the water contaminants in relation to the natural environment cannot be found.
The results of the principal component analysis (PCA) of water quality parameters for both GW and RFP water are shown in Table S6 which explained the cumulative variances of 91.30% and 91.12%, respectively. In the PCA of water quality parameters for both GW and RFP, four principal components/factors were identified (Fig. 4a, b) as responsible for controlling the water geochemistry. In GW, PC1 represents 30.15% of the total variance, which was strongly loaded with conductivity, salinity, and Na while moderately loaded with Fe, Pb, and As. The loading of principal components is referred to as weak, moderate, and strong for the component scores of 0.30–0.50, 0.50–0.75, and ˃ 0.75, respectively (Ahsan et al. 2019). The strong loading of conductivity, salinity, and Na on PC1 can be corroborated by the intrusion of saline water in the groundwater aquifer of the studied area (Hasan et al. 2021). The strong significant loadings of salinity-affiliated parameters may have contributed to raising the dissolution of higher co-contaminants in the littoral aquifer. The loading of Fe, Pb, and As indicated their essential geogenic source of origin in the water. The PC2 accounts for 25.89% of the total variance where strong positive loadings were observed for Mg and moderate positive loadings were observed for Fe and Ca, indicating the geogenic source of origin of Mg, Fe, and Ca in water. The Fe and As showed positive loadings on three components, PC1, PC2, and PC3, indicating their common distribution in the groundwater aquifer. The third component, PC3, covers 17.75% of the total variance where strong positive loadings were found for TDS and Co. The strong significant loadings of TDS-affiliated components can have empowered the dissolution of more co-contaminants in the littoral aquifer. The fourth component, PC4, explained 17.52% of the total variance in which Zn is strongly loaded and moderate positive loadings were observed for temperature, K, and Cu. The elemental loadings of K and Cu are corroborated by the common geogenic source of origin in the water of the study area. Higher contents of saline water raise the dissolution rate of co-contaminants in the drinking water, whereas TDS and EC levels demonstrated positive correlations among the co-contaminants (e.g., trace elements). In GW, conductivity and salinity loaded positively in three components. Thus, the pollutants in this water can be positively triggered by conductivity and salinity, which would elevate the abundance of each component in the water.
a) Principal component analysis of the analyzed water quality parameters for GW of the study area by scree plot of the characteristic roots (eigenvalues) and component plot in rotated space. b) Principal component analysis of the analyzed water quality parameters for RFP water of the study area by scree plot of the characteristic roots (eigenvalues) and component plot in rotated space
In RFP water, PC1 accounted for 34.66% of the total variance in which the governing variables were temperature, Fe, Zn, Ca, and Cu, indicating the common source of origin of these elements in the water. The PC2 represents 29.39% of the total variance. Here, the major variables are TDS, salinity, Mg, and Pb, which indicate the common source of origin of these elements in the water and could be positively empowered by the intrusion of saline water (Hasan et al. 2021). The third component PC3 covers 18.81% of the total variance in which the governing variables are conductivity, K, and Co which indicates the common source(s) of origin of K. The K is assumed to be positively triggered by EC, which can raise the K-content in the water. The fourth component PC4 explained 8.27% of the total variance and loaded positively with Mn and As. Loading of these elements confirmed their geogenic source of origin in the area (Islam et al. 2019). The pH and temperature loaded positively in three components. Thus, most of the pollutants in RFP water could be positively triggered by pH and temperature which would elevate the abundance of each component in the coastal aquifer.
The cluster analysis (CA) of the water quality parameters for both GW and RFP water demonstrated four major clusters (with the phenon line set to a rescaled distance of about 15) in the dendrogram (Fig. 5) and the outcomes are consistent with the results of their PCA. For GW samples, the parameters such as conductivity, salinity, Na, and Pb are grouped into cluster 1 (Fig. 5a), which are also loaded positively on PC1 (Table S6). The other parameters forming cluster 2, 3, and 4 also showed similar types of association in PC2, PC3, and PC4, respectively. Similarly, the water quality parameters for RFP water which form clusters 1, 2, 3, and 4 (Fig. 5b) also loaded positively on PC1, PC2, PC3, and PC4, respectively. The similar group of loadings on PCA as well as similar clustering of the same water quality parameters in the dendrogram obtained from the cluster analysis indicate their common source of origin in the studied water samples (Hossain et al. 2022; Hasan et al. 2021; Siddique et al. 2021).
In the correlation analysis of water quality parameters of GW samples (Table S7), conductivity, salinity, Na, and Pb showed a significant positive relationship with each other. The Mn revealed a strong correlation with Ca. TDS is positively correlated with Co, Fe, and As. Temperature showed a positive correlation with pH, Zn, Mg, K, and Cu. In RFP water, temperature, Fe, Zn, Ca, and Cu showed a positive correlation among them. TDS is positively correlated with the salinity, Mg, Na, and Pb. The conductivity is found to be associated with Co and Pb, while Mn and As are correlated with each other. All the associations among the water quality parameters of GW and RFP water are supported by the outcomes of the PCA and CA. The internal relationships among water quality parameters disclosed that some of the chemical elements originated from their common source or geogenic source. But the elevated levels of saline water raised the dissolution rate of these elements in the littoral aquifer as well as surface water, while TDS, EC, pH, and temperature generally demonstrated positive correlations with the chemical elements.
The overall outcome of multivariate analyses indicates a significant impact of saline water intrusion on the water quality of the study area along with other potential geogenic contributions. In a nutshell, the intrusion of seawater impacts significantly the coastal aquifer, water quality, water suitability for irrigation, and possible human health risks (Kabir et al. Kabir and Golder 2017; Salehin et al. 2018; Ashrafuzzaman et al. 2022).
Hydro-geochemical evaluation
Hydro-geochemical processes, e.g., evaporation and water–rock interaction, can be categorized by a wide range of lithogenic influences, e.g., silicate weathering, carbonate dissolution, evaporate dissolution (Kumar et al. 2014), etc. These kinds of lithogenic influences can be evaluated by the bivariate plots. To recognize and categorize the diverse water geochemistry, GW and RFP water samples were considered since these water samples have a direct connection with the sediments. The measured water quality parameters are processed and represented on the scatter diagrams to enable the assessment of evolutionary trends. The molar ratio of Ca/Mg implies the dolomite and calcite dissolution in groundwater (Ca/Mg = 1: demonstrates dolomite dissolution; 1 < Ca/Mg < 2: calcite dissolution; and Ca/Mg > 2: silicate dissolution) (Paul et al. 2019). The weathering processes of calcite (CaCO3) and dolomite [CaMg(CO3)2] can be written as (Mohamed and Zineb 2015; Nasher and Ahmed 2021).
An overall depiction of hydrogeochemical data could be understood possibly by the hydrochemical facies of water, usually known as water-type using ionic composition (Mohamed and Zineb 2015). The scatter diagram of samples and Ca/Mg showed that most of the ratios of GW and RFP were > 2 and some ratios were between 1 and 2 (Fig. S4), suggesting the dominating factor of silicate weathering as well as calcite weathering. The earlier research (Nasher and Ahmed 2021) also stated that hydrogeochemical properties in water samples of the GBM delta areas were dominated by silicate weathering.
Water pollution and quality assessment
To get an inclusive assessment of water quality, influences of all quality parameters should be considered. About 67% of samples had a pH above the Bangladesh drinking water standard (6.5 and 8.5, ECR 1997) (Tables 1 and S8). Around 71, 67, and 100% of samples of RFP, PSF, and GW, respectively, possess higher EC compared to that of Bangladesh drinking water standard (Table S8). Considering TDS values, about 29, 17, and 43% of samples of RFP, PSF, and GW, respectively, were higher than the standard of drinking water quality for Bangladesh (1000 mgL−1, ECR 1997) (Tables 1 and S8). About 57, 67, and 100% of samples of RFP, PSF, and GW had brackish water, respectively (Table S8), and about 29% of both RFP and GW samples possess higher Fe contents than the Bangladesh drinking water standard (0.3 mgL−1, ECR 1997) (Table S8). About 71 and 14% of samples of RFP and GW, respectively, had Mn contents above the Bangladesh drinking water standard of 0.1 mgL−1 (ECR 1997) (Table S8). About 57, 50, and 86% of samples of RFP, PSF, and GW, respectively, exceeded the Ca contents mentioned in the Bangladesh drinking water quality standard (75 mgL−1, ECR 1997) as well as WHO recommendation (100 mgL−1, WHO 2011) (Table S8). About 57, 67, and 86% of samples of RFP, PSF, and GW, respectively, had Mg levels above the Bangladesh drinking water quality standard (Table S8). Considering the mean value of Pb, about 71, 33, 43, and 67% of samples of RFP, PSF, GW, and HRW, respectively, were above the standard of drinking water quality of Bangladesh (0.05 mgL−1, ECR 1997) and WHO (2011) recommended values (0.01 mgL−1) (Table S8). For this reason, weightage-based WQI was used in this study. It can be observed that all water samples of this study had WQI ranging from 18.16 to 429.7 (Table S9) reflecting an “excellent” to “unsuitable” category of water quality. Estimated WQI demonstrated that water samples from different sources possess diverse qualities. The poor to unsuitable natures of water samples were collected from RFP, PSF, and GW, although two samples from PSF had good quality for consumption. Though RFP, PSF, and GW had a poor to unsuitable nature of water samples, HRW and PGW showed excellent to good quality water. Similarly, Chakraborty et al. (2007) demonstrated that in terms of WQI values (range: 68–621), 26 and 17% of groundwater samples from West Bengal (India) can be categorized as “very poor” and “water unsuitable for drinking,” respectively.
Irrigation feasibility study
The status of irrigation water existing for irrigation purposes has momentous impacts on the growth and yield of crops. Climate change empowered sea-level rise and anthropogenic activities can introduce an additional load of salinity to the freshwater reservoirs of the littoral and estuarine areas (Rashid et al. 2013; Kabir et al. Kabir and Golder 2017; Salehin et al. 2018; Lam et al. 2021; Ashrafuzzaman et al. 2022; Rashid 2023; Rashid et al. 2023), which invoke the study of irrigation water assessment. Irrigation water with high salinity may change the chemical and physical characteristics of agricultural soil which can affect the crop’s yields and quality by degrading soil structure, excess Na-toxicity in plants, and depleting K and Ca in agricultural fields (Rawat et al. 2018). Irrigation indices such as SAR, SSP, KR, MHR, TH, and TDS were used in this study to calculate the water value for irrigation. Only GW and RFP water samples were utilized for the irrigation feasibility study since these waters were used for irrigation purposes. Sampling location-wise average irrigation indices for RFP and GW samples with their uncertainties (1σ) are shown in Fig. S5 with their corresponding categorizations (by dotted lines).
SAR is a primary assessment of probable Na accumulation by agricultural soil at the expense of K, Mg, and Ca as a result of sodic water utilization for irrigation. SAR values for most sampling locations were less than 10 and other values were less than 18, which implies little or no adverse effect on irrigation. SSP values were > 40 to < 80, which positioned in the “doubtful” region among which only one groundwater sample was categorized as a “good” source of irrigation water. Likewise, KR values were > 1 for most of the samples which ascribed to their direct interaction of them with the seawater. Considering the uncertainties (SD: 1σ) of MHR, all samples can be recommended for irrigation. Furthermore, water samples from all the locations were hard to very hard, and three water samples showed the best irrigation quality and the rest of the samples remained at moderate irrigation quality in terms of TDS.
Moreover, the water classifications by the US Salinity Laboratory (USSL) were presented (Fig. S6) to assess the appropriateness of the water samples of the studied area for irrigation purposes (Xiao et al. 2020). As shown in Fig. S6, most of the water samples fall in the category of high to very high salinity and medium to very high alkalinity (SAR), demonstrating their comparable nature and almost the same sorts of contamination source(s). Hence, most of the water samples were unsuitable for irrigation use. Only one sample (S1 from RFP water) falls in the category of medium salinity and two samples (S1 from RFP water and G2 from GW) fall in the category of low alkalinity (SAR). This unsuitable nature of water for irrigation use is attributed to the impact of the salinity water intrusion in the study area.
Assessment of human health risks
Human health risk assessment from seven toxic elements (Fe, Mn, Zn, Cu, Co, Pb, and As) was conducted for both children and adult inhabitants, and data were presented in Fig. S7 and Table S10. This attempt is the first-ever study to conduct a human health risk assessment by the potentially hazardous elements in the drinking water of the study area.
Non-carcinogenic risks
The hazard index (HI) is utilized in this study to understand the overall potential health risks instigated by elemental contents in water, which is the sum of hazard quotients (HQs) calculated for all probable routes of elemental exposure. HQ and HI values for the specific elements owing to dermal and ingestion contacts for children and adults are presented in Fig. S7a–g and Table S10. Overall, considering the uncertainties (SD: 1σ), the average HI values (Mn of RFP and GW; Cu of PGW; Pb from all sources of water; and As of RFP water) for both age groups surpassed the safe limits (> 1), indicating that all sources of water possessed potential health risks. The Mn, Cu, Pb, and As were the foremost contributors to the dermal and ingestion exposures, while the other elements of this study were comparatively less important with respect to health risks.
Element-specific major influences on HI values followed the descending order of Pb > As > Mn > Cu > Fe > Zn > Co, for both children and adults, where HI of Mn, Cu, Pb, and As surpassed 1, which can be responsible for the severe health risks to the residents. Though Mn is essential for human health, long-term exposure to a high concentration of this element in drinking water may cause neurological disorders (WHO 2011). Infants are exceedingly susceptible to high Mn contents in drinking water as it interrupts their intellectual growth during pregnancy. Bouchard et al. (2007) demonstrated that children with Mn exposure through drinking water were positively correlated with hyperactive classroom behaviors. An earlier study (Wasserman et al. 2006) also presented that high Mn content in drinking water can affect the intellectual development of school-aged children. Manganese concentrations in groundwater are mostly governed by the reductive dissolution of Mn (IV) oxyhydroxides which primarily depends on numerous environmental factors such as redox potential, pH level, type and distribution of ions in water, precipitation of secondary mineral phases, and microbial activities (Halim et al. 2014; Hasan et al. 2007; Huang et al. 2015). Copper is a “probable human carcinogen” because of an inconclusive sign of human carcinogenicity (WHO 2004). Lead is also a “possible human carcinogen” because of inconclusive evidence of humans and sufficient evidence of animal carcinogenicity (WHO 1996). Arsenic was also a potential pollutant in water where high levels of As in potable water cause dermal lesions such as hypopigmentation and hyperpigmentation, peripheral neuropathy, skin cancer, peripheral vascular disease, and bladder and lung cancers (WHO 2011).
However, the calculation of such health risks consists of uncertainties owing to (1) potential variations of RfD values (USEPA 2004) to the considered population, (2) dissimilarities of ages and exposure frequencies, and (3) alteration of risk elements by the interactions with physicochemical properties of aquatic body (Wu et al. 2009). Hence, this study represents a preliminary health risk evaluation, and more accurate assessments of the toxic elements should be conducted.
Carcinogenic risks
The carcinogenic risks (CR) of As from water are presented in Fig. S7h and Table S10. Considering the uncertainties (SD: 1σ), the CRingestion values of As of all sources of water (except PGW) for both adults and children were higher compared to the permissible limit (1 × 10−6 to 1 × 10−4) of cancer risks recommended by the USEPA (2004). These findings appeal that As may cause harmful risks to the local inhabitants via water consumption. Minor CRdermal values compared to the permissible limit (1 × 10−6) indicate insignificant risks of carcinogenicity through dermal exposure. Therefore, a sensitive group of local occupants should be taken more care of high As in waters in the southwestern coastal area of Bangladesh. In the future, a more elaborated study on different water sources should be carried out in these areas with high abundances of potentially toxic elements that induce severe health problems.
Recommendations
To overcome the fresh water scarcity in the studied southwestern coastal area of Bangladesh and achieve the UN SDGs, it is essential to have an integrated plan. The following recommendations are made from this study to mitigate drinking water scarcity and improve the overall water quality in the study area, and for the policymakers to take appropriate steps in this regard.
-
Contamination-free freshwater aquifers should be identified in the study areas and their water quality should be checked periodically before use by the residents.
-
Sufficient rain-fed freshwater ponds should be excavated in the areas under government control and also the high enough embankments of the ponds should be ensured to protect them from tidal surges or saline water intrusion during cyclone surges in consideration of climate change and sea level rise.
-
Installation of rainwater harvesting systems (HRW) should be ensured in every family in the area and the government can take proper steps in this regard by providing funds and technical support through an HRW-installation project.
-
Installation of modern water filtering machines and their easy access to the local dwellers can ensure the safe drinking water supply in the area, especially for those that are now exposed to the risk.
-
Proper measures should be adopted by carrying out more fruitful research regarding the remediation and mitigation of water contaminants in the study area to prevent health risks from higher levels of potentially toxic elements in differential water sources of the area.
-
Considering the diverse water sources in the study area, it is suggestive for continuous monitoring of water quality to determine the accepted level of contaminants prior to use by the local habitants.
-
In all cases, the collaborative involvement of local government, people, and policymakers should be ensured for regular monitoring of these works regarding safe water management in the study area.
Conclusions
The physicochemical parameters, viz., pH, EC, TDS, salinity, and elemental concentrations of Fe, Mn, Ca, Mg, Na, K, and Pb, in different drinking water samples from the southwestern coastal area of Bangladesh exceeded their corresponding recommended standard limits. The maximum values of physicochemical parameters (e.g., EC, TDS, and salinity) in RFP, PSF, and GW were 1.3 to 7.8 times higher than the Bangladesh standard limits. However, the elemental contents of Na, Ca, Pb, Mg, Fe, and K were 1.2 to 32.6 times higher compared to the corresponding recommended limits. Multivariate analyses of the water quality parameters implied a potential impact of seawater intrusion on the studied aquifers along with some other geogenic contributions. The study suggested that high salinity increased the dissolution rate of trace elements and major metals in the water sources. The ionic composition of surface and groundwater indicates the silicate as well as calcite dominating weathering in the study area, which in turn affects the irrigation suitability of the studied water sources. The SSP values of the surface and groundwater were > 40 to < 80, and these fall in the doubtful region of irrigation. The SSL indicates that most of the samples were high to very high salinity category and medium to very high alkalinity (SAR), demonstrating their similar nature and almost the same types of contamination sources. On the other hand, element-specific predominant contributions to HI values for non-carcinogenic risks followed the order of Pb > As > Mn > Cu > Fe > Zn > Co, for both adults and children, where Mn of RFP and GW, Cu of PGW, Pb from of all sources of water, and As of RFP water for both age groups exceeded the safe limits (> 1). The CRingestion values of As from all sources of water (except PGW) for both adults and children are greater than the permissible limit (1 × 10−6 to 1 × 10−4) of cancer risks. Hence, this study reveals the unsuitability of water for drinking and domestic uses due to the higher levels of physicochemical and potentially toxic elements in differential water sources. Therefore, appropriate filtration and regular monitoring are invoked to ensure public health safety in the study area (southwestern Bangladesh).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Change history
12 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11356-023-29227-9
References
Abedin MJ, Khan R, Siddique MAB, Khan AHAN, Islam MT, Rashid MB (2023) Metal(loid)s in tap-water from schools in Central Bangladesh (Mirpur): source apportionment, water quality, and health risks appraisals. Heliyon 9(5):e15747. https://doi.org/10.1016/j.heliyon.2023.e15747
Ahmed FT, Khan AHAN, Khan R, Saha SK, Alam FA, Dafader NC, Sultana S, Elius IB, Mamun SA (2021) Characterization of Arsenic contaminated groundwater from central Bangladesh: irrigation feasibility and preliminary health risks assessment. Environ Nanotechnol Monit Manag 15:100433. https://doi.org/10.1016/j.enmm.2021.100433
Ahsan MA, Satter F, Siddique MAB, Akbor MA, Shamim A, Shajahan M, Khan R (2019) Chemical and physicochemical characterization of effluents from the tanning and textile industries in Bangladesh with multivariate statistical approach. Environ Monit Assess 191:575. https://doi.org/10.1007/s10661-019-7654-2
Ahsan K, Rashid MB, Talukder S (2022) Tidal River siltation and its impact in the coastal parts of Bangladesh. Int J Econ Environ Geol 13(2):35–41
Ahsan K, Rashid MB, Habib MA, Alam MF (2009) The changing geometry of the Cox’s Bazar-Badarmokam Coast, Bangladesh. Bangladesh J Geol 26–28:25-36
APHA (American Public Health Association) (2017) In: Baird, R.B., Eaton, A.D., Rice, E. W. (Eds.), Standard methods for the examination of water and wastewater, 23rd ed. American Public Health Association, American Water Works Association, Water Environment Federation, Washington, DC, USA https://secure.apha.org/imis/ ItemDetail?iProductCode=978–087553–2875&CATEGORY=BK
Ashrafuzzaman M, Artemi C, Santos FD, Schmidt L (2022) Current and future salinity intrusion in the south-western coastal region of Bangladesh. Span J Soil Sci 12:10017. https://doi.org/10.3389/sjss.2022.10017
Bahar MM, Reza MS (2010) Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environ Earth Sci 61(5):1065–1073
Barakat A, El Baghdadi M, Rais J, Aghezzaf B, Slassi M (2016) Assessment of spatial and seasonal water quality variation of OumErRbia River (Morocco) using multivariate statistical techniques. Int Soil Water Conserv Res 4(4):284–292. https://doi.org/10.1016/j.iswcr.2016.11.002
BBS (Bangladesh Bureau of Statistics) (2011) Bangladesh population and housing census.Statistics and information division, ministry of the people republic of Bangladesh
BGS and DPHE (2001) Arsenic contamination of groundwater in Bangladesh. BGS Technical Report, WC/00/19
Bhattacharya AK (2015) Saline water intrusion in coastal aquifers of Bangladesh. J Appl Hydrogeol XXVIII 1&2:37–45
BIS (Bureau of Indian Standards) (1991) Indian standard for drinking water – Specification IS 10500:1991
Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D (2007) Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect 115(1):122–127
Carretero S, Kruse E (2015) Iron and manganese content in groundwater on the northeastern coast of the Buenos Aires Province, Argentina. Environ Earth Sci 73(5):1983–1995. https://doi.org/10.1007/s12665-014-3546-5
Chakraborty S, Sikdar PK, Paul PK (2007) Hydrogeochemical framework of quaternary aquifer of English Bazaar Block, Malda District, West Bengal. The Icfai J Earth Sci 1:61–74
Chakraborty R, Khan KM, Dibaba DT, Khan MA, Ahmed A, Islam MZ (2019) Health implications of drinking water salinity in coastal areas of Bangladesh. Int J Environ Res Public Health 16(19):3746. https://doi.org/10.3390/ijerph16193746
Damanik-Ambarita MN, Lock K, Boets P, Everaert G, Nguyen THT, Forio MAE, Musonge PLS, Suhareva N, Bennetsen E, Landuyt D, Dominguez-Granda L, Goethals PLM (2016) Ecological water quality analysis of the Guayas River basin (Ecuador) based on macroinvertebrates indices. Limnologica 57:27–59
Das TK, Choudhury M, Sultana M (2017) Determination of drinking water quality: a case study on saline prone south-west coastal belt of Bangladesh. J Environ Sci Nat Reso 10(1):101–108. https://doi.org/10.3329/jesnr.v10i1.34702
ECR (The Environment Conservation Rules) (1997) Department of Environment, Ministry of Environment and Forest, Government of the People’s Republic of Bangladesh
Egbueri JC, Ezugwu CK, Ameh PD, Unigwe CO, Ayejoto DA (2020) Appraising drinking water quality in Ikem rural area (Nigeria) based on chemometrics and multiple indexical methods. Environ Monit Assess 192:1–18. https://doi.org/10.1007/s10661-020-08277-3
El-Sayed SM, Hegab MH, Mola HR, Ahmed NM, GoherME, (2020) An integrated water quality assessment of Damietta and Rosetta branches (Nile River, Egypt) using chemical and biological indices. Environ Monit Assess 192(4):1–16. https://doi.org/10.1007/s10661-020-8195-4
EU (European Union) (1998) Council Directive 98/83/EC on the quality of water intended for human consumption
Ghosh GC, Khan MJH, Chakraborty TK, Zaman S, Kabir AE, Tanaka H (2020) Human health risk assessment of elevated and variable iron and manganese intake with arsenic-safe groundwater in Jashore, Bangladesh. Sci Rep 10(1):1–9
Gray S, Cohen Y (2011) Water-quality engineering in treatise on water science
Gromaire MC, Chebbo G, Constant A (2002) Impact of zinc roofing on urban runoff pollutant load, the case of Paris. Water Sci Technol 45:113–121
Habib MA, Islam ARMT, Bodrud-Doza M, Mukta FA, Khan R, Siddique MAB, Phoungthong K, Techato K (2020) Simultaneous appraisals of pathway and probable health risk associated with trace metals contamination in groundwater from Barapukuria coal basin, Bangladesh. Chemosphere 242:125183. https://doi.org/10.1016/j.chemosphere.2019.125183
Halim MA, Majumder RK, Rasul G, Hirosiro Y, Sasaki K, Shimada J, Jinno K (2014) Geochemical evaluation of arsenic and manganese in shallow groundwater and core sediment in Singair Upazila, Central Bangladesh. Arab J Sci Eng 39(7):5585–5601
Handia L (2005) Comparative study of rainwater quality in urban Zambia. J Water Suppl: Res Technol AQUA 54:55–64
Haring BS, Delft VW (1981) Changes in the mineral composition of food as a result of cooking in hard and soft waters. Arch Environ Health 36:33–35
Hasan MA, Ahmed KM, Sracek O, Bhattacharya P, Von Broemssen M, Broms S, Fogelström J, Mazumder ML, Jacks G (2007) Arsenic in shallow groundwater of Bangladesh: investigations from three different physiographic settings. Hydrogeol J 15(8):1507–1522
Hasan AB, Reza AHMS, Kabir S, Siddique MAB, Ahsan MA, Akbor MA (2020) Accumulation and distribution of heavy metals in soil and food crops around the ship breaking area in southern Bangladesh and associated health risk assessment. SN Appl Sci 2(2):1–18. https://doi.org/10.1007/s42452-019-1933-y
Hasan MN, Siddique MAB, Reza AHMS, Khan R, Akbor MA, Elius IB, Hasan AB, Hasan M (2021) Vulnerability assessment of seawater intrusion in coastal aquifers of southern Bangladesh: water quality appraisals. Environ Nanotechnol Monit Manage 16:100498. https://doi.org/10.1016/j.enmm.2021.100498
Horton RK (1965) An index system for rating water quality. J Water Pollut Control Ed 37:300–306
Hossain S, Anik AH, Khan R, Ahmed FT, Siddique MAB, Khan AHAN, Saha N, Idris AM, Alam M (2022) Public health vulnerability due to the exposure of dissolved metal(oid)s in tap water from a mega city (Dhaka, Bangladesh): source and quality appraisals. Expo Health 14:713–732. https://doi.org/10.1007/s12403-021-00446-0
Howard G, Ahmed MF, Shamsuddin AF, Mahmud SG, Deere D (2006) Risk assessment of arsenic mitigation options in Bangladesh. J Health Popul Nutr 24:346–355
Huang B, Li Z, Chen Z, Chen G, Zhang C, Huang J, Nie X, Xiong W, Zeng G (2015) Study and health risk assessment of the occurrence of iron and manganese in groundwater at the terminal of the Xiangjiang River. Environ Sci Pollut Res 22(24):19912–19921
Huang G, SunJ, Zhang Y, Che Z, Liu F (2013) Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Sci Total Environ 463–464:209–221
IPCC (2007) Summary for policymakers. In: Climate Change 2007. Synthesis Report. represents the formally agreed statement of the IPCC concerning key findings and uncertainties contained in the Working Group contributions to the Fourth Assessment Report. Valencia, Spain, 12–17 November 2007. https://www.ipcc.ch/site/assets/uploads/2018/02/ar4_syr_spm.pdf. Accessed 1 Jul 2019
Islam MA, Majlis ABK, Rashid MB (2011) Changing face of Bangladesh coast. J NOAMI 28(1):1–17
Islam MS, Han S, Ahmed MK, Masunaga S (2014) Assessment of trace metal contamination in water and sediment of some rivers in Bangladesh. J Water Environ Technol 12(2):109–121. https://doi.org/10.2965/jwet.2014.109
Islam MA, Mitra D, Dewan A, Akhter SH (2016) Coastal multi-hazard vulnerability assessment along the Ganges deltaic coast of Bangladesh–a geospatial approach. Ocean Coast Manag 127:1–15
Islam MA, Zahid A, Rahman MM, Rahman MS, Islam MJ, Akter Y, Shammi M, Bodrud-Doza M, Roy B (2017a) Investigation of groundwater quality and its suitability for drinking and agricultural use in the south-central part of the coastal region in Bangladesh. Exposure Health 9(1):27–41
Islam SMD, Majumder RK, Uddin MJ, Khalil MI, Alam MF (2017b) Hydrochemical characteristics and quality assessment of groundwater in Patuakhali district, southern coastal region of Bangladesh. Expo Health 9(1):43–60. https://doi.org/10.1007/s12403-016-0221-y
Islam AT, Shen S, Haque MA, Bodrud-Doza M, Maw KW, Habib MA (2018) Assessing ground water quality and its sustainability in Joypurhat district of Bangladesh using GIS and multivariate statistical approaches. Environ Dev Sustainability 20(5):1935–1959. https://doi.org/10.1007/s10661-020-8195-4
Islam ME, Reza AHMS, Sattar GS, Ahsan MA, Akbor MA, Siddique MAB (2019) Distribution of arsenic in core sediments and groundwater in the Chapainawabganj district, Bangladesh. Arab J Geosci 12(3):99. https://doi.org/10.1007/s12517-019-4272-9
Islam ARMT, Hasanuzzaman M, Islam HMT, Mia MU, Khan R, Habib MA, Rahman MM, Siddique MAB, Moniruzzama M, Rashid MB (2020) Quantifying source apportionment, co-occurrence and ecotoxicological risk of metals from up-mid-downstream river segments, Bangladesh. Environ Toxicol Chem. https://doi.org/10.1002/etc.4814
Jigyasu DK, Singh M, Singh S, Singh S, Singh IB (2020) Trace element mobility, regional significance and global implication of Gomati river basin, northern India. SN Appl Sci 2(8):1–13. https://doi.org/10.1007/s42452-020-03204-0
Kabir H, Golder J (2017) Rainfall variability and its impact on crop agriculture in southwest region of Bangladesh. Climatol Weather Forecast 5:1. https://doi.org/10.4172/2332-2594.1000196
Karim MR (2010) Quality and suitability of harvested rainwater for drinking in Bangladesh. Water Sci Technol: Water Suppl 10:359–366
Kelley WP (1963) Use of saline irrigation water. Soil Sci 95:385–391
Khan A, Ireson A, Kovats S, Mojumder S, Khusru A, Rahman A, Vineis P (2011) Drinking water salinity and maternal health in coastal Bangladesh: implications of climate change. Environ Health Perspect 119:1328–1332
Khan SR (2001) Geomorphic and geologic characteristics of the coastal plains of Bangladesh. In: Proceedings of the International Seminar on- Quaternary Developments and Coastal Hydrodynamics of the Ganges Delta in Bangladesh. Printsasia, Bangladesh, pp 240–245
Kim Y, Kong I, Park H, Kim HJ, Kim IJ, Um MJ, Green PA, Vörösmarty CJ (2018) Assessment of regional threats to human water security adopting the global framework: a case study in South Korea. Sci Total Environ 637–638:1413–1422. https://doi.org/10.1016/j.scitotenv.2018.04.420
Kormoker T, Idris AM, Khan MM, Tusher TR, Proshad R, Islam MS, Khadka S, Rahman S, Kabir MH, Kundu S (2020) Spatial distribution, multivariate statistical analysis, and health risk assessment of some parameters controlling drinking water quality at selected primary schools located in the southwestern coastal region of Bangladesh. Toxin Rev. https://doi.org/10.1080/15569543.2020.1866012
Kumar PS, Jegathambal P, James EJ (2014) Chemometric evaluation of nitrate contamination in the groundwater of a hard rock area in Dharapuram, south India. Appl Water Sci 4:397–405
Lam Y, Winch PJ, Nizame FA, Broaddus-Shea ET, Harun M, Dostogir G et al (2021) Salinity and food security in southwest coastal Bangladesh: impacts on household food production and strategies for adaptation. Food Sec 14:229–248. https://doi.org/10.1007/s12571-021-01177-5
Livens FR (1991) Chemical reactions of metals with humic material. Environ Pollut 70:183–208
Macomber PSH (2001) Guidelines on rainwater catchment systems for Hawaii. In: College of Tropical Agriculture and Human Resources, University of Hawaii, Manoa, Publication no RM-12
Magyar MI, Mitchell VG, Ladson AR, Diaper C (2008) Lead and other heavy metals common contaminants of rainwater tanks in Melbourne. In: Proceedings of Water Down Under 2008, Adelaide, Australia. Engineers Australia: Causal Productions, Modbury, S. Aust., pp 409–417
Martinez-Tavera E, Rodriguez-Espinosa PF, Shruti VC, Sujitha SB, Morales-Garcia SS, Muñoz-Sevilla NP (2017) Monitoring the seasonal dynamics of physicochemical parameters from Atoyac River basin (Puebla), Central Mexico: multivariate approach. Environ Earth Sci 76(2):95. https://doi.org/10.1007/s12665-017-6406-2
Miller RL, Bradford WL, Peters NE (1988) Specific conductance: theoretical considerations and application to analytical quality control, In USGS Water-Supply Paper, http://pubs.usgs.gov/wsp/2311/report, pdf
Ministry of Environment and Forest (2006) Coastal land zoning in the southwest: report on ‘‘impact of sea level rise on land use suitability and adaptation options. Ministry of Environment and Forest, Dhaka, Bangladesh
Mohamed C, Zineb A (2015) Geochemistry and hydrogeochemical process of groundwater in the Souf valley of Low Septentrional Sahara, Algeria. Afr J Environ Sci Technol 9(3):261–273. https://doi.org/10.5897/AJEST2014.1710
Murshed SB, Rahman MR, Kaluarachchi JJ (2019) Changes in hydrology of the Ganges delta of Bangladesh and corresponding impacts on water resources. J Am Water Resour Assoc 55(4):800–823. https://doi.org/10.1111/1752-1688.12775
Nasher NMR, Ahmed MH (2021) Groundwater geochemistry and hydrogeochemical processes in the Lower Ganges-Brahmaputra-Meghna River Basin areas, Bangladesh. J Asian Earth Sci: X 6(2021):100062. https://doi.org/10.1016/j.jaesx.2021.100062
Nguyen BT, Nguyen TMT, Bach QV (2020) Assessment of groundwater quality based on principal component analysis and pollution source-based examination: a case study in Ho Chi Minh City, Vietnam. Environ Monit Assess 192(6):395–395
Nishida C, Uauy R, Kumanyika S, Shetty P (2004) The joint WHO/ FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr 7(1A):245–250. https://doi.org/10.1079/PHN2003592
Paliwal KV (1967) Effect of gypsum application on the quality of irrigation water. Madras Agric J 59:646–647
Paul R, Brindha K, Gowrisankar G, Tan ML, Singh MK (2019) Identification of hydrogeochemical processes controlling groundwater quality in Tripura, Northeast India using evaluation indices, GIS and multivariate statistical methods. Environ Earth Sci 78(15):470
Penning-Rowsell EC, Sultana P, Thompson PM (2013) The “last resort”? Population movement in response to climate-related hazards in Bangladesh. Environ Sci Policy 27:1–16. https://doi.org/10.1016/j.envsci.2012.03.009
Pethick J, Orford JD (2013) Rapid rise in effective sea-level in southwest Bangladesh: its causes and contemporary rates. Global Planet Change 111:237–245. https://doi.org/10.1016/j.gloplacha.2013.09.019
Raghunath IIM (1987) Groundwater.2nd edition; Wiley Eastern Ltd., New Delhi, India
Rahman MM, Islam MA, Bodrud-Doza M, Muhib MI, Zahid A, Shammi M, Tareq SM, Kurasaki M (2017) Spatiotemporal assessment of groundwater quality and human health risk: a case study in Gopalganj, Bangladesh. Expo Health 10(3):167–188
Rahman M, Khan MR, Ahmed KM, Zahid A, Siddique MAM (2018) An assessment of nitrate concentration in the groundwater of the central coastal region of Bangladesh. J NOAMI 35(1–2):107–116
Rahman M, Al Noor M, Tushar Zahid A, Mustafa MG, Siddique MAM, Ahmed KM (2021a) Spatial distribution of manganese in groundwater and associated human health risk in the southern part of the Bengal Basin. Environ Sci Pollut Res 28:41061–41070. https://doi.org/10.1007/s11356-021-13577-3
Rahman M, Tushar MAN, Zahid A, Ahmed KMU, Siddique MAM, Mustafa MG (2021b) Spatiotemporal distribution of boron in the groundwater and human health risk assessment from the coastal region of Bangladesh. Environ Sci Pollut Res 28:21964–21977. https://doi.org/10.1007/s11356-020-11682-3
Rahman MA, Siddique MAB, Khan R, Reza AHMS, Khan AHAN, Akbor MA, Islam MS, Hasan AB, Hasan MI, Elius IB (2022) Mechanism of arsenic enrichment and mobilization in groundwater from southeastern Bangladesh: water quality and preliminary health risks assessment. Chemosphere 294:133556. https://doi.org/10.1016/j.chemosphere.2022.133556
Rakib MA, Sasaki J, Matsuda H, Fukunaga M (2019) Severe salinity contamination in drinking water and associated human health hazards increase migration risk in the southwestern coastal part of Bangladesh. J Environ Manage 240:238–248. https://doi.org/10.1016/j.jenvman.2019.03.101
Raknuzzaman M, Ahmed MK, Islam MS, Habibullah-Al-Mamun M, Tokumura M, Sekine M, Masunaga S (2016) Assessment of trace metals in surface water and sediment collected from polluted coastal areas of Bangladesh. J Water Environ Technol 14(4):247–259. https://doi.org/10.2965/jwet.15-038
Rashid MB (2023) Monitoring of drainage system and waterlogging area in the human-induced Ganges-Brahmaputra tidal delta plain of Bangladesh using MNDWI index. Heliyon 9(6):e17412. https://doi.org/10.1016/j.heliyon.2023.e17412
Rashid MB, Mahmud A (2011) Longshore Currents and Its Effect on Kuakata Beach, Bangladesh. Bangladesh J Geol 29–30:30–40
Rashid MB, Mahmud A, Ahsan MK, Khasru MH, Islam MA (2013) Drainage congestion and its impact on environment in the south-western coastal part of Bangladesh. Bangladesh J Geol 31–32:43–55
Rashid MB, Mahmud A, Ahsan MK, Khasru MH, Ahsan K, Habib MA, Hossain MA, Alam MF (2014) Role of major rivers for the development of Ganges-Brahmaputra Delta. Int J Econ Environ Geol 5(1):25–32. https://doi.org/10.46660/ijeeg.Vol0.Iss0.0.108
Rashid MB, Sheikh MR, Haque AE, Patwary MAA (2022a) Climate change impact and frequency of cyclone surge in bangladesh coast: proper plan to address sustainably. Int J Econ Environ Geol 13(2):15–20. https://doi.org/10.46660/ijeeg.v13i2.25
Rashid MB, Ahsan K, Majlis ABK, Ahsan MK, Mahmud A (2022b) Sedimentation and coastal area management in the human-modified Ganges–Brahmaputra tidal delta plain of Bangladesh. Int J River Basin Manag 1–13. https://doi.org/10.1080/15715124.2022.2092489
Rashid MB, Sheik MR, Haque AJME, Siddique MAB, Habib MA, Patwary MAA (2023) Salinity-induced change in green vegetation and land use patterns using remote sensing, NDVI, and GIS techniques: a case study on the southwestern coast of Bangladesh. Case Stud Chem Environ Eng 7:100314. https://doi.org/10.1016/j.cscee.2023.100314
Rawat KS, Singh SK, Gautam SK (2018) Assessment of groundwater quality for irrigation use: a peninsular case study. Appl Water Sci 8:233. https://doi.org/10.1007/s13201-018-0866-8
Richard LA (US Salinity Laboratory) (1954) Diagnosis and improvement of saline and alkaline soils. US Department of Agriculture handbook (p. 60)
Saha N, Rahman MS, Ahmed MB, Zhou JL, Ngo HH, Guo W (2017) Industrial metal pollution in water and probabilistic assessment of human health risk. J Environ Manage 185:70–78
Saha R, Dey NC, Rahman S, Galagedara L, Bhattacharya P (2018) Exploring suitable sites for installing safe drinking water wells in coastal Bangladesh. Groundw Sustain Dev 7:91–100. https://doi.org/10.1016/j.gsd.2018.03.002
Salehin M, Chowdhury M, Arefin M, Clarke D, Mondal S, Nowreen S et al (2018) Mechanisms and drivers of soil salinity in coastal Bangladesh. Ecosystem services for well-being in Deltas: integrated assessment for policy analysis. Palgrave Macmillan, Cham, pp 333–347
Sarkar R, Vogt J (2015) Drinking water vulnerability in rural coastal areas of Bangladesh during and after natural extreme events. Int J Disaster Risk Red 14:411–423. https://doi.org/10.1016/j.ijdrr.2015.09.007
SDWF (Safe Drinking Water Foundation) (2018) Water and human health. https://www.safewater.org/fact-sheets-1/2017/1/23/water-and-human-health, Accessed date: November 19, 2019
Siddique MAB, Alam MK, Islam S, Diganta MTM, Akbor MA, Bithi UH, Chowdhury AI, Ullah AKMA (2020) Apportionment of some chemical elements in soils around the coal mining area in northern Bangladesh and associated health risk assessment. Environ Nanotechnol Monit Manage 14:100366. https://doi.org/10.1016/j.enmm.2020.100366
Siddique MAB, Khan R, Islam ARMT, Alam MK, Islam MS, Hossain MS, Habib MA, Akbor MA, Bithi UH, Rashid MB, Hossain M, Rahman IMM, Elius IB, Islam MS (2021) Quality assessment of freshwaters from a coastal city of southern Bangladesh: irrigation feasibility and preliminary health risks appraisal. Environ Nanotechnol Monit Manage 16:100524. https://doi.org/10.1016/j.enmm.2021.100524
Siddique MAB, Islam ARMT, Hossain MS, Khan R, Akbor MA, Hasanuzzaman M, Sajid MWM, Mia MY, Mallick J, Rahman MS, Rahman MM, Bodrud-Doza M (2022) Multivariate statistics and entropy theory for irrigation water quality and entropy-weighted index development in a subtropical urban river, Bangladesh. Environ Sci Pollut Res 29:8577–8596. https://doi.org/10.1007/s11356-021-16343-7
Simmons G, Hope V, Lewis G, Whitmore J, Gao W (2001) Contamination of roof-collected rainwater in Auckland, New Zealand. Water Res 35:1518–1524
Sojobi AO (2016) Evaluation of groundwater quality in a rural community in North Central of Nigeria. Environ Monit Assess 188:192. https://doi.org/10.1007/s10661-016-5149-y
Stevens RG (1990) Iron and the risk of cancer. Med Oncol Tumor Pharmacother 7:177–181
Sun Y, Bricheno LM, Payo-Payo M, Rahman MM, Burns NM (2022) Simulation of freshwater transport network and salt flux in the Bangladesh delta. Estuarine Coastal Shelf Sci. https://doi.org/10.1016/j.ecss.2022.107839
Talukder MRR, Rutherford S, Phung D, Islam MZ, Chu C (2016) The effect of drinking water salinity on blood pressure in young adults of coastal Bangladesh. Environ Pollut 214:248–254. https://doi.org/10.1016/j.envpol.2016.03.074
Todd DK (1980) Groundwater hydrology. Wiley International Edition, John Wiley and Sons. Inc., New York
Uba BN, Aghogho O (2000) Rainwater quality from different roof catchment in the Port Harcourt District, Rivers State, Nigeria. J Water Suppl: Res Technol AQUA 49:281–288
USEPA (US Environmental Protection Agency) (1989) Risk assessment guidance for superfund, vol. I: human health evaluation manual (Part A). US Environmental Protection Agency, Office of Emergency and Remedial Response, EPA 540/1–89/ 002, Washington, DC
USEPA (US Environmental Protection Agency) (1992) Guidelines for exposure assessment. USEPA 600Z-92/001, 29 May 1992, US Environmental Protection Agency, Risk Assessment Forum, Washington, DC
USEPA (2004) US Environmental Protection Agency. Risk assessment guidance for superfund volume I: human health evaluation manual (part E, supplemental guidance for dermal risk assessment) final. EPA/540/R/99/005 OSWER 9285.7–02EP PB99–963312 July 2004, Office of Superfund Remediation and Technology Innovation
Venkatramanan S, Chung SY, Selvam S, Lee SY, Elzain HE (2017) Factors controlling groundwater quality in the Yeonjegu District of Busan City, Korea, using the hydrogeochemical processes and fuzzy GIS. Environ Sci Pollut Res 24(30):23679–23693. https://doi.org/10.1007/s11356-017-9990-5
Vineis P, Chan Q, Khan A (2011) Climate change impacts on water salinity and health. J Epid Global Health 1(1):5–10. https://doi.org/10.1016/j.jegh.2011.09.001
Vu CT, Lin C, Shern CC, Yeh G, Tran HT (2017) Contamination, ecological risk and source apportionment of heavy metals in sediments and water of a contaminated river in Taiwan. Ecol Indic 82:32–42. https://doi.org/10.1016/j.ecolind.2017.06.008
Wang J, Liu G, Liu H, Lam PKS (2017) Multivariate statistical evaluation of dissolved trace elements and a water quality assessment in the middle reaches of Huaihe River, Anhui, China. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.01.088
Ward S, Memon FA, Butter D (2010) Harvested rainwater quality: the importance of appropriate design. Water Sci Technol 61:1707–1714
Wasserman G, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, An Geen A, Slavkovich V, Lolacono N, Cheng Z, Zheng Y, Graziano J (2006) Water manganese exposure and children’s intellectual functions in Araihazar, Bangladesh. Environ Health Perspect 114:124–129
WHO (1996) Guidelines for drinking-water quality-health criteria and other supporting information. WHO, Geneva
WHO (2011) In: Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
WHO and UNICEF (2017) Progress on drinking water, sanitation and hygiene: update and SDG baselines. Licence, Geneva. CC BY-NC-SA 3.0 IGO
WHO (2004) Copper in drinking-water background document for development of WHO Guidelines for Drinking-water Quality, https://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf
Wu B, Zhao DY, Jia HY, Zhang Y, Zhang XX, Cheng SP (2009) Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing section, China. Bull Environ Contam Toxicol 82:405–409. https://doi.org/10.1007/s00128-008-9497-3
Xiao Y, Yin S, Hao Q, Gu X, Pei Q, Zhang Y (2020) Hydrogeochemical appraisal of groundwater quality and health risk in a near-suburb area of North China. J Water Supply Res Technol Aqua 69(1):55–69. https://doi.org/10.2166/aqua.2019.101
Yang CY, Chang CC, Tsai SS, Chiu HF (2006) Calcium and magnesium in drinking water and risk of death from acute myocardial infarction in Taiwan. Environ Res 101:407–411
Zahid A, Hassan MQ, Balke KD, Flegr M, Clark DW (2008) Groundwater chemistry and occurrence of arsenic in the Meghna floodplain aquifer, southeastern Bangladesh. Environ Geol 54:1247–1260. https://doi.org/10.1007/s00254-007-0907-3
Zahid A, Hassan MQ, Breit GN, Balke KD, Flegr M (2009) Accumulation of iron and arsenic in the Chandina alluvium of the lower delta plain, Southeastern Bangladesh. Environ Geochem Health 31(Suppl. 1):69–84. https://doi.org/10.1007/s10653-008-9226-1
Zeinalzadeh K, Rezaei E (2017) Determining spatial and temporal changes of surface water quality using principal component analysis. J Hydrol: Reg Stud 13:1–10. https://doi.org/10.1016/j.ejrh.2017.07.002
Acknowledgements
The authors would like to express their deep gratitude and cordial thanks to Mohammad Elius Hossain, Director General, Geological Survey of Bangladesh (GSB), for allowing carrying out the work. The authors are grateful to the authority of the Institute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific, and Industrial Research (BCSIR), Dhaka, Bangladesh, for providing analytical laboratory facilities. Many local people helped the authors by giving a lot of valuable information. So, the authors also acknowledged their kind support with deep satisfaction.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through small group Research Project under grant number RGP1/120/44.
Author information
Authors and Affiliations
Contributions
Md Bazlar Rashid, Md Abu Bakar Siddique, and Rahat Khan: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation, writing—reviewing and editing, software, and visualization. Md Ahosan Habib and Md Saiful Islam: methodology, investigation, and writing—reviewing and editing. Md Ahedul Akbor: investigation and resources. A. J. M. Emdadul Haque, Md Rubel Sheik, Mohammad Alamgir Kabir, and Md Nuruddin Sarker: methodology, formal analysis, and investigation. Abubakr M Idris: writing—reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: V.V.S.S. Sarma
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rashid, M.B., Siddique, M.A.B., Khan, R. et al. Human exposures to multiple water sources in the southwestern coastal region of Bangladesh: water quality, pollution sources, and preliminary health risks appraisals. Environ Sci Pollut Res 30, 88132–88154 (2023). https://doi.org/10.1007/s11356-023-28108-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28108-5