Abstract
Exercise results in increased left ventricular contractility in normal individuals. Similar changes can also be seen with vasodilator stress. This article discusses the physiologic basis of these changes as well as reviews the clinical data supporting the use of these parameters for diagnostic and prognostic evaluation. Methodologic limitations as well as other concomitant pathologic processes which may confound interpretation of stress-induced changes in LVEF are also reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The concept of an ischemic cascade has been well refined and validated in the medical literature (Figure 1). In this model, abnormalities of myocardial perfusion trigger a series of downstream changes, both adaptive and maladaptive. Metabolic changes such as a shift towards glycolysis, lactate production, and increased adenosine are followed by relaxation abnormalities and subsequently systolic dysfunction.1,2 Electrocardiographic changes, symptoms, and tissue infarction generally occur later in the cascade.
Illustration of ischemic cascade model in which increasing severity and duration of ischemia arising from abnormalities of myocardial perfusion lead in turn to disturbances in myocardial metabolism, altered myocardial relaxation, decreased myocardial contractility, electrocardiographic repolarization abnormalities, clinical symptoms, and eventually myocardial infarction. Adapted from Farhad and Murthy.10
Supporting this model, exercise-induced deterioration of global left ventricular function was documented during the 1970s in patients with coronary artery disease (CAD) using contrast ventriculography.3,4 Within a few years, these results were replicated non-invasively with radionuclide cineangiography.5 The diagnostic6,7 and prognostic8 value of this finding was validated in the early 1980s.
Mechanisms for increased global systolic function with stress
Exercise increases demand for oxygen and nutrients in skeletal muscle, through a variety of mechanisms including increased sympathetic tone, vagal withdrawal, and nitric oxide- and metabolite-mediated pathways.9 These collectively cause coronary vasodilation as well as positive chronotropy and inotropy, all resulting in increased cardiac output due predominantly to increased heart rate but also by increased ejection fraction. Dobutamine, a sympathomimetic agent, recapitulates many of these effects, although perhaps less completely than exercise.10
All three currently used vasodilators (i.e., dipyridamole, adenosine, and regadenoson) also increase ejection fraction with stress in individuals without coronary artery disease.11,12,13,14,15 There are several possible mechanisms for this. First, adenosine results increased venous return (i.e., preload) causing increased contractility via the Frank-Starling mechanism.12 Second, increased coronary perfusion itself increases myocardial contractility16 via the Gregg mechanism.17 Third, regadenoson, and presumably the other agents, increases heart rate in a manner which can be abrogated by hexamethonium ganglionic blockade in rats despite decreased blood pressure, indicating sympathetic activation beyond simple baroreflex response.18 Further, regadenoson administration nearly doubled plasma norepinephrine levels. This sympathetic activation is likely to also contribute to increased contractility. Failure to augment left ventricular ejection fraction (LVEF) in response to the inotropic effects of sympathetic drive during acute vasodilator stress may reflect advanced coronary disease and/or other serious abnormalities.
Global systolic function in non-nuclear stress testing
Although the initial application of peak stress systolic function was performed using nuclear techniques in the 1970s, evaluation of regional and global systolic function in response to exercise now forms the basis of stress echocardiography Evaluation of contractile response using echocardiographic imaging in response to dipyridamole stress has been well validated, with numerous diagnostic and prognostic studies.19,20 Importantly, the higher specificity of vasodilator stress echocardiography compared to dobutamine may reflect a higher threshold in the extent and/or severity of coronary disease required to induce contractile abnormalities in response to vasodilators.19
In clinical practice, stress cardiac magnetic resonance imaging is generally performed by evaluating first-pass contrast enhancement of the myocardium under vasodilator stress. However, contractile function increases acutely with vasodilator administration13 and failure to increase global and regional function has diagnostic and prognostic value.21,22,23
Systolic function during stress in cardiac PET
Interest in stress-induced changes in LVEF among the nuclear cardiology community was renewed after it was observed in an analysis of 510 patients referred for 82Rb rest/stress PET and coronary angiography that LVEF reserve in response to adenosine or dipyridamole was a strong independent predictor of left main or 3-vessel CAD.24 An LVEF reserve of >5% had a negative predictive value of 97% for severe left main or 3-vessel CAD. Conversely, an LVEF <−5% had a positive predictive value of 77%. Similarly, regadenoson stress LVEF reserve was significantly greater in individuals with normal compared to mild, or moderately to severely abnormal myocardial perfusion imaging.14 A follow-up analysis of 1432 patients undergoing vasodilator rest/stress 82Rb PET demonstrated that LVEF reserve <0 compared to ≥0 was associated with a much higher rate of cardiac events (5.3%/year vs 2.1%/year, P < .001) and all-cause mortality (9.2%/year vs 4.3%/year, P < .001). Even after adjusting for clinical, rest LVEF, and rest and stress perfusion findings, LVEF was an independent predictor of cardiac events (hazard ratio 0.79/5% increase, P = .03).
Early post-stress systolic function with SPECT
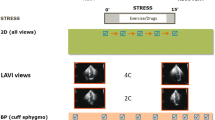
An international multi-center trial has established that early post-stress imaging at 15 minutes post-exercise leads to equivalent image quality compared to delayed imaging at 60 minutes with greater detection of post-ischemic stunning as manifested by decreased post-stress LVEF.25 Although early post-stress imaging with vasodilator SPECT is feasible,26 it is not commonly done in practice. Further, only a few studies have evaluated the clinical utility of post-stress LVEF with SPECT, but have generally found that post-stress stunning in the form of decreased LVEF is an important predictor of events.27,28,29 However, when stress imaging is done late after vasodilator administration, after the effects are likely to have worn off, the diagnostic and prognostic value are likely to be attenuated.30
Important limitations and caveats
Importantly, stress echocardiography has enabled the evaluation of the time course of stress-induced wall motion abnormalities.31 Although these are generally relatively transient, they may persist for as long as 25 minutes after termination of dobutamine stress in patients with three-vessel coronary disease. However, using cardiac MRI global increases in LVEF in response to adenosine generally resolved within 3-4 minutes after adenosine infusion.32 This underscores the greater importance of imaging during or immediately after stress in order to capture vasodilator-induced contractile abnormalities. Unfortunately, most vasodilator SPECT images are obtained at approximately 40-60 minutes post stress, when ischemia-induced changes in global and regional function should almost certainly have resolved.30 While the value of early SPECT imaging has been established for patients undergoing exercise stress testing,25 early imaging is not widely used with vasodilator stress but is potentially feasible.26 Further, because many laboratories collect images over longer periods of up to 20 minutes or longer when using 13N-ammonia, underestimation of LVEF reserve compared to protocols reconstructing gated images from the first 3-5 minutes of imaging is likely.
Arrhythmias may also have a significant impact on observed contractile reserve. Generally, vasodilators result in increased heart rate, although response is somewhat variable and is itself maybe related to prognosis.33 Either resolution of arrhythmia, as might occur in some patients with resting premature ventricular contractions, or induced arrhythmias during stress could change average global LVEF without directly reflecting underlying ischemia. Along these lines, errors in gating due to incorrect placement of R-R acceptance window or inappropriate triggering based on T-waves or artifacts need to be excluded as part of routine quality control.
Although the implications for vasodilator stress are not known, changes in LVEF with exercise may not be specific for obstructive CAD. Increased afterload, aging, or valve disease may attenuate LVEF responses to exercise. Mental stress induced a decline in LVEF via an increase in afterload, in middle-aged men and women without evidence of coronary artery disease.34 Increasing age may attenuate the changes in LVEF with exercise. In a study of 77 asymptomatic volunteers (ages 20-95 years), the change in LVEF from rest to upright bicycle exercise was inversely related to age (R = −.71, P < .001).35 Chronic regurgitation of the mitral or aortic valves may also decrease LVEF with exercise and portends adverse prognosis.36
Finally, the implications of failure to augment LVEF or even modest decreases in LVEF in small ventricles remain unclear. Given spatial resolution limitations of nuclear methodologies, it is unlikely that a decline in LVEF from 70% to 65% in a small ventricle, as is common in smaller women, is as concerning as a drop from 55% to 45% in a normal-sized ventricle.
Conclusions
The concept of vasodilator-induced changes in LVEF is well grounded in theoretical, animal, and human data including clinical validation for diagnostic and prognostic assessment. However, reproduction of clinical validation in larger cohorts in multiple centers would be an important future direction.
References
Kassiotis C, Rajabi M, Taegtmeyer H. Metabolic reserve of the heart: the forgotten link between contraction and coronary flow. Prog Cardiovasc Dis 2008;51(1):74–88.
Taegtmeyer H. Tracing cardiac metabolism in vivo: one substrate at a time. J Nucl Med 2010;1(51 Suppl 1):80S–7S.
Dwyer EM. Left ventricular pressure-volume alterations and regional disorders of contraction during myocardial ischemia induced by atrial pacing. Circulation 1970;42(6):1111–22.
Sharma B, Goodwin JF, Raphael MJ, Steiner RE, Rainbow RG, Taylor SH. Left ventricular angiography on exercise. A new method of assessing left ventricular function in ischaemic heart disease. Br Heart J 1976;38(1):59–70.
Borer JS, Bacharach SL, Green MV, Kent KM, Epstein SE, Johnston GS, et al. Real-time radionuclide cineangiography in the noninvasive evaluation of global and regional left ventricular function at rest and during exercise in patients with coronary-artery disease. N Engl J Med 1977;296(15):839–44.
Jones RH, McEwan P, Newman GE, Port S, Rerych SK, Scholz PM, et al. Accuracy of diagnosis of coronary artery disease by radionuclide management of left ventricular function during rest and exercise. Circulation 1981;64(3):586–601.
Dehmer GJ, Lewis SE, Hillis LD, Corbett J, Parkey RW, Willerson JT. Exercise-induced alterations in left ventricular volumes and the pressure-volume relationship: A sensitive indicator of left ventricular dysfunction in patients with coronary artery disease. Circulation 1981;63(5):1008–18.
Corbett JR, Dehmer GJ, Lewis SE, Woodward W, Henderson E, Parkey RW, et al. The prognostic value of submaximal exercise testing with radionuclide ventriculography before hospital discharge in patients with recent myocardial infarction. Circulation 1981;64(3):535–44.
Farhad H, Murthy VL. Pharmacologic manipulation of coronary vascular physiology for the evaluation of coronary artery disease. Pharmacol Ther [Internet]. 2013. Available from: http://www.sciencedirect.com/science/article/pii/S0163725813001307
Attenhofer CH, Pellikka PA, Oh JK, Roger VL, Sohn DW, Seward JB. Comparison of ischemic response during exercise and dobutamine echocardiography in patients with left main coronary artery disease. J Am Coll Cardiol 1996;27(5):1171–7.
Cates CU, Kronenberg MW, Collins HW, Sandler MP. Dipyridamole radionuclide ventriculography: A test with high specificity for severe coronary artery disease. J Am Coll Cardiol 1989;13(4):841–51.
Nussbacher A, Ariê S, Kalil R, Horta P, Feldman MD, Bellotti G, et al. Mechanism of adenosine-induced elevation of pulmonary capillary wedge pressure in humans. Circulation 1995;92(3):371–9.
Kawel N, Santini F, Haas T, Zellweger MJ, Streefkerk HJ, Bremerich J. Normal response of cardiac flow and function to adenosine stress as assessed by cardiac MR. J Cardiovasc Med (Hagerstown). 2012;13(11):720–6.
Hsiao E, Ali B, Blankstein R, Skali H, Ali T, Bruyere J, et al. Detection of obstructive coronary artery disease using regadenoson stress and 82Rb PET/CT myocardial perfusion imaging. J Nucl Med. 2013;54(10):1748–54.
Ogilby JD, Iskandrian AS, Untereker WJ, Heo J, Nguyen TN, Mercuro J. Effect of intravenous adenosine infusion on myocardial perfusion and function. Hemodynamic/angiographic and scintigraphic study. Circulation 1992;86(3):887–95.
Iwamoto T, Bai XJ, Downey HF. Coronary perfusion related changes in myocardial contractile force and systolic ventricular stiffness. Cardiovasc Res 1994;28(9):1331–6.
Gregg DE. Effect of coronary perfusion pressure or coronary flow on oxygen usage of the myocardium. Circ Res 1963;13(6):497–500.
Dhalla AK, Wong M-Y, Wang W-Q, Biaggioni I, Belardinelli L. Tachycardia caused by A2A adenosine receptor agonists is mediated by direct sympathoexcitation in awake rats. J Pharmacol Exp Ther. 2006;316(2):695–702.
Kim C, Kwok YS, Heagerty P, Redberg R. Pharmacologic stress testing for coronary disease diagnosis: A meta-analysis. Am Heart J. 2001;142(6):934–44.
Cortigiani L, Bigi R, Landi P, Bovenzi F, Picano E, Sicari R. Prognostic implication of stress echocardiography in 6214 hypertensive and 5328 normotensive patients. Eur Heart J. 2011;32(12):1509–18.
Bodi V, Sanchis J, Lopez-Lereu MP, Nunez J, Mainar L, Monmeneu JV, et al. Prognostic and therapeutic implications of dipyridamole stress cardiovascular magnetic resonance on the basis of the ischaemic cascade. Heart. 2009;95(1):49–55.
Hojjati MR, Muthupillai R, Wilson JM, Preventza OA, Cheong BYC. Assessment of perfusion and wall-motion abnormalities and transient ischemic dilation in regadenoson stress cardiac magnetic resonance perfusion imaging. Int J Cardiovasc Imaging. 2014;30(5):949–57.
Thomas D, Strach K, Meyer C, Naehle CP, Schaare S, Wasmann S, et al. Combined myocardial stress perfusion imaging and myocardial stress tagging for detection of coronary artery disease at 3 Tesla. J Cardiovasc Magn Reson. 2008;18(10):59.
Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: A 82Rb PET/CT study. J Nucl Med. 2007;48(3):349–58.
Mut F, Giubbini R, Vitola J, Lusa L, Sobic-Saranovic D, Peix A, et al. Detection of post-exercise stunning by early gated SPECT myocardial perfusion imaging: Results from the IAEA multi-center study. J Nucl Cardiol. 2014;21(6):1168–76.
Brodov Y, Fish M, Rubeaux M, Otaki Y, Gransar H, Lemley M, et al. Quantitation of left ventricular ejection fraction reserve from early gated regadenoson stress Tc-99 m high-efficiency SPECT. J Nucl Cardiol. 2016;23(6):1251–61.
Carvalho PA, Aguiar PM, Grossman GB, Moraes JF, Baptista IS, Hirakata VN, et al. Prognostic implications of the difference between left ventricular ejection fractions after stress and at rest in addition to the quantification of myocardial perfusion abnormalities obtained with gated SPECT. Clin Nucl Med. 2012;37(8):748–54.
Dona M, Massi L, Settimo L, Bartolini M, Giannì G, Pupi A, et al. Prognostic implications of post-stress ejection fraction decrease detected by gated SPECT in the absence of stress-induced perfusion abnormalities. Eur J Nucl Med Mol Imaging. 2011;38(3):485–90.
Kato M, Matsumoto N, Nakano Y, Suzuki Y, Yoda S, Sato Y, et al. Combined assessment of myocardial perfusion and function by ECG-gated myocardial perfusion single-photon emission computed tomography for the prediction of future cardiac events in patients with type 2 diabetes mellitus. Circ J. 2011;75(2):376–82.
Gomez J, Golzar Y, Fughhi I, Olusanya A, Doukky R. The significance of post-stress decrease in left ventricular ejection fraction in patients undergoing regadenoson stress gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2017. doi:10.1007/s12350-017-0802-6.
Tsoukas A, Ikonomidis I, Cokkinos P, Nihoyannopoulos P. Significance of persistent left ventricular dysfunction during recovery after dobutamine stress echocardiography. J Am Coll Cardiol 1997;30(3):621–6.
Sievers B, Jacobi L, Sommer P, Speiser U, Schoen S, Strasser RH. Influence of adenosine on ventricular function measurements as part of a comprehensive stress perfusion magnetic resonance imaging study. Acta Radiol. 2011;52(6):624–31.
Bellam N, Veledar E, Dorbala S, Di Carli MF, Shah S, Eapen D, et al. Prognostic significance of impaired chronotropic response to pharmacologic stress Rb-82 PET. J Nucl Cardiol. 2014;21(2):233–44.
Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, et al. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) Study. Circulation 1996;94(11):2768–77.
Port S, Cobb FR, Coleman RE, Jones RH. Effect of age on the response of the left ventricular ejection fraction to exercise. N Engl J Med 1980;303(20):1133–7.
Tamás E, Broqvist M, Olsson E, Franzén S, Nylander E. Exercise radionuclide ventriculography for predicting post-operative left ventricular function in chronic aortic regurgitation. JACC Cardiovasc Imaging 2009;2(1):48–55.
Disclosures
Dr. Murthy has received speaker fees from Bracco Diagnostics and Ionetix and also received monetary and non-monetary research support from INVIA Medical Imaging Solutions. Dr. Dorbala has received research Grant from Astellas Global Pharma Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murthy, V.L., Dorbala, S. Clinical value of hyperemic left ventricular systolic function in vasodilator stress testing. J. Nucl. Cardiol. 24, 1002–1006 (2017). https://doi.org/10.1007/s12350-017-0836-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-017-0836-9